The Impact of an Anchoring Layer on the Formation of Tethered Bilayer Lipid Membranes on Silver Substrates
Abstract
:1. Introduction
2. Results and Discussion
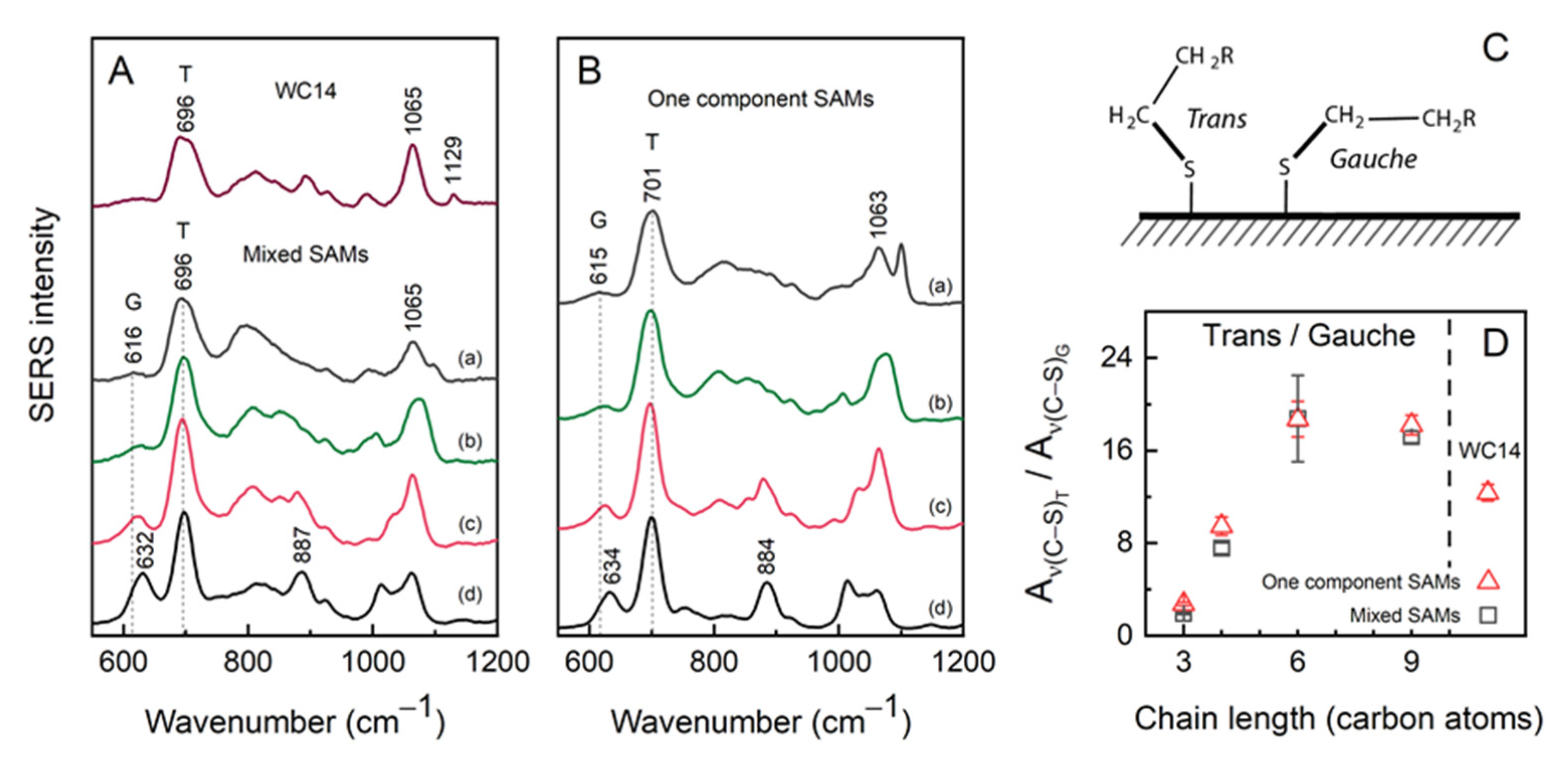
2.1. Spectroscopic Characterisations of Anchoring SAMs
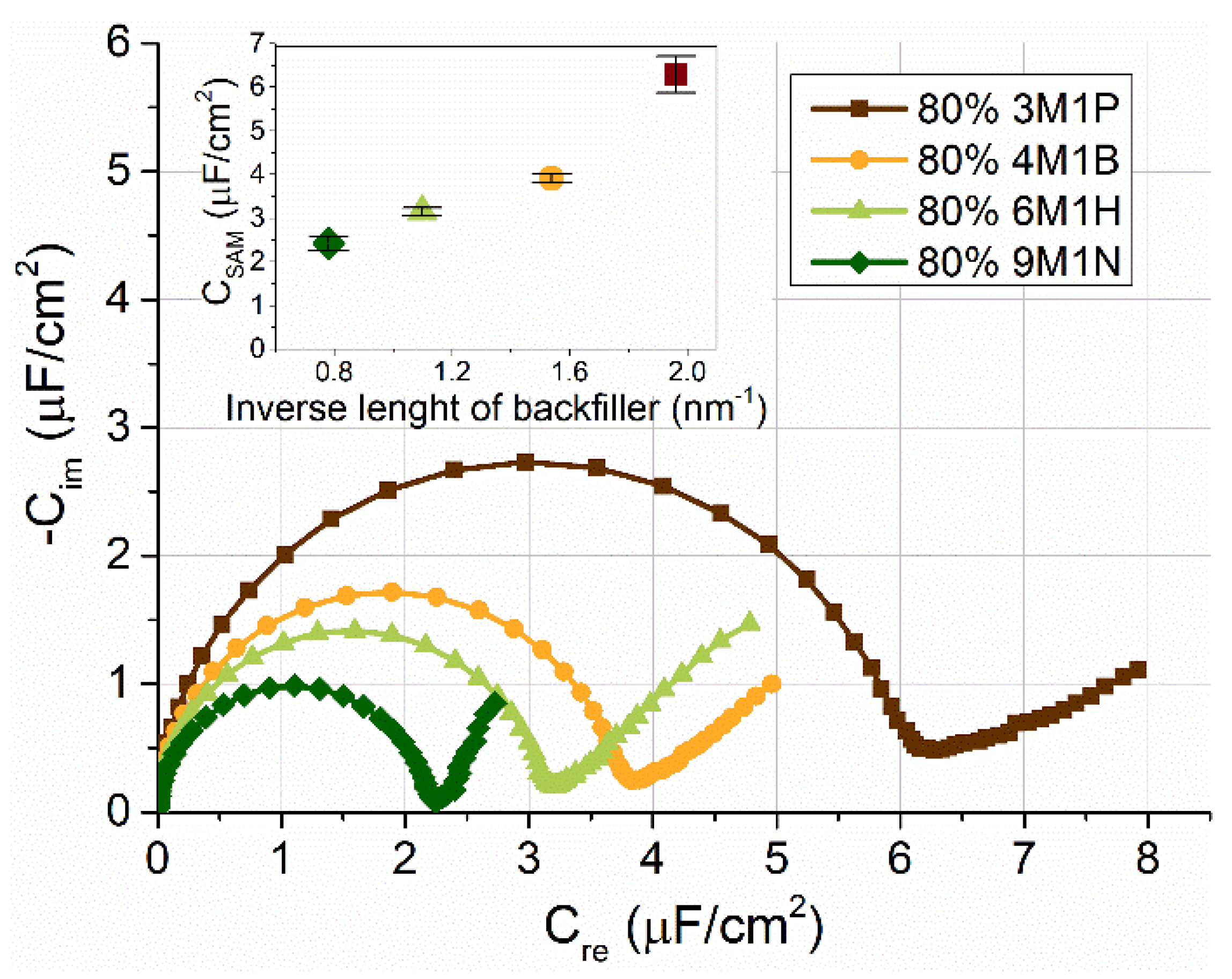
2.2. EIS Analysis of Mixed SAM‘s
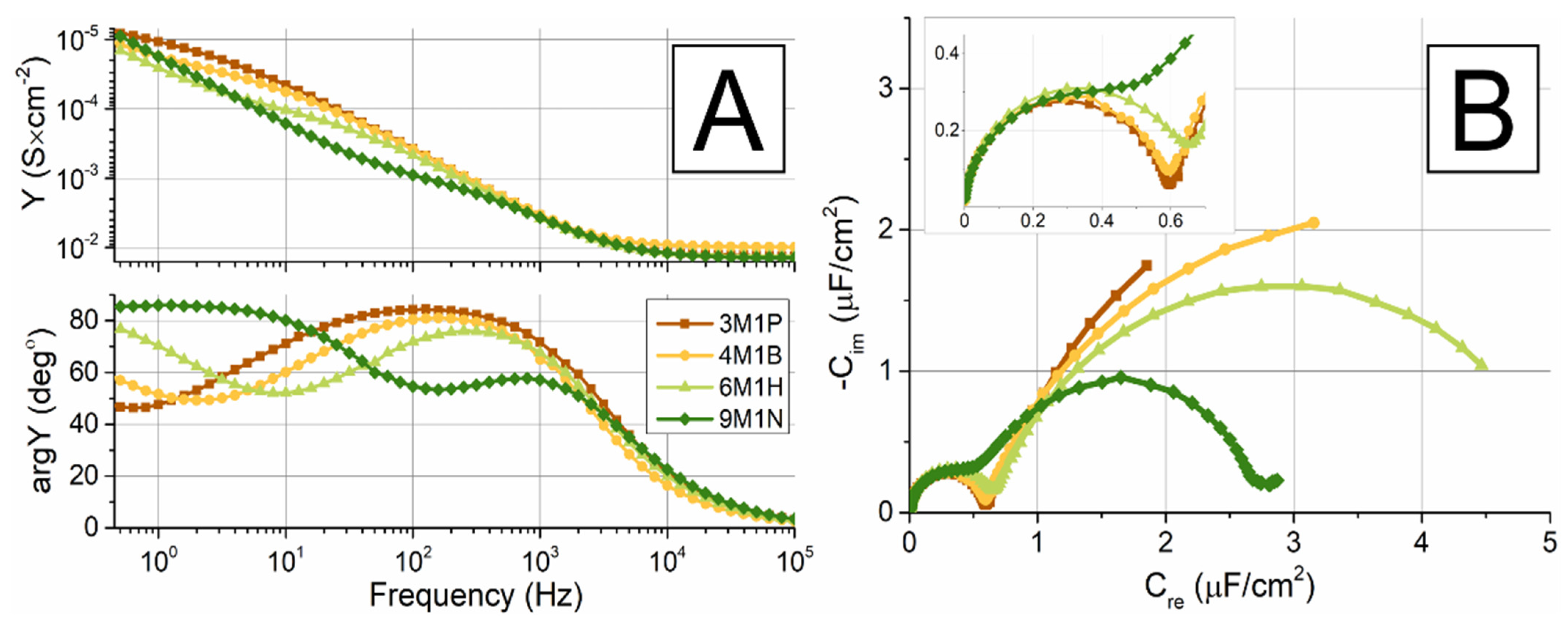
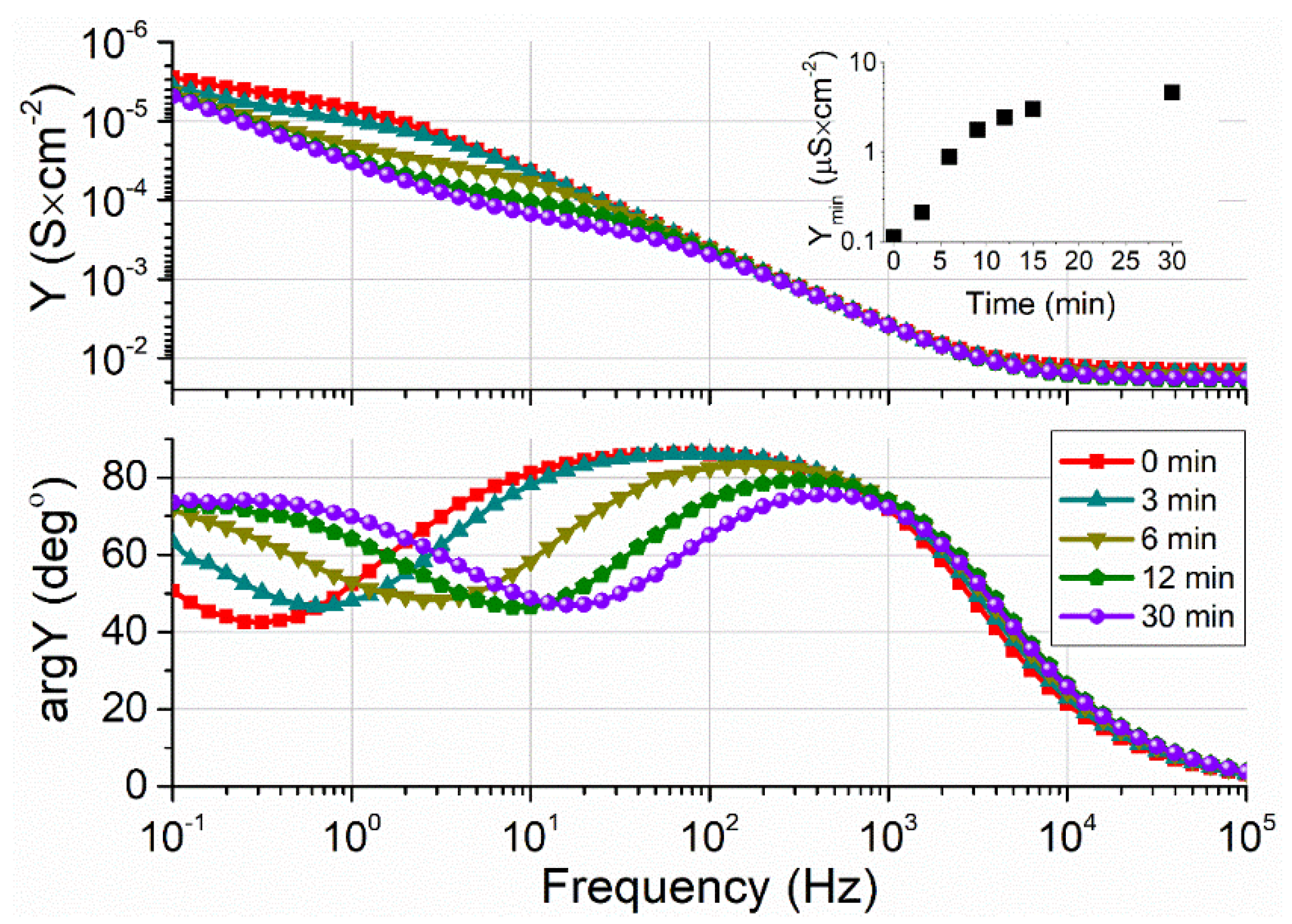
2.3. EIS Analysis of tBLMs
2.4. A Biological Relevance of tBLM on Silver Substrates Containing Different Short-Chain Backfiller Molecules
3. Materials and Methods
3.1. Fabrication of Silver SERS Sensors
3.2. Deposition of Silver Film
3.3. Self-Assembled Monolayers Preparation
3.4. Tethered Bilayer Lipid Membrane Preparation
3.5. Electrochemical Reductive Desorption
3.6. SERS Measurements
3.7. Reflection-Adsorption Infrared Spectroscopy (RAIRS)
3.8. EIS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chan, Y.-H.M.; Boxer, S.G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penkauskas, T.; Preta, G. Biological applications of tethered bilayer lipid membranes. Biochimie 2019, 157, 131–141. [Google Scholar] [CrossRef]
- Valincius, G.; Meškauskas, T.; Ivanauskas, F. Electrochemical Impedance Spectroscopy of Tethered Bilayer Membranes. Langmuir 2012, 28, 977–990. [Google Scholar] [CrossRef]
- Budvytyte, R.; Valincius, G.; Niaura, G.; Voiciuk, V.; Mickevicius, M.; Chapman, H.; Goh, H.-Z.; Shekhar, P.; Heinrich, F.; Shenoy, S.; et al. Structure and Properties of Tethered Bilayer Lipid Membranes with Unsaturated Anchor Molecules. Langmuir 2013, 29, 8645–8656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakovska, B.; Ragaliauskas, T.; Mickevicius, M.; Jankunec, M.; Niaura, G.; Vanderah, D.J.; Valincius, G. Structure and Function of the Membrane Anchoring Self-Assembled Monolayers. Langmuir 2015, 31, 846–857. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Robertson, J.W.F.; Li, J.; Kärcher, I.; Schiller, S.M.; Knoll, W.; Naumann, R. Tethered Bilayer Lipid Membranes Based on Monolayers of Thiolipids Mixed with a Complementary Dilution Molecule. 1. Incorporation of Channel Peptides. Langmuir 2005, 21, 11666–11672. [Google Scholar] [CrossRef] [PubMed]
- McGillivray, D.J.; Valincius, G.; Vanderah, D.J.; Febo-Ayala, W.; Woodward, J.T.; Heinrich, F.; Kasianowicz, J.J.; Lösche, M. Molecular-scale structural and functional characterization of sparsely tethered bilayer lipid membranes. Biointerphases 2007, 2, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Lang, H.; Duschl, C.; Vogel, H. A new class of thiolipids for the attachment of lipid bilayers on gold surfaces. Langmuir 1994, 10, 197–210. [Google Scholar] [CrossRef]
- Vockenroth, I.K.; Atanasova, P.P.; Jenkins, A.T.A.; Köper, I. Incorporation of α-Hemolysin in Different Tethered Bilayer Lipid Membrane Architectures. Langmuir 2008, 24, 496–502. [Google Scholar] [CrossRef]
- Junghans, A.; Köper, I. Structural analysis of tethered bilayer lipid membranes. Langmuir 2010, 26, 11035–11040. [Google Scholar] [CrossRef]
- McGillivray, D.J.; Valincius, G.; Heinrich, F.; Robertson, J.W.F.; Vanderah, D.J.; Febo-Ayala, W.; Ignatjev, I.; Lösche, M.; Kasianowicz, J.J. Structure of Functional Staphylococcus aureus α-Hemolysin Channels in Tethered Bilayer Lipid Membranes. Biophys. J. 2009, 96, 1547–1553. [Google Scholar] [CrossRef] [Green Version]
- Schiller, S.M.; Naumann, R.; Lovejoy, K.; Kunz, H.; Knoll, W. Archaea Analogue Thiolipids for Tethered Bilayer Lipid Membranes on Ultrasmooth Gold Surfaces. Angew. Chemie Int. Ed. 2003, 42, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Talaikis, M.; Valincius, G.; Niaura, G. Potential-Induced Structural Alterations in the Tethered Bilayer Lipid Membrane-Anchoring Monolayers Revealed by Electrochemical Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2020, 124, 19033–19045. [Google Scholar] [CrossRef]
- Cheng, Y.; Boden, N.; Bushby, R.J.; Clarkson, S.; Evans, S.D.; Knowles, P.F.; Marsh, A.; Miles, R.E. Attenuated Total Reflection Fourier Transform Infrared Spectroscopic Characterization of Fluid Lipid Bilayers Tethered to Solid Supports. Langmuir 1998, 14, 839–844. [Google Scholar] [CrossRef]
- Naumann, R.; Schiller, S.M.; Giess, F.; Grohe, B.; Hartman, K.B.; Kärcher, I.; Köper, I.; Lübben, J.; Vasilev, K.; Knoll, W. Tethered Lipid Bilayers on Ultraflat Gold Surfaces. Langmuir 2003, 19, 5435–5443. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Intact Vesicle Adsorption and Supported Biomembrane Formation from Vesicles in Solution: Influence of Surface Chemistry, Vesicle Size, Temperature, and Osmotic Pressure. Langmuir 2003, 19, 1681–1691. [Google Scholar] [CrossRef]
- Han, X.; Pradeep, S.N.D.; Critchley, K.; Sheikh, K.; Bushby, R.J.; Evans, S.D. Supported Bilayer Lipid Membrane Arrays on Photopatterned Self-Assembled Monolayers. Chem. A Eur. J. 2007, 13, 7957–7964. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Ruželė, Ž.; Malysheva, L.; Onipko, A.; Gutés, A.; Björefors, F.; Valiokas, R.; Liedberg, B. Long-Chain Alkylthiol Assemblies Containing Buried In-Plane Stabilizing Architectures. Langmuir 2009, 25, 13959–13971. [Google Scholar] [CrossRef]
- Atanasov, V.; Atanasova, P.P.; Vockenroth, I.K.; Knorr, N.; Köper, I. A molecular toolkit for highly insulating tethered bilayer lipid membranes on various substrates. Bioconjug. Chem. 2006, 17, 631–637. [Google Scholar] [CrossRef]
- Lee, H.-H.; Gavutis, M.; Ruželė, Ž.; Valiokas, R.; Liedberg, B. Mixed Self-Assembled Monolayers with Terminal Deuterated Anchors: Characterization and Probing of Model Lipid Membrane Formation. J. Phys. Chem. B 2018, 122, 8201–8210. [Google Scholar] [CrossRef]
- Talaikis, M.; Eicher-Lorka, O.; Valincius, G.; Niaura, G. Water-Induced Structural Changes in the Membrane-Anchoring Monolayers Revealed by Isotope-Edited SERS. J. Phys. Chem. C 2016, 120, 22489–22499. [Google Scholar] [CrossRef]
- Valincius, G.; Mickevicius, M. Tethered Phospholipid Bilayer Membranes. In Advances in Planar Lipid Bilayers and Liposomes; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 21, pp. 27–61. [Google Scholar]
- Krishna, G.; Schulte, J.; Cornell, B.A.; Pace, R.J.; Osman, P.D. Tethered Bilayer Membranes Containing Ionic Reservoirs: Selectivity and Conductance. Langmuir 2003, 19, 2294–2305. [Google Scholar] [CrossRef]
- Aleknavičienė, I.; Jankunec, M.; Penkauskas, T.; Valincius, G. Electrochemical properties of tethered lipid bilayers on thin film silver substrates. Electrochim. Acta 2021, 389, 138726. [Google Scholar] [CrossRef]
- Valincius, G.; Heinrich, F.; Budvytyte, R.; Vanderah, D.J.; McGillivray, D.J.; Sokolov, Y.; Hall, J.E.; Lösche, M. Soluble Amyloid β-Oligomers Affect Dielectric Membrane Properties by Bilayer Insertion and Domain Formation: Implications for Cell Toxicity. Biophys. J. 2008, 95, 4845–4861. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-F.; Lee, Y.-L. Adsorption characteristics of OH-terminated alkanethiol and arenethiol on Au(111) surfaces. Nanoscale 2012, 4, 2093. [Google Scholar] [CrossRef]
- Imabayashi, S.-i.; Iida, M.; Hobara, D.; Feng, Z.Q.; Niki, K.; Kakiuchi, T. Reductive desorption of carboxylic-acid-terminated alkanethiol monolayers from Au(111) surfaces. J. Electroanal. Chem. 1997, 428, 33–38. [Google Scholar] [CrossRef]
- Harder, P.; Grunze, M.; Dahint, R.; Whitesides, G.M.; Laibinis, P.E. Molecular Conformation in Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers on Gold and Silver Surfaces Determines Their Ability To Resist Protein Adsorption. J. Phys. Chem. B 1998, 102, 426–436. [Google Scholar] [CrossRef]
- Leitch, J.; Kunze, J.; Goddard, J.D.; Schwan, A.L.; Faragher, R.J.; Naumann, R.; Knoll, W.; Dutcher, J.R.; Lipkowski, J. In Situ PM-IRRAS Studies of an Archaea Analogue Thiolipid Assembled on a Au(111) Electrode Surface. Langmuir 2009, 25, 10354–10363. [Google Scholar] [CrossRef]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors that Determine the Protein Resistance of Oligoether Self-Assembled Monolayers − Internal Hydrophilicity, Terminal Hydrophilicity, and Lateral Packing Density. J. Am. Chem. Soc. 2003, 125, 9359–9366. [Google Scholar] [CrossRef]
- Vanderah, D.J.; Arsenault, J.; La, H.; Gates, R.S.; Silin, V.; Meuse, C.W.; Valincius, G. Structural Variations and Ordering Conditions for the Self-Assembled Monolayers of HS(CH2CH2O)3-6CH3. Langmuir 2003, 19, 3752–3756. [Google Scholar] [CrossRef]
- Nelson, P.N. Chain Length and Thermal Sensitivity of the Infrared Spectra of a Homologous Series of Anhydrous Silver(I) n-Alkanoates. Int. J. Spectrosc. 2016, 2016, 3068430. [Google Scholar] [CrossRef] [Green Version]
- Glazier, S.A.; Vanderah, D.J.; Plant, A.L.; Bayley, H.; Valincius, G.; Kasianowicz, J.J. Reconstitution of the Pore-Forming Toxin α-Hemolysin in Phospholipid/18-Octadecyl-1-thiahexa(ethylene oxide) and Phospholipid/n-Octadecanethiol Supported Bilayer Membranes. Langmuir 2000, 16, 10428–10435. [Google Scholar] [CrossRef]
- Raila, T.; Ambrulevičius, F.; Penkauskas, T.; Jankunec, M.; Meškauskas, T.; Vanderah, D.J.; Valincius, G. Clusters of protein pores in phospholipid bilayer membranes can be identified and characterized by electrochemical impedance spectroscopy. Electrochim. Acta 2020, 364, 137179. [Google Scholar] [CrossRef]
- Raila, T.; Penkauskas, T.; Jankunec, M.; Dreižas, G.; Meškauskas, T.; Valincius, G. Electrochemical impedance of randomly distributed defects in tethered phospholipid bilayers: Finite element analysis. Electrochim. Acta 2019, 299, 863–874. [Google Scholar] [CrossRef]
- Tumenas, S.; Ragaliauskas, T.; Penkauskas, T.; Valanciute, A.; Ambrulevicius, F.; Valincius, G. Solvent effects on composition and structure of thiolipid molecular anchors for tethering phospholipid bilayers. Appl. Surf. Sci. 2020, 509, 145268. [Google Scholar] [CrossRef]
- Budvytyte, R.; Pleckaityte, M.; Zvirbliene, A.; Vanderah, D.J.; Valincius, G. Reconstitution of Cholesterol-Dependent Vaginolysin into Tethered Phospholipid Bilayers: Implications for Bioanalysis. PLoS ONE 2013, 8, e82536. [Google Scholar] [CrossRef]
- Aleknavičienė, I.; Pabrėža, E.; Talaikis, M.; Jankunec, M.; Račiukaitis, G. Low-cost SERS substrate featuring laser-ablated amorphous nanostructure. Appl. Surf. Sci. 2022, 571, 151248. [Google Scholar] [CrossRef]
- Ragaliauskas, T.; Mickevicius, M.; Rakovska, B.; Penkauskas, T.; Vanderah, D.J.; Heinrich, F.; Valincius, G. Fast formation of low-defect-density tethered bilayers by fusion of multilamellar vesicles. Biochim. Biophys. Acta Biomembr. 2017, 1859, 669–678. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleknavičienė, I.; Talaikis, M.; Budvytyte, R.; Valincius, G. The Impact of an Anchoring Layer on the Formation of Tethered Bilayer Lipid Membranes on Silver Substrates. Molecules 2021, 26, 6878. https://doi.org/10.3390/molecules26226878
Aleknavičienė I, Talaikis M, Budvytyte R, Valincius G. The Impact of an Anchoring Layer on the Formation of Tethered Bilayer Lipid Membranes on Silver Substrates. Molecules. 2021; 26(22):6878. https://doi.org/10.3390/molecules26226878
Chicago/Turabian StyleAleknavičienė, Indrė, Martynas Talaikis, Rima Budvytyte, and Gintaras Valincius. 2021. "The Impact of an Anchoring Layer on the Formation of Tethered Bilayer Lipid Membranes on Silver Substrates" Molecules 26, no. 22: 6878. https://doi.org/10.3390/molecules26226878
APA StyleAleknavičienė, I., Talaikis, M., Budvytyte, R., & Valincius, G. (2021). The Impact of an Anchoring Layer on the Formation of Tethered Bilayer Lipid Membranes on Silver Substrates. Molecules, 26(22), 6878. https://doi.org/10.3390/molecules26226878







