Preparation and Application of a Hydrochar-Based Palladium Nanocatalyst for the Reduction of Nitroarenes
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials and Methods
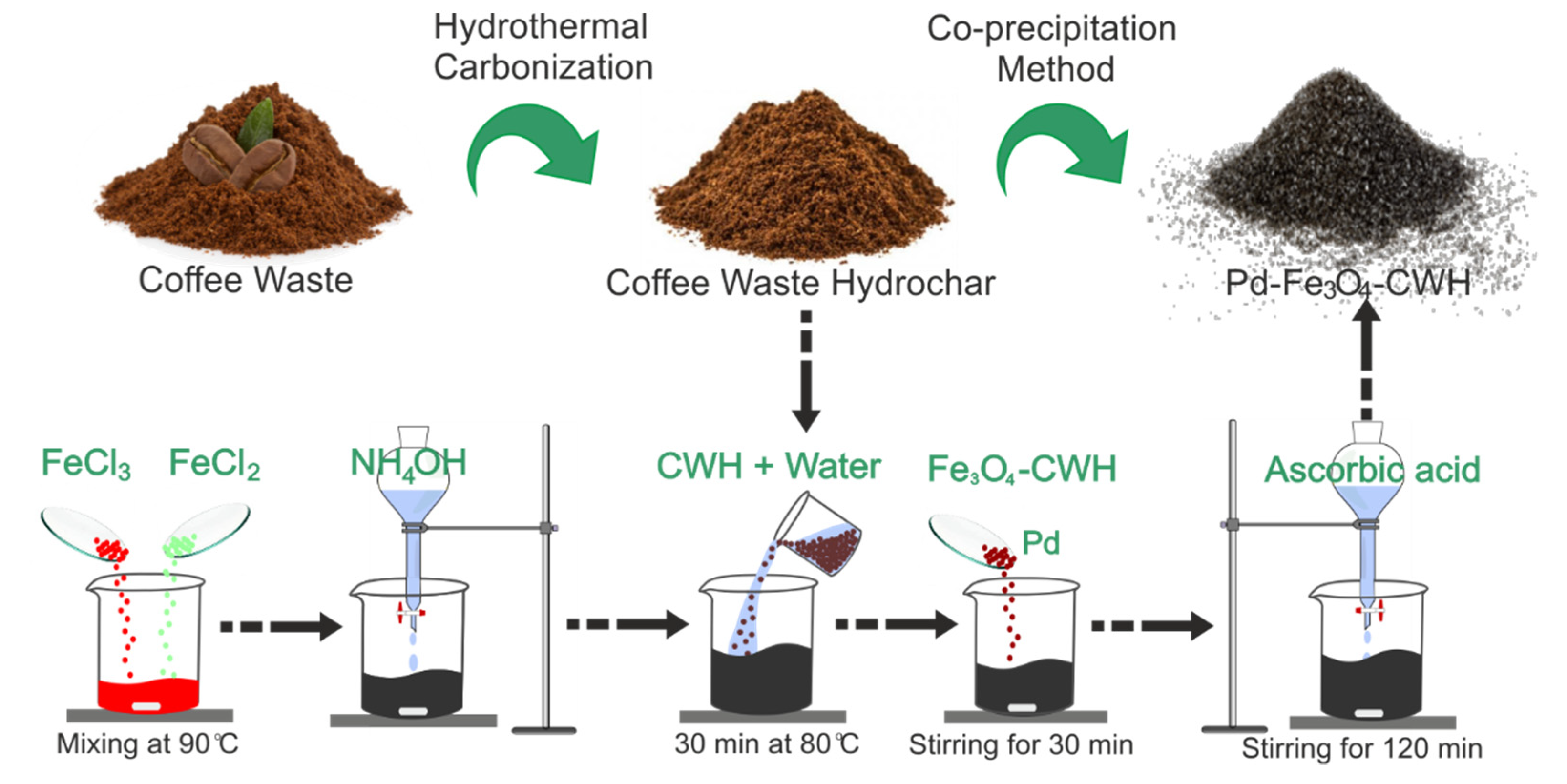
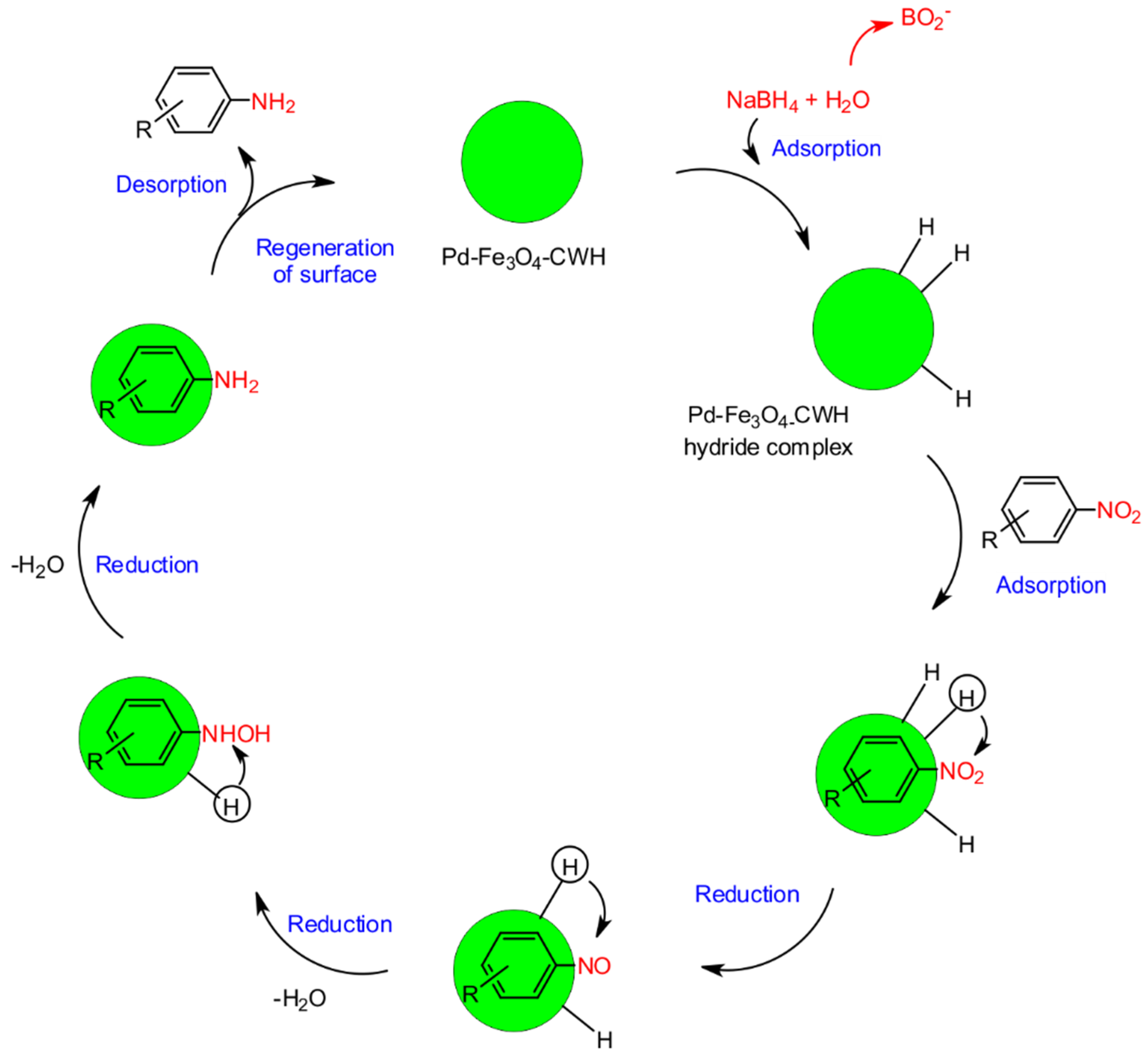
2.2. Preparation and Characterization of Pd-Fe3O4-CWH Nanocatalyst
2.3. Reduction in Nitro Compounds to Anilines
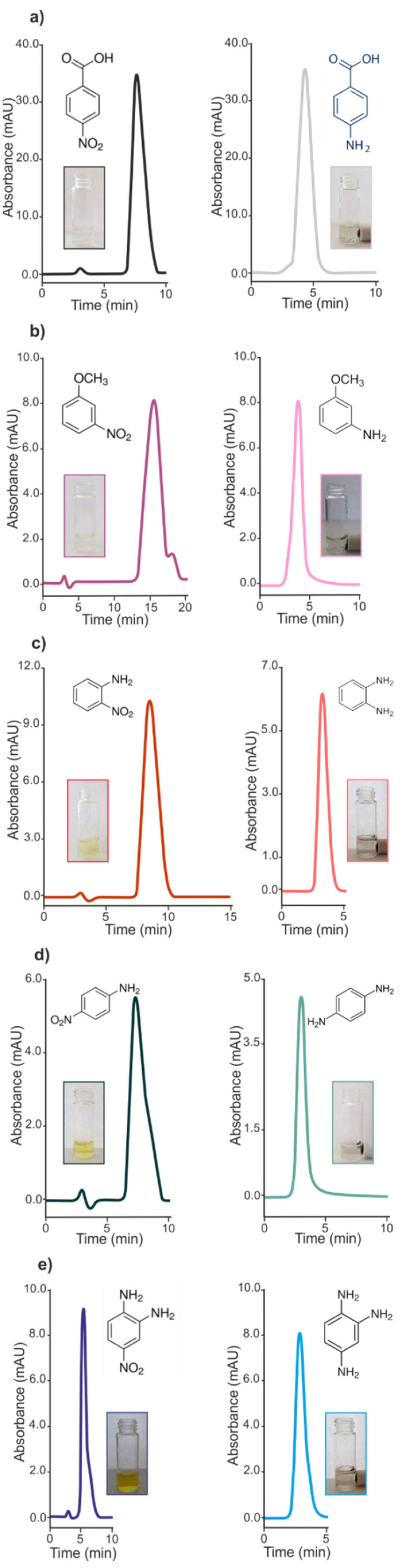
2.4. HPLC Analysis
3. Results and Discussion
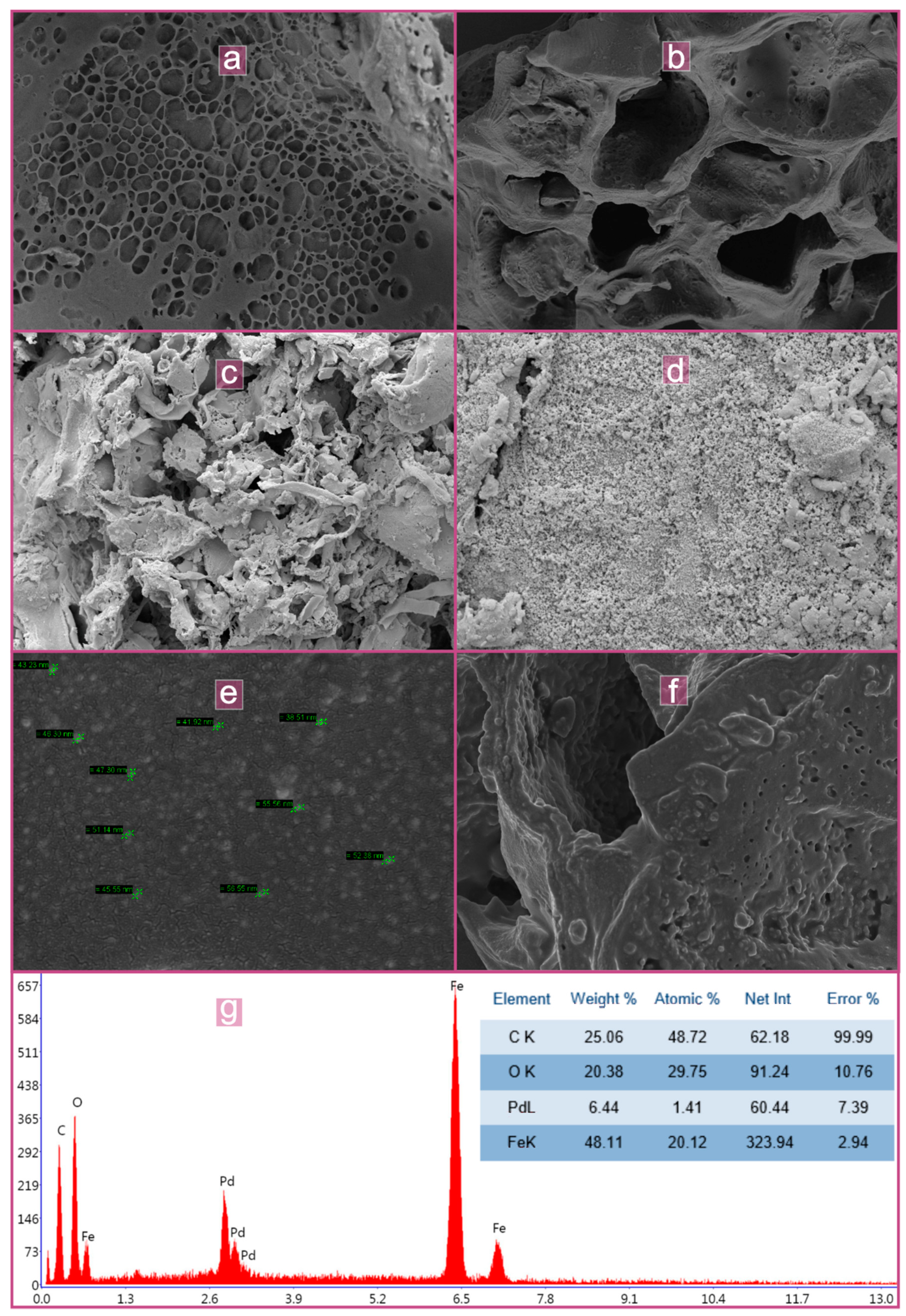
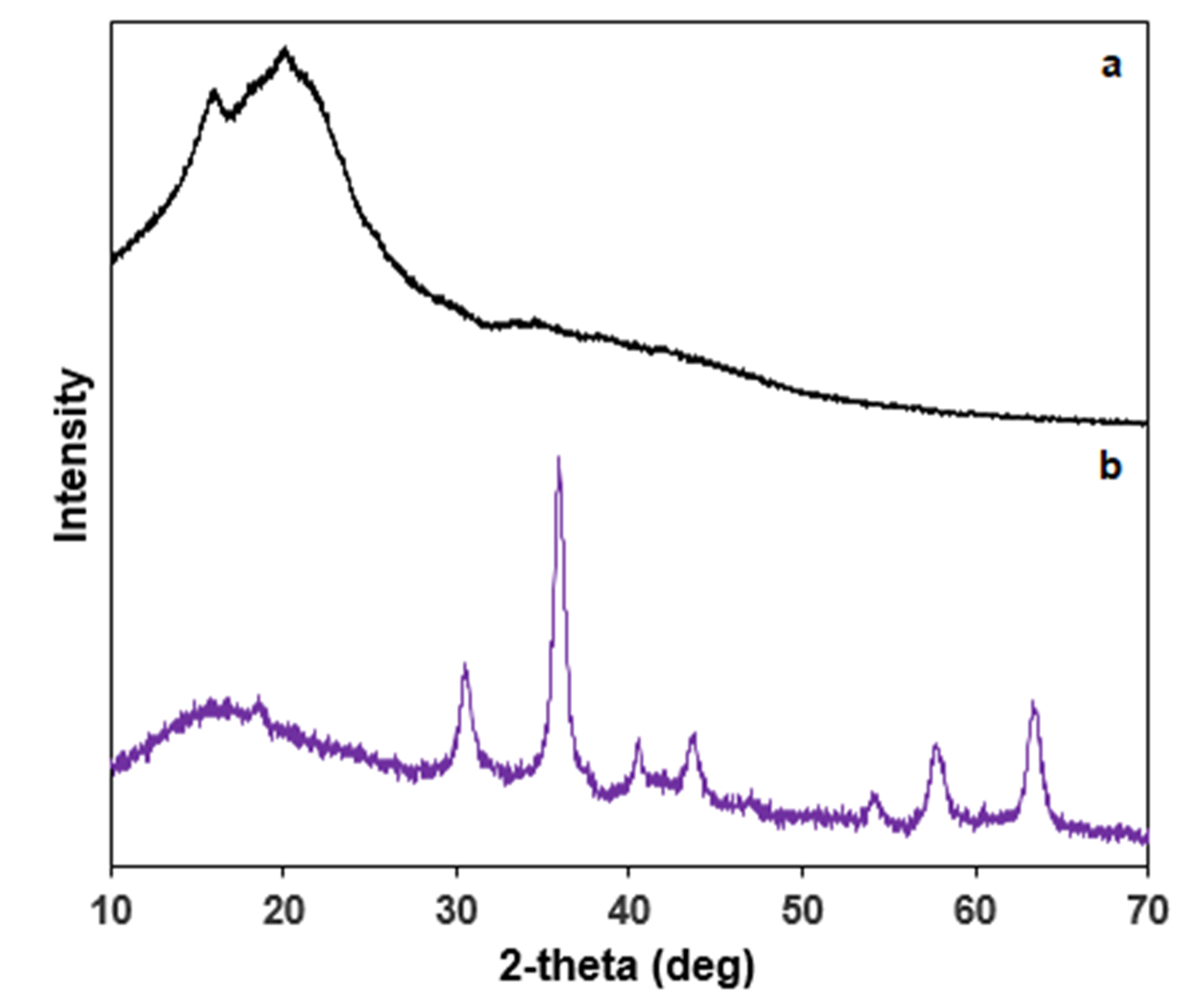
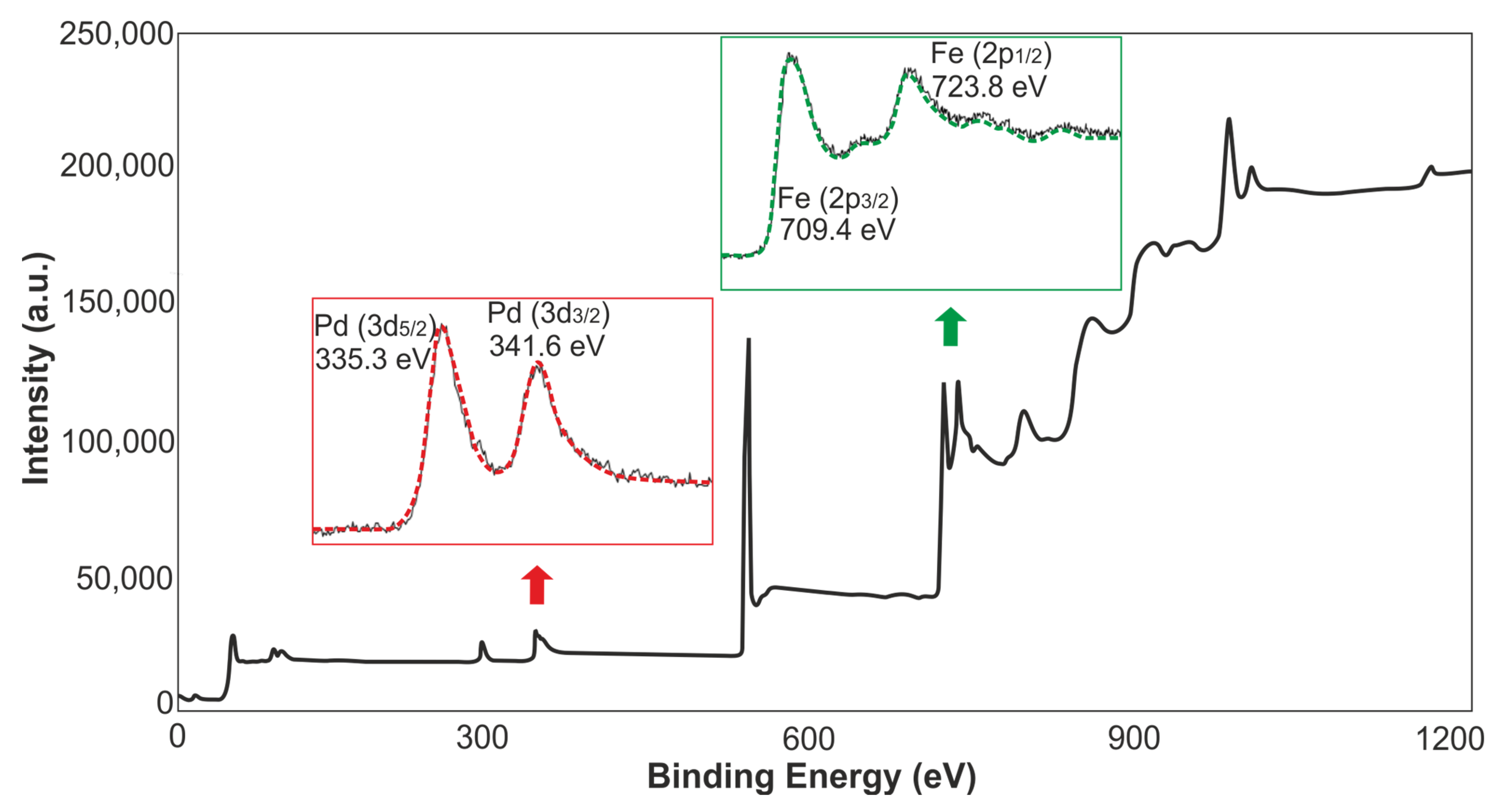
3.1. Characterization
3.2. Catalytic Activity Test
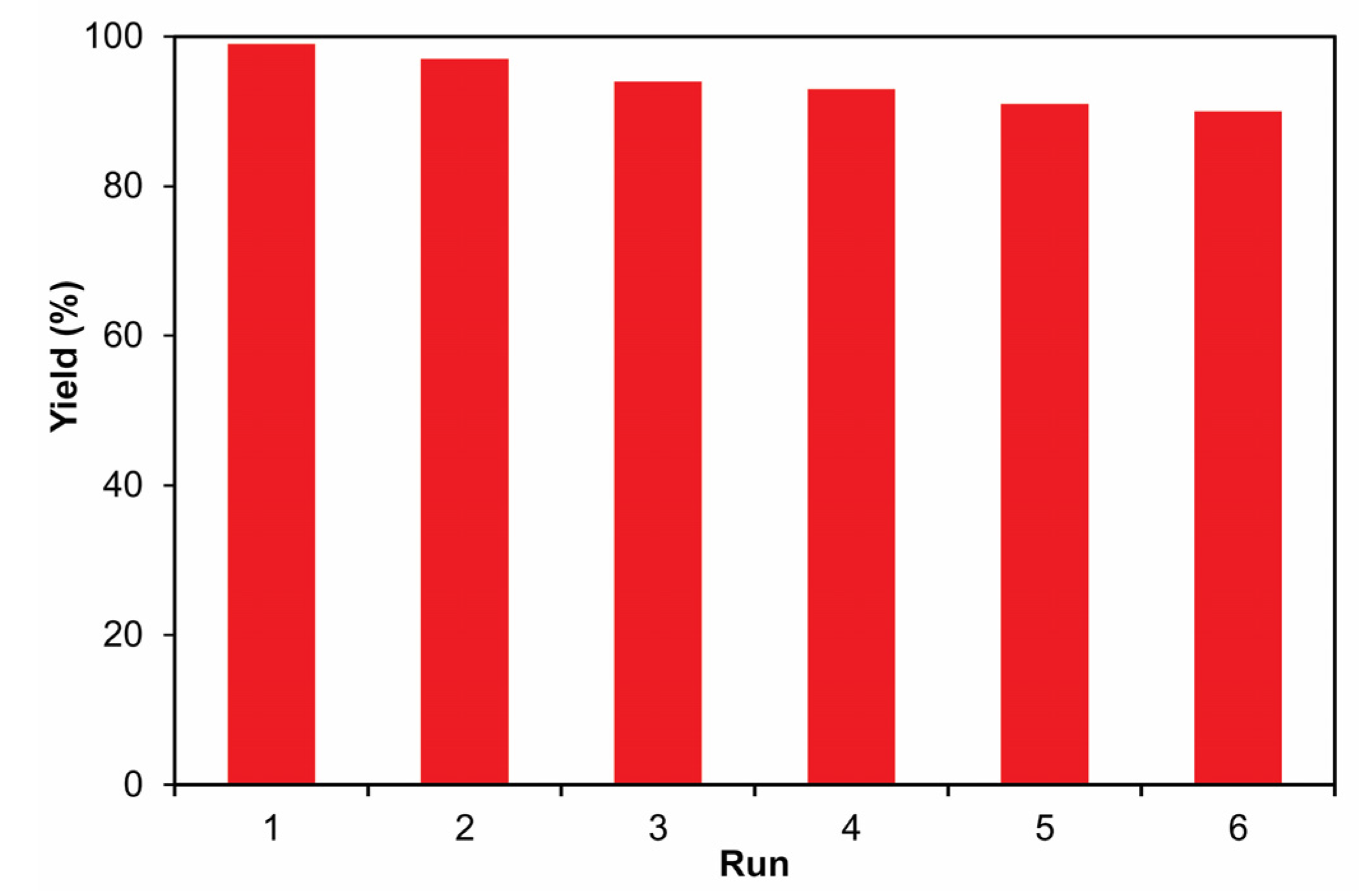
3.3. Recyclability of Pd-Fe3O4-CWH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cho, D.-W.; Park, J.; Kwon, G.; Lee, J.; Yim, G.-J.; Jung, W.; Cheong, Y.-W. Zirconia-Assisted pyrolysis of coffee waste in CO2 environment for the Simultaneous production of fuel gas and composite adsorbent. J. Hazard. Mater. 2020, 386, 121989. [Google Scholar] [CrossRef]
- Santana, M.S.; Alves, R.P.; da Silva Borges, W.M.; Francisquini, E.; Guerreiro, M.C. Hydrochar production from defective coffee beans by hydrothermal carbonization. Bioresour. Technol. 2020, 300, 122653. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Li, C.; Qi, L. Characterization and sulfonamide antibiotics adsorption capacity of spent coffee grounds based biochar and hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef]
- Saberian, M.; Li, J.; Donnoli, A.; Bonderenko, E.; Oliva, P.; Gill, B.; Lockrey, S.; Siddique, R. Recycling of spent coffee grounds in construction materials: A review. J. Clean. Prod. 2021, 289, 125837. [Google Scholar] [CrossRef]
- Khataee, A.; Kayan, B.; Kalderis, D.; Karimi, A.; Akay, S.; Konsolakis, M. Ultrasound-assisted removal of Acid Red 17 using nanosized Fe3O4-loaded coffee waste hydrochar. Ultrason. Sonochem. 2017, 35, 72–80. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Nguyen, T.-B.; Chen, C.-W.; Hung, C.-M.; Huang, C.; Dong, C.-D. Cobalt-impregnated biochar (Co-SCG) for heterogeneous activation of peroxymonosulfate for removal of tetracycline in water. Bioresour. Technol. 2019, 292, 121954. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Nguyen, T.-B.; Chen, C.-W.; Hung, C.-M.; Chang, J.-H.; Dong, C.-D. Influence of pyrolysis temperature on polycyclic aromatic hydrocarbons production and tetracycline adsorption behavior of biochar derived from spent coffee ground. Bioresour. Technol. 2019, 284, 197–203. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Akbari, R.; Sakhaei, S.; Nezafat, Z.; Banazadeh, S.; Orooji, Y.; Hegde, G. Polymer supported copper complexes/nanoparticles for treatment of environmental contaminants. J. Mol. Liq. 2021, 115668. [Google Scholar] [CrossRef]
- Begildayeva, T.; Lee, S.J.; Yu, Y.; Park, J.; Kim, T.H.; Theerthagiri, J.; Ahn, A.; Jung, H.J.; Choi, M.Y. Production of copper nanoparticles exhibiting various morphologies via pulsed laser ablation in different solvents and their catalytic activity for reduction of toxic nitroaromatic compounds. J. Hazard. Mater. 2021, 409, 124412. [Google Scholar] [CrossRef]
- Ramu, A.; Kumari, M.A.; Elshikh, M.S.; Alkhamis, H.H.; Alrefaei, A.F.; Choi, D. A facile and green synthesis of CuO/NiO nanoparticles and their removal activity of toxic nitro compounds in aqueous medium. Chemosphere 2021, 271, 129475. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xie, Q.; Tang, L.; Yu, J.; Wang, J.; Yang, Z.; Fan, C.; Zhang, S. The reduction of nitrobenzene by extracellular electron transfer facilitated by Fe-bearing biochar derived from sewage sludge. J. Hazard. Mater. 2021, 403, 123682. [Google Scholar] [CrossRef]
- Ghadermazi, M.; Moradi, S.; Mozafari, R. Rice husk-SiO2 supported bimetallic Fe–Ni nanoparticles: As a new, powerful magnetic nanocomposite for the aqueous reduction of nitro compounds to amines. RSC Adv. 2020, 10, 33389–33400. [Google Scholar] [CrossRef]
- Georgiou, E.; Mihajlovic, M.; Petrovic, J.; Anastopoulos, I.; Dosche, C.; Pashalidis, I.; Kalderis, D. Single-stage production of miscanthus hydrochar at low severity conditions and application as adsorbent of copper and ammonium ions. Bioresour. Technol. 2021, 337, 125458. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakis, T.N.; Vardiambasis, I.O.; Nikolopoulos, C.D.; Konstantaras, A.I.; Trang, T.K.; Khuong, D.A.; Tsubota, T.; Keyikoglu, R.; Khataee, A.; Kalderis, D. Towards engineered hydrochars: Application of artificial neural networks in the hydrothermal carbonization of sewage sludge. Energies 2021, 14, 3000. [Google Scholar] [CrossRef]
- Sener, M.; Kayan, B.; Akay, S.; Gozmen, B.; Kalderis, D. Fe-modified sporopollenin as a composite biosorbent for the removal of Pb2+ from aqueous solutions. Desalin. Water Treat. 2016, 57, 28294–28312. [Google Scholar] [CrossRef]
- Lestari, W.W.; Prajanira, L.B.; Putra, R.; Purnawan, C.; Prihadiyono, F.I.; Arrozi, U.S.F. Green-synthesized MIL-100(Fe) modified with palladium as a selective catalyst in the hydrogenation of citronellal to citronellol. Mater. Res. Express 2021, 8, 045504. [Google Scholar] [CrossRef]
- Lotfi, S.; Veisi, H. Pd nanoparticles decorated poly-methyldopa@ GO/Fe3O4 nanocomposite modified glassy carbon electrode as a new electrochemical sensor for simultaneous determination of acetaminophen and phenylephrine. Mater. Sci. Eng. C 2019, 105, 110112. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S.; Malmir, M.; Heravi, M.M.; Ghoreyshi Kahangi, F. Magnetic covalent hybrid of graphitic carbon nitride and graphene oxide as an efficient catalyst support for immobilization of Pd nanoparticles. Inorg. Chim. Acta 2019, 488, 62–70. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Green synthesis of palladium nanoparticles using gum ghatti (Anogeissus latifolia) and its application as an antioxidant and catalyst. Arab. J. Chem. 2018, 11, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Veisi, H.; Ghorbani, M.; Hemmati, S. Sonochemical in situ immobilization of Pd nanoparticles on green tea extract coated Fe3O4 nanoparticles: An efficient and magnetically recyclable nanocatalyst for synthesis of biphenyl compounds under ultrasound irradiations. Mater. Sci. Eng. C 2019, 98, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, S.; Zhou, H.; Lieberwirth, I.; Feng, X.; Müllen, K. 3D graphene foams cross-linked with pre-encapsulated Fe3O4 nanospheres for enhanced lithium storage. Adv. Mater. 2013, 25, 2909–2914. [Google Scholar] [CrossRef]
- Deka, J.R.; Saikia, D.; Chen, P.-H.; Chen, K.-T.; Kao, H.-M.; Yang, Y.-C. N-functionalized mesoporous carbon supported Pd nanoparticles as highly active nanocatalyst for Suzuki-Miyaura reaction, reduction of 4-nitrophenol and hydrodechlorination of chlorobenze. J. Ind. Eng. Chem. 2021, 104, 529–543. [Google Scholar] [CrossRef]
- Layek, K.; Kantam, M.L.; Shirai, M.; Nishio-Hamane, D.; Sasaki, T.; Maheswaran, H. Gold nanoparticles stabilized on nanocrystalline magnesium oxide as an active catalyst for reduction of nitroarenes in aqueous medium at room temperature. Green Chem. 2012, 14, 3164–3174. [Google Scholar] [CrossRef]
- Rangraz, Y.; Nemati, F.; Elhampour, A. Selenium-doped graphitic carbon nitride decorated with Ag NPs as a practical and recyclable nanocatalyst for the hydrogenation of nitro compounds in aqueous media. Appl. Surf. Sci. 2020, 507, 145164. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Aboo, A.H.; Ahmed, E.; Sharif, A.; Xiao, J. Reduction of nitroarenes catalyzed by microgel-stabilized silver nanoparticles. J. Hazard. Mater. 2019, 377, 399–408. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mohammad Sajadi, S.; Rostami-Vartooni, A.; Alizadeh, M.; Bagherzadeh, M. Green synthesis of the Pd nanoparticles supported on reduced graphene oxide using barberry fruit extract and its application as a recyclable and heterogeneous catalyst for the reduction of nitroarenes. J. Colloid Interface Sci. 2016, 466, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zeynizadeh, B.; Rahmani, S.; Tizhoush, H. The immobilized Cu nanoparticles on magnetic montmorillonite (MMT@Fe3O4@Cu): As an efficient and reusable nanocatalyst for reduction and reductive-acetylation of nitroarenes with NaBH4. Polyhedron 2020, 175, 114201. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, R.; Jain, Y. Fe3O4–Glutathione stabilized Ag nanoparticles: A new magnetically separable robust and facile catalyst for aqueous phase reduction of nitroarenes. Appl. Organomet. Chem. 2019, 33, e5223. [Google Scholar] [CrossRef]
- Niakan, M.; Asadi, Z. Selective reduction of nitroarenes catalyzed by sustainable and reusable DNA-supported nickel nanoparticles in water at room temperature. Catal. Lett. 2019, 149, 2234–2246. [Google Scholar] [CrossRef]
- Sharma, R.K.; Monga, Y.; Puri, A. Magnetically separable silica@Fe3O4 core–shell supported nano-structured copper(II) composites as a versatile catalyst for the reduction of nitroarenes in aqueous medium at room temperature. J. Mol. Catal. A Chem. 2014, 393, 84–95. [Google Scholar] [CrossRef]
- Zelekew, O.A.; Kuo, D.-H. Facile synthesis of SiO2@CuxO@TiO2 heterostructures for catalytic reductions of 4-nitrophenol and 2-nitroaniline organic pollutants. Appl. Surf. Sci. 2017, 393, 110–118. [Google Scholar] [CrossRef]
- Le, X.; Dong, Z.; Zhang, W.; Li, X.; Ma, J. Fibrous nano-silica containing immobilized Ni@Au core–shell nanoparticles: A highly active and reusable catalyst for the reduction of 4-nitrophenol and 2-nitroaniline. J. Mol. Catal. A Chem. 2014, 395, 58–65. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Zhu, Y.; Tan, H.; Chen, X.; Lu, Z.-H. Core–shell structured nanocomposites Ag@ CeO2 as catalysts for hydrogenation of 4-nitrophenol and 2-nitroaniline. RSC Adv. 2016, 6, 47966–47973. [Google Scholar] [CrossRef]
- Rocha, M.; Pereira, C.; Freire, C. Au/Ag nanoparticles-decorated TiO2 with enhanced catalytic activity for nitroarenes reduction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126614. [Google Scholar] [CrossRef]
- Sypu, V.S.; Bhaumik, M.; Raju, K.; Maity, A. Nickel hydroxide nanoparticles decorated napthalene sulfonic acid-doped polyaniline nanotubes as efficient catalysts for nitroarene reduction. J. Colloid Interface Sci. 2021, 581, 979–989. [Google Scholar] [CrossRef]
- Du, J.-T.; Qiao, M.; Pu, Y.; Wang, J.-X.; Chen, J.-F. Aqueous dispersions of monodisperse Pt, Pd, and Au nanoparticles stabilized by thermosensitive polymer for the efficient reduction of nitroarenes. Appl. Catal. A Gen. 2021, 624, 118323. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M. Catalytic Activity of Palladium Nanoparticles Encapsulated in Spherical Polyelectrolyte Brushes and Core-Shell Microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Catalytic hydrogenation of nitrophenols and nitrotoluenes over a palladium/graphene nanocomposite. Catal. Sci. Technol. 2014, 4, 1742. [Google Scholar] [CrossRef]


| 4-NA (M) | NaBH4 (M) | Pd-Fe3O4-CWH (mg) | Reaction Time (s) |
|---|---|---|---|
| 3 × 10−4 | 0.08 | 10 | 300 |
| 3 × 10−4 | 0.08 | 15 | 180 |
| 3 × 10−4 | 0.08 | 20 | 82 |
| 3 × 10−4 | 0.04 | 20 | 145 |
| 3 × 10−4 | 0.06 | 20 | 115 |
| 3.5 × 10−4 | 0.08 | 20 | 140 |
| 4 × 10−4 | 0.08 | 20 | 190 |
| Substrate | Product | Reduction Time (s) | Retention Time (rtmin) a | Retention Time (rtmin) b |
|---|---|---|---|---|
| 4-Nitrobenzoic Acid | 4-Aminobenzoic acid | 60 | 7.74 | 4.27 |
| 4-Nitroaniline | 4-Phenylenediamine | 82 | 7.33 | 2.95 |
| 2-Nitroaniline | 2-Phenylenediamine | 90 | 8.51 | 3.20 |
| 4-Nitro-o-phenylenediamine | 1,2,4-Triaminobenzene | 168 | 5.30 | 2.95 |
| 3-Nitroanisole | 3-Anisidine | 428 | 15.87 | 3.79 |
| Entry | Catalyst | Time | Ref. |
|---|---|---|---|
| 1 | Ag-PNA-BIS-2 | 8 h | [26] |
| 2 | Pd NPs/RGO | 1.5 h | [27] |
| 3 | MMT@Fe3O4@Cu | 6 min | [28] |
| 4 | Fe3O4-Glu-Ag | 12 min | [29] |
| 5 | NiNPs/DNA | 3 h | [30] |
| 6 | Cu–Acac@Am–Si–Fe3O4 | 5 min | [31] |
| 7 | SiO2@CuxO@TiO2 | 150 s | [32] |
| 8 | Ni@Au/KCC-1 | 660 s | [33] |
| 9 | Ag@CeO2 NCs | 240 s | [34] |
| 10 | Pd-Fe3O4-CWH nanocatalyst | 90 s | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çalışkan, M.; Akay, S.; Kayan, B.; Baran, T.; Kalderis, D. Preparation and Application of a Hydrochar-Based Palladium Nanocatalyst for the Reduction of Nitroarenes. Molecules 2021, 26, 6859. https://doi.org/10.3390/molecules26226859
Çalışkan M, Akay S, Kayan B, Baran T, Kalderis D. Preparation and Application of a Hydrochar-Based Palladium Nanocatalyst for the Reduction of Nitroarenes. Molecules. 2021; 26(22):6859. https://doi.org/10.3390/molecules26226859
Chicago/Turabian StyleÇalışkan, Melike, Sema Akay, Berkant Kayan, Talat Baran, and Dimitrios Kalderis. 2021. "Preparation and Application of a Hydrochar-Based Palladium Nanocatalyst for the Reduction of Nitroarenes" Molecules 26, no. 22: 6859. https://doi.org/10.3390/molecules26226859
APA StyleÇalışkan, M., Akay, S., Kayan, B., Baran, T., & Kalderis, D. (2021). Preparation and Application of a Hydrochar-Based Palladium Nanocatalyst for the Reduction of Nitroarenes. Molecules, 26(22), 6859. https://doi.org/10.3390/molecules26226859








