Anticancer Activity of Continentalic Acid in B-Cell Lymphoma
Abstract
:1. Introduction
2. Results
2.1. Aralia continentalis Extract Induces Apoptosis of Human Lymphoma Cell Line
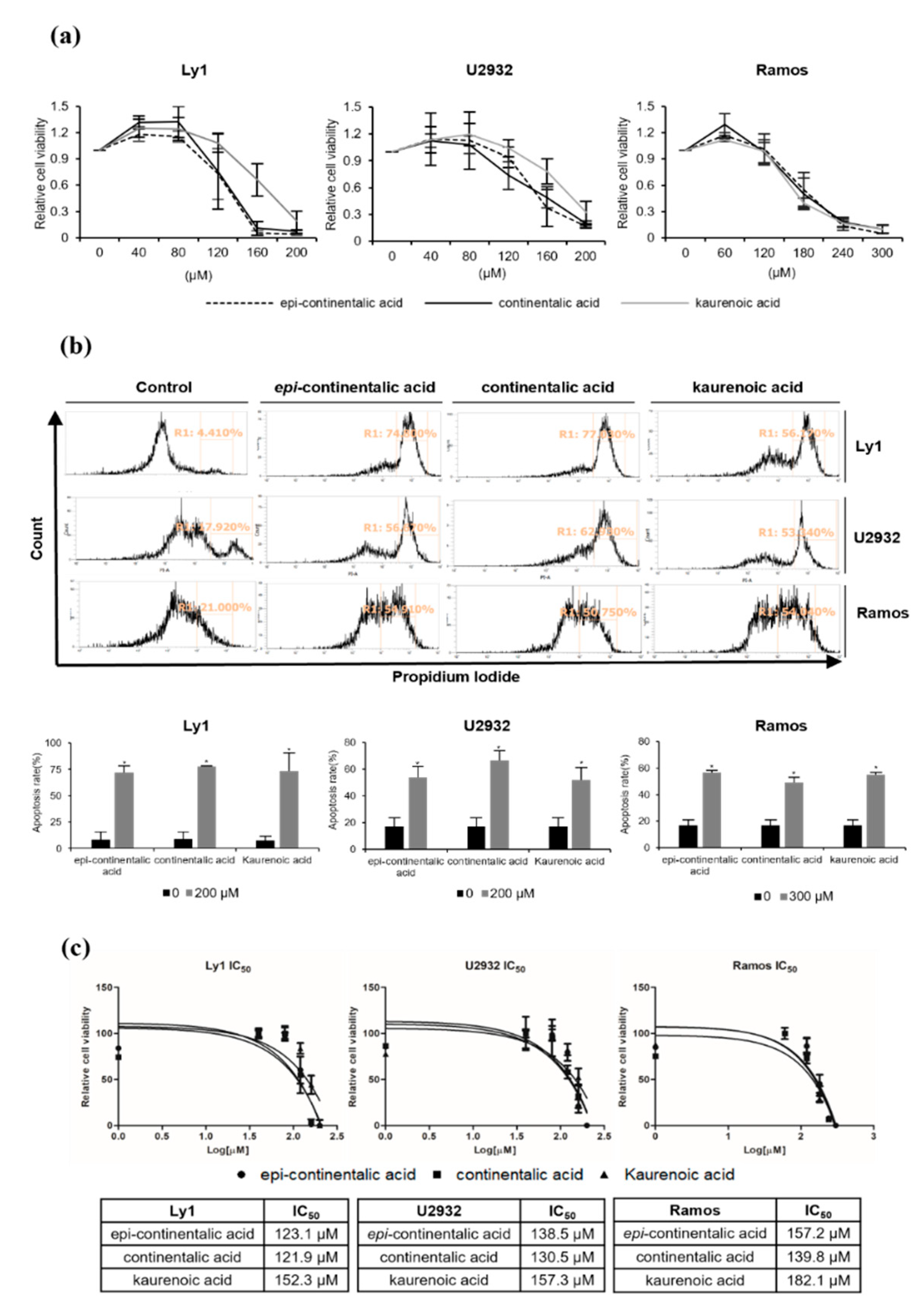
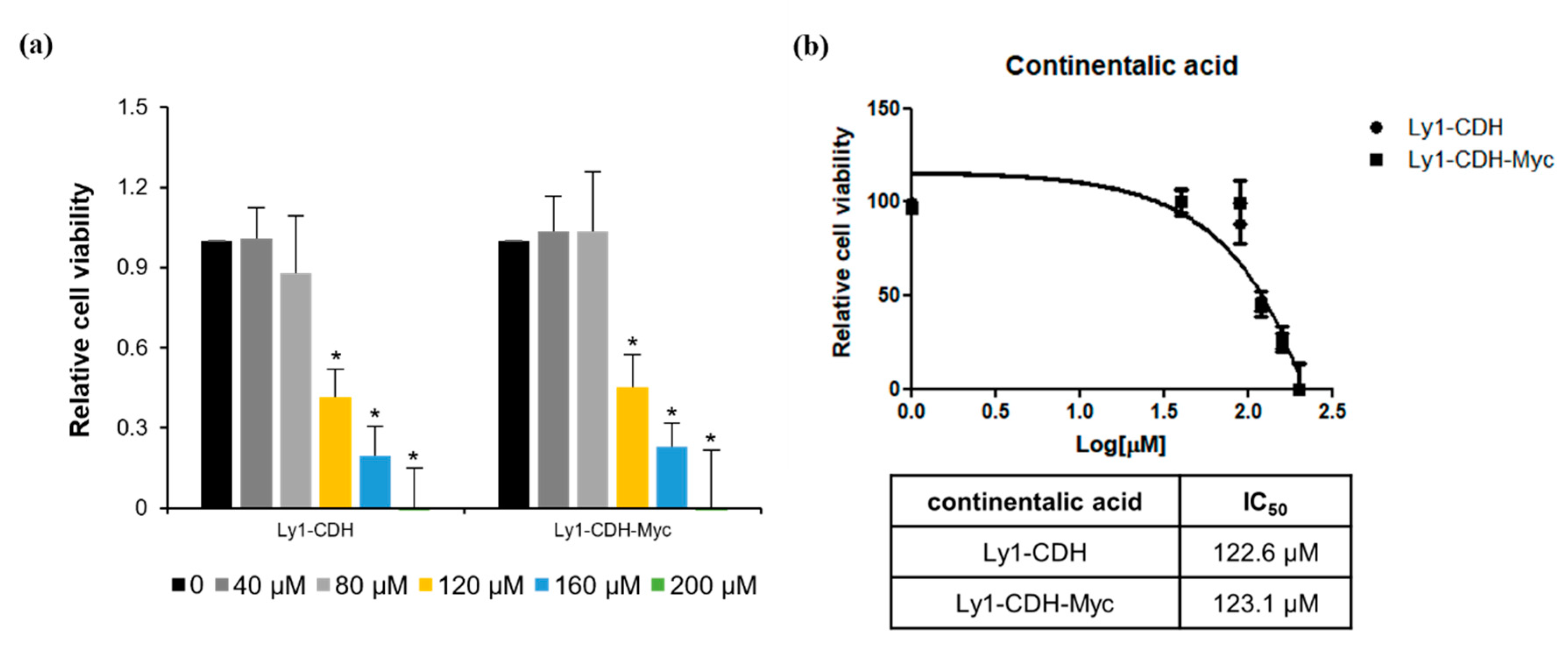
2.2. Three Diterpenes from A. continentalis Extracts Induce Apoptosis in Human Lymphoma Cell Lines
2.3. Three Dipertenes from A. continentalis Extracts Have Minimal Toxicity towards Normal Cells
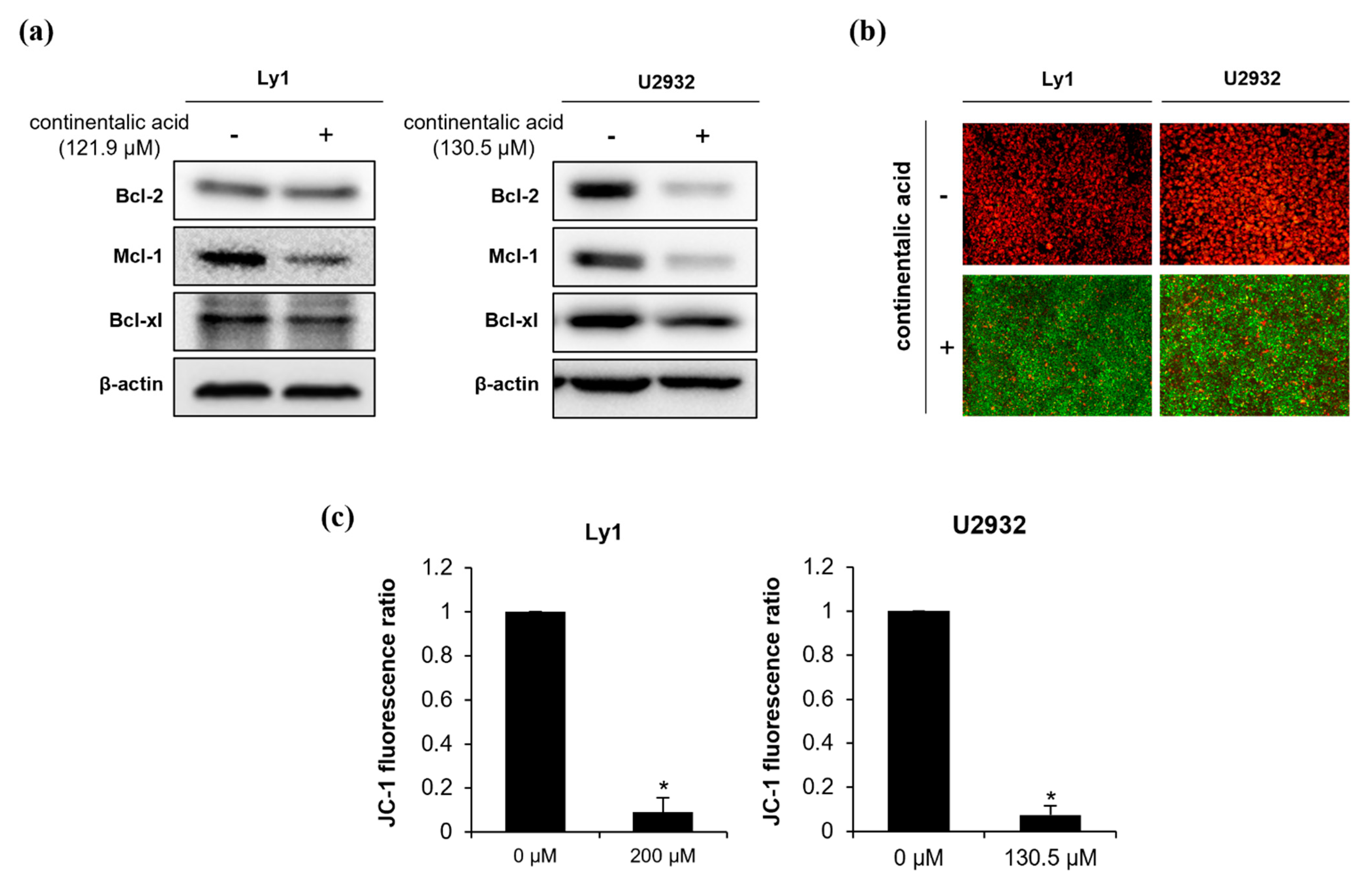
2.4. Continentalic Acid Induces Apoptosis Depending on Caspase and Mitochondria by Regulating Bcel-2 Family Members
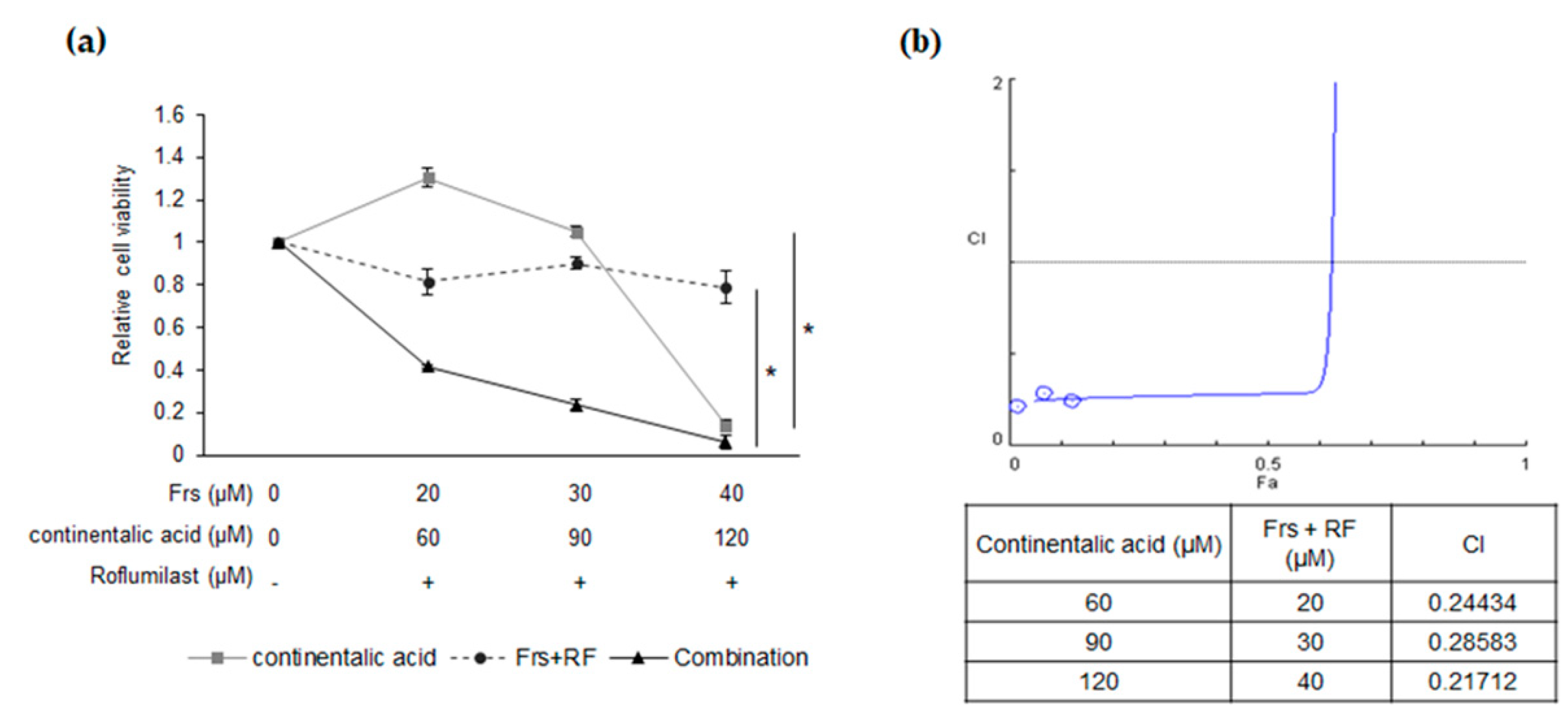
2.5. Synergy between Roflumilast and Continentalic Acid
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Antibodies
4.2. Preparation of Aralia continentalis Extracts and Isolation of Single Compounds
4.3. Cell Viability and Apoptosis Assays
4.4. Analysis of Caspase 3/7 Activity and Mitochondrial Membrane Potential
4.5. Western Blot Analysis
4.6. Animal Studies
4.7. Hematoxylin and Eosin (H&E) Staining
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F.J.T.L. Non-hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef]
- Rabbitts, T.; Hamlyn, P.H.; Baer, R. Altered nucleotide sequences of a translocated c-myc gene in Burkitt lymphoma. Nature 1983, 306, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Dalla-Favera, R.; Bregni, M.; Erikson, J.; Patterson, D.; Gallo, R.C.; Croce, C.M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc. Natl. Acad. Sci. USA 1982, 79, 7824–7827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Young, K.H.; Medeiros, L.J.J.P. Diffuse large B-cell lymphoma. Diagn. Histopathol. 2018, 50, 74–87. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, J.M.; Johnson, N.A.; Morin, R.D.; Scott, D.W.; Tan, K.; Ben-Nierah, S.; Boyle, M.; Slack, G.W.; Marra, M.A.; Connors, J.M.; et al. BCL2 mutations in diffuse large B-cell lymphoma. Leukemia 2011, 26, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.-H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011, 470, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Lenz, G.; Davis, R.E.; Ngo, V.N.; Lam, L.; George, T.C.; Wright, G.W.; Dave, S.S.; Zhao, H.; Xu, W.; Rosenwald, A.; et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 2008, 319, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.-K.; Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003, 21, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D.R. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, G.J. Rituximab: Mechanism of action. In Seminars in Hematology; WB Saunders: Philadelphia, PA, USA, 2010; Volume 47, pp. 115–123. [Google Scholar]
- Lee, B.; Hong, R.; Lim, P.; Cho, D.; Yeom, M.; Lee, S.; Kang, K.S.; Lee, S.C.; Shim, I.; Lee, H.; et al. The ethanolic extract of Aralia continentalis ameliorates cognitive deficits via modifications of BDNF expression and anti-inflammatory effects in a rat model of post-traumatic stress disorder. BMC Complement. Altern. Med. 2019, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, I.; Khan, A.-U.; Khan, S. Pharmacological evaluation of continentalic acid for antidiabetic potential. Biomed. Pharmacother. 2021, 138, 111411. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Lee, S.-J.; Adam, G.O.; Kim, S.-J. Aralia continentalis kitagawa extract attenuates the fatigue induced by exhaustive exercise through inhibition of oxidative stress. Antioxidants 2020, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Cho, S.-G.; Kim, S.-W.; Kim, J.N. Anticariogenic potential of Korean native plant extracts against Streptococcus mutans. Planta Medica 2019, 85, 1242–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mongelli, E.; Pomilio, A.B.; Sánchez, J.B.; Guerra, F.M.; Massanet, G.M. ent-Kaur-16-en-19-oic acid, a KB cells cytotoxic diterpenoid from Elaeoselinum foetidum. Phytother. Res. 2002, 16, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Arora, B.; Sharma, E.; Agrawal, S.; Agrawal, M. In vitro cytotoxicity of methanol extract from aerial parts of Aralia cachemirica and purified continentalic acid. Indian J. Pharm. Sci. 2015, 77, 792–795. [Google Scholar] [CrossRef]
- Kwon, T.O.; Jeong, S.-I.; Kwon, J.W.; Kim, Y.C.; Jang, S.I. Continentalic acid from Aralia continentalis induces growth inhibition and apoptosis in HepG2 cells. Arch. Pharmacal Res. 2008, 31, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Lizarte Neto, F.S.; Tirapelli, D.P.; Ambrosio, S.R.; Tirapelli, C.R.; Oliveira, F.M.; Novais, P.C.; Peria, F.M.; Oliveira, H.F.; Carlotti Junior, C.G.; Tirapelli, L.F. Kaurene diterpene induces apoptosis in U87 human malignant glioblastoma cells by suppression of anti-apoptotic signals and activation of cysteine proteases. Braz. J. Med. Biol. Res. 2013, 46, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hueso-Falcon, I.; Giron, N.; Velasco, P.; Amaro-Luis, J.M.; Ravelo, A.G.; de las Heras, B.; Hortelano, S.; Estevez-Braun, A. Synthesis and induction of apoptosis signaling pathway of ent-kaurane derivatives. Bioorg. Med. Chem. 2010, 18, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Kim, D.U.; Kim, E.; Kwak, B.; Ko, M.J.; Oh, A.-Y.; Park, B.-J.; Kim, Y.W.; Kim, A.; Sun, H.; et al. Disruption of the Myc-PDE4B regulatory circuitry impairs B-cell lymphoma survival. Leukemia 2019, 33, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Wuillemetoumi, S.; Robillard, N.; Gomez, P.; Moreau, P.; le Gouill, S.; Avet-Loiseau, H.; Harousseau, J.-L.; Amiot, M.; Bataille, R. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 2005, 19, 1248–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, R.W. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia 2002, 16, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, S.P.; Lee, E.; Trounson, E.; Bouillet, P.; Wei, A.; Fairlie, W.; Izon, D.J.; Zuber, J.; Rappaport, A.R.; Herold, M.; et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012, 26, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Xiang, W.; Yang, C.-Y.; Bai, L. MCL-1 inhibition in cancer treatment. OncoTargets Ther. 2018, 11, 7301–7314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hird, A.W.; Tron, A.E. Recent advances in the development of Mcl-1 inhibitors for cancer therapy. Pharmacol. Ther. 2019, 198, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wilson, D.; Jayaram, H.N.; Pankiewicz, K.W. Dual inhibitors of inosine monophosphate dehydrogenase and histone deacetylases for cancer treatment. J. Med. Chem. 2007, 50, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Guo, Q.; Wang, Z.; Wang, H.; Yang, Y.; Huang, Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials 2012, 33, 6155–6161. [Google Scholar] [CrossRef]
- National Institute of Food and Drug Safety Evaluation. Araliae Continentalis Radix; National Institute of Food and Drug Safety Evaluation: Osong, Korea, 2018; pp. 1–58.
- Moon, K.; Cha, J. Enhancement of antioxidant and antibacterial activities of Salvia miltiorrhiza roots fermented with Aspergillus oryzae. Foods 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, K.; Lee, S.; Park, H.; Cha, A.J. Enzymatic synthesis of resveratrol α-glucoside by amylosucrase of Deinococcus geothermalis. J. Microbiol. Biotechnol. 2021, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Nam, J.; Chang, W.; Zulfugarov, I.S.; Okhlopkova, Z.; Olennikov, D.; Chirikova, N.K.; Kim, S.-W. Angelica gigas nakai and decursin downregulate myc expression to promote cell death in b-cell lymphoma. Sci. Rep. 2018, 8, 10590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, H.; Nam, J.; Kim, S.-W.J.G. NF-κB p65 represses microRNA-124 transcription in diffuse large B-cell lymphoma. Genes Genomics. 2020, 42, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.N.; Park, I.; Sivtseva, S.; Okhlopkova, Z.; Zulfugarov, I.S.; Kim, S.-W. Dracocephalum palmatum Stephan extract induces caspase-and mitochondria-dependent apoptosis via Myc inhibition in diffuse large B cell lymphoma. Oncol. Rep. 2020, 44, 2746–2756. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, B.-E.; Kwon, C.-S.; Lee, J.-E.; Moon, K.; Cha, J.; Park, I.; Koh, S.; Yoon, M.; Kim, S.-W.; Kim, J.N. Anticancer Activity of Continentalic Acid in B-Cell Lymphoma. Molecules 2021, 26, 6845. https://doi.org/10.3390/molecules26226845
Jeon B-E, Kwon C-S, Lee J-E, Moon K, Cha J, Park I, Koh S, Yoon M, Kim S-W, Kim JN. Anticancer Activity of Continentalic Acid in B-Cell Lymphoma. Molecules. 2021; 26(22):6845. https://doi.org/10.3390/molecules26226845
Chicago/Turabian StyleJeon, Byeol-Eun, Chan-Seong Kwon, Ji-Eun Lee, Keumok Moon, Jaeho Cha, Inmyoung Park, Sara Koh, Myunghee Yoon, Sang-Woo Kim, and Jeong Nam Kim. 2021. "Anticancer Activity of Continentalic Acid in B-Cell Lymphoma" Molecules 26, no. 22: 6845. https://doi.org/10.3390/molecules26226845
APA StyleJeon, B.-E., Kwon, C.-S., Lee, J.-E., Moon, K., Cha, J., Park, I., Koh, S., Yoon, M., Kim, S.-W., & Kim, J. N. (2021). Anticancer Activity of Continentalic Acid in B-Cell Lymphoma. Molecules, 26(22), 6845. https://doi.org/10.3390/molecules26226845






