Synthesis and Physico-Chemical Properties of Homoleptic Copper(I) Complexes with Asymmetric Ligands as a DSSC Dye
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Grätzel, M. Solar cells to dye for. Nature 2003, 421, 586–587. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Nazeeruddin, M.K.; Sekiguchi, T.; Grätzel, M. A stable quasi-solid-state dye-sensitized solar cell with an amphiphilic ruthenium sensitizer and polymer gel electrolyte. Nat. Mater. 2003, 2, 402–407. [Google Scholar] [CrossRef]
- Son, H.-J.; Prasittichai, C.; Mondloch, J.E.; Luo, L.; Wu, J.; Kim, D.W.; Farha, O.K.; Hupp, J.T. Dye stabilization and enhanced photoelectrode wettability in water-based dye-sensitized solar cells through post-assembly atomic layer deposition of TiO2. J. Am. Chem. Soc. 2013, 135, 11529–11532. [Google Scholar] [CrossRef]
- Manfredi, N.; Bianchi, A.; Causin, V.; Ruffo, R.; Simonutti, R.; Abbotto, A. Electrolytes for quasi solid-state dye-sensitized solar cells based on block copolymers. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 719–727. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Bessho, T.; Gratzel, M. Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2013, 127, 16835–16847. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Bozic-Weber, B.; Constable, E.C.; Hostettler, N.; Housecroft, C.E.; Schmitt, R.; Schonhofer, E. The d10 route to dye-sensitized solar cells: Step-wise assembly of zinc(II) photosensitizers on TiO2 surfaces. Chem. Commun. 2012, 48, 5727–5729. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Gratzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.; Lee, J.H.; Park, C.; Lee, H.; Kim, C.; Park, C.; Lee, M.H.; Lee, W.; Park, J.; Kim, K.; et al. A highly efficient organic sensitizer for dye-sensitized solar cells. Chem. Commun. 2007, 46, 4887–4889. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Cui, Y.; Dan-oh, Y.; Kasada, C.; Shinpo, A.; Hara, K. Thiophene-Functionalized Coumarin Dye for Efficient Dye-Sensitized Solar Cells: Electron Lifetime Improved by Coadsorption of Deoxycholic Acid. J. Phys. Chem. C 2007, 111, 7224–7230. [Google Scholar] [CrossRef]
- Choi, H.; Baik, C.; Kang, S.O.; Ko, J.; Kang, M.S.; Nazeeruddin, M.K.; Gratzel, M. Highly efficient and thermally stable organic sensitizers for solvent-free dye-sensitized solar cells. Angew. Chem. Int. Ed. Engl. 2008, 47, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari Astani, N.; Teuscher, J.; Polander, L.E.; Mathew, S.; Moser, J.-E.; Tavernelli, I.; Rothlisberger, U.; et al. Molecular Engineering of a Fluorene Donor for Dye-Sensitized Solar Cells. Chem. Mater. 2013, 25, 2733–2739. [Google Scholar] [CrossRef]
- Bessho, T.; Constable, E.C.; Graetzel, M.; Hernandez Redondo, A.; Housecroft, C.E.; Kylberg, W.; Nazeeruddin, M.K.; Neuburger, M.; Schaffner, S. An element of surprise—efficient copper-functionalized dye-sensitized solar cells. Chem. Commun. 2008, 32, 3717–3719. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Hernandez Redondo, A.; Housecroft, C.E.; Neuburger, M.; Schaffner, S. Copper(I) complexes of 6,6′-disubstituted 2,2′-bipyridine dicarboxylic acids: New complexes for incorporation into copper-based dye sensitized solar cells (DSCs). Dalton Trans. 2009, 33, 6634–6644. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Price, J.R. Sticky complexes: Carboxylic acid-functionalized N-phenylpyridin-2-ylmethanimine ligands as anchoring domains for copper and ruthenium dye-sensitized solar cells. Dalton Trans. 2010, 39, 3585–3594. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Chaurin, V.; Constable, E.C.; Housecroft, C.E.; Meuwly, M.; Neuburger, M.; Rudd, J.A.; Schonhofer, E.; Siegfried, L. Exploring copper(I)-based dye-sensitized solar cells: A complementary experimental and TD-DFT investigation. Dalton Trans. 2012, 41, 14157–14169. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Yu, Z.T.; Zhang, J.Y.; Zou, Z.G. A copper(I) dye-sensitised TiO2-based system for efficient light harvesting and photoconversion of CO2 into hydrocarbon fuel. Dalton Trans. 2012, 41, 9594–9597. [Google Scholar] [CrossRef]
- Ashbrook, L.N.; Elliott, C.M. Dye-Sensitized Solar Cell Studies of a Donor-Appended Bis(2,9-dimethyl-1,10-phenanthroline) Cu(I) Dye Paired with a Cobalt-Based Mediator. J. Phys. Chem. C 2013, 117, 3853–3864. [Google Scholar] [CrossRef]
- Wills, K.A.; Mandujano-Ramírez, H.J.; Merino, G.; Mattia, D.; Hewat, T.; Robertson, N.; Oskam, G.; Jones, M.D.; Lewis, S.E.; Cameron, P.J. Investigation of a copper(i) biquinoline complex for application in dye-sensitized solar cells. RSC Adv. 2013, 3, 23361–23369. [Google Scholar] [CrossRef] [Green Version]
- Bozic-Weber, B.; Brauchli, S.Y.; Constable, E.C.; Furer, S.O.; Housecroft, C.E.; Wright, I.A. Hole-transport functionalized copper(I) dye sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 4500–4504. [Google Scholar] [CrossRef]
- Brauchli, S.Y.; Bozic-Weber, B.; Constable, E.C.; Hostettler, N.; Housecroft, C.E.; Zampese, J.A. Factors controlling the photoresponse of copper(i) diimine dyes containing hole-transporting dendrons in dye-sensitized solar cells: Substituent and solvent effects. RSC Adv. 2014, 4, 34801–34815. [Google Scholar] [CrossRef] [Green Version]
- Hewat, T.E.; Yellowlees, L.J.; Robertson, N. Neutral copper(I) dipyrrin complexes and their use as sensitizers in dye-sensitized solar cells. Dalton Trans. 2014, 43, 4127–4136. [Google Scholar] [CrossRef] [Green Version]
- Sandroni, M.; Favereau, L.; Planchat, A.; Akdas-Kilig, H.; Szuwarski, N.; Pellegrin, Y.; Blart, E.; Le Bozec, H.; Boujtita, M.; Odobel, F. Heteroleptic copper(i)–polypyridine complexes as efficient sensitizers for dye sensitized solar cells. J. Mater. Chem. A 2014, 2, 9944–9947. [Google Scholar] [CrossRef]
- Brauchli, S.Y.; Constable, E.C.; Housecroft, C.E. Concentration effects on the performance of bis(diimine) copper(I) dyes in dye-sensitized solar cells. Dyes Pigment. 2015, 113, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Housecroft, C.E.; Constable, E.C. The emergence of copper(I)-based dye sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8386–8398. [Google Scholar] [CrossRef] [Green Version]
- Tsaturyan, A.; Machida, Y.; Akitsu, T.; Gozhikova, I.; Shcherbakov, I. Binaphthyl-containing Schiff base complexes with carboxyl groups for dye sensitized solar cell: An experimental and theoretical study. J. Mol. Struct. 2018, 1162, 54–62. [Google Scholar] [CrossRef]
- Liu, Y.; Yiu, S.-C.; Ho, C.-L.; Wong, W.-Y. Recent advances in copper complexes for electrical/light energy conversion. Coord. Chem. Rev. 2018, 375, 514–557. [Google Scholar] [CrossRef]
- Armaroli, N. Photoactive mono- and polynuclear Cu(i)–phenanthrolines. A viable alternative to Ru(ii)–polypyridines? Chem. Soc. Rev. 2001, 30, 113–124. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D.; Demartin, F.; Caramori, S.; Bignozzi, C.A. Efficient copper mediators based on bulky asymmetric phenanthrolines for DSSCs. ACS Appl. Mater. Interfaces 2014, 6, 13945–13955. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.; Dragonetti, C.; Roberto, D.; Fagnani, F. Copper Complexes as Alternative Redox Mediators in Dye-Sensitized Solar Cells. Molecules 2021, 26, 194. [Google Scholar] [CrossRef] [PubMed]
- Dragonetti, C.; Magni, M.; Colombo, A.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P.; Fantacci, S. Towards efficient sustainable full-copper dye-sensitized solar cells. Dalton Trans. 2019, 48, 9703–9711. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P. Improving the efficiency of copper-dye-sensitized solar cells by manipulating the electrolyte solution. Dalton Trans. 2019, 48, 9818–9823. [Google Scholar] [CrossRef]
- Hara, K.; Tachibana, Y.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. Dye-sensitized nanocrystalline TiO2 solar cells based on novel coumarin dyes. Sol. Energy Mater. Sol. Cells 2003, 77, 89–103. [Google Scholar] [CrossRef]
- Hara, K.; Kurashige, M.; Ito, S.; Shinpo, A.; Suga, S.; Sayama, K.; Arakawa, H. Novel polyene dyes for highly efficient dye-sensitized solar cells. Chem. Commun. 2003, 2, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, X.; Cao, K.; Cui, J.; Zhang, Y.; Shen, Y.; Shi, X.; Liao, L.; Cheng, Y.; Wang, M. D-π-A structured porphyrins for efficient dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 10008–10015. [Google Scholar] [CrossRef]
- Yum, J.H.; Baranoff, E.; Kessler, F.; Moehl, T.; Ahmad, S.; Bessho, T.; Marchioro, A.; Ghadiri, E.; Moser, J.E.; Yi, C.; et al. A cobalt complex redox shuttle for dye-sensitized solar cells with high open-circuit potentials. Nat. Commun. 2012, 3, 631. [Google Scholar] [CrossRef] [Green Version]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef] [PubMed]
- Noviandri, I.; Brown, K.N.; Fleming, D.S.; Gulyas, P.T.; Lay, P.A.; Masters, A.F.; Phillips, L. The Decamethylferrocenium/Decamethylferrocene Redox Couple: A Superior Redox Standard to the Ferrocenium/Ferrocene Redox Couple for Studying Solvent Effects on the Thermodynamics of Electron Transfer. J. Comput. Chem. B 1999, 101, 6713–6722. [Google Scholar] [CrossRef]
- Pavlishchuk, V.V.; Addison, A.W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 2000, 298, 97–102. [Google Scholar] [CrossRef]
- Daeneke, T.; Mozer, A.J.; Uemura, Y.; Makuta, S.; Fekete, M.; Tachibana, Y.; Koumura, N.; Bach, U.; Spiccia, L. Dye regeneration kinetics in dye-sensitized solar cells. J. Am. Chem. Soc. 2012, 134, 16925–16928. [Google Scholar] [CrossRef]
- Zervaki, G.E.; Roy, M.S.; Panda, M.K.; Angaridis, P.A.; Chrissos, E.; Sharma, G.D.; Coutsolelos, A.G. Efficient sensitization of dye-sensitized solar cells by novel triazine-bridged porphyrin-porphyrin dyads. Inorg. Chem. 2013, 52, 9813–9825. [Google Scholar] [CrossRef]
- Funaki, T.; Otsuka, H.; Onozawa-Komatsuzaki, N.; Kasuga, K.; Sayama, K.; Sugihara, H. Systematic evaluation of HOMO energy levels for efficient dye regeneration in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 15945–15951. [Google Scholar] [CrossRef]
- Mishra, A.; Pootrakulchote, N.; Fischer, M.K.; Klein, C.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Bauerle, P.; Gratzel, M. Design and synthesis of a novel anchoring ligand for highly efficient thin film dye-sensitized solar cells. Chem. Commun. 2009, 46, 7146–7148. [Google Scholar] [CrossRef] [PubMed]
- Feldt, S.M.; Lohse, P.W.; Kessler, F.; Nazeeruddin, M.K.; Gratzel, M.; Boschloo, G.; Hagfeldt, A. Regeneration and recombination kinetics in cobalt polypyridine based dye-sensitized solar cells, explained using Marcus theory. Phys. Chem. Chem. Phys. 2013, 15, 7087–7097. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Dennis, R.S.; Michael, C.Z. The Challenge of d and f Electrons. Theory and Computation; ACS: Washington, DC, USA, 1989. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Wachters, A.J.H. Gaussian Basis Set for Molecular Wavefunctions Containing Third-Row Atoms. J. Chem. Phys. 1970, 52, 1033–1036. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W. Highly correlated systems. Excitation energies of first row transition metals Sc–Cu. J. Chem. Phys. 1989, 91, 1062–1065. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chem. Acc. 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Dreuw, A.; Head-Gordon, M. Single-Reference ab Initio Methods for the Calculation of Excited States of Large Molecules. Chem. Rev. 2005, 105, 4009–4037. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. II. Overlap Populations, Bond Orders, and Covalent Bond Energies. J. Chem. Phys. 1955, 23, 1841–1846. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. III. Effects of Hybridization on Overlap and Gross AO Populations. J. Chem. Phys. 1955, 23, 2338–2342. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. IV. Bonding and Antibonding in LCAO and Valence-Bond Theories. J. Chem. Phys. 1955, 23, 2343–2346. [Google Scholar] [CrossRef]
- Wasada, H.; Tsutsui, Y. Bull. Fac. General Educ.; Gifu University: Gifu, Japan, 1996; Volume 33, pp. 145–158. [Google Scholar]
- Takahashi, I.; Wasada, H.; Tsutsui, Y. MOVIEW; Representing Molecular Orbitals and Electron Density Maps by Isosurfaces A Program; Nagoya University Information Technology Center: Nagoya, Japan, 1996. [Google Scholar]
- Gorelsky, S.I. AOMix Version 6.85. Available online: http://www.sg-chem.net/ (accessed on 16 October 2021).
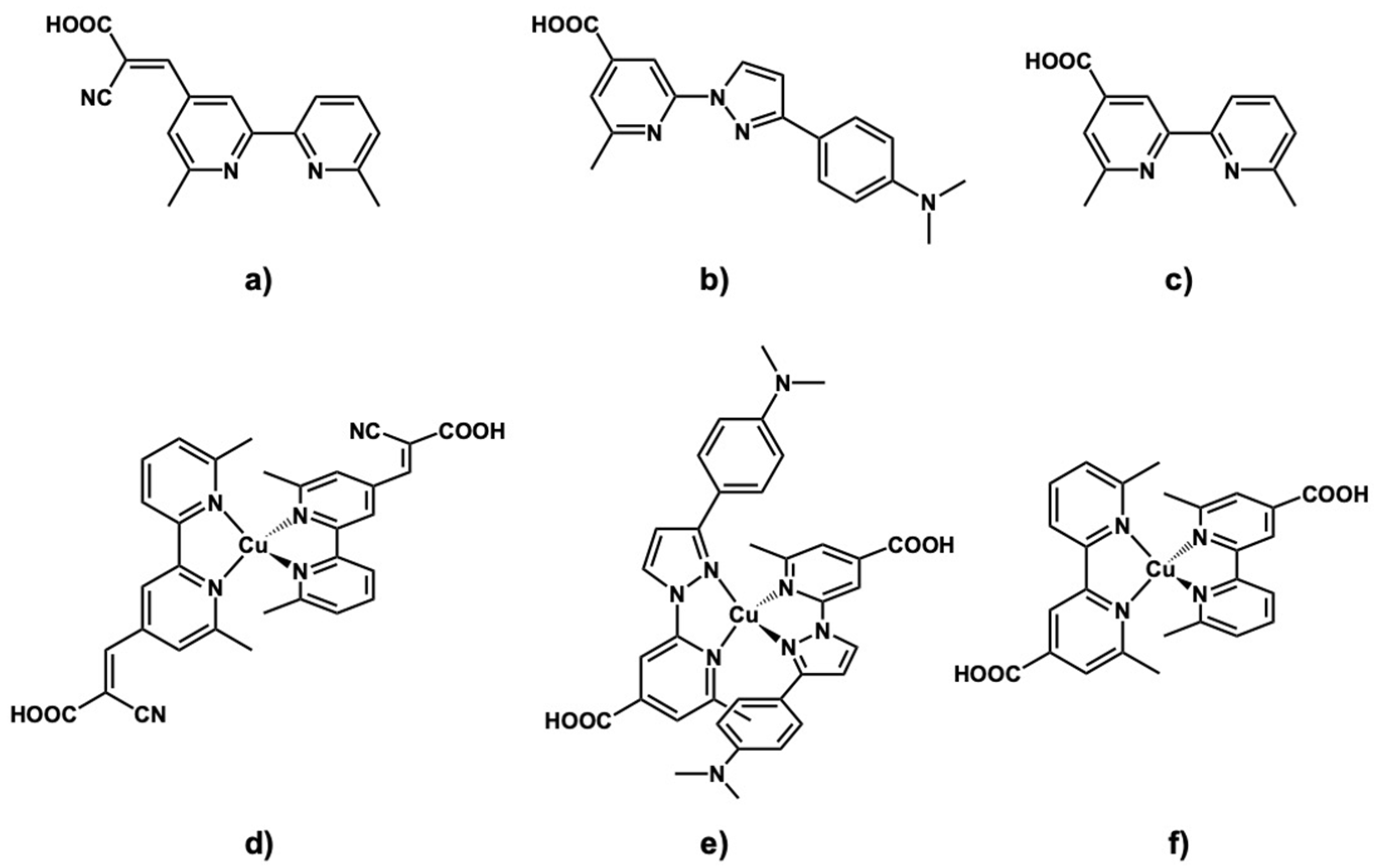
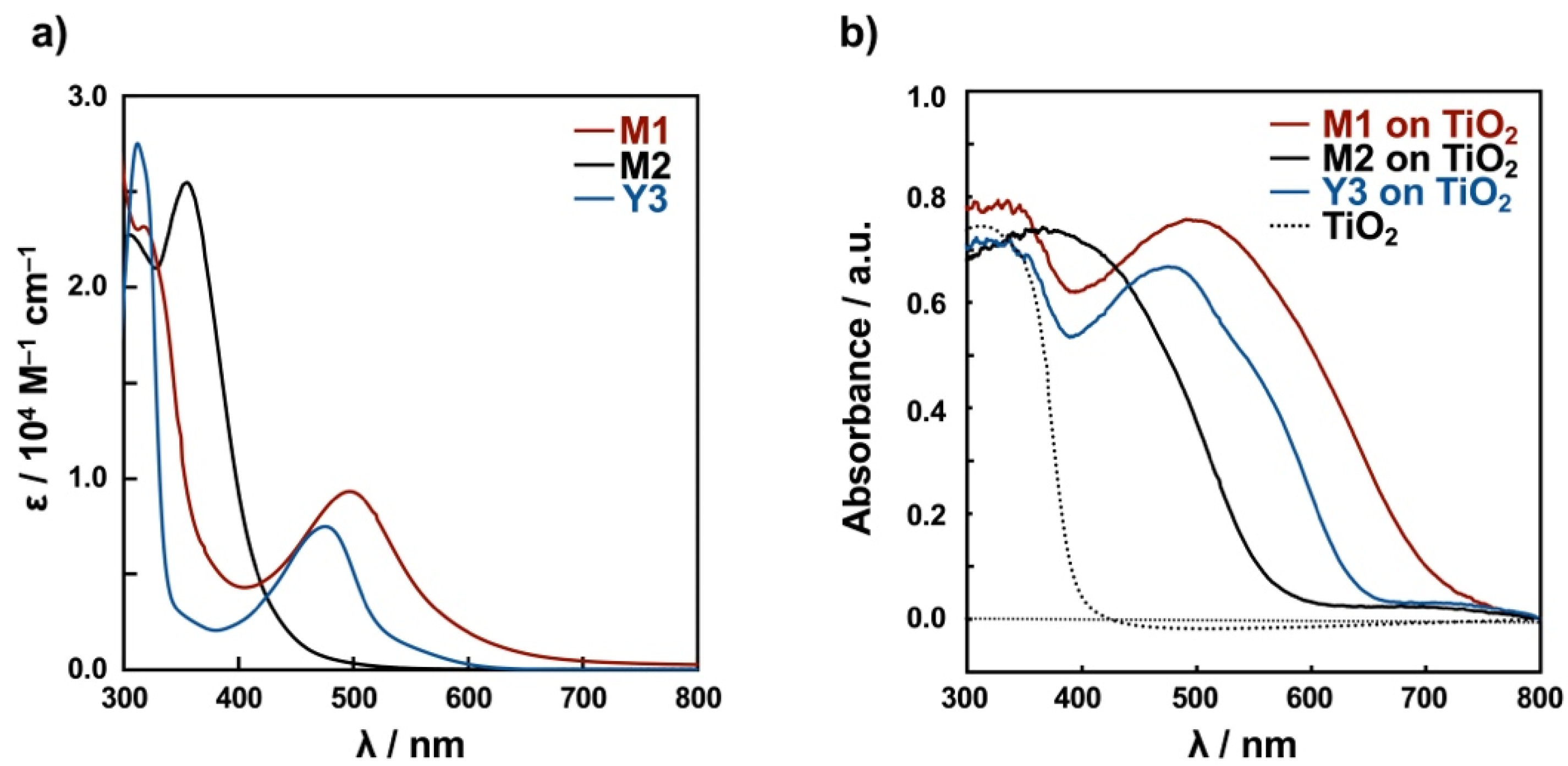
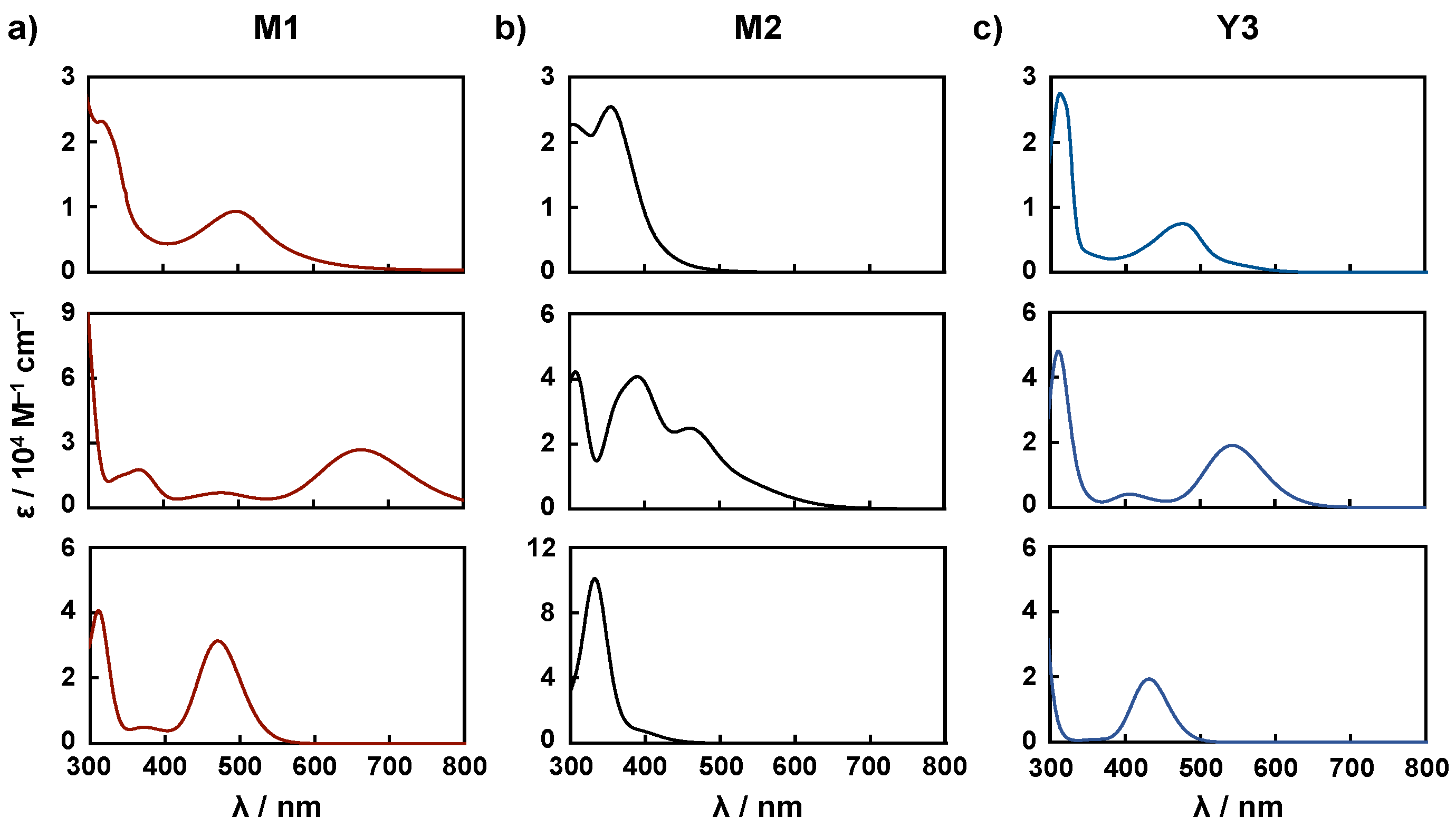
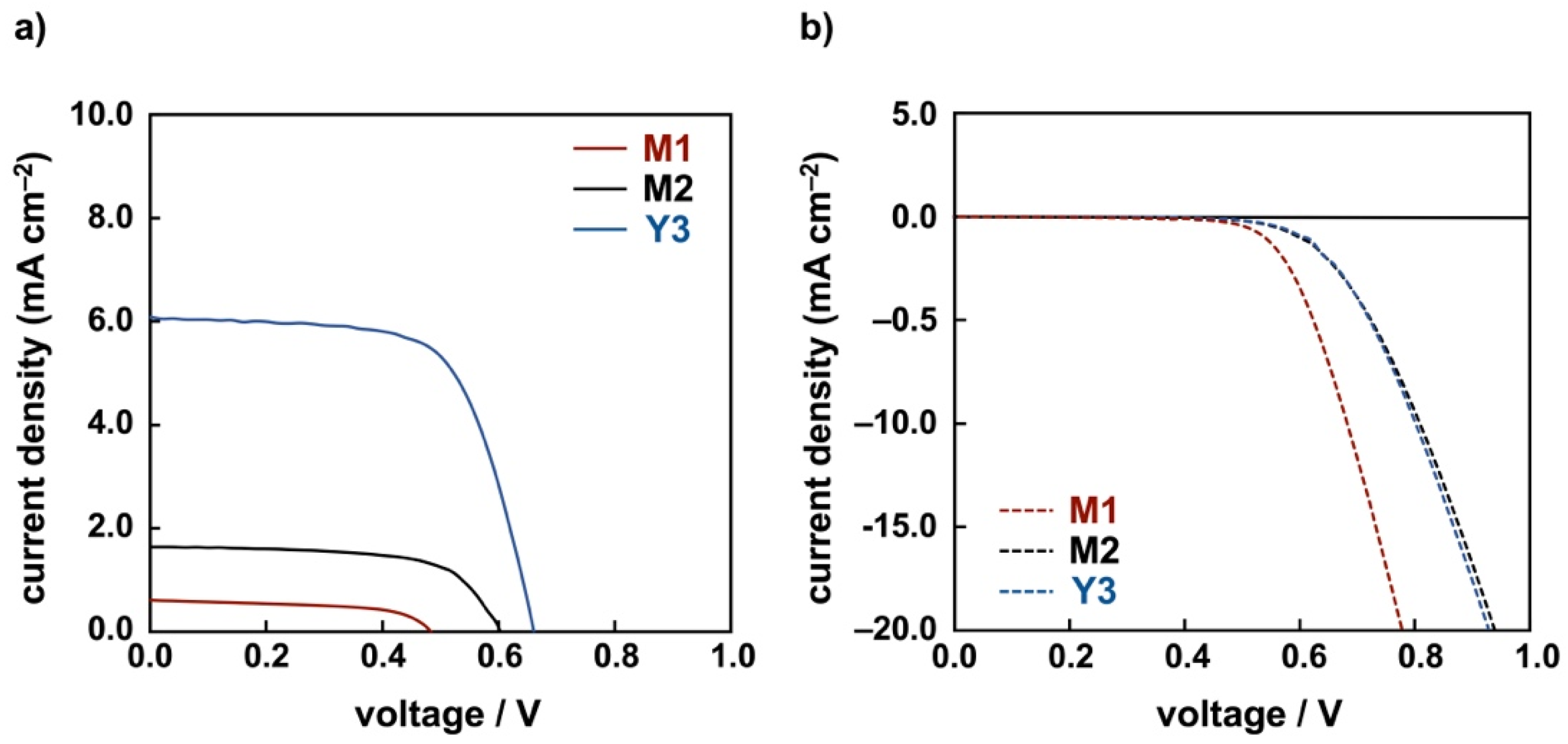
| Dye | in EtOH | on TiO2 | |
|---|---|---|---|
| λ/nm (ε/M−1·cm−1) | Assignment | λmax/nm | |
| M1 | 317 (23,200) 497 (9330) | π-π* (L1) MLCT (Cu(I)→L1) | 491 |
| M2 | 355 (25,500) | π-π* (L2) MLCT (Cu(I)→L2) | 366 |
| Y3 | 312 (27,500) 475 (7480) | π-π* (L3) MLCT (Cu(I)→L3) | 475 |
| Dye | in Solution 1 | on TiO2 2 | ||||
|---|---|---|---|---|---|---|
| E1/2/V vs. Fc/Fc+ | E1/2/V vs. NHE | ΔG/V 3 | E1/2/V vs. Fc/Fc+ | E1/2/V vs. NHE | ΔG/V 3 | |
| M1 | −0.09 | 0.49 | 0.09 | −0.10 | 0.52 | 0.12 |
| M2 | 0.33 | 0.91 | 0.51 | 0.04 | 0.66 | 0.26 |
| Y3 | −0.03 | 0.55 | 0.15 | −0.05 | 0.57 | 0.17 |
| Functional | Dye | λcalc. nm | f | Assignment | Character |
|---|---|---|---|---|---|
| B3LYP | M1 | 665 | 0.37 | HOMO−1→LUMO (44%) HOMO→LUMO+1 (41%) | MLCT |
| M2 | 469 | 0.23 | HOMO−2→LUMO (44%) HOMO–3→LUMO+1 (35%) | MLCT π-π* | |
| Y3 | 543 | 0.26 | HOMO→LUMO (50%) HOMO−1→LUMO+1 (49%) | MLCT | |
| CAM-B3LYP | M1 | 471 | 0.43 | HOMO→LUMO (23%) HOMO−1→LUMO (22%) HOMO−1→LUMO+1 (21%) HOMO→LUMO+1 (17%) | MLCT |
| M2 | 334 | 0.79 | HOMO−1→LUMO+1 (22%) HOMO−2→LUMO (20%) HOMO→LUMO (15%) HOMO−3→LUMO+1 (14%) | MLCT π-π* | |
| Y3 | 431 | 0.27 | HOMO→LUMO (47%) HOMO−1→LUMO+1 (47%) | MLCT |
| Dye | Voc/V | Jsc/mA·cm−2 | FF | η/% |
|---|---|---|---|---|
| M1 | 0.48 | 0.61 | 0.58 | 0.17 |
| M2 | 0.60 | 1.64 | 0.65 | 0.64 |
| Y3 | 0.66 | 6.08 | 0.66 | 2.66 |
| N719 | 0.69 | 16.5 | 0.69 | 7.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inomata, T.; Hatano, M.; Kawai, Y.; Matsunaga, A.; Kitagawa, T.; Wasada-Tsutsui, Y.; Ozawa, T.; Masuda, H. Synthesis and Physico-Chemical Properties of Homoleptic Copper(I) Complexes with Asymmetric Ligands as a DSSC Dye. Molecules 2021, 26, 6835. https://doi.org/10.3390/molecules26226835
Inomata T, Hatano M, Kawai Y, Matsunaga A, Kitagawa T, Wasada-Tsutsui Y, Ozawa T, Masuda H. Synthesis and Physico-Chemical Properties of Homoleptic Copper(I) Complexes with Asymmetric Ligands as a DSSC Dye. Molecules. 2021; 26(22):6835. https://doi.org/10.3390/molecules26226835
Chicago/Turabian StyleInomata, Tomohiko, Mayuka Hatano, Yuya Kawai, Ayaka Matsunaga, Takuma Kitagawa, Yuko Wasada-Tsutsui, Tomohiro Ozawa, and Hideki Masuda. 2021. "Synthesis and Physico-Chemical Properties of Homoleptic Copper(I) Complexes with Asymmetric Ligands as a DSSC Dye" Molecules 26, no. 22: 6835. https://doi.org/10.3390/molecules26226835
APA StyleInomata, T., Hatano, M., Kawai, Y., Matsunaga, A., Kitagawa, T., Wasada-Tsutsui, Y., Ozawa, T., & Masuda, H. (2021). Synthesis and Physico-Chemical Properties of Homoleptic Copper(I) Complexes with Asymmetric Ligands as a DSSC Dye. Molecules, 26(22), 6835. https://doi.org/10.3390/molecules26226835







