Enhanced Efficiency of the Removal of Cytostatic Anthracycline Drugs Using Immobilized Mycelium of Bjerkandera adusta CCBAS 930
Abstract
1. Introduction
2. Results
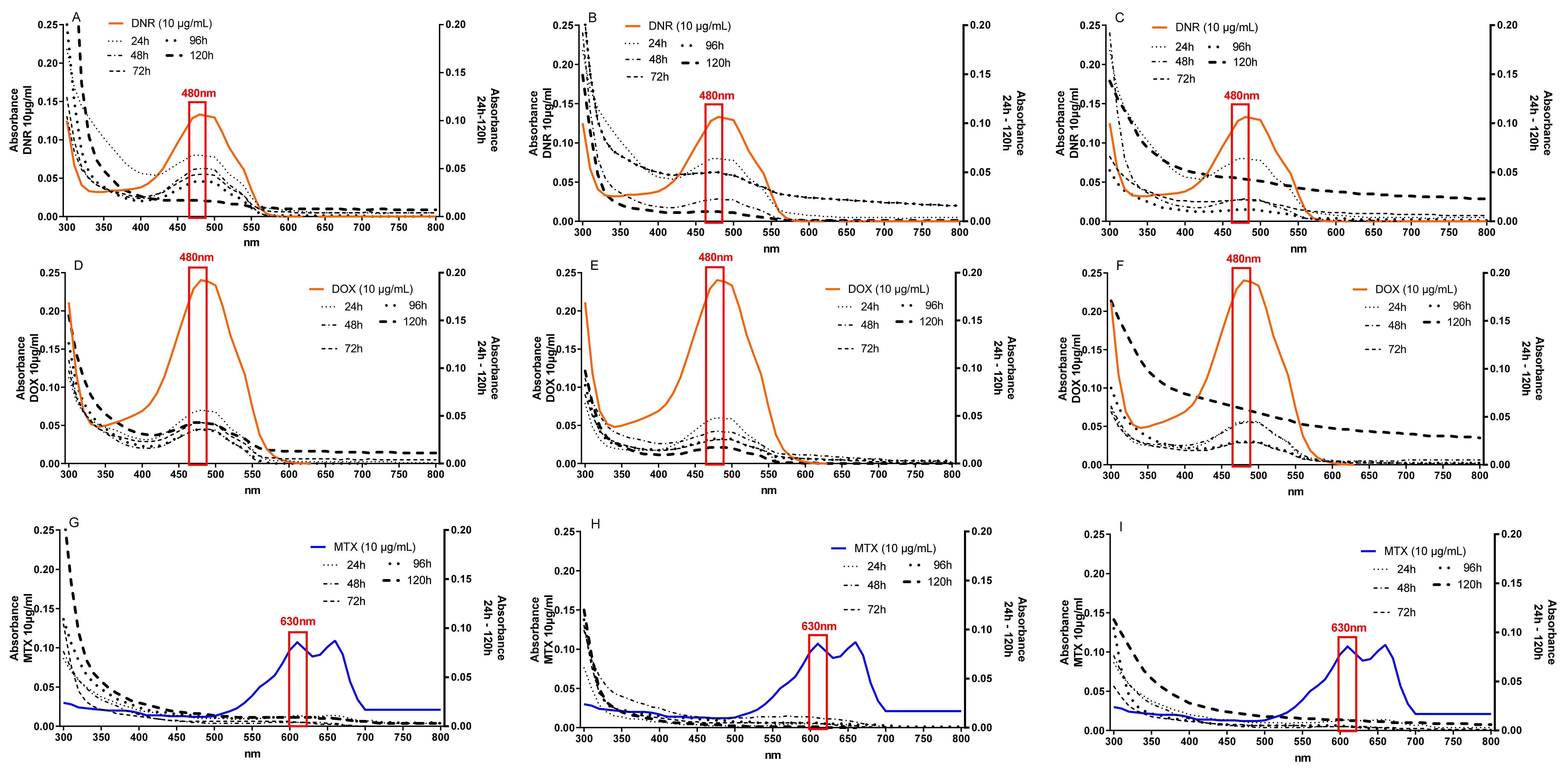
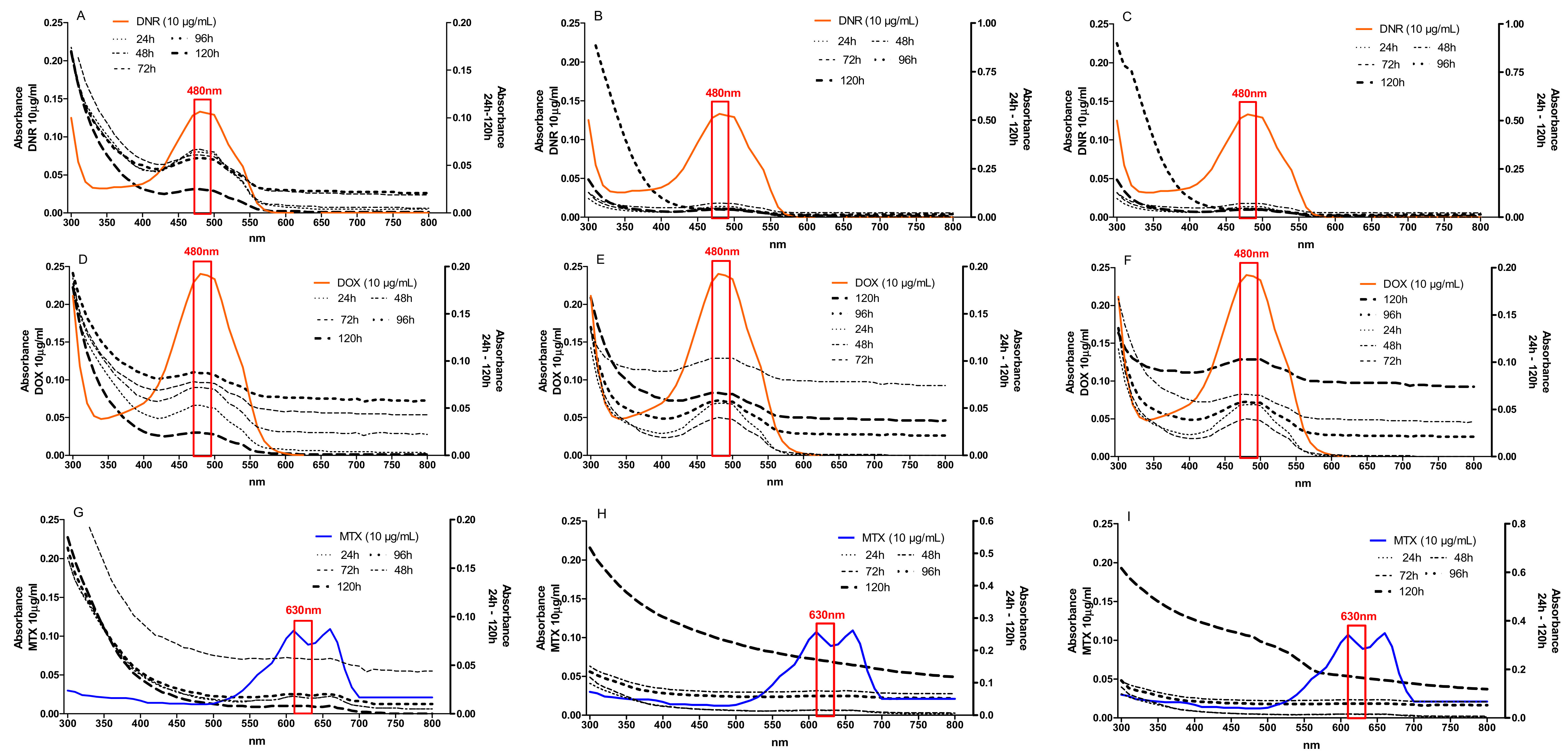
2.1. Efficiency of Anthracycline Removal Using Immobilized Mycelium of B. adusta CCAS 930
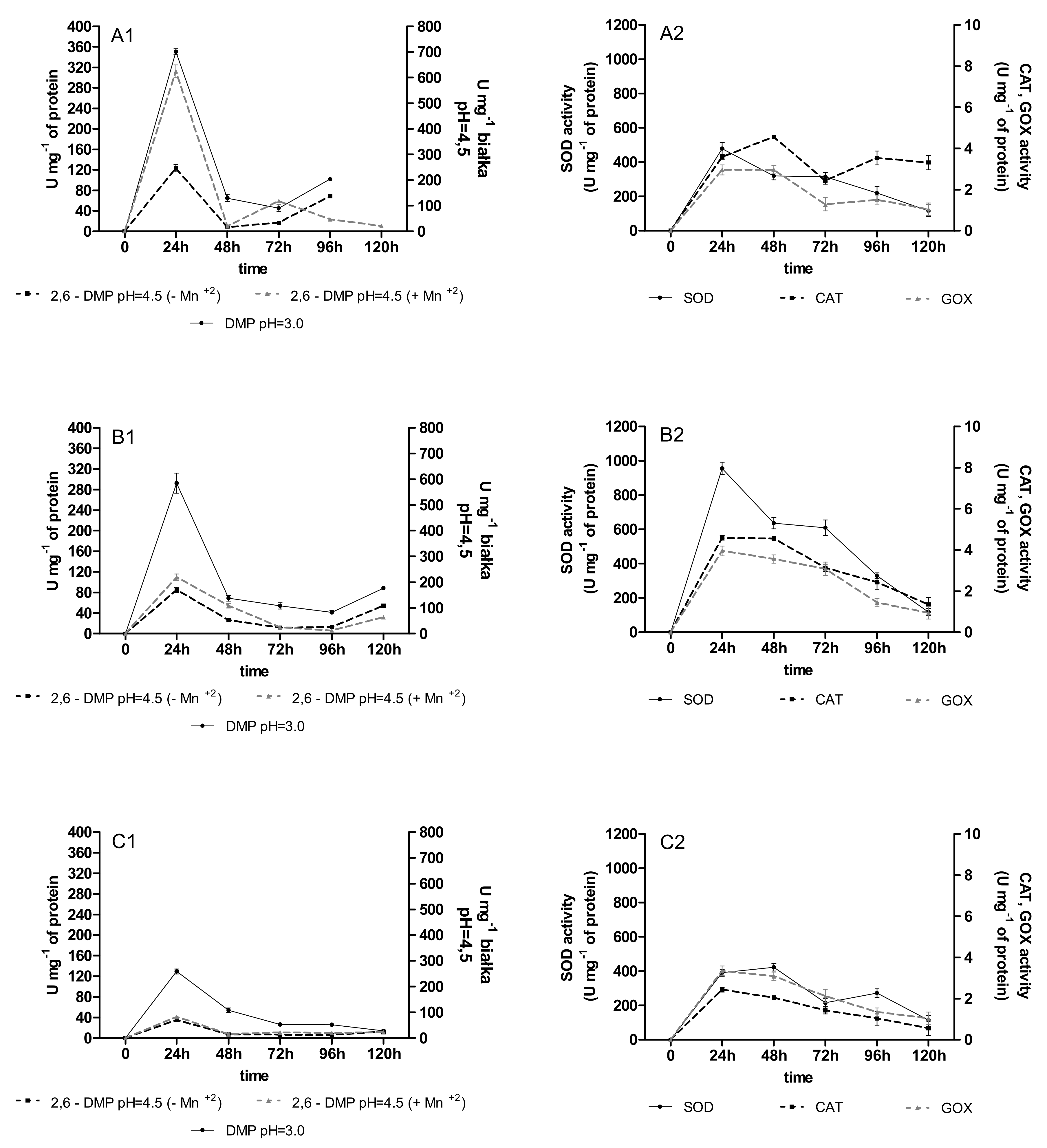
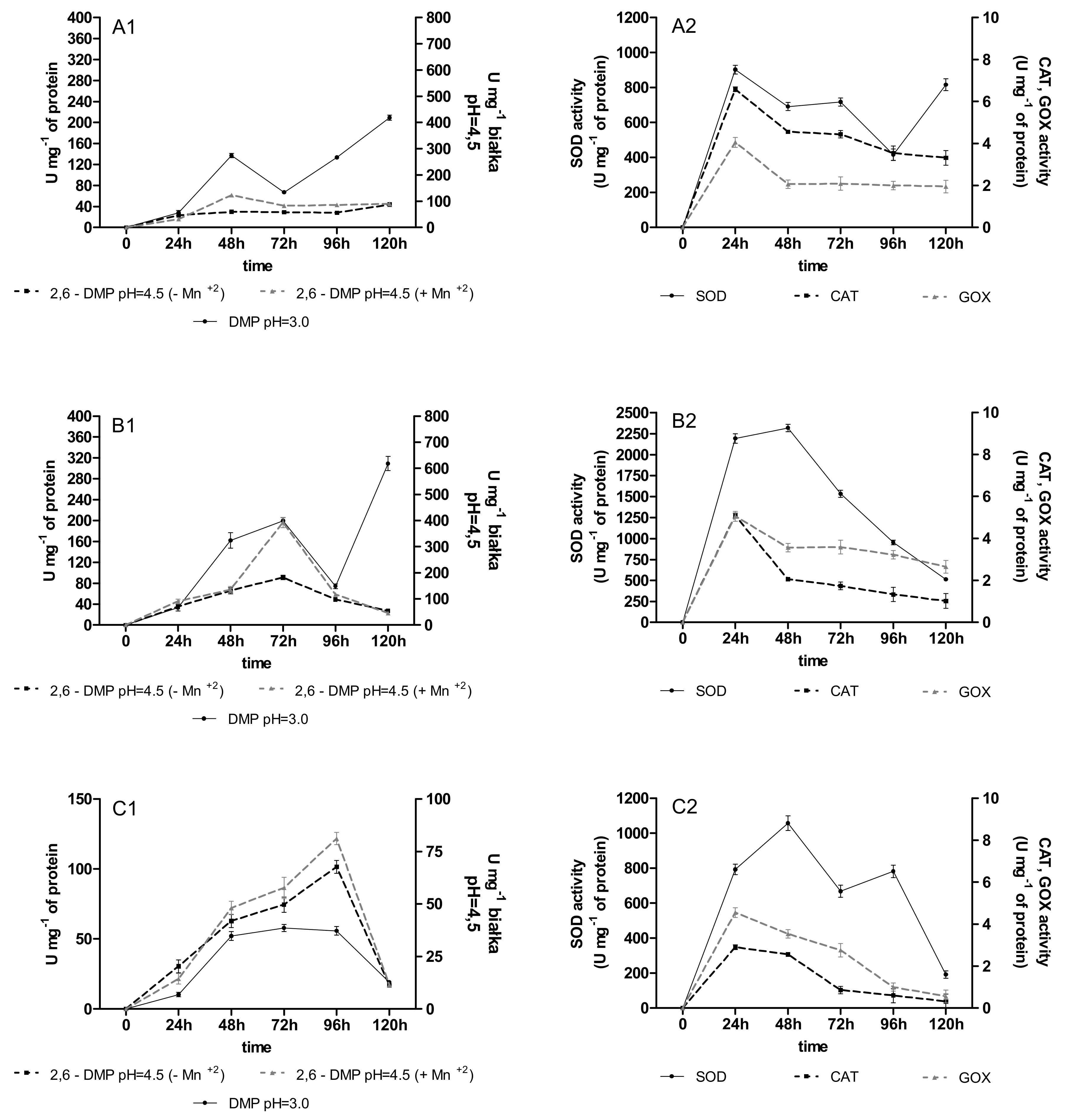
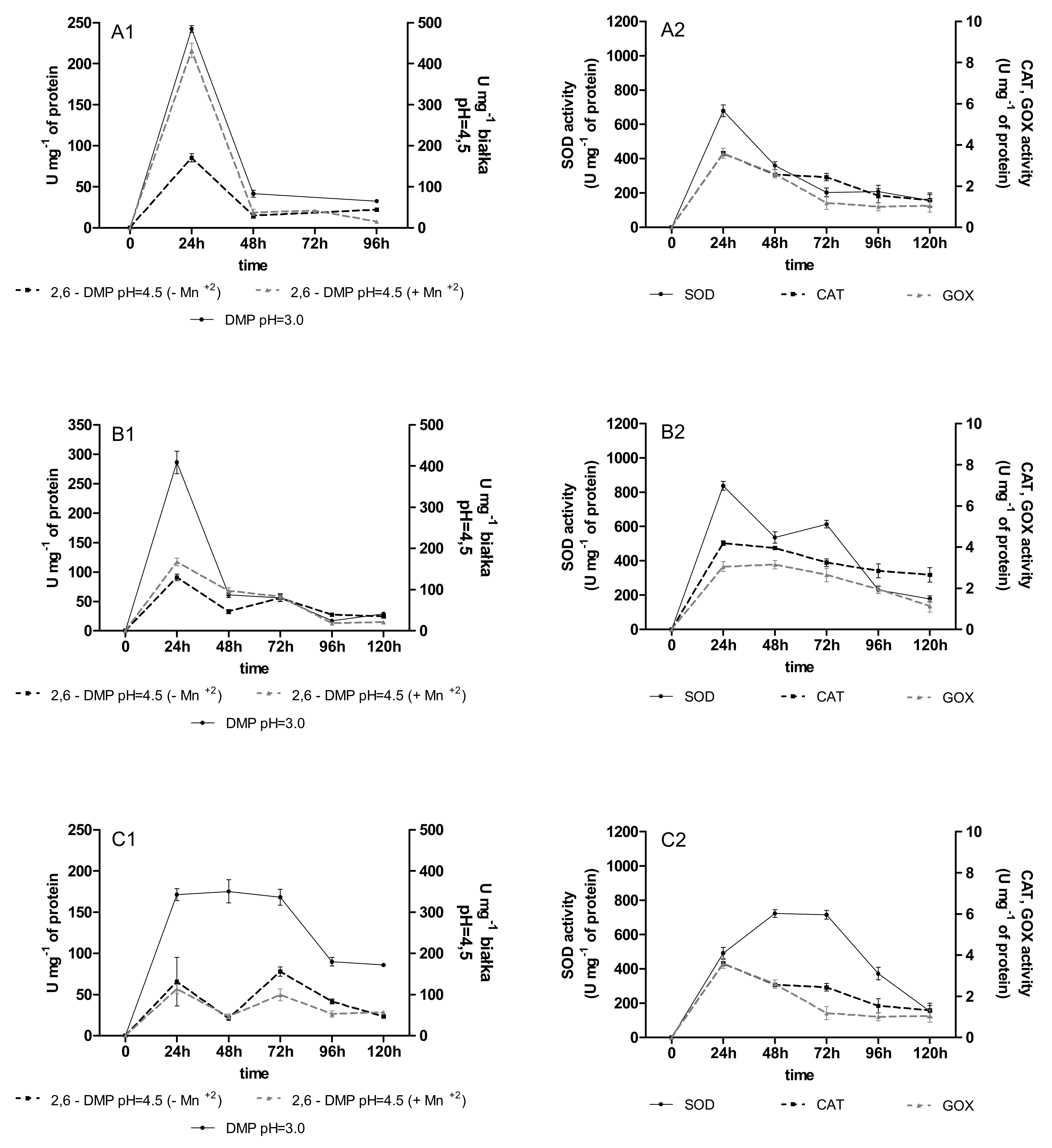
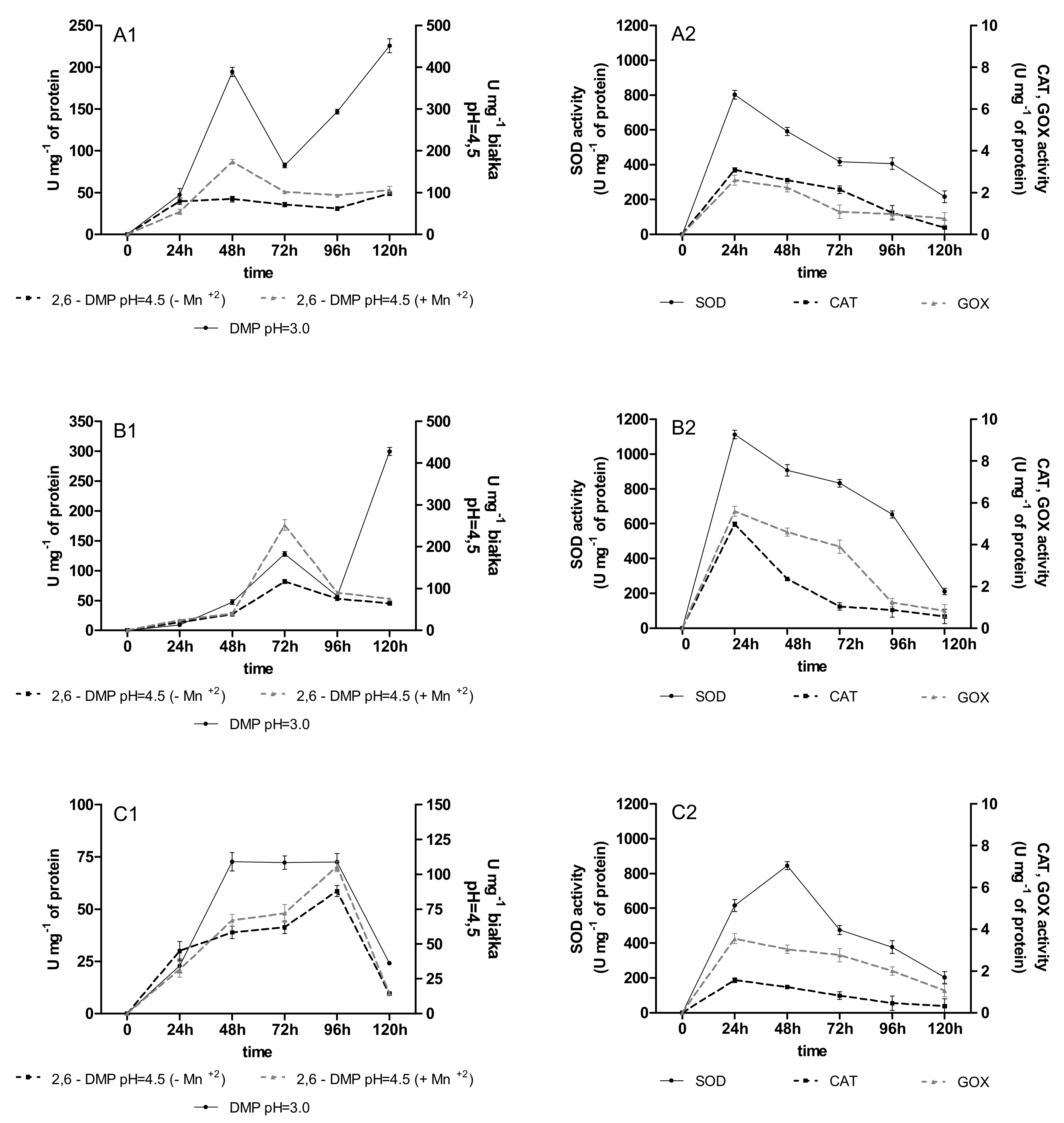
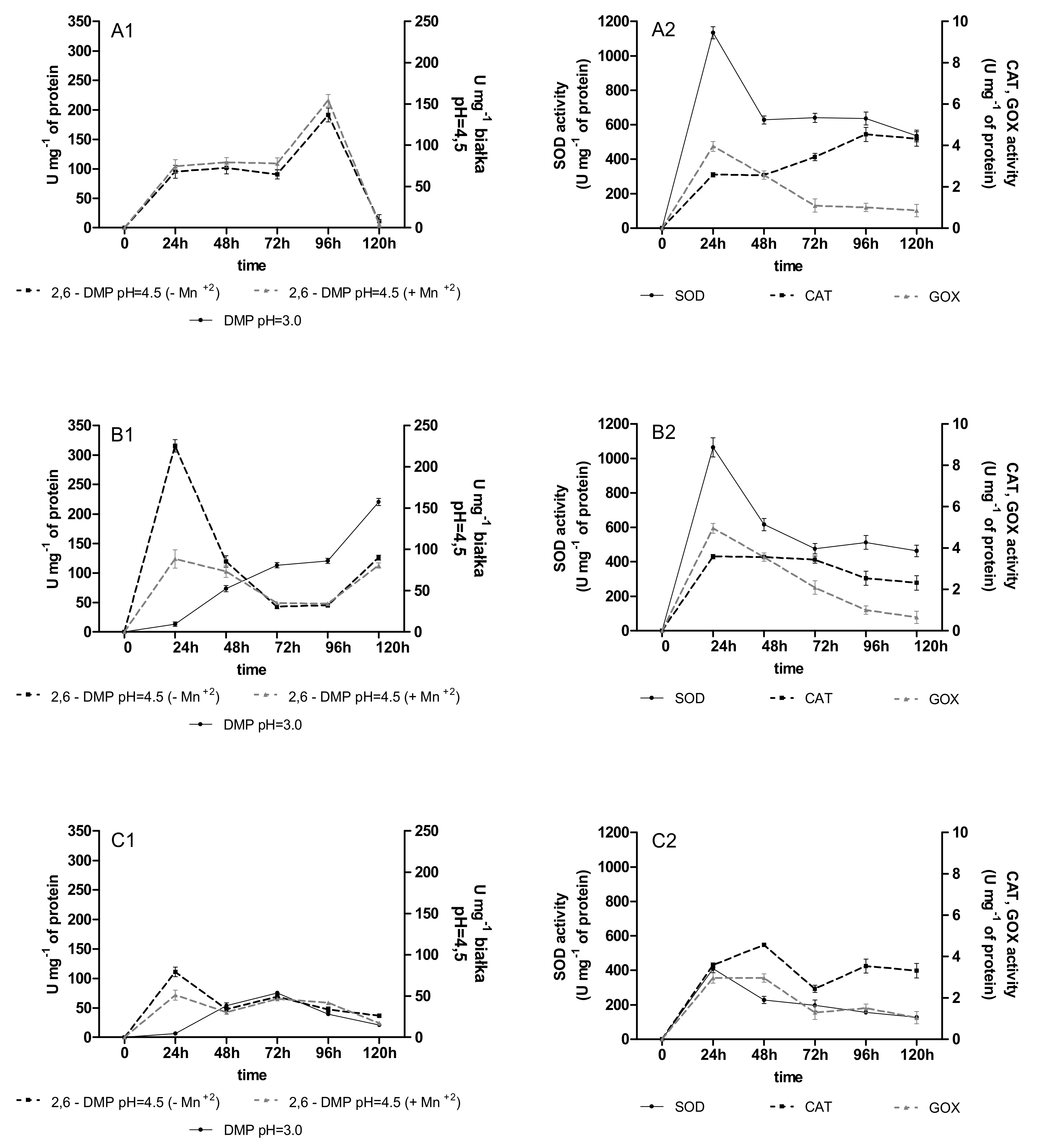
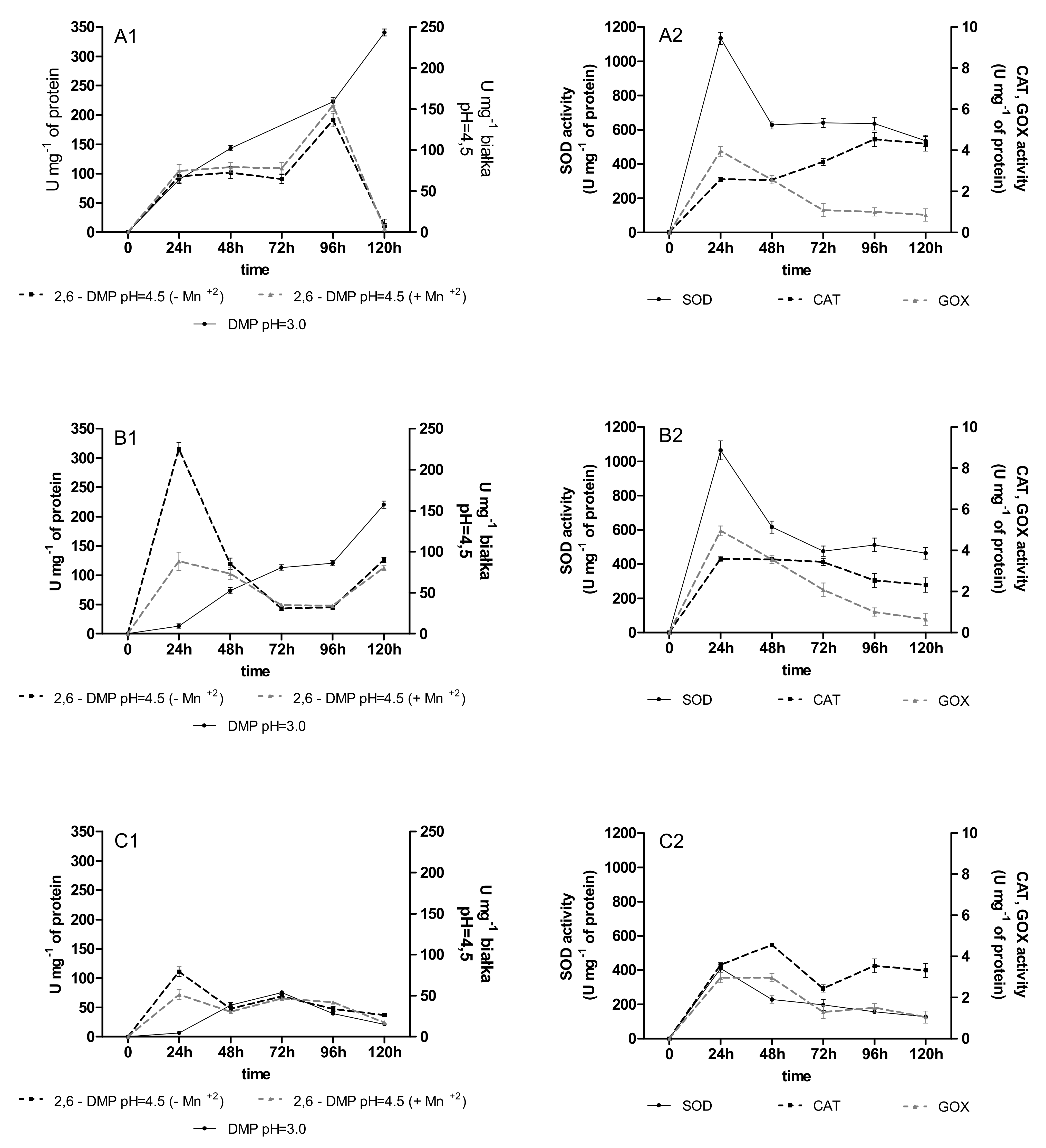
2.2. Activities of Oxidoreductases
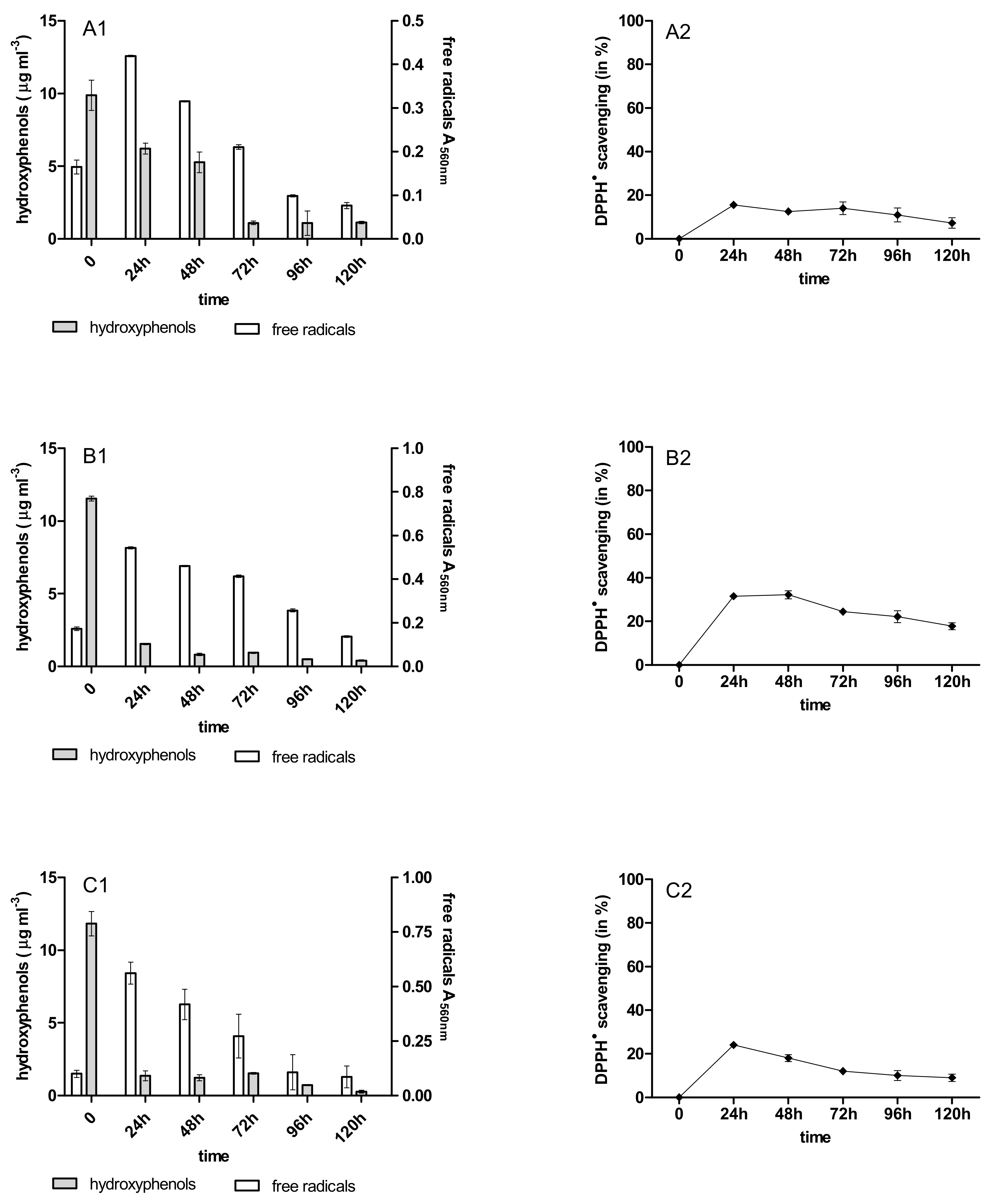
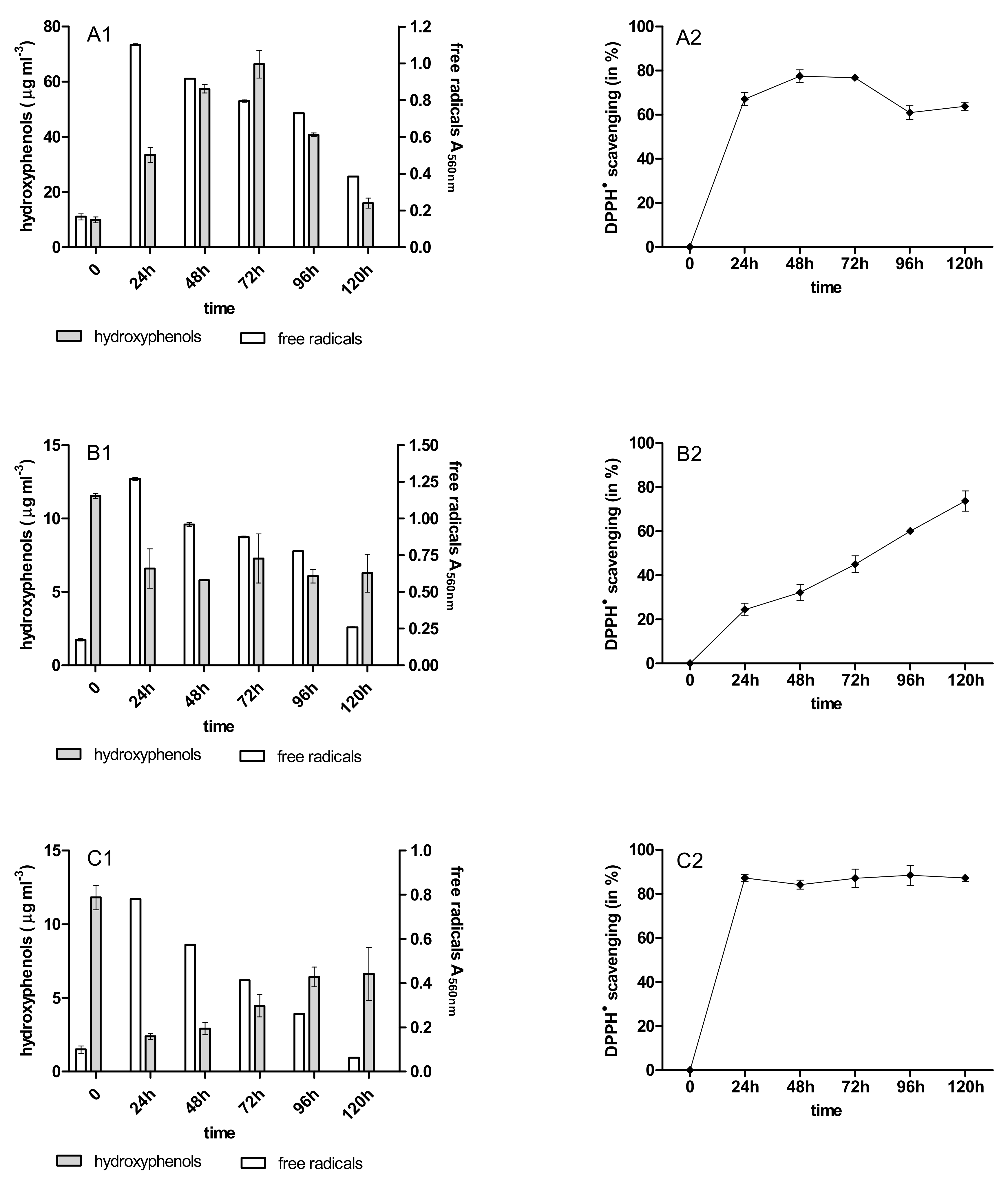
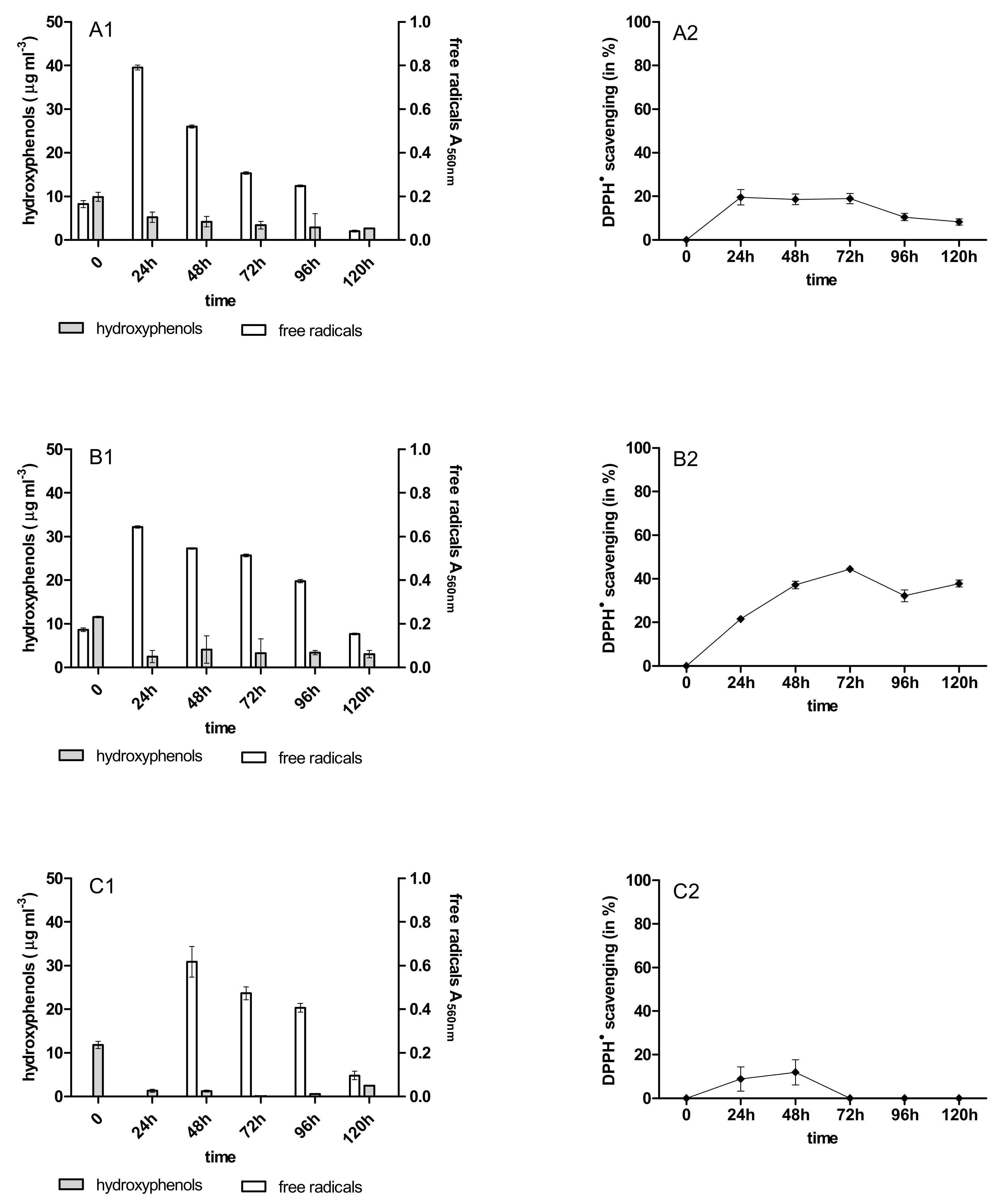
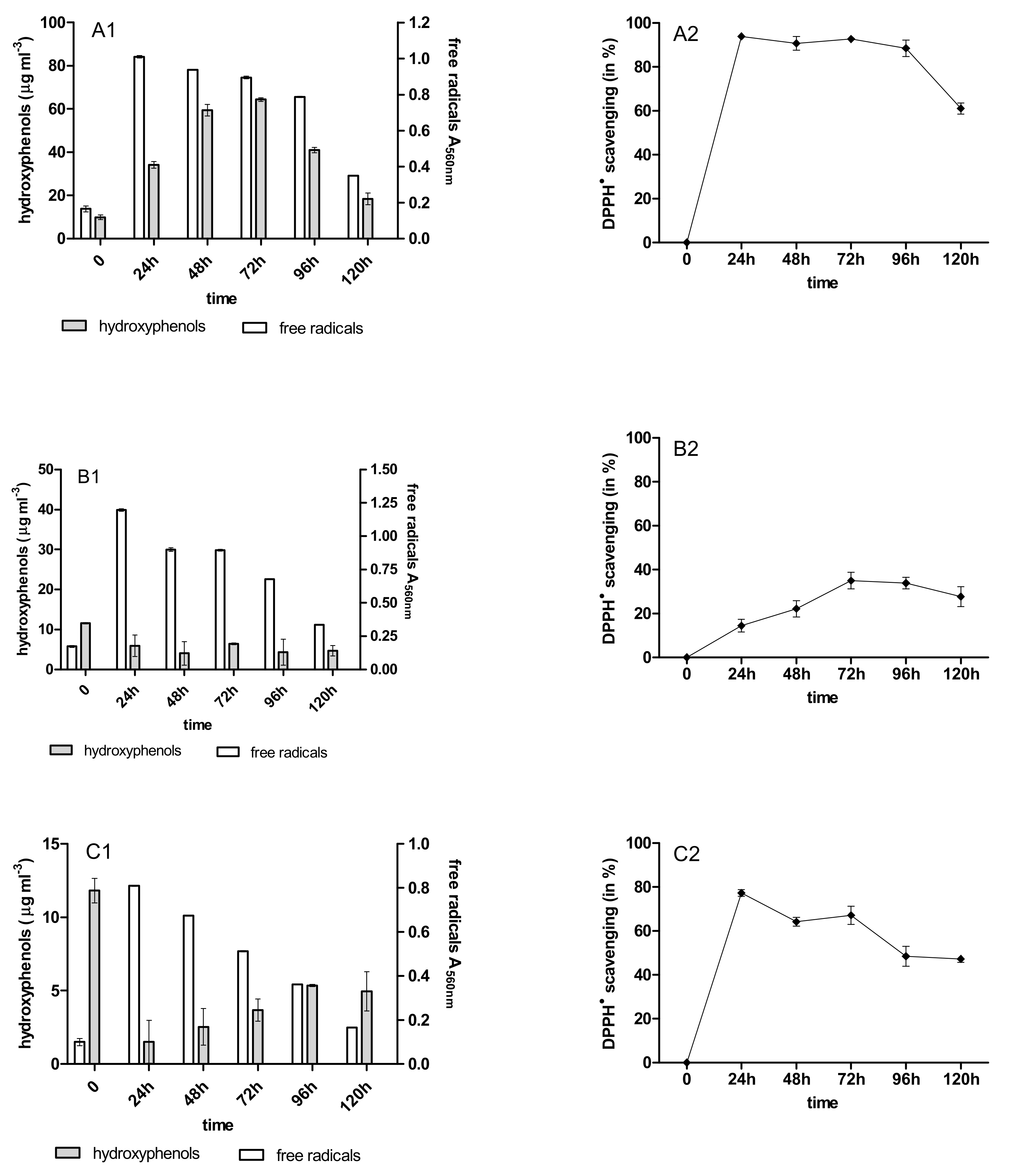
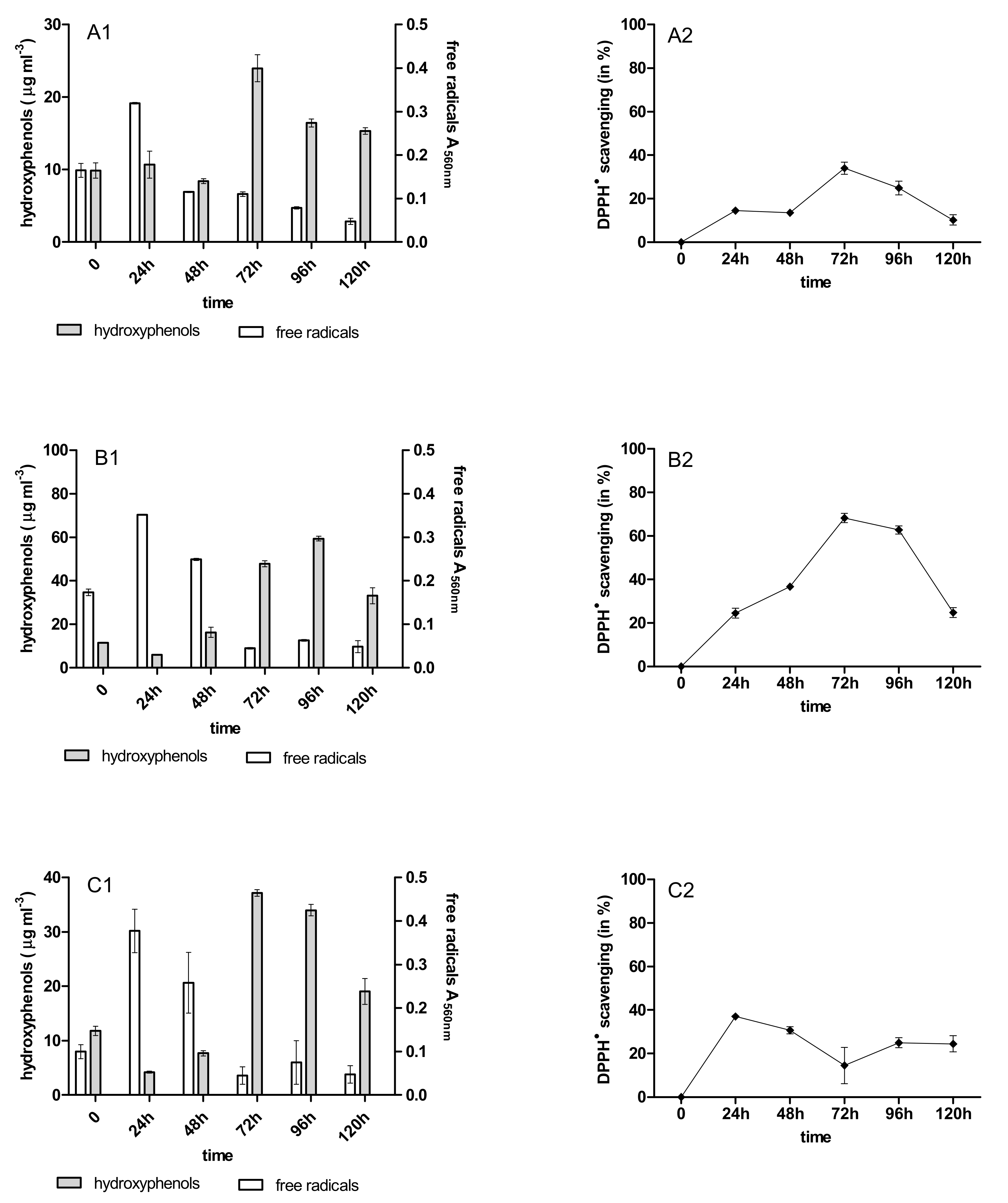
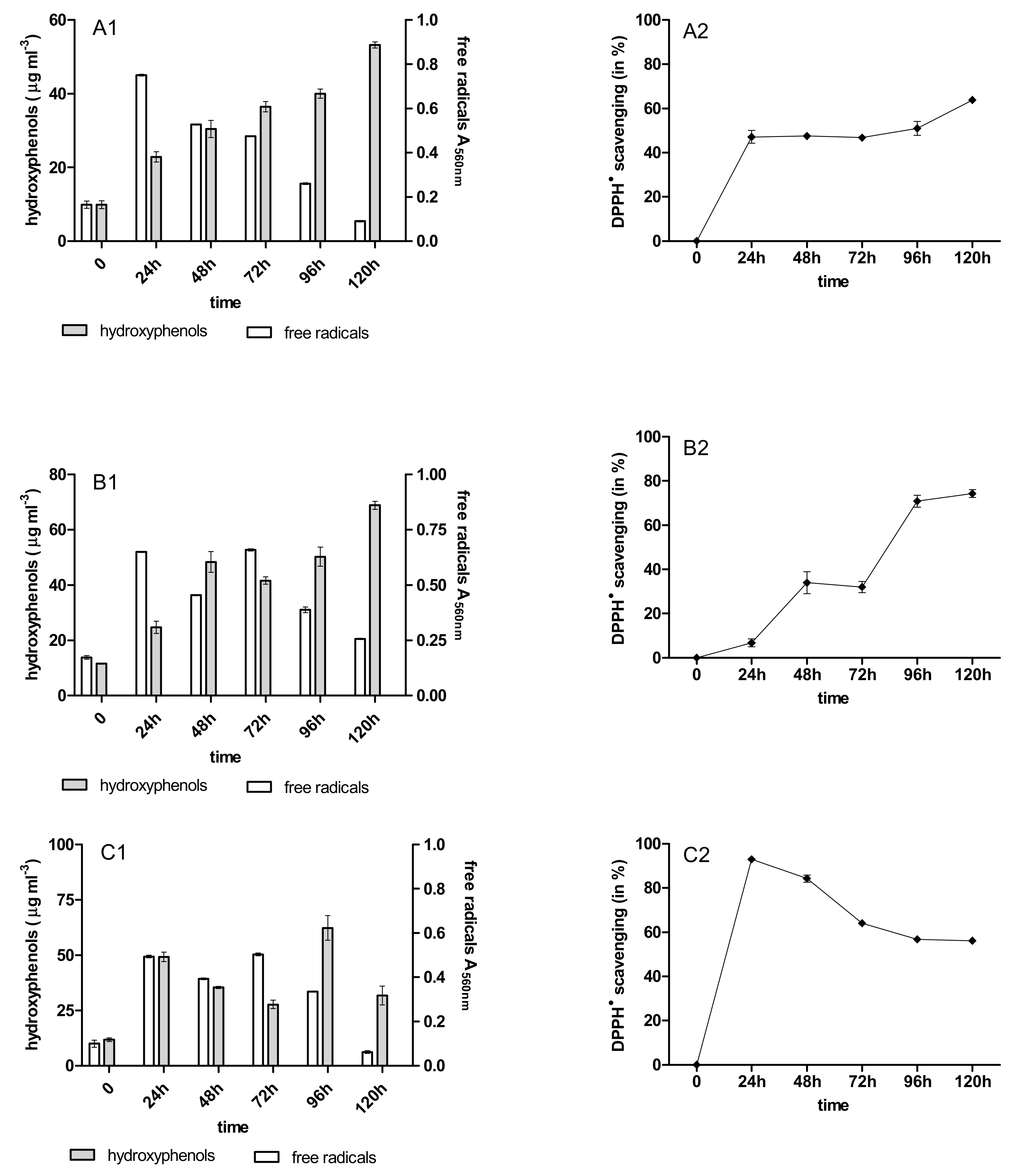
2.3. Content of Phenolic Acids (PhC) and Free Radicals (SOR)
2.4. Antioxidant Activity of PhC after Anthracycline Biotreatment
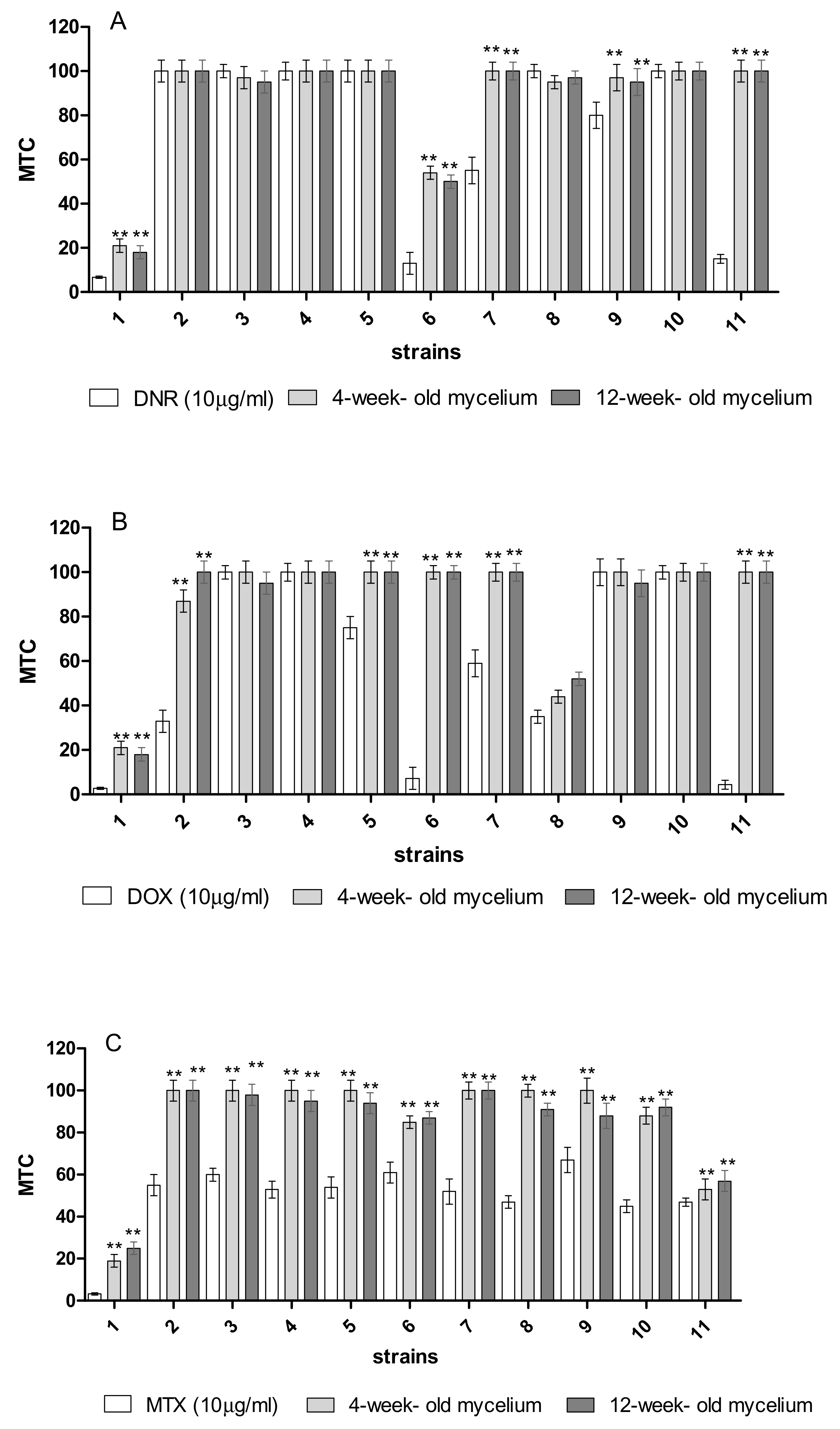
2.5. Detoxification of Anthracyclines by Immobilized Mycelium of B. adusta CCBAS 930
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cultures of B. adusta CCBAS 930
4.3. Immobilization of B. adusta CCBAS 930 Mycelium
4.4. Determination of Storage Conditions and Reusability of Immobilized B. adusta CCBAS 930 Mycelium
4.5. Anthracycline Removal Using Immobilized Cells of B. adusta CCAS 930
4.6. Estimation of Oxidoreductase Activity
4.7. Estimation of PhC and SOR
4.8. Determination of the Antioxidative Activity of Initial Anthracycline Solutions and Post-Culture Fluids of B. adusta CCBAS 930
4.9. Estimation of the Toxicity of Anthracyclines in Immobilized Cultures of B. adusta CCBAS 930
4.9.1. Phytotoxicity Assay
4.9.2. Multi-Species Microbial Assay (MARA)
4.9.3. Genotoxicity Assay
4.10. Data Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A

References
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Chugun, A.; Uchide, T.; Tsurimaki, C.; Nagasawa, H.; Sasaki, T.; Ueno, S.; Takagishi, K.; Hara, Y.; Temma, K. Mechanisms responsible for reduced cardiotoxicity of mitoxantrone compared to doxorubicin examined in isolated guinea-pig heart preparations. J. Vet. Med. Sci. 2008, 70, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.; Mahnik, S.N.; Weissenbacher, N.; Mader, R.M.; Krenn, P.; Hann, S.; Koellensperger, G.; Uhl, M.; Knasmüller, S.; Ferk, F.; et al. Monitoring, removal and risk assessment of cytostatic drugs in hospital wastewater. Water Sci. Technol. 2007, 56, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mahnik, S.N.; Lenz, K.; Weissenbacher, N.; Mader, R.M.; Fuerhacker, M. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 2007, 66, 30–37. [Google Scholar] [CrossRef]
- Malakar, A.; Snow, D.D.; Ray, C. Irrigation water quality-A contemporary perspective. Water 2019, 11, 1482. [Google Scholar] [CrossRef]
- Pereira, C.S.; Kelbert, M.; Daronch, N.A.; Michels, C.; de Oliveira, D.; Soares, H.M. Potential of enzymatic process as an innovative technology to remove anticancer drugs in wastewater. Appl. Microbiol. Biotechnol. 2020, 104, 23–31. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Korniłłowicz-Kowalska, T.; Szychowski, K.A. Possibility to Biotransform Anthracyclines by Peroxidases Produced by Bjerkandera adusta CCBAS 930 with Reduction of Geno- and Cytotoxicity and Pro-Oxidative Activity. Molecules 2021, 26, 462. [Google Scholar] [CrossRef]
- Jureczko, M.; Kalka, J. Cytostatic pharmaceuticals as water contaminants. Eur. J. Pharmacol. 2020, 866, 172816. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologie developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Jafarizad, A.; Rostamizadeh, M.; Zarei, M.; Gharibian, S. Mitoxantrone removal by electrochemical method: A comparison of homogenous and heterogenous catalytic reactions. Environ. Health Eng. Manag. 2017, 4, 185–193. [Google Scholar] [CrossRef][Green Version]
- Stenglová-Netíková, I.R.; Petruzelka, L.; Stastny, M.; Stengl, V. Anthracycline antibiotics derivate mitoxantrone—Destructive sorption and photocatalytic degradation. PLoS ONE 2018, 13, e0193116. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef]
- Prieto, A.; Möder, M.; Rodil, R.; Adrian, L.; Marco-Urrea, E. Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour. Technol. 2011, 102, 10987–10995. [Google Scholar] [CrossRef]
- Muter, O.; Perkons, I.; Selga, T.; Berzins, A.; Gudra, D.; Radovica-Spalvina, I.; Fridmanis, D.; Bartkevics, V. Removal of pharmaceuticals from municipal wastewaters at laboratory scale by treatment with activated sludge and biostimulation. Sci. Total Environ. 2017, 584–585, 402–413. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Rybczyńska-Tkaczyk, K. Decolorization and biodegradation of melanoidin contained in beet molasses by an anamorphic strain of Bjerkandera adusta CCBAS930 and its mutants. World J. Microbiol. Biotechnol. 2021, 37, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.U. In vitro degradation of carbamazepine and diclofenac by crude lignin peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092. [Google Scholar] [CrossRef]
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J. Clean. Prod. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Taboada-Puig, R.; Lu-Chau, T.A.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Continuous removal of endocrine disruptors by versatile peroxidase using a two-stage system. Biotechnol. Prog. 2015, 31, 908–916. [Google Scholar] [CrossRef]
- Torres, E.; Bustos-Jaimes, I.; Le Borgne, S. Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl. Catal. B Environ. 2003, 46, 1–15. [Google Scholar] [CrossRef]
- Pan, H.; Xu, X.; Wen, Z.; Kang, Y.; Wang, X.; Ren, Y.; Huang, D. Decolorization pathways of anthraquinone dye Disperse Blue 2BLN by Aspergillus sp. XJ-2 CGMCC12963. Bioengineered 2017, 8, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Bergsten-Torralba, L.R.; Zamith, H.P.S.; Conde, T.R.; Aiub, C.A.F.; Felzenszwalb, I.; Da Silva, M. Dye detoxification by Lentinula edodes INCQS 40220. Vigilância Sanitária Em Debate 2016, 4, 92–99. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Korniłłowicz-Kowalska, T.; Szychowski, K.A.; Gmiński, J. Biotransformation and toxicity effect of monoanthraquinone dyes during Bjerkandera adusta CCBAS 930 cultures. Ecotoxicol. Environ. Saf. 2020, 191, 110203. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agric. Water Manag. 2015, 155, 113–124. [Google Scholar] [CrossRef]
- Dantas, A.; Valério, A.; Ninow, J.L.; de Oliveira, J.V.; de Oliveira, D. Potential application of Thermomyces lanuginosus lipase (TLL) immobilized on nonporous polystyrene particles. Environ. Prog. Sustain. Energy 2019, 38, 608–613. [Google Scholar] [CrossRef]
- Ürek, R.Ö.; Pazarlioǧlu, N.K. Purification and partial characterization of manganese peroxidase from immobilized Phanerochaete chrysosporium. Process Biochem. 2004, 39, 2061–2068. [Google Scholar] [CrossRef]
- Rahmani, K.; Faramarzi, M.A.; Mahvi, A.H.; Gholami, M.; Esrafili, A.; Forootanfar, H.; Farzadkia, M. Elimination and detoxification of sulfathiazole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int. Biodeterior. Biodegrad. 2015, 97, 107–114. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging contaminants of high concern and their enzyme-assisted biodegradation—A review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Šekuljica, N.; Prlainović, N.; Jakovetić, S.M.; Grbavčić, S.; Ognjanović, N.D.; Knežević-Jugović, Z.D.; Mijin, D. Removal of Anthraquinone Dye by Cross-Linked Enzyme Aggregates From Fresh Horseradish Extract. Clean Soil Air Water 2016, 44, 891–900. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M. Dye decolorization and detoxification potential of Ca-alginate beads immobilized manganese peroxidase. BMC Biotechnol. 2015, 15, 111. [Google Scholar] [CrossRef]
- Arikan, E.B.; Isik, Z.; Bouras, H.D.; Dizge, N. Investigation of immobilized filamentous fungi for treatment of real textile industry wastewater using up flow packed bed bioreactor. Bioresour. Technol. Rep. 2019, 7, 100197. [Google Scholar] [CrossRef]
- Akerman-Sanchez, G.; Rojas-Jimenez, K. Fungi for the bioremediation of pharmaceutical-derived pollutants: A bioengineering approach to water treatment. Environ. Adv. 2021, 4, 100071. [Google Scholar] [CrossRef]
- Del Álamo, A.C.; Pariente, M.I.; Vasiliadou, I.; Padrino, B.; Puyol, D.; Molina, R.; Martínez, F. Removal of pharmaceutical compounds from urban wastewater by an advanced bio-oxidation process based on fungi Trametes versicolor immobilized in a continuous RBC system. Environ. Sci. Pollut. Res. 2018, 25, 34884–34892. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Korniłłowicz-Kowalska, T. Biodecolorization of anthraquinone dyes using immobilised mycelium of Bjerkandera adusta CCBAS930. E3S Web Conf. 2020, 171, 01013. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sungusia, M.G.; Sawada, T.; Kuwahara, M. Lignin-degrading enzyme production by Bjerkandera adusta immobilized on polyurethane foam. J. Biosci. Bioeng. 1999, 88, 41–47. [Google Scholar] [CrossRef]
- Dosoretz, C.G.; Rothschild, N.; Hadar, Y. Overproduction of lignin peroxidase by Phanerochaete chrysosporium (BKM-F-1767) under nonlimiting nutrient conditions. Appl. Environ. Microbiol. 1993, 59, 1919–1926. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Rybczyńska-Tkaczyk, K. Growth conditions, physiological properties, and selection of optimal parameters of biodegradation of anticancer drug daunomycin in industrial effluents by Bjerkandera adusta CCBAS930. Int. Microbiol. 2020, 23, 287–301. [Google Scholar] [CrossRef]
- Eibes, G.; Debernardi, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Oxidation of pharmaceutically active compounds by a ligninolytic fungal peroxidase. Biodegradation 2011, 22, 539–550. [Google Scholar] [CrossRef]
- Sridhar, M. Versatile Peroxidases: Super Peroxidases with Potential Biotechnological Applications—A Mini Review. J. Dairy Vet. Anim. Res. 2016, 4, 2–7. [Google Scholar] [CrossRef][Green Version]
- Taboada-Puig, R.; Lú-Chau, T.; Moreira, M.T.; Feijoo, G.; Martínez, M.J.; Lema, J.M. A new strain of Bjerkandera sp. production, purification and characterization of versatile peroxidase. World J. Microbiol. Biotechnol. 2011, 27, 115–122. [Google Scholar] [CrossRef]
- Ruiz-Dueñas, F.J.; Morales, M.; García, E.; Miki, Y.; Martínez, M.J.; Martínez, A.T. Substrate oxidation sites in versatile peroxidase and other basidiomycete peroxidases. J. Exp. Bot. 2009, 60, 441–452. [Google Scholar] [CrossRef]
- Gutierrez-Zetina, S.M.; Gonzalez-Manzano, S.; Perez-Alonso, J.J.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Pellati, F.; Mercolini, L.; Sardella, R. Preparation and Characterization of Protocatechuic Acid Sulfates. Molecules 2019, 24, 307. [Google Scholar] [CrossRef]
- Soliman, E.R.S.; El-Sayed, H. Molecular identification and antimicrobial activities of some wild Egyptian mushrooms: Bjerkandera adusta as a promising source of bioactive antimicrobial phenolic compounds. J. Genet. Eng. Biotechnol. 2021, 19, 106. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, Antiradical Potential and Phenolic Compounds of Thirty-One Polish Mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Bu’skobu’sko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. Trans-Cinnamic and Chlorogenic Acids Affect the Secondary Metabolic Profiles and Ergosterol Biosynthesis by Fusarium culmorum and F. graminearum Sensu Stricto. Toxins 2017, 9, 198. [Google Scholar] [CrossRef]
- Jaszek, M.; Kos, K.; Matuszewska, A.; Grąz, M.; Stefaniuk, D.; Osińska-Jaroszuk, M.; Prendecka, M.; Jóźwik, E.; Grzywnowicz, K.; Jaszek, M.; et al. Effective Stimulation of the Biotechnological Potential of the Medicinal White Rot Fungus: Phellinus pini by Menadione-Mediated Oxidative Stress. Appl. Biochem. Biotechnol. 2014, 174, 644–656. [Google Scholar] [CrossRef]
- Alzagameem, A.; El Khaldi-Hansen, B.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M.; Xu, C.; Paleologou, M. Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef]
- Belinky, P.A.; Flikshtein, N.; Dosoretz, C.G. Induction of lignin peroxidase via reactive oxygen species in manganese-deficient cultures of Phanerochaete chrysosporium. Enzym. Microb. Technol. 2006, 39, 222–228. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Liu, H.; Schlenk, D.; Mu, J.; Lacorte, S.; Ying, G.G.; Xie, L. Anticancer drugs in the aquatic ecosystem: Environmental occurrence, ecotoxicological effect and risk assessment. Environ. Int. 2021, 153, 106543. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Kijeńska, M.; Wrzosek, J.; Graniewska, M. Pharmaceuticals in the Soil and Plant Environment: A Review. Water Air Soil Pollut. 2021, 232, 145. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Święciło, A.; Szychowski, K.A.; Korniłłowicz-Kowalska, T. Comparative study of eco- and cytotoxicity during biotransformation of anthraquinone dye Alizarin Blue Black B in optimized cultures of microscopic fungi. Ecotoxicol. Environ. Saf. 2018, 147, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.; Dubrovskaya, E.; Chernyshova, M.; Makarov, O.; Golubev, S.; Balandina, S.; Turkovskaya, O. The degradation of three-ringed polycyclic aromatic hydrocarbons by wood-inhabiting fungus Pleurotus ostreatus and soil-inhabiting fungus Agaricus bisporus. Fungal Biol. 2018, 122, 363–372. [Google Scholar] [CrossRef]
- Fu, Q.; Malchi, T.; Carter, L.J.; Li, H.; Gan, J.; Chefetz, B. Pharmaceutical and Personal Care Products: From Wastewater Treatment into Agro-Food Systems. Environ. Sci. Technol. 2019, 53, 14083–14090. [Google Scholar] [CrossRef]
- Kim, B.S.; Moon, S.S.; Hwang, B.K. Structure elucidation and antifungal activity of an anthracycline antibiotic, daunomycin, isolated from Actinomadura roseola. J. Agric. Food Chem. 2000, 48, 1875–1881. [Google Scholar] [CrossRef]
- Sangnier, M.; Bouguéon, G.; Berroneau, A.; Dubois, V.; Crauste-Manciet, S. Removal of bacterial growth inhibition of anticancer drugs by using complexation materials. Pharm. Technol. Hosp. Pharm. 2020, 5, 20190018. [Google Scholar] [CrossRef]
- Parrella, A.; Lavorgna, M.; Criscuolo, E.; Russo, C.; Isidori, M. Eco-genotoxicity of six anticancer drugs using comet assay in daphnids. J. Hazard. Mater. 2015, 286, 573–580. [Google Scholar] [CrossRef]
- Jolibois, B.; Guerbet, M. Hospital wastewater genotoxicity. Ann. Occup. Hyg. 2006, 50, 189–196. [Google Scholar] [CrossRef]
- Kümmerer, K.; Balcerzak, W.; Rezka, P.; Szuławska, A.; Czyż, M.; Doddapaneni, H.; Subramanian, V.; Fu, B.; Cullen, D.; Zounková, R.; et al. Microbial cytochromes P450: Biodiversity and biotechnology. Where do cytochromes P450 come from, what do they do and what can they do for US? Sci. Total Environ. 2013, 75, 417–434. [Google Scholar] [CrossRef]
- Zounková, R.; Odráska, P.; Dolezalová, L.; Hilscherová, K.; Marsálek, B.; Bláha, L. Ecotoxicity and genotoxicity assessment of cytostatic pharmaceuticals. Environ. Toxicol. Chem. 2007, 26, 2208–2214. [Google Scholar] [CrossRef]
- Kupczewska-Dobecka, M. Doxorubicine and doxorubicine hydrochloride–inhalable fraction. Documentation of proposed values of occupational exposure limits (OELs). Podstawy i Metod. Oceny Sr. Pr. 2020, 36, 5–39. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Święciło, A.; Rybczyńska-Tkaczyk, K.; Najda, A.; Krzepiłko, A.; Prażak, R.; Zawiślak, G. Application of growth tests employing a Δsod1 mutant of Saccharomyces cerevisiae to study the antioxidant activity of berry fruit extracts. LWT 2018, 94, 96–102. [Google Scholar] [CrossRef]
- Paździoch-Czochra, M.; Malarczyk, E.; Sielewiesiuk, J. Relationship of demethylation processes to veratric acid concentration and cell density in cultures of Rhodococcus erythropolis. Cell Biol. Int. 2003, 27, 325–336. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]

| Samples | %vol. | CIF (4-Week Old Mycelium) | CIF (12-Week Old Mycelium) |
|---|---|---|---|
| DNR after treatment by IM/Ba | 100 | 0.90 (±0.04) | 1.02 (±0.03) |
| 50 | 0.67 (±0.05) | 0.54 (±0.04) | |
| 25 | 0.52 (±0.05) | 0.51 (±0.04) | |
| 12.50 | 0.12 (±0.02) | 0.17 (±0.02) | |
| 6.25 | 0.41 (±0.01) | 0.32 (±0.01) | |
| 3.12 | 0.13 (±0.02) | 0.15 (±0.01) | |
| 1.56 | 0.14 (±0.02) | 0.16 (±0.01) | |
| DOX after treatment by IM/Ba | 100 | 0.90 (±0.04) | 0.90 (±0.02) |
| 50 | 1.04 (±0.02) | 1.02 (±0.02) | |
| 25 | 1.09 (±0.02) | 0.93 (±0.02) | |
| 12.50 | 1.03 (±0.02) | 0.85 (±0.02) | |
| 6.25 | 0.48 (±0.01) | 0.32 (±0.01) | |
| 3.12 | 1.05 (±0.02) | 0.56 (±0.02) | |
| 1.56 | 1.03 (±0.06) | 0.48 (±0.02) | |
| MTX after treatment by IM/Ba | 100 | 1.03 (±0.03) | 0.76 (±0.04) |
| 50 | 0.42 (±0.03) | 0.53 (±0.05) | |
| 25 | 1.07 (±0.03) | 0.75 (±0.05) | |
| 12.50 | 1.04 (±0.02) | 0.78 (±0.01) | |
| 6.25 | 0.92 (±0.04) | 0.80 (±0.04) | |
| 3.12 | 0.94 (±0.02) | 0.82 (±0.03) | |
| 1.56 | 1.02 (±0.02) | 0.32 (±0.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybczyńska-Tkaczyk, K. Enhanced Efficiency of the Removal of Cytostatic Anthracycline Drugs Using Immobilized Mycelium of Bjerkandera adusta CCBAS 930. Molecules 2021, 26, 6842. https://doi.org/10.3390/molecules26226842
Rybczyńska-Tkaczyk K. Enhanced Efficiency of the Removal of Cytostatic Anthracycline Drugs Using Immobilized Mycelium of Bjerkandera adusta CCBAS 930. Molecules. 2021; 26(22):6842. https://doi.org/10.3390/molecules26226842
Chicago/Turabian StyleRybczyńska-Tkaczyk, Kamila. 2021. "Enhanced Efficiency of the Removal of Cytostatic Anthracycline Drugs Using Immobilized Mycelium of Bjerkandera adusta CCBAS 930" Molecules 26, no. 22: 6842. https://doi.org/10.3390/molecules26226842
APA StyleRybczyńska-Tkaczyk, K. (2021). Enhanced Efficiency of the Removal of Cytostatic Anthracycline Drugs Using Immobilized Mycelium of Bjerkandera adusta CCBAS 930. Molecules, 26(22), 6842. https://doi.org/10.3390/molecules26226842






