High-Reflective Templated Cholesteric Liquid Crystal Filters
Abstract
:1. Introduction
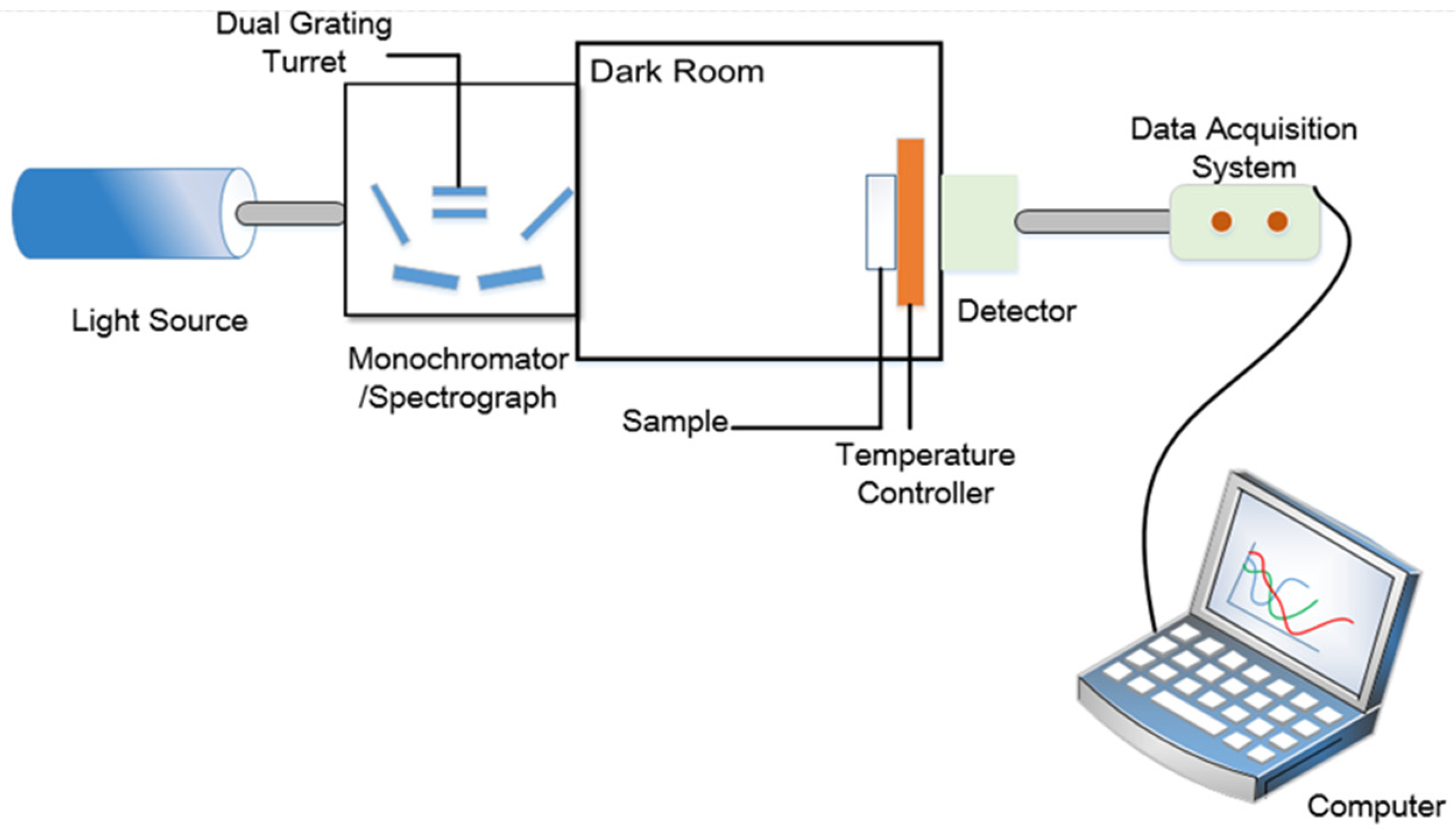
2. Experimental Design and Sample Fabrication
2.1. Experimental Design
2.2. Sample Fabrication
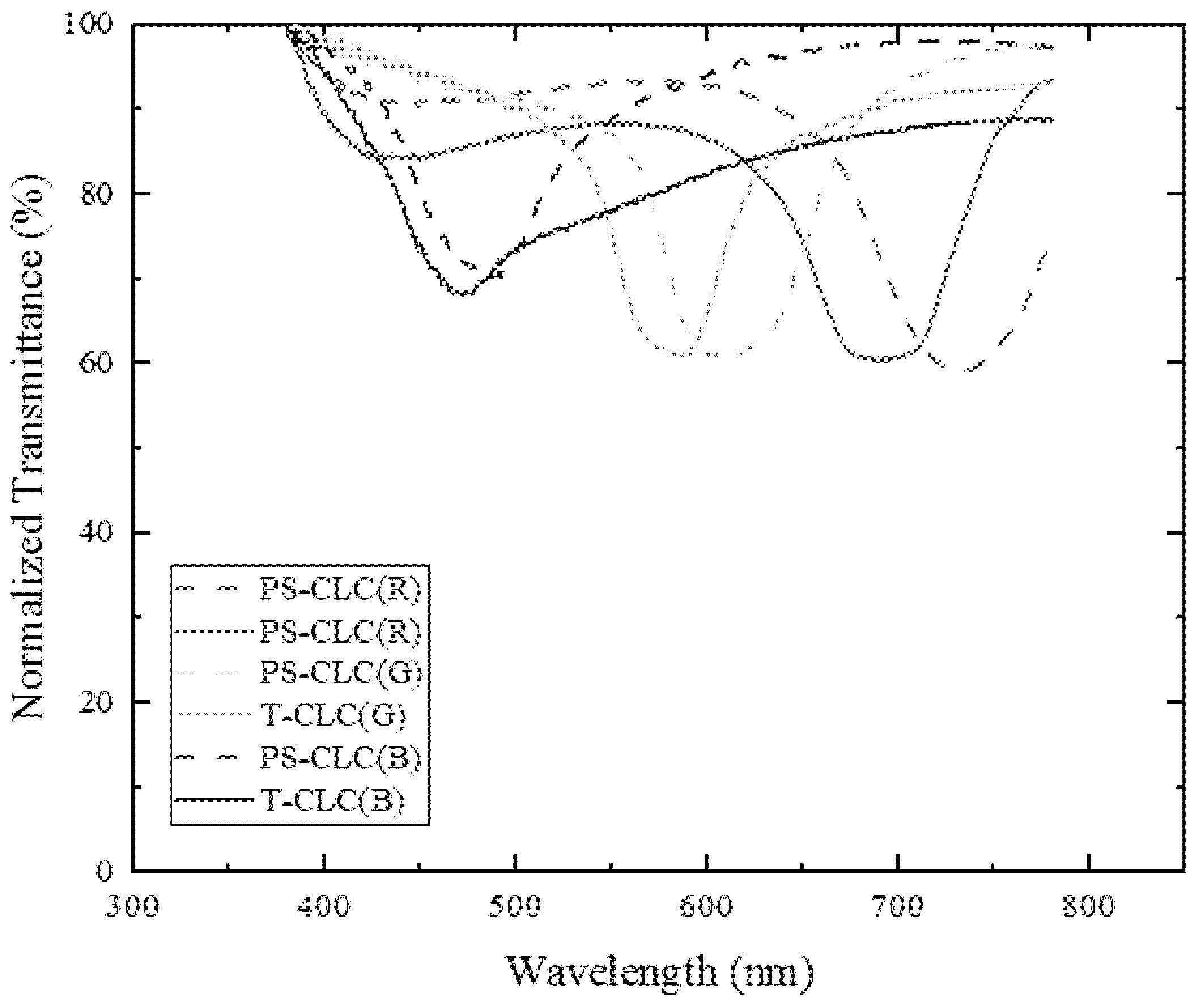
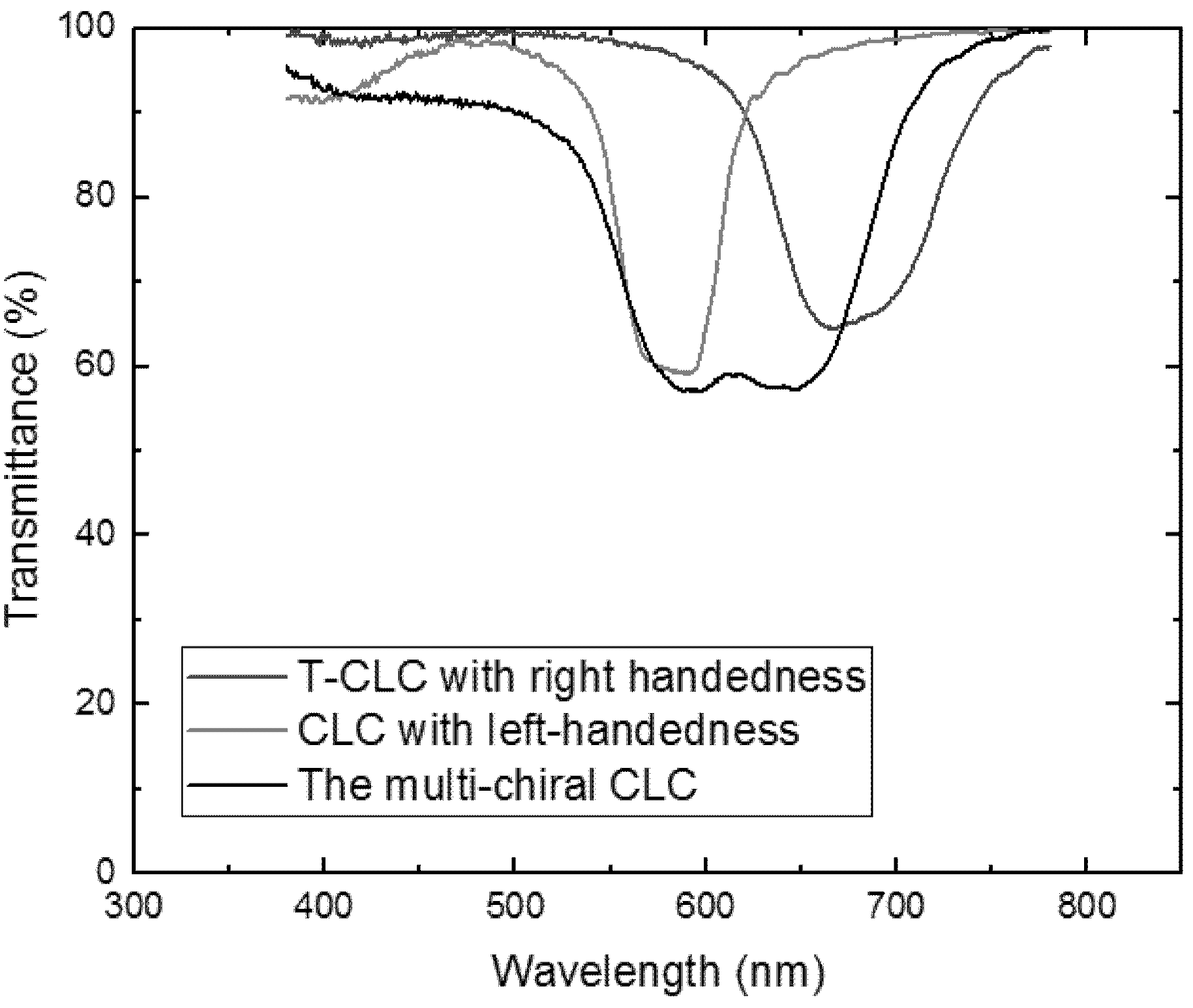
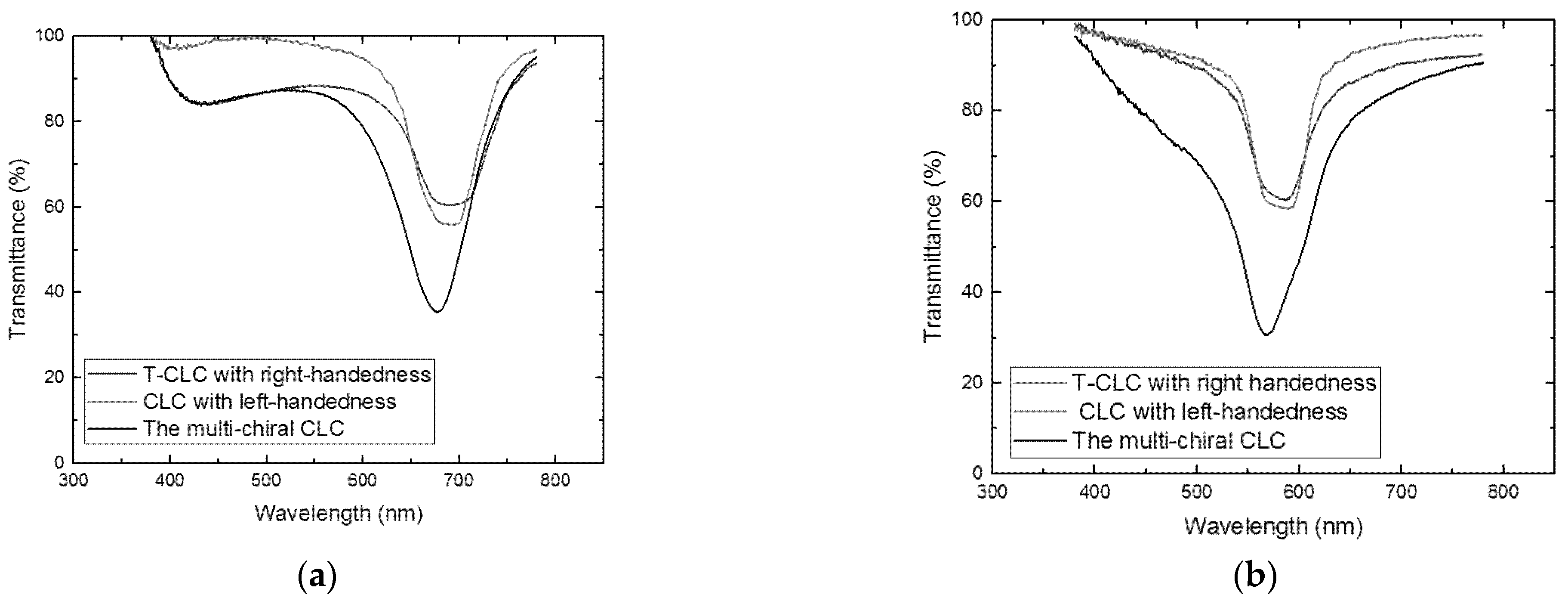
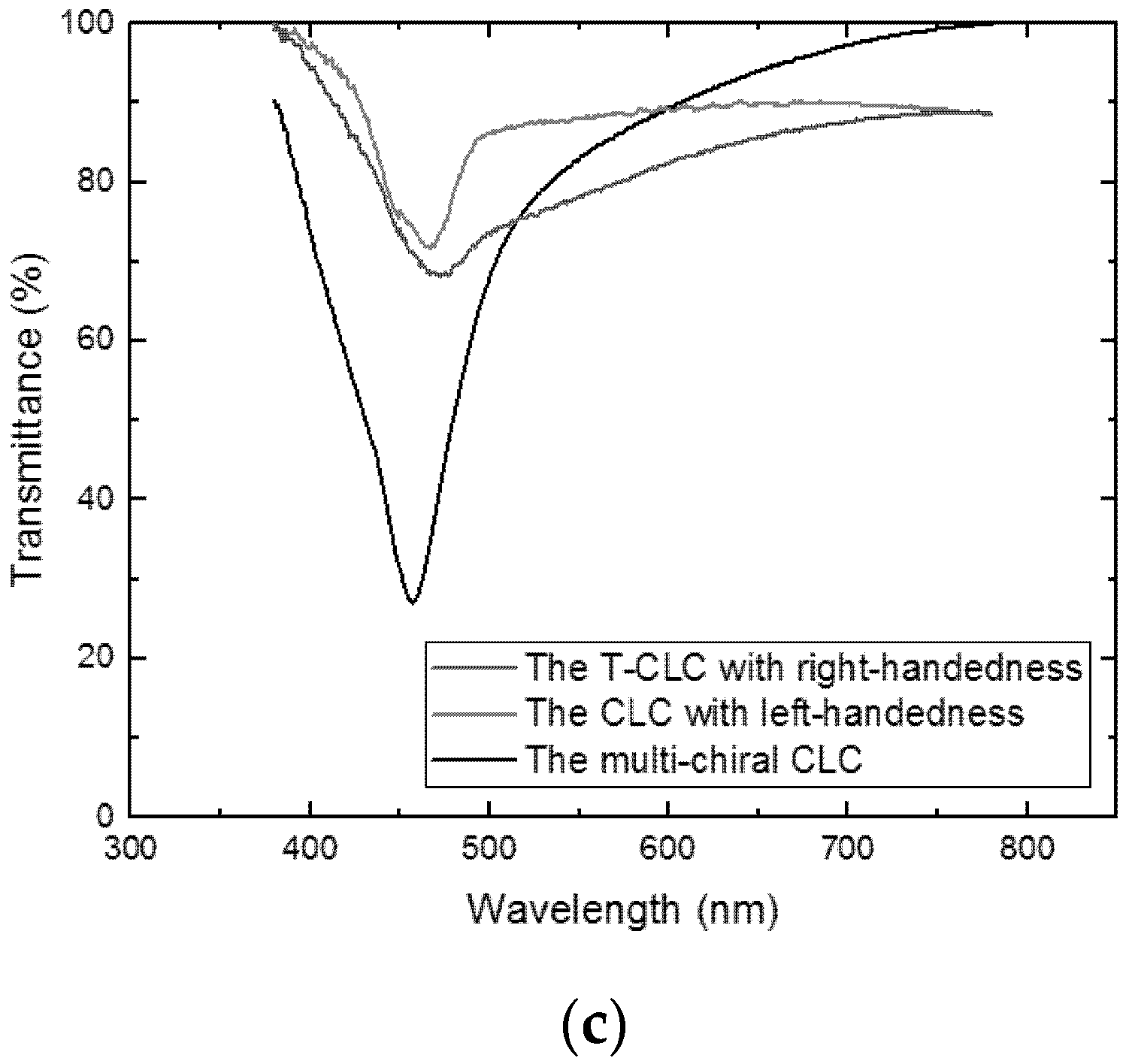
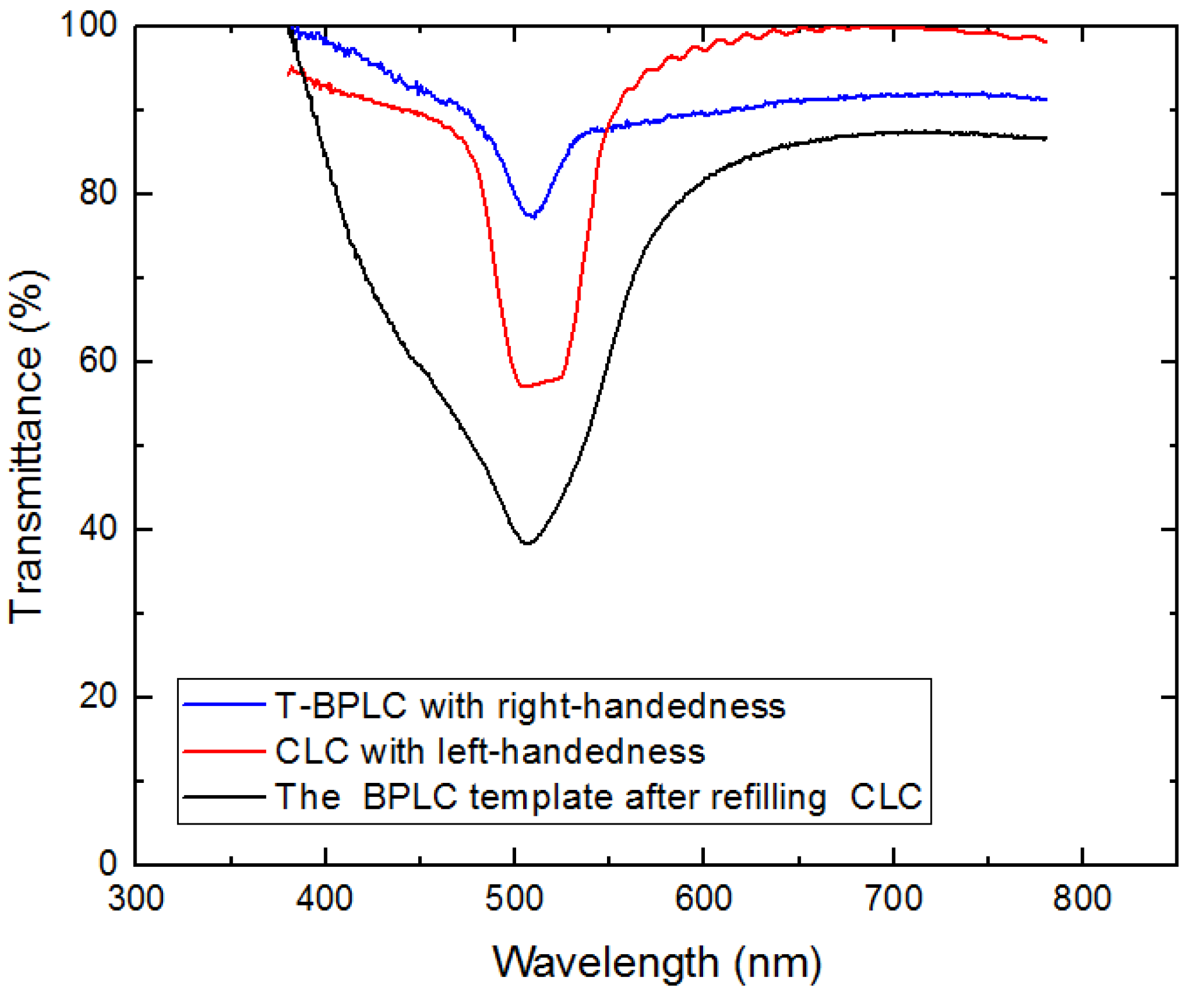
3. Measurement and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hikmet, R.A.M.; Kemperman, H. Switchable mirrors of chiral liquid crystal gels. Liquid Cryst. 1999, 26, 1645–1653. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S. Optical filter with tunable wavelength and bandwidth based on cholesteric liquid crystals. Opt. Lett. 2011, 36, 4563–4565. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan, A.H. Resonant interaction of light with a stack of alternating layers of a cholesteric liquid crystal and an isotropic medium. Phys. Rev. E 2015, 92, 062501. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lu, J. A Tunable NIR Filter with Sphere Phase Liquid Crystal. Crystals 2019, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, S. Widely tunable optical filter with variable bandwidth based on the thermal effect on cholesteric liquid crystals. Appl. Opt. 2012, 51, 5780–5784. [Google Scholar] [CrossRef]
- Atia, A.; Williams, A. Narrow-bandpass waveguide filters. IEEE Trans. Microw. Theory Tech. 1972, 20, 258–265. [Google Scholar] [CrossRef]
- Zha, S.; Zhang, H.; Sun, C.; Feng, Y.; Lu, J. Multi-Wavelength Filters of Templated Blue Phase Liquid Crystal. Crystals 2019, 9, 451. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, H.; Kim, S.; Choi, S.; Jang, W.; Kim, J.; Lee, J.H. Broadening the reflection bandwidth of polymer-stabilized cholesteric liquid crystal via a reactive surface coating layer. Appl. Opt. 2017, 56, 5731–5735. [Google Scholar] [CrossRef]
- Ogiwara, A.; Kakiuchida, H. Thermally tunable light filter composed of cholesteric liquid crystals with different temperature dependence. Sol. Energy Mater. Sol. Cells 2016, 157, 250–258. [Google Scholar] [CrossRef]
- Guo, J.; Cao, H.; Wei, J.; Zhang, D.; Liu, F.; Pan, G.; Zhao, D.; He, W.; Yang, H. Polymer stabilized liquid crystal films reflecting both right- and left-circularly polarized light. Appl. Phys. Lett. 2008, 93, 201901. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric liquid crystals with a broad light reflection band. Adv. Mater. 2012, 24, 6260–6276. [Google Scholar] [CrossRef]
- Mitov, M.; Dessaud, N. Going beyond the reflectance limit of cholesteric liquid crystals. Nat. Mater. 2006, 5, 361–364. [Google Scholar] [CrossRef]
- Ranjkesh, A.; Yoon, T.H. Thermal and electrical wavelength tuning of Bragg reflection with ultraviolet light absorbers in polymer-stabilized cholesteric liquid crystals. J. Mater. Chem. C 2018, 6, 12377–12385. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Luo, D. Optical thermal sensor based on cholesteric film refilled with mixture of toluene and ethanol. Opt. Express 2017, 25, 26349–26355. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.C.; Wu, G.W.; Timofeev, I.V.; Zyryanov, V.Y.; Lee, W. Electro-thermally tunable reflective colors in a self-organized cholesteric helical superstructure. Photon. Res. 2018, 6, 1094–1100. [Google Scholar] [CrossRef]
- Mcconney, M.E.; Tondiglia, V.P.; Hurtubise, J.M.; White, T.J.; Bunning, T.J. Photoinduced hyperreflective cholesteric liquid crystals enabled via surface initiated photopolymerization. Chem. Commun. 2011, 47, 505–507. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Yang, Z.H.; Shen, D.; Lee, W. Red, green, and blue reflections enabled in an electrically tunable helical superstructure. Adv. Opt. Mater. 2018, 6, 5050–5054. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, Z.; Liu, Y.; Zhang, X.; Zheng, Z.; Luo, D.; Sun, X. Electrically switchable, hyper-reflective blue phase liquid crystals films. Adv. Opt. Mater. 2018, 6, 1700891. [Google Scholar] [CrossRef]
- Mitov, M.; Dessaud, N. Cholesteric liquid crystalline materials reflecting more than 50% of unpolarized incident light intensity. Liq. Cryst. 2007, 34, 183–193. [Google Scholar] [CrossRef]
- Makow, D.M. Peak reflectance and color gamut of superimposed left- and right-handed cholesteric liquid crystals. Appl. Opt. 1980, 19, 1274–1277. [Google Scholar] [CrossRef] [Green Version]
- Mccony, M.E.; Tondiglia, V.P.; Hurtubise, J.M.; Natarajan, L.V.; White, T.J.; Bunning, T.J. Thermally induced, multicolored hyper-reflective cholesteric liquid. Adv. Mater. 2011, 23, 1453–1457. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.J.; Dai, H.T.; Zhang, X.H.; Luo, D.; Sun, X.W. Flexible cholesteric films with super-reflectivity and high stability based on a multi-layer helical structure. J. Mater. Chem. C 2017, 5, 10828–10833. [Google Scholar] [CrossRef]
- Caveney, S. Cuticle reflectivity and optical activity in scarab beetles: The role of uric acid. Proc. R. Soc. Lond. 1971, 178, 205–225. [Google Scholar]
- Wang, J.H.; Song, M.H.; Park, B.; Nishimura, S.; Toyooka, T.; Wu, J.W.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Electro-tunable optical diode based on photonic bandgap liquid-crystal heterojunctions. Nat. Mater. 2005, 4, 383. [Google Scholar] [CrossRef]
- Ranjkesh, A.; Choi, Y.; Huh, J.W.; Oh, S.W.; Yoon, T.H. Flexible, broadband, super-reflective infrared reflector based on cholesteric liquid crystal polymer. Sol. Energy Mater. Sol. Cells 2021, 230, 111137. [Google Scholar] [CrossRef]
- Guo, J.; Wu, H.; Chen, E.; Zhang, L.; He, W.; Yang, H.; Wei, J. Fabrication of multi-pitched photonic structure in cholesteric liquid crystals based on a polymer template with helical structure. J. Mater. Chem. 2010, 20, 4094–4102. [Google Scholar] [CrossRef]
- Guo, J.; Yang, H.; Li, R.; Ji, N.; Dong, X.; Wu, H.; Wei, J. Effect of Network Concentration on the Performance of Polymer-Stabilized Cholesteric Liquid Crystals with a Double-Handed Circularly Polarized Light Reflection Band. J. Phys. Chem. 2009, 113, 16538–16543. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, T.; Lu, J. Template Effect of Multi-Phase Liquid Crystals. Crystals 2021, 11, 602. [Google Scholar] [CrossRef]

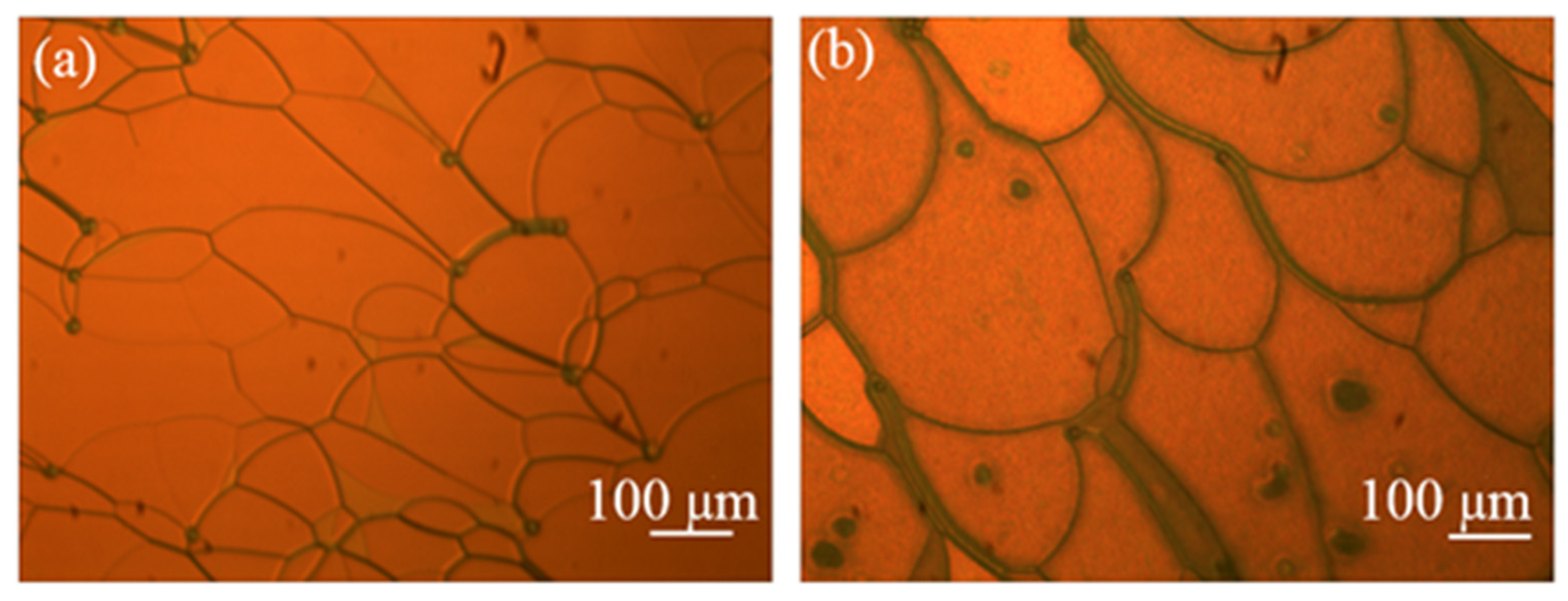
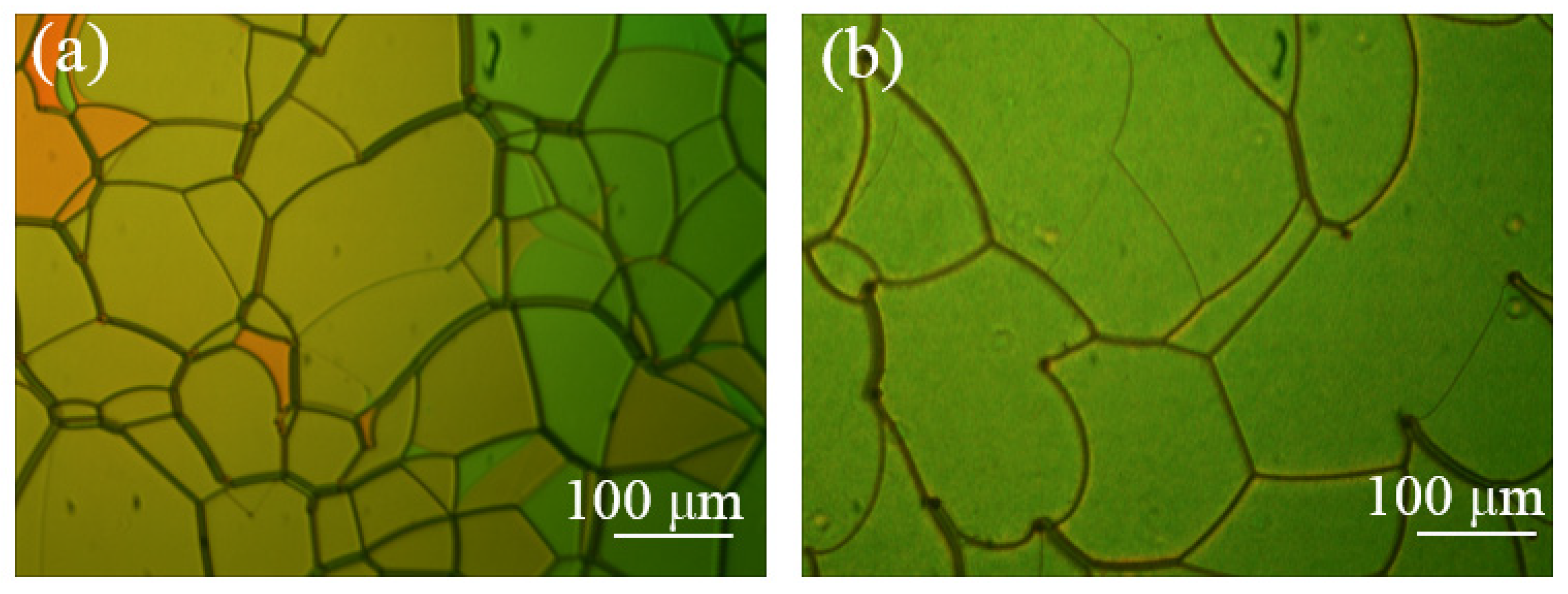
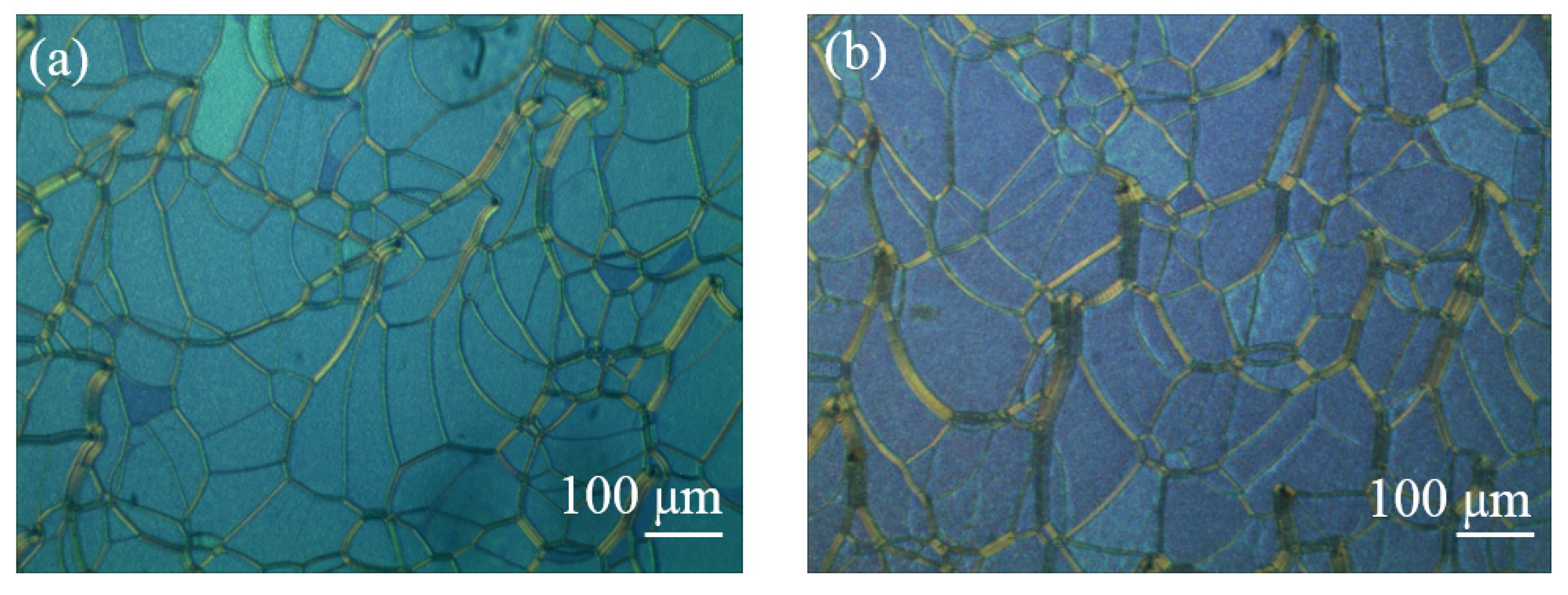
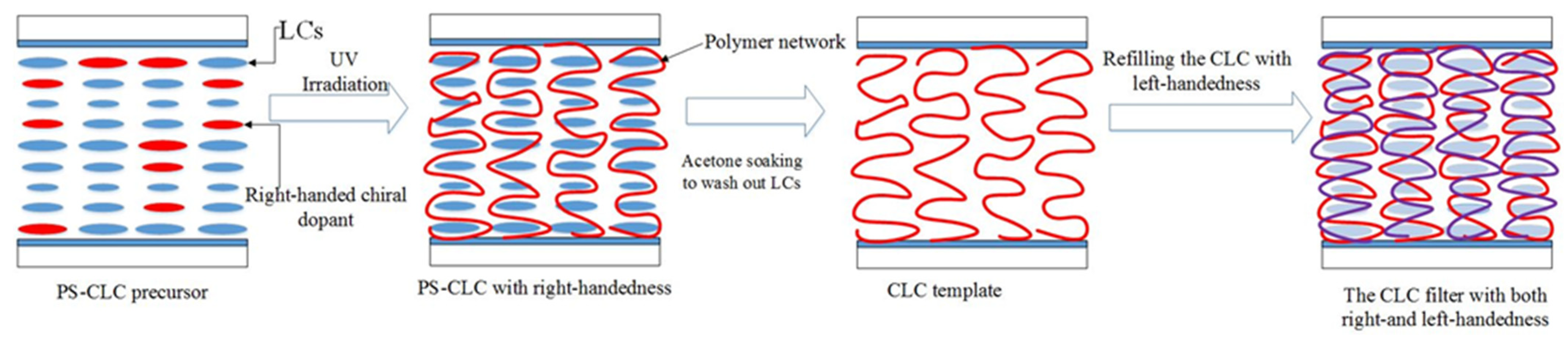

| Color | Sample | BPH006 [wt%] | R5011 [wt%] | S811 [wt%] | TMPTA [wt%] | C3M [wt%] | IRG184 [wt%] |
|---|---|---|---|---|---|---|---|
| Red | PS-CLC | 86 | 1.45 | 5.00 | 7.45 | 0.10 | |
| CLC | 81.4 | 16.8 | |||||
| Green | PS-CLC | 86.13 | 1.8 | 5.13 | 6.84 | 0.10 | |
| CLC | 79.6 | 20.4 | |||||
| Blue | PS-CLC | 85.63 | 2.2 | 5.43 | 6.64 | 0.10 | |
| CLC | 74.6 | 25.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Luo, Y.; Lu, J. High-Reflective Templated Cholesteric Liquid Crystal Filters. Molecules 2021, 26, 6889. https://doi.org/10.3390/molecules26226889
Gao Y, Luo Y, Lu J. High-Reflective Templated Cholesteric Liquid Crystal Filters. Molecules. 2021; 26(22):6889. https://doi.org/10.3390/molecules26226889
Chicago/Turabian StyleGao, Yao, Yuxiang Luo, and Jiangang Lu. 2021. "High-Reflective Templated Cholesteric Liquid Crystal Filters" Molecules 26, no. 22: 6889. https://doi.org/10.3390/molecules26226889
APA StyleGao, Y., Luo, Y., & Lu, J. (2021). High-Reflective Templated Cholesteric Liquid Crystal Filters. Molecules, 26(22), 6889. https://doi.org/10.3390/molecules26226889







