Construction of a Luminescent Cadmium-Based Metal–Organic Framework for Highly Selective Discrimination of Ferric Ions
Abstract
:1. Introduction
2. Results and Discussion
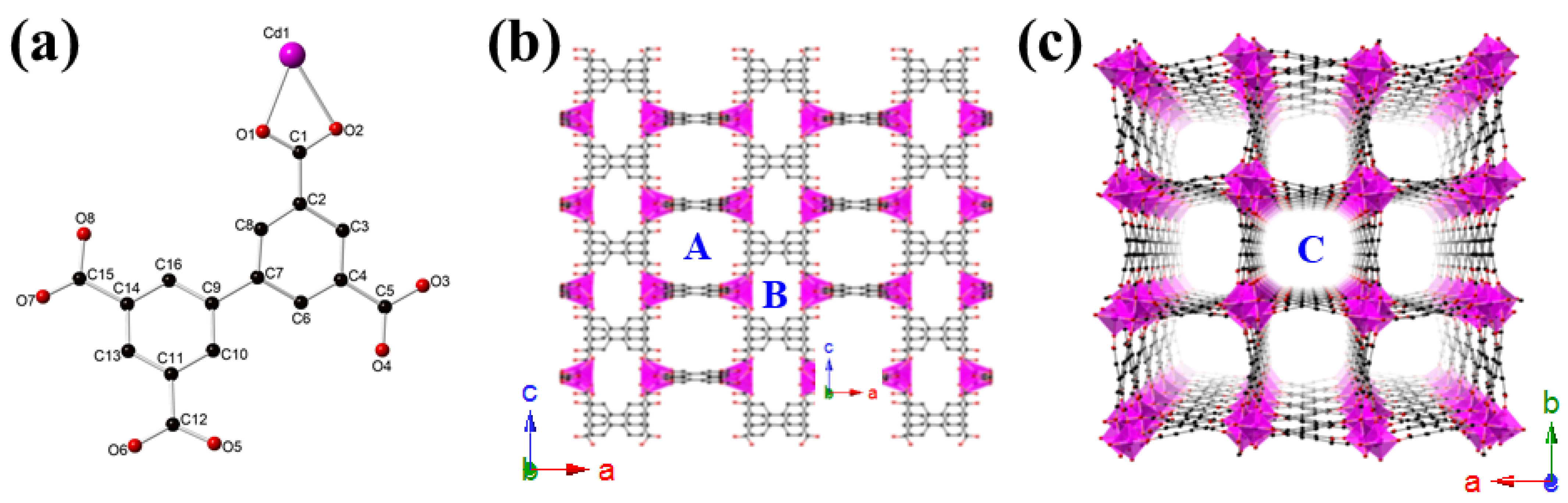
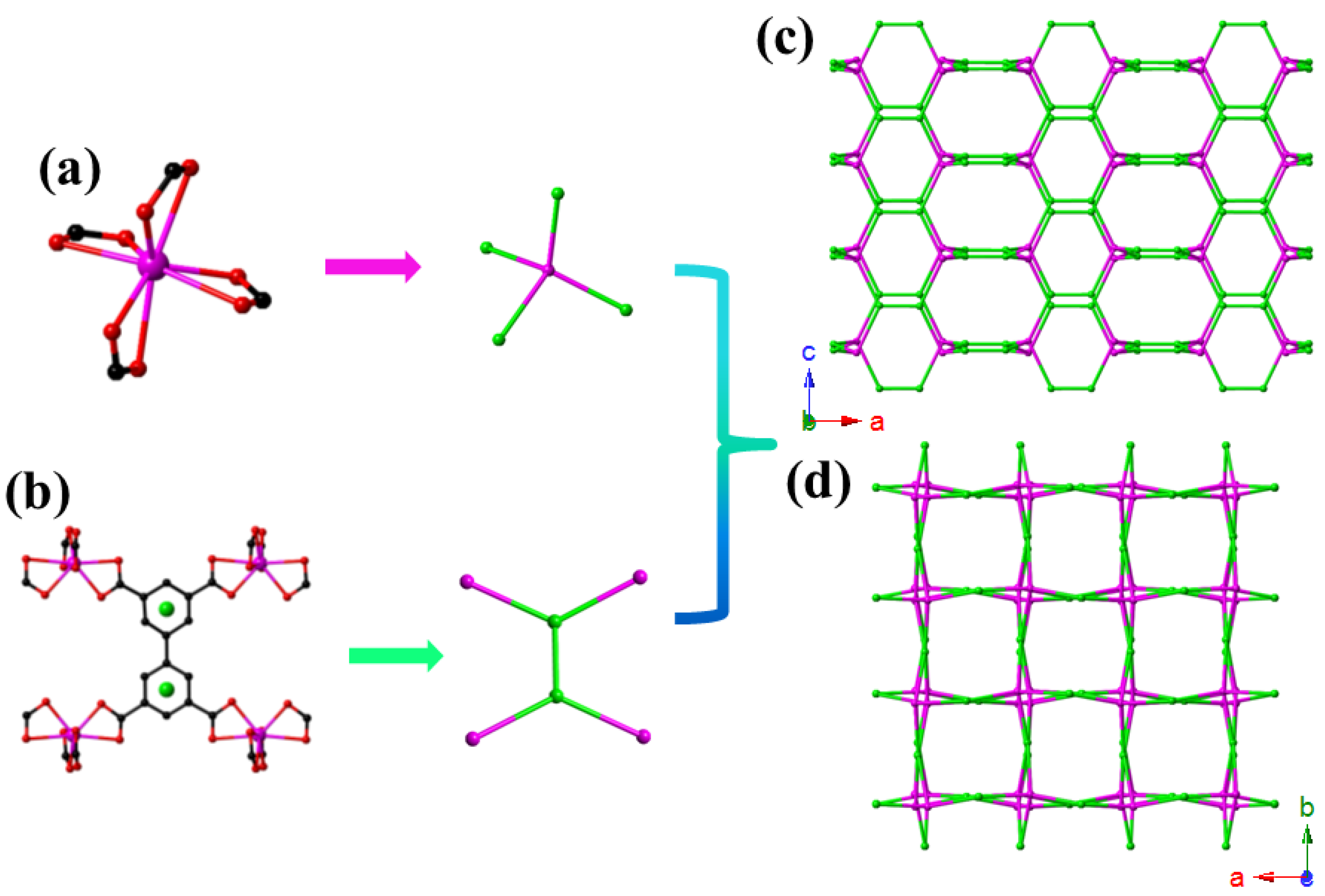
2.1. Crystal Structure Description of {[Me2NH2]2[CdBPTC]·6H2O}n (CdBPTC)
2.2. Framework Stability
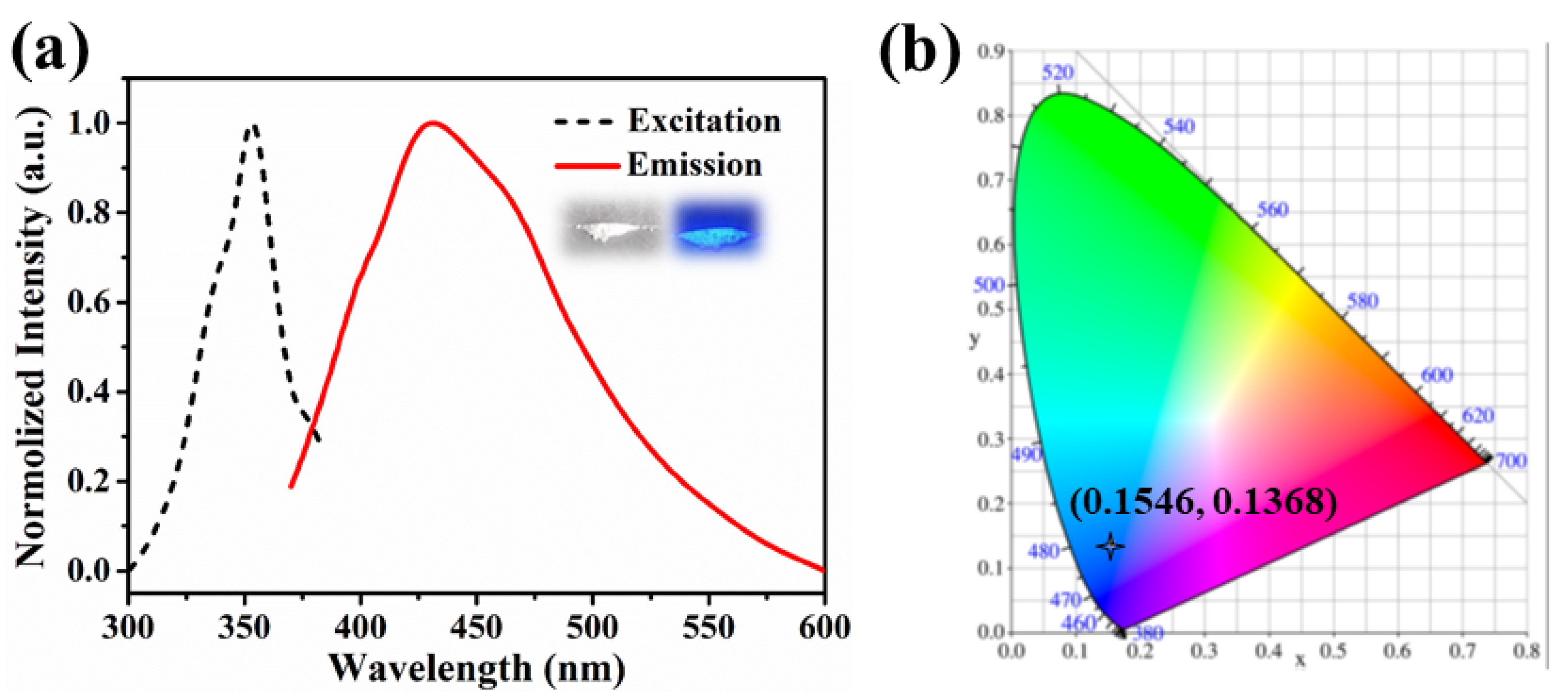
2.3. Luminescent and Sensing Properties
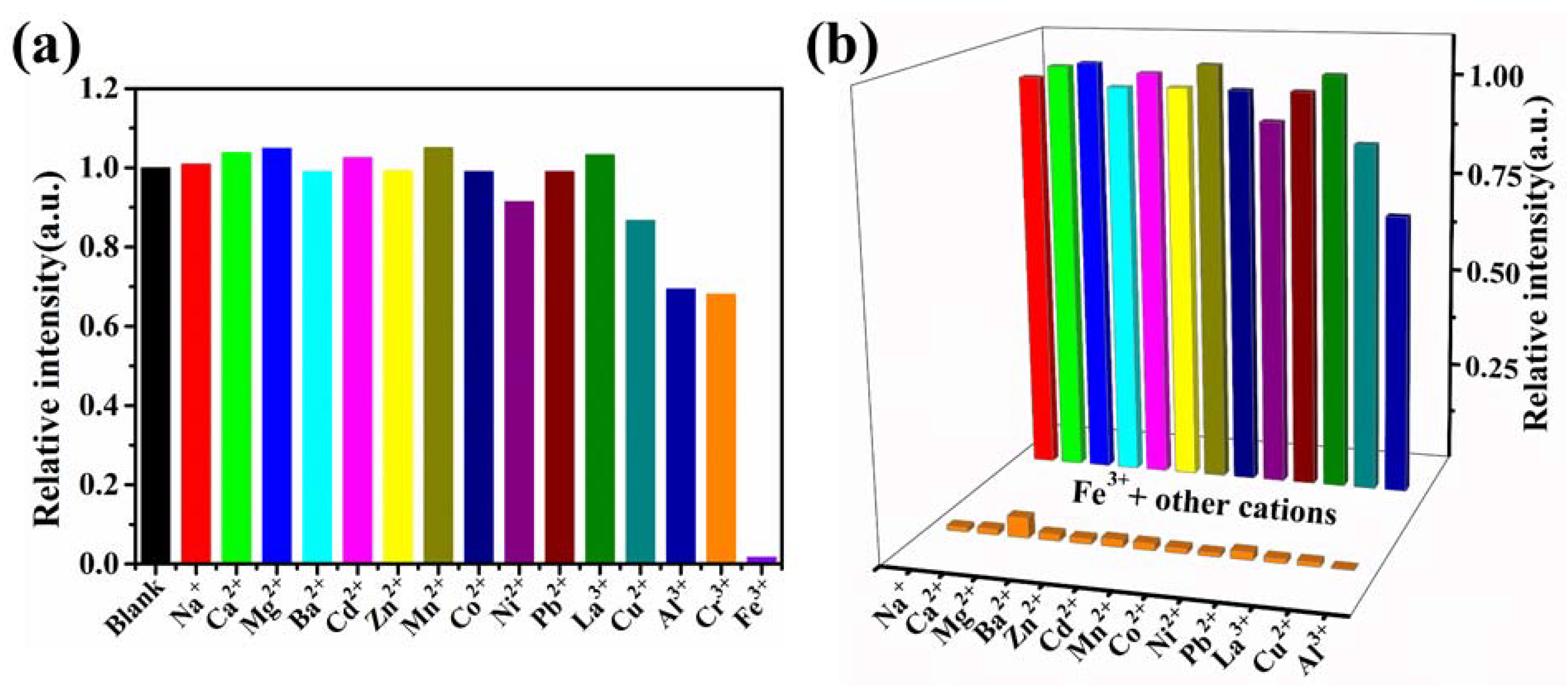
2.3.1. Fluorescence Detection of Metal Ions
2.3.2. Fluorescence-Sensing Mechanism of the CdBPTC Probe
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Synthesis of {[Me2NH2]2[CdBPTC]·6H2O}n
3.4. Determination of the Crystal Structures
3.5. Sensing Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, D.M.; Zhang, N.N.; Liu, C.S.; Du, M. Template-directed synthesis of a luminescent Tb-MOF material for highly selective Fe3+ and Al3+ ion detection and VOC vapor sensing. J. Mater. Chem. C 2017, 5, 2311–2317. [Google Scholar] [CrossRef]
- Yu, C.; Sun, X.; Zou, L.; Li, G.; Zhang, L.; Liu, Y. A pillar-layered Zn-LMOF with uncoordinated carboxylic acid sites: High performance for luminescence sensing Fe3+ and TNP. Inorg. Chem. 2019, 58, 4026–4032. [Google Scholar] [CrossRef]
- Wang, J.; Yu, M.; Chen, L.; Li, Z.; Li, S.; Jiang, F.; Hong, M. Construction of a stable lanthanide Metal-Organic Framework as a luminescentprobe for rapid naked-eye recognition of Fe3+ and acetone. Molecules 2021, 26, 1695. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Theil, E.C. Ferritins: Dynamic Management of biological Iron and oxygen chemistry. Acc. Chem. Res. 2005, 38, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Chen, Z.; Fu, X.; Yang, B.; Li, H.; Su, J.; Gu, W.; Liu, X. A highly selective and fast-response fluorescent probe based on Cd-MOF for the visual detection of Al3+ ion and quantitative detection of Fe3+ ion. J. Solid State Chem. 2018, 259, 67–72. [Google Scholar] [CrossRef]
- He, J.; Xu, J.; Yin, J.; Li, N.; Bu, X.H. Recent advances in luminescent metal-organic frameworks for chemical sensors. Sci. China Mater. 2019, 62, 1655–1678. [Google Scholar] [CrossRef] [Green Version]
- Pamei, M.; Puzari, A. Luminescent transition metal–organic frameworks: An emerging sensor for detecting biologically essential metal ions. Nano-Struct. Nano-Objects 2019, 19, 100364. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Fang, X.; Zong, B.; Mao, S. Metal–Organic Framework-based sensors for environmental contaminant sensing. Nano-Micro Lett. 2018, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, J.; Kirillov, A.M.; Dou, W.; Xu, C.; Xu, C.; Yang, L.; Fang, R.; Liu, W. Multifunctional Ln–MOF luminescent probe for efficient sensing of Fe3+, Ce3+, and acetone. ACS Appl. Mater. Inter. 2018, 10, 23976–23986. [Google Scholar] [CrossRef]
- Yao, Z.Q.; Xu, J.; Zou, B.; Hu, Z.; Wang, K.; Yuan, Y.J.; Chen, Y.P.; Feng, R.; Xiong, J.B.; Hao, J. A dual-stimuli-responsive coordination network featuring reversible wide-range luminescence-tuning behavior. Angew. Chem. Int. Ed. 2019, 58, 5614–5618. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the art and prospects in Metal–Organic Framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, M.; Wang, T.; Wei, R.J.; Xie, X.J.; Zhao, Y.; Lu, W.; Li, D. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature 2021, 595, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Qiu, P.L.; Bai, J.P.; Luo, D.; Lu, W.; Li, D. Exclusive recognition of acetone in a luminescent BioMOF through multiple hydrogen-bonding interactions. Inorg. Chem. 2019, 58, 7667–7671. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Zhang, X.; Zhou, X.P.; Li, D. Robust heterometallic MOF catalysts for the cyanosilylation of aldehydes. Inorg. Chem. Front. 2018, 5, 2772–2776. [Google Scholar] [CrossRef]
- Wang, X.N.; Zhang, P.; Kirchon, A.; Li, J.L.; Chen, W.M.; Zhao, Y.M.; Li, B.; Zhou, H.C. Crystallographic visualization of postsynthetic nickel clusters into Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 13654–13663. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Kim, K.H.; Deep, A. MOF–bacteriophage biosensor for highly sensitive and specific detection of staphylococcus aureus. ACS Appl. Mater. Inter. 2017, 9, 33589–33598. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.L.; Li, D. Biological metal-organic frameworks: Structures, host-guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Hou, S.L.; Dong, J.; Jiang, X.L.; Jiao, Z.H.; Wang, C.M.; Zhao, B. Interpenetration-dependent luminescent probe in Indium-Organic Frameworks for selectively detecting nitrofurazone in water. Anal. Chem. 2018, 90, 1516–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.L.; Zhang, H.F.; Li, M.A.; Ng, S.W.; Feng, J.H.; Mao, J.G.; Li, D. Chiroptical activity from an achiral biological Metal-Organic Framework. J. Am. Chem. Soc. 2018, 140, 11569–11572. [Google Scholar] [CrossRef]
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal-organic frameworks. Coord. Chem. Rev. 2021, 426, 213544. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, G. Porous aromatic frameworks as a platform for multifunctional applications. ACS Central Sci. 2019, 5, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Yang, Y.; Ma, X.; Meng, Q.; Wang, L.; Zhao, S.; Zhu, G. Molecularly imprinted porous aromatic frameworks and their composite components for celective extraction of uranium ions. Adv. Mater. 2018, 30, 1706507. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, Z.; Wang, L.; Meng, Q.; Yuan, Y.; Zhu, G. Constructing synergistic groups in porous aromatic frameworks for the selective removal and recovery of lead(II) ions. J. Mater. Chem. A 2018, 6, 5202–5207. [Google Scholar] [CrossRef]
- Tian, X.M.; Yao, S.L.; Qiu, C.Q.; Zheng, T.F.; Chen, Y.-Q.; Huang, H.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Turn-on luminescent sensor toward Fe3+, Cr3+, and Al3+ based on a Co(II) Metal–Organic Framework with open functional sites. Inorg. Chem. 2020, 59, 2803–2810. [Google Scholar] [CrossRef]
- Fu, C.; Sun, X.; Zhang, G.; Shi, P.; Cui, P. Porphyrin-based Metal–Organic Framework probe: Highly selective and sensitive fluorescent turn-on sensor for M3+ (Al3+, Cr3+, and Fe3+) ions. Inorg. Chem. 2021, 60, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Ma, E.; Sun, Z.M.; Zhang, H. A layer-structured Eu-MOF as a highly selective fluorescent probe for Fe3+ detection through a cation-exchange approach. J. Mater. Chem. 2012, 22, 16920–16926. [Google Scholar] [CrossRef]
- Spek, A. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Lu, R.; Tang, S.; Liu, X. Highly cross-linked fluorescent poly(cyclotriphosphazene-co-curcumin) microspheres for the selective detection of picric acid in solution phase. J. Mater. Chem. A 2015, 3, 4604–4611. [Google Scholar] [CrossRef]
- Weng, H.; Yan, B. A flexible Tb(III) functionalized cadmium metal organic framework as fluorescent probe for highly selectively sensing ions and organic small molecules. Sens. Actuators B Chem. 2016, 228, 702–708. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.H.; Zhao, Y.; Wang, p.; Liu, Y.; Azam, M.; Al-Resayes, S.I.; Lu, Y.; Sun, W.Y. Luminescent Cd (II)–organic frameworks with chelating NH2 sites for selective detection of Fe (III) and antibiotics. J. Mater. Chem. A 2017, 5, 15797–1580732. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Z.Q.; Ren, G.J.; Han, S.D.; Hu, T.L.; Bu, X.H. A luminescent metal–organic framework for selective sensing of Fe3+ with excellent recyclability. Inorg. Chem. Commun. 2016, 65, 9–12. [Google Scholar] [CrossRef]
- Hu, F.l.; Shi, Y.X.; Chen, H.H.; Lang, J.P. A Zn (II) coordination polymer and its photocycloaddition product: Syntheses, structures, selective luminescence sensing of iron (III) ions and selective absorption of dyes. Dalton Trans. 2015, 44, 18795–18803. [Google Scholar] [CrossRef]
- Chen, M.; Xu, W.M.; Tian, J.Y.; Cui, H.; Zhang, J.X.; Liu, C.S.; Du, M. A Terbium(III) lanthanide–organic framework as a platform for a recyclable multi-responsive luminescent sensor. J. Mater. Chem. C 2017, 5, 2015–2021. [Google Scholar] [CrossRef]
- Yang, C.X.; Ren, H.B.; Yan, X.P. Fluorescent Metal–Organic Framework MIL-53(Al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal. Chem. 2013, 85, 7441–7446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.T.; Yang, J.; Wu, H.; Liu, Y.Y.; Ma, J.F. Systematic Investigation of high-sensitivity luminescent sensing for polyoxometalates and iron(III) by MOFs assembled with a new resorcin[4] arene-Functionalized Tetracarboxylate. Chem. Eur. J. 2015, 21, 15806–15819. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Compound | CdBPTC |
|---|---|
| Empirical formula | C16H6CdO8 |
| Formula weight | 438.61 |
| Temperature (K) | 100.15 |
| Crystal system | Tetragonal |
| Space group | I41cd |
| a/Å | 20.07520 (10) |
| c/Å | 36.7826 (3) |
| Volume (Å3) | 14,823.89 (19) |
| Z | 16 |
| ρcalc (g·cm−3) | 0.786 |
| μ (mm−1) | 4.897 |
| F (000) | 3424.0 |
| Independent reflections | 5869 [Rint = 0.0332] |
| Data/restraints/parameters | 5869/25/215 |
| Goodness of fit on F2 | 1.087 |
| R1 [I ≥ 2σ(I)] a | 0.0399 |
| wR2 [all data] b | 0.1365 |
| Flack parameter | 0.478 (16) |
| CCDC number | 2,112,156 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.-L.; Zhang, Q.-F.; Wang, D.; Wu, G.-W.; Cai, H. Construction of a Luminescent Cadmium-Based Metal–Organic Framework for Highly Selective Discrimination of Ferric Ions. Molecules 2021, 26, 6847. https://doi.org/10.3390/molecules26226847
Xu L-L, Zhang Q-F, Wang D, Wu G-W, Cai H. Construction of a Luminescent Cadmium-Based Metal–Organic Framework for Highly Selective Discrimination of Ferric Ions. Molecules. 2021; 26(22):6847. https://doi.org/10.3390/molecules26226847
Chicago/Turabian StyleXu, Li-Li, Qiu-Feng Zhang, Dong Wang, Guang-Wei Wu, and Hong Cai. 2021. "Construction of a Luminescent Cadmium-Based Metal–Organic Framework for Highly Selective Discrimination of Ferric Ions" Molecules 26, no. 22: 6847. https://doi.org/10.3390/molecules26226847
APA StyleXu, L.-L., Zhang, Q.-F., Wang, D., Wu, G.-W., & Cai, H. (2021). Construction of a Luminescent Cadmium-Based Metal–Organic Framework for Highly Selective Discrimination of Ferric Ions. Molecules, 26(22), 6847. https://doi.org/10.3390/molecules26226847






