Strawberry Juice Powders: Effect of Spray-Drying Conditions on the Microencapsulation of Bioactive Components and Physicochemical Properties
Abstract
:1. Introduction
2. Results and Discussion
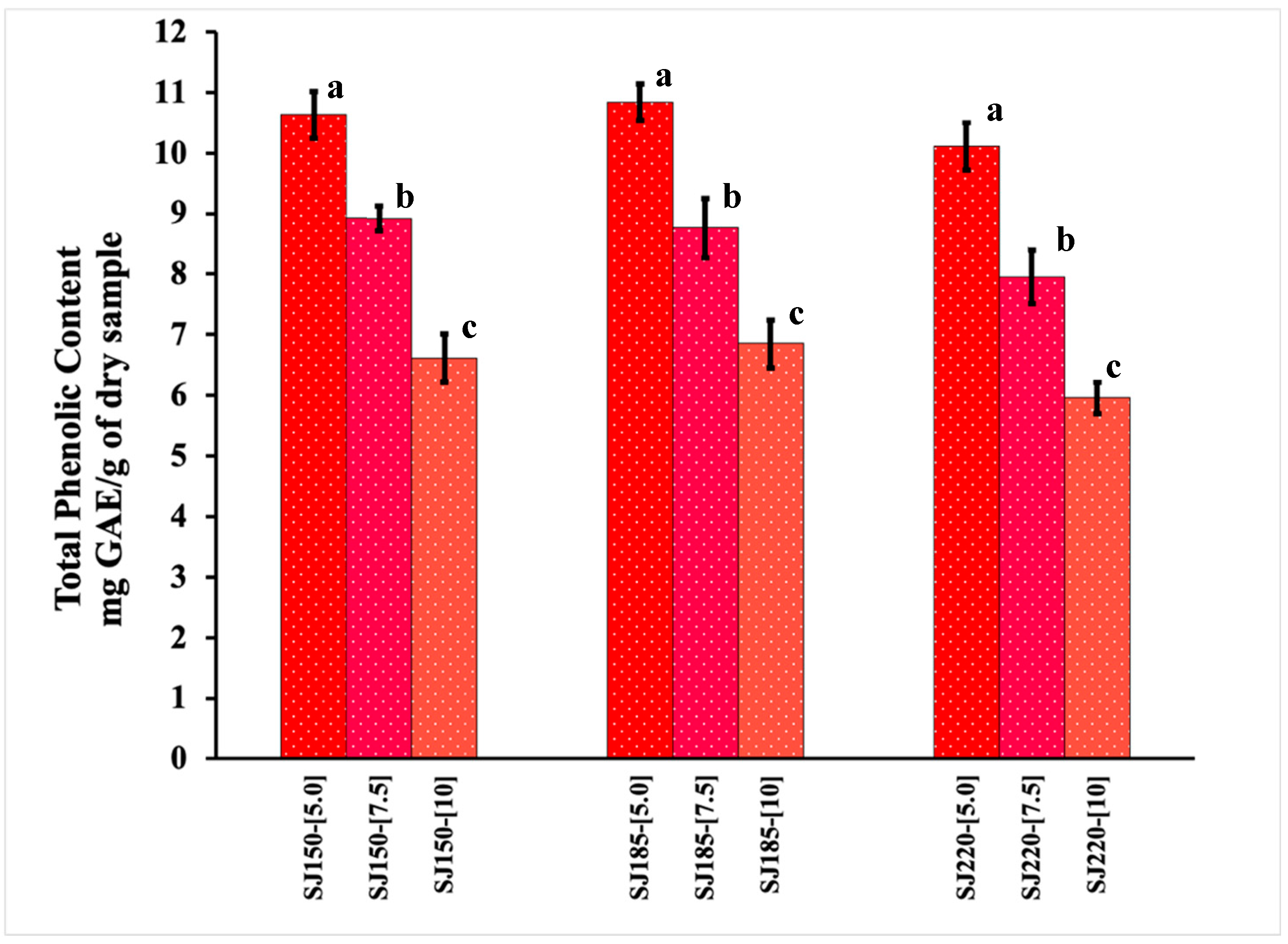
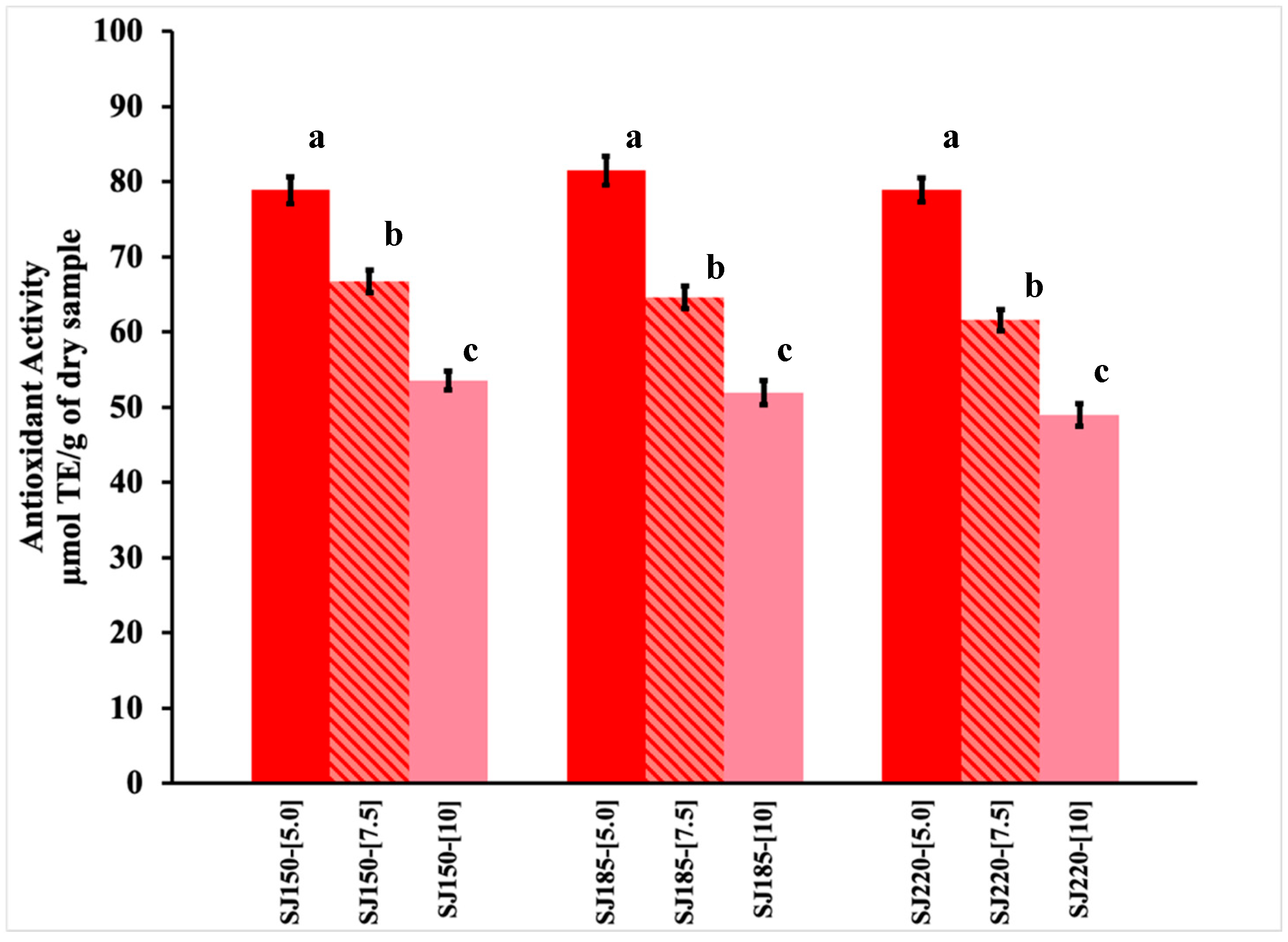
2.1. Total Phenolic Content and Antioxidant Activity
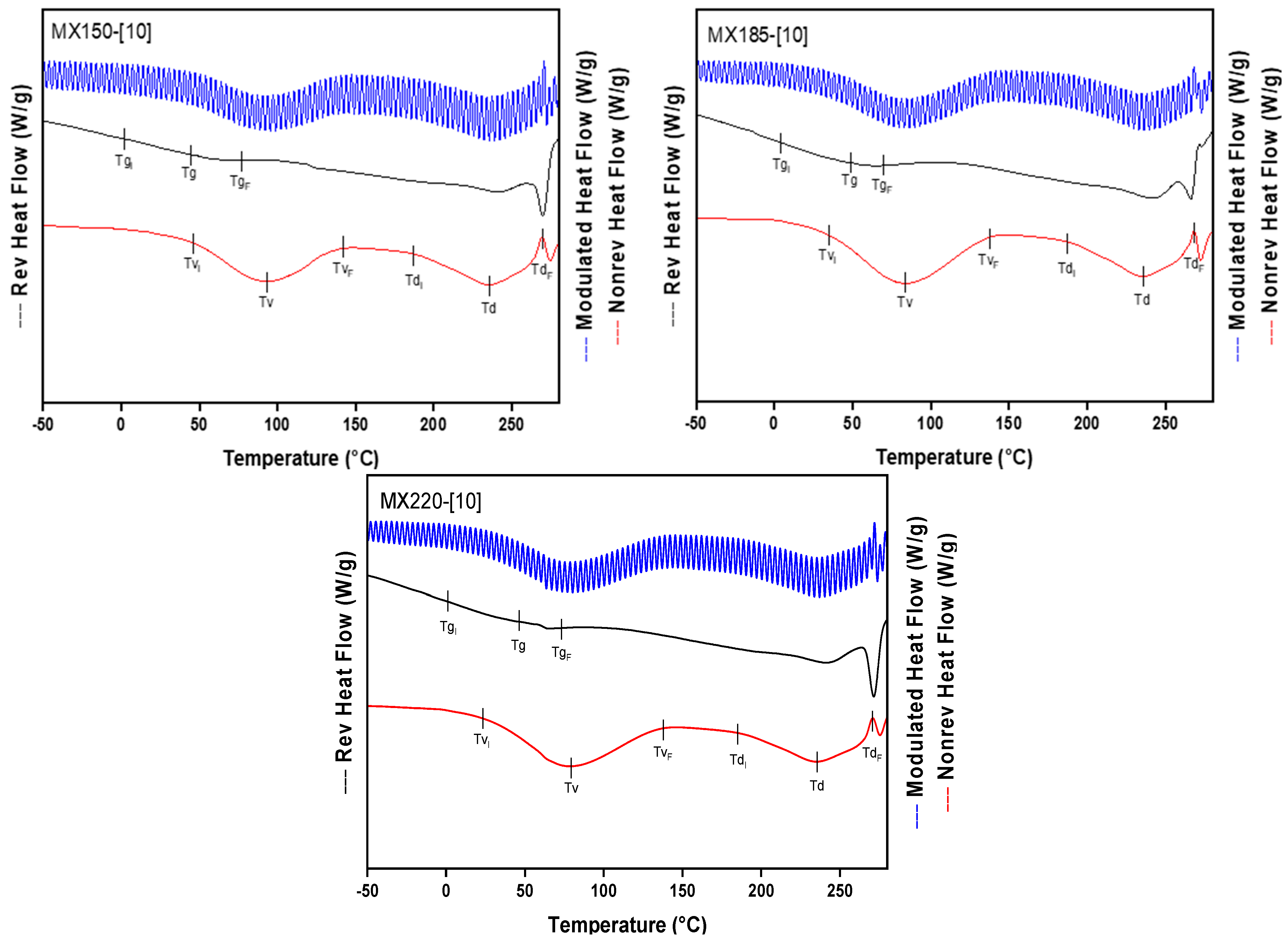
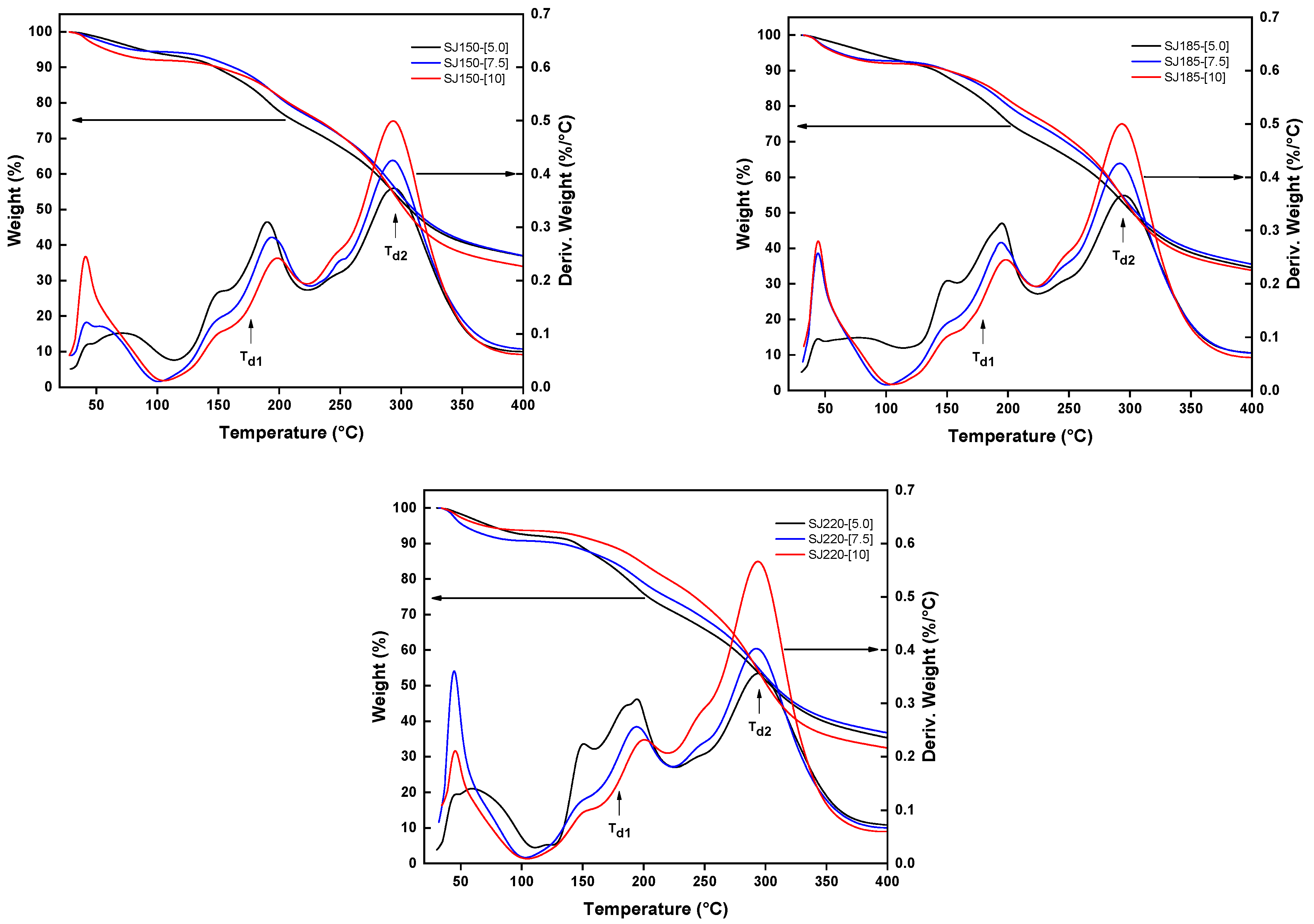
2.2. Thermal Characterization
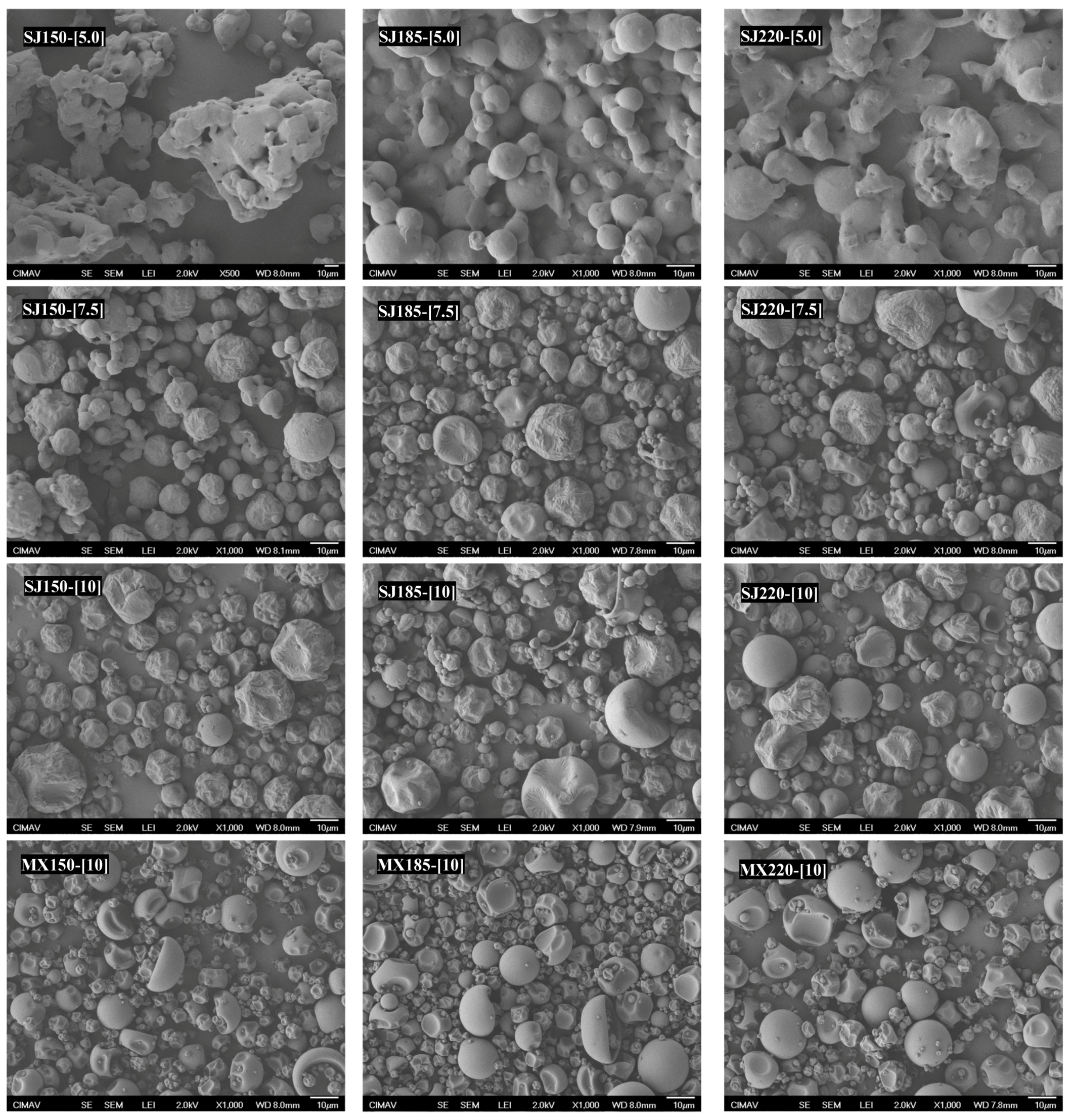
2.3. Microstructural Analysis
2.4. Microestructural Analysis by X-ray Driffraction (XRD)
3. Materials and Methods
3.1. Materials
3.2. Extraction of Strawberry Juice
3.3. Preparation of Spray-Dried Powders
3.4. Extraction of Bioactive Compounds from the SJ-MX Powder
3.4.1. Determination of the Total Phenolic Compounds
3.4.2. Antioxidant Activity by DPPH
3.5. Thermal Analysis
3.5.1. MDSC
3.5.2. TGA-DSC-SDT
3.6. Physicochemical Characterization
3.6.1. Scanning Electron Microscopy
3.6.2. X-ray Diffraction
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FAOSTAT. Crop and Livestock Products. 2019. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 30 July 2021).
- Li, C.; Huang, W.-Y.; Wang, X.-N.; Liu, W.-X. Oxygen Radical Absorbance Capacity of Different Varieties of Strawberry and the Antioxidant Stability in Storage. Molecules 2013, 18, 1528–1539. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H. Mass Spectrometry Imaging of Flavonols and Ellagic Acid Glycosides in Ripe Strawberry Fruit. Molecules 2020, 25, 4600. [Google Scholar] [CrossRef] [PubMed]
- Dzhanfezova, T.; Barba-Espín, G.; Müller, R.; Joernsgaard, B.; Hegelund, J.N.; Madsen, B.; Larsen, D.H.; Vega, M.M.; Toldam-Andersen, T.B. Anthocyanin profile, antioxidant activity and total phenolic content of a strawberry (Fragaria × ananassa Duch) genetic resource collection. Food Biosci. 2020, 36, 100620. [Google Scholar] [CrossRef]
- Sadowska, A.; Świderski, F.; Hallmann, E. Bioactive, Physicochemical and Sensory Properties as Well as Microstructure of Organic Strawberry Powders Obtained by Various Drying Methods. Appl. Sci. 2020, 10, 4706. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Román-Aguirre, M.; Cruz-Alcantar, P.; Pérez-Urizar, J.T.; Saavedra-Leos, M.Z. Application of Antioxidants as an Alternative Improving of Shelf Life in Foods. Polysaccharides 2021, 2, 594–607. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; López-Martínez, L.A.; González-García, R.; Martínez, J.O.; Compeán-Martínez, I.; Toxqui-Terán, A. Evaluation of the Spray Drying Conditions of Blueberry Juice-Maltodextrin on the Yield, Content, and Retention of Quercetin 3-d-Galactoside. Polymers 2019, 11, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Toxqui-Terán, A.; Espinosa-Solis, V. Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica oleracea var Italica) Stalk and Floret Juice Powders. Molecules 2021, 26, 1973. [Google Scholar] [CrossRef]
- Santhalakshmy, S.; Bosco, S.J.D.; Francis, S.; Sabeena, M. Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technol. 2015, 274, 37–43. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Martínez-Guerra, E.; Pérez-García, S.A.; Aguilar-Martínez, J.A.; Álvarez-Salas, C. Physical properties of inulin and inulin–orange juice: Physical characterization and technological application. Carbohydr. Polym. 2014, 105, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Yu, M.; Wang, W.; Shi, X. Functionality of spray-dried strawberry powder: Effects of whey protein isolate and maltodextrin. Int. J. Food Prop. 2018, 21, 2229–2238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, C.; Chen, X.; Quek, S.-Y. Effect of spray drying on phenolic compounds of cranberry juice and their stability during storage. J. Food Eng. 2020, 269, 109744. [Google Scholar] [CrossRef]
- Araujo-Díaz, S.B.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chaves, V.C.; Calvete, E.; Reginatto, F.H. Quality properties and antioxidant activity of seven strawberry (Fragaria x ananassa Duch) cultivars. Sci. Hortic. 2017, 225, 293–298. [Google Scholar] [CrossRef]
- Martinsen, B.K.; Aaby, K.; Skrede, G. Effect of temperature on stability of anthocyanins, ascorbic acid and color in strawberry and raspberry jams. Food Chem. 2020, 316, 126297. [Google Scholar] [CrossRef]
- Álvarez-Fernández, M.A.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; García-Parrilla, M.C. Effects of the strawberry (Fragaria ananassa) purée elaboration process on non-anthocyanin phenolic composition and antioxidant activity. Food Chem. 2014, 164, 104–112. [Google Scholar] [CrossRef]
- Żary-Sikorska, E.; Fotschki, B.; Jurgoński, A.; Kosmala, M.; Milala, J.; Kołodziejczyk, K.; Majewski, M.; Ognik, K.; Juśkiewicz, J. Protective Effects of a Strawberry Ellagitannin-Rich Extract against Pro-Oxidative and Pro-Inflammatory Dysfunctions Induced by a High-Fat Diet in a Rat Model. Molecules 2020, 25, 5874. [Google Scholar] [CrossRef]
- Daza, L.D.; Fujita, A.; Granato, D.; Fávaro-Trindade, C.S.; Genovese, M.I. Functional properties of encapsulated Cagaita (Eugenia dysenterica DC.) fruit extract. Food Biosci. 2017, 18, 15–21. [Google Scholar] [CrossRef]
- López-Belchí, M.D.; Caamaño, E.F.; Pascual, G.; Noriega, F.; Fierro-Morales, P.; Romero-Román, M.E.; Jara, P.; Schoebitz, M.; Serra, I.; Moreno, D.A. Spray-Dried Formulations Rich in Malvidin from Tintorera Grape Wastes: Characterization, Stability, and Storage. Processes 2021, 9, 518. [Google Scholar] [CrossRef]
- Suriñach, S.; Baro, M.; Bordas, S.; Clavaguera, N.; Clavaguera-Mora, M.T. La calorimetría diferancial de barrido y su aplicación a la ciencia de materiales. Bol. De La Soc. Española De Ceram. Y Vidr. 1992, 31, 11–17. [Google Scholar]
- Verdonck, E.; Schaap, K.; Thomas, L.C. A discussion of the principles and applications of modulated temperature DSC (MTDSC). Int. J. Pharm. 1999, 192, 3–20. [Google Scholar] [CrossRef]
- Sablani, S.S.; Syamaladevi, R.M.; Swanson, B.G. A review of methods, data and applications of state diagrams of food systems. Food Eng. Rev. 2010, 2, 168–203. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compeán-Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Saavedra-Leos, Z.; Leyva-Porras, C.; Araujo-Díaz, S.B.; Toxqui-Terán, A.; Borrás-Enríquez, A.J. Technological Application of Maltodextrins According to the Degree of Polymerization. Molecules 2015, 20, 21067–21081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra–Leos, M.Z.; Leyva-Porras, C.; Alvarez-Salas, C.; Longoria-Rodríguez, F.; López-Pablos, A.L.; González-García, R.; Pérez-Urizar, J.T. Obtaining orange juice–maltodextrin powders without structure collapse based on the glass transition temperature and degree of polymerization. CyTA-J. Food 2018, 16, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Nemzer, B.; Vargas, L.; Xia, X.; Sintara, M.; Feng, H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef]
- Hashib, S.A.; Rahman, N.A.; Suzihaque, M.U.H.; Ibrahim, U.K.; Hanif, N.E. Effect of Slurry Concentration and Inlet Temperature Towards Glass Temperature of Spray Dried Pineapple Powder. Procedia-Soc. Behav. Sci. 2015, 195, 2660–2667. [Google Scholar] [CrossRef] [Green Version]
- Tonon, R.V.; Baroni, A.F.; Brabet, C.; Gibert, O.; Pallet, D.; Hubinger, M.D. Water sorption and glass transition temperature of spray dried açai (Euterpe oleracea Mart.) juice. J. Food Eng. 2009, 94, 215–221. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A new technique for spray drying orange juice concentrate. Innov. Food Sci. Emerg. Technol. 2010, 11, 342–351. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Grajales, A.; González, R.; Toxqui-Teran, A.; Pérez, S.; Abud, M.; Ruiz, M. Glass Transition Study in Model Food Systems Prepared with Mixtures of Fructose, Glucose, and Sucrose. J. Food Sci. 2012, 77, E118–E126. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Yekdane, N.; Goli, S.A.H. Effect of Pomegranate Juice on Characteristics and Oxidative Stability of Microencapsulated Pomegranate Seed Oil Using Spray Drying. Food Bioprocess Technol. 2019, 12, 1614–1625. [Google Scholar] [CrossRef]
- Przybył, K.; Gawałek, J.; Koszela, K.; Wawrzyniak, J.; Gierz, L. Artificial neural networks and electron microscopy to evaluate the quality of fruit and vegetable spray-dried powders. Case study: Strawberry powder. Comput. Electron. Agric. 2018, 155, 314–323. [Google Scholar] [CrossRef]
- Vázquez-Maldonado, D.; Espinosa-Solis, V.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Martinez-Gutierrez, F.; Román-Aguirre, M.; Saavedra-Leos, M.Z. Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol. Processes 2020, 8, 849. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]


| SJ150-[5.0] | SJ150-[7.5] | SJ150-[10] | SJ185-[5.0] | SJ185-[7.5] | SJ185-[10] | SJ220-[5.0] | SJ220-[7.5] | SJ220-[10] | |
|---|---|---|---|---|---|---|---|---|---|
| Tgi (°C) | −13.85 | −14.96 | −8.03 | −7.67 | −2.33 | −8.25 | −9.41 | −9.96 | −11.35 |
| Tg (°C) | 10 | 13.06 | 16.38 | 10.33 | 20.39 | 21 | 12.5 | 24.43 | 21.93 |
| Tgf (°C) | 28.87 | 37.19 | 43.29 | 25.98 | 38.2 | 38.93 | 25.26 | 44.96 | 44.96 |
| Run | MX Concentration (%) | Inlet Temperature (°C) | Identification |
|---|---|---|---|
| 1 | 5.0 | 150 | SJ150-[5.0] |
| 2 | 5.0 | 185 | SJ185-[5.0] |
| 3 | 5.0 | 220 | SJ220-[5.0] |
| 4 | 7.5 | 150 | SJ150-[7.5] |
| 5 | 7.5 | 185 | SJ185-[7.5] |
| 6 | 7.5 | 220 | SJ220-[7.5] |
| 7 | 10.0 | 150 | SJ150-[10] |
| 8 | 10.0 | 185 | SJ185-[10] |
| 9 | 10.0 | 220 | SJ220-[10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Porras, C.; Saavedra-Leos, M.Z.; López-Martinez, L.A.; Espinosa-Solis, V.; Terán-Figueroa, Y.; Toxqui-Terán, A.; Compeán-Martínez, I. Strawberry Juice Powders: Effect of Spray-Drying Conditions on the Microencapsulation of Bioactive Components and Physicochemical Properties. Molecules 2021, 26, 5466. https://doi.org/10.3390/molecules26185466
Leyva-Porras C, Saavedra-Leos MZ, López-Martinez LA, Espinosa-Solis V, Terán-Figueroa Y, Toxqui-Terán A, Compeán-Martínez I. Strawberry Juice Powders: Effect of Spray-Drying Conditions on the Microencapsulation of Bioactive Components and Physicochemical Properties. Molecules. 2021; 26(18):5466. https://doi.org/10.3390/molecules26185466
Chicago/Turabian StyleLeyva-Porras, César, María Zenaida Saavedra-Leos, Laura Araceli López-Martinez, Vicente Espinosa-Solis, Yolanda Terán-Figueroa, Alberto Toxqui-Terán, and Isaac Compeán-Martínez. 2021. "Strawberry Juice Powders: Effect of Spray-Drying Conditions on the Microencapsulation of Bioactive Components and Physicochemical Properties" Molecules 26, no. 18: 5466. https://doi.org/10.3390/molecules26185466
APA StyleLeyva-Porras, C., Saavedra-Leos, M. Z., López-Martinez, L. A., Espinosa-Solis, V., Terán-Figueroa, Y., Toxqui-Terán, A., & Compeán-Martínez, I. (2021). Strawberry Juice Powders: Effect of Spray-Drying Conditions on the Microencapsulation of Bioactive Components and Physicochemical Properties. Molecules, 26(18), 5466. https://doi.org/10.3390/molecules26185466









