Therapeutic Screening of Herbal Remedies for the Management of Diabetes
Abstract
1. Introduction
2. Diabetes Mellitus
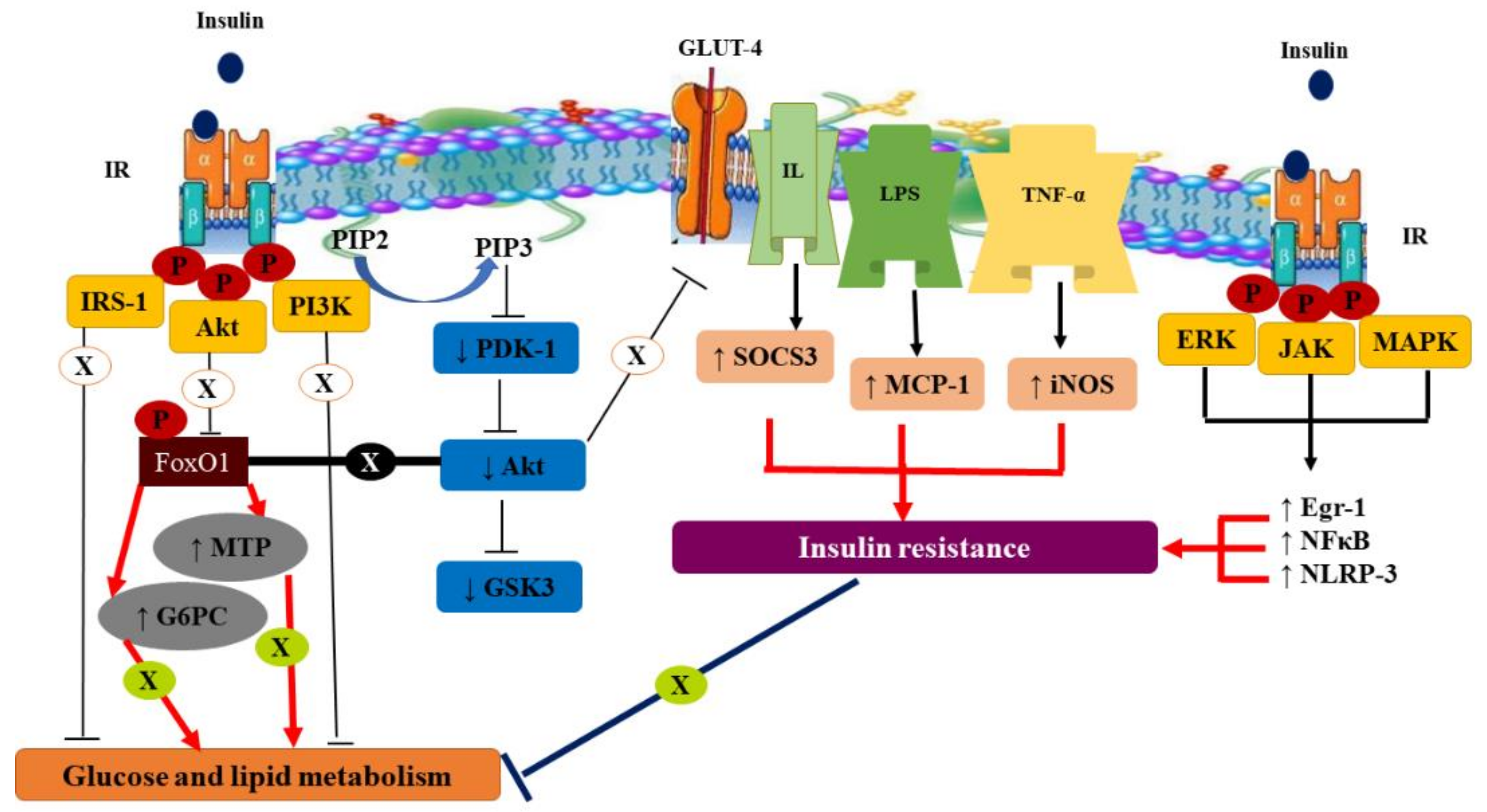
2.1. Prevalence, Types, Symptoms, Pathophysiology, and Molecular Mechanism of DM
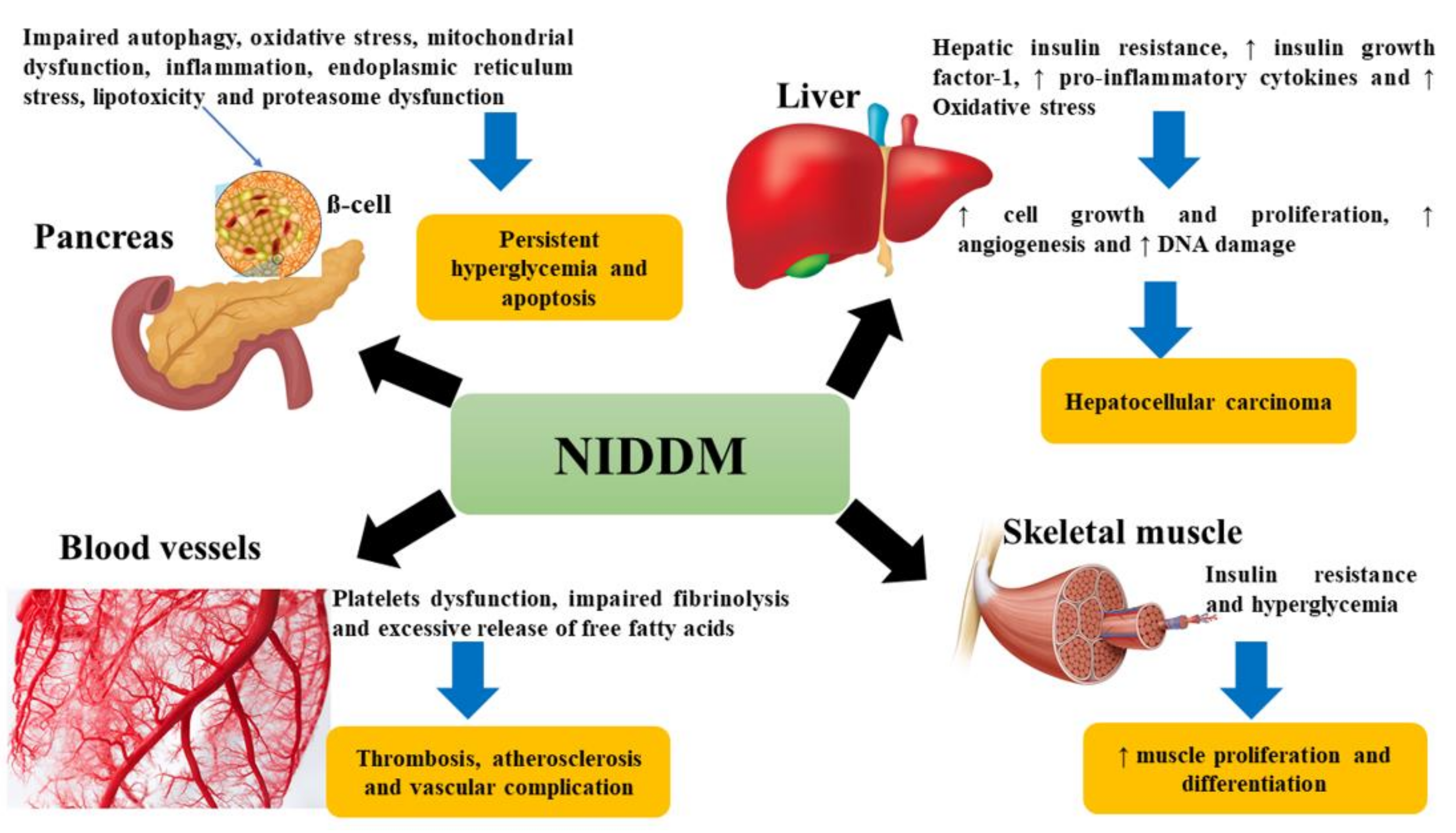
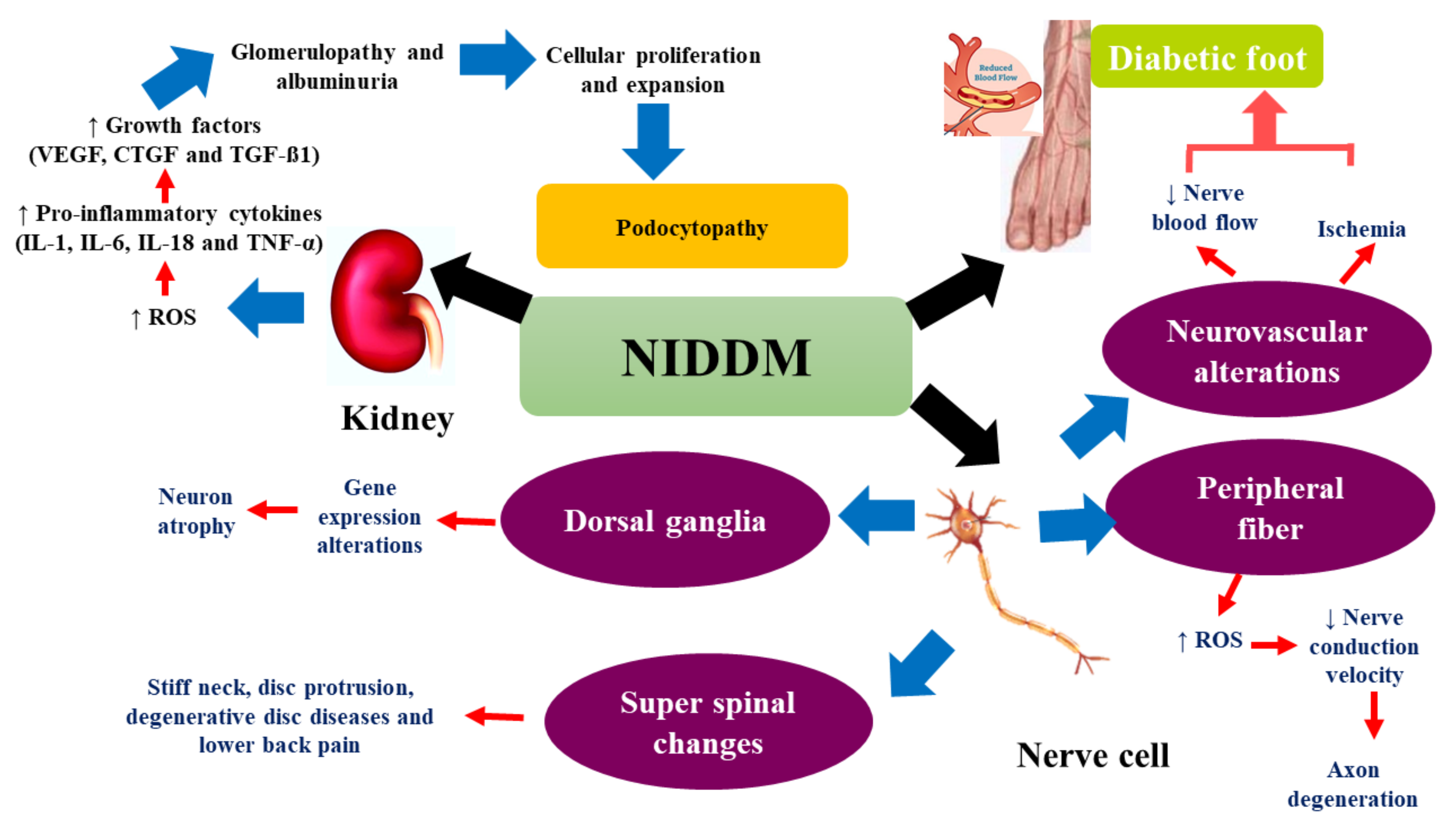
2.2. Complications of DM
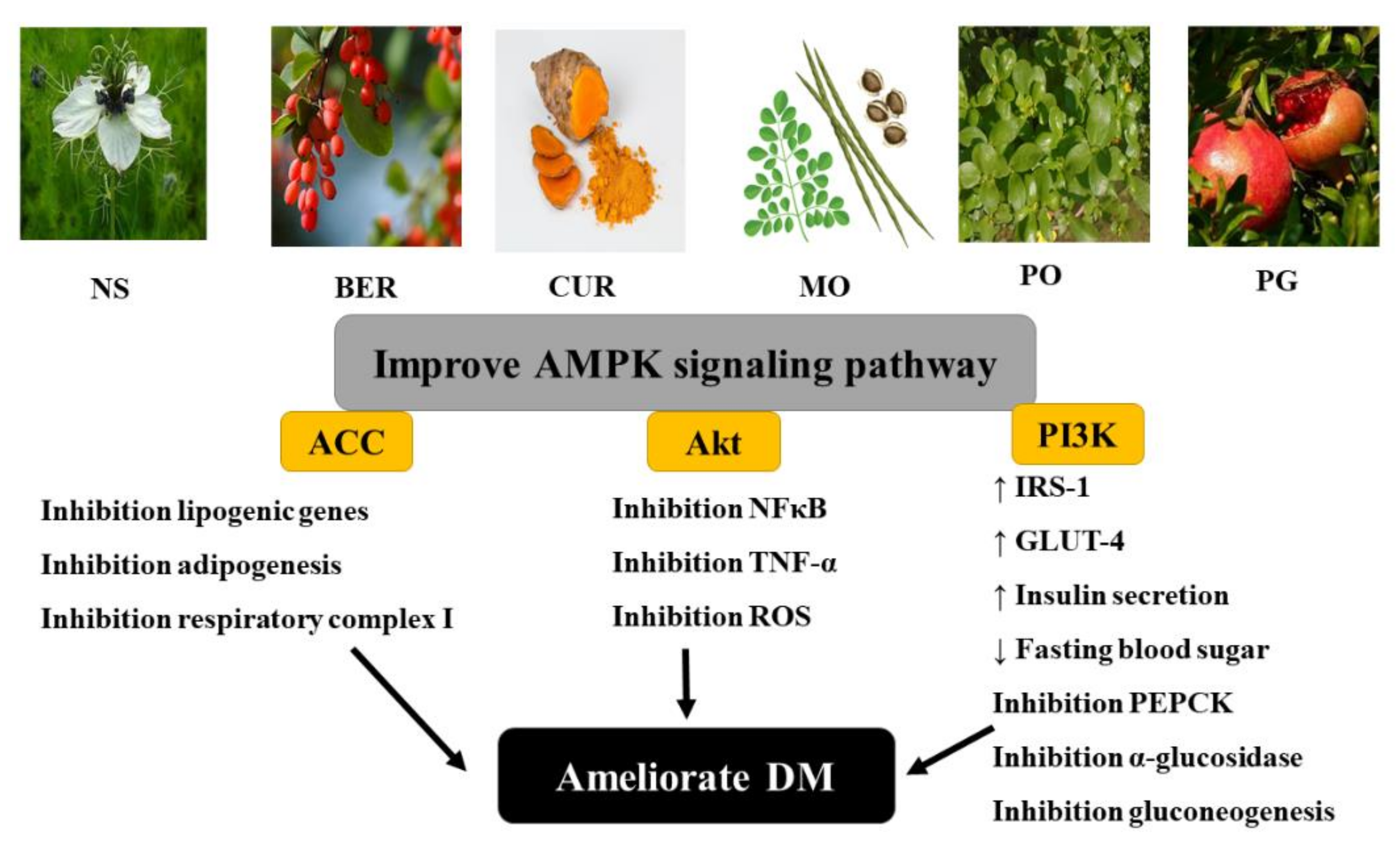
3. Natural Therapy: A Safe Tool for DM Management
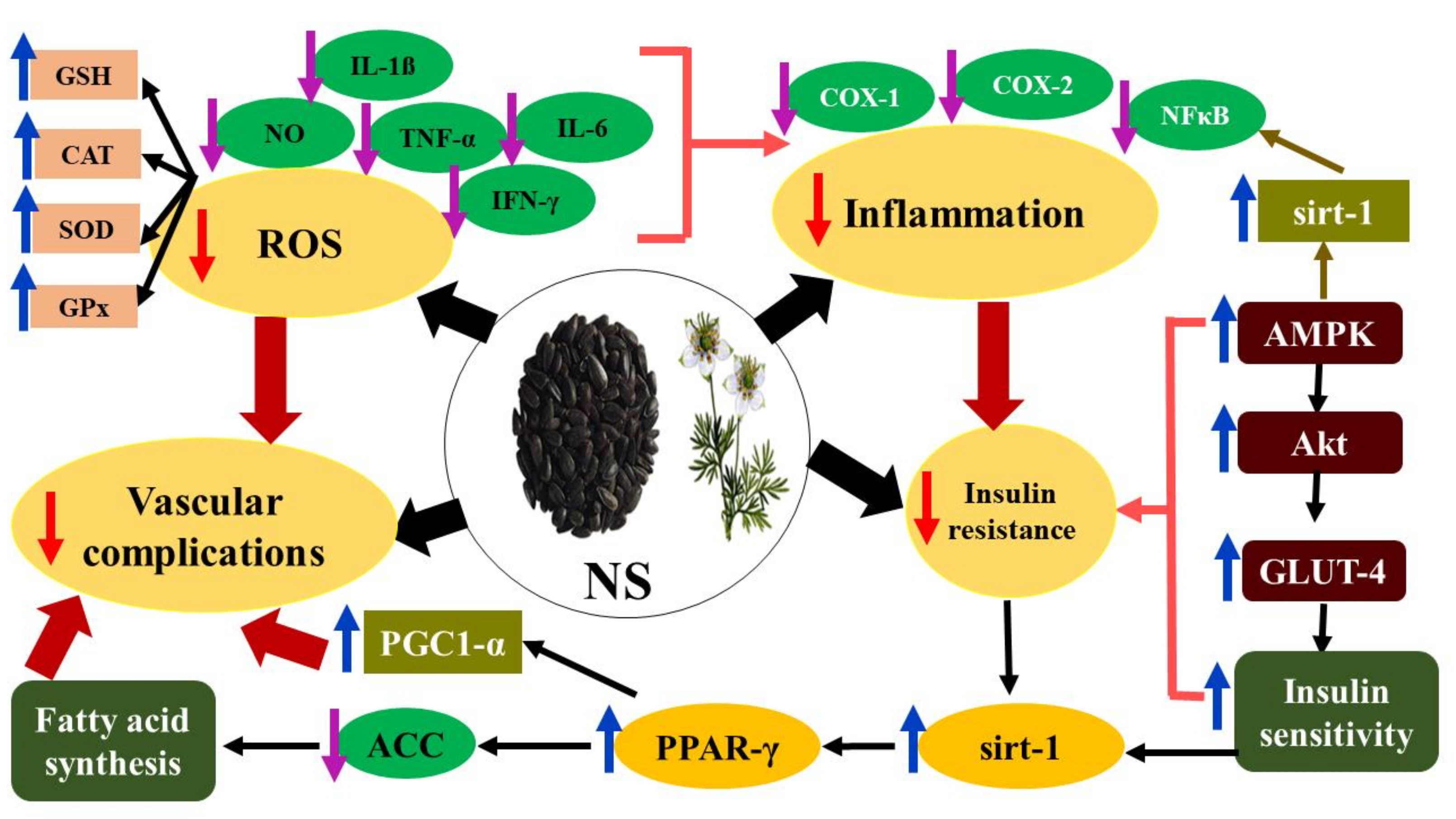
3.1. Nigella Sativa (NS)
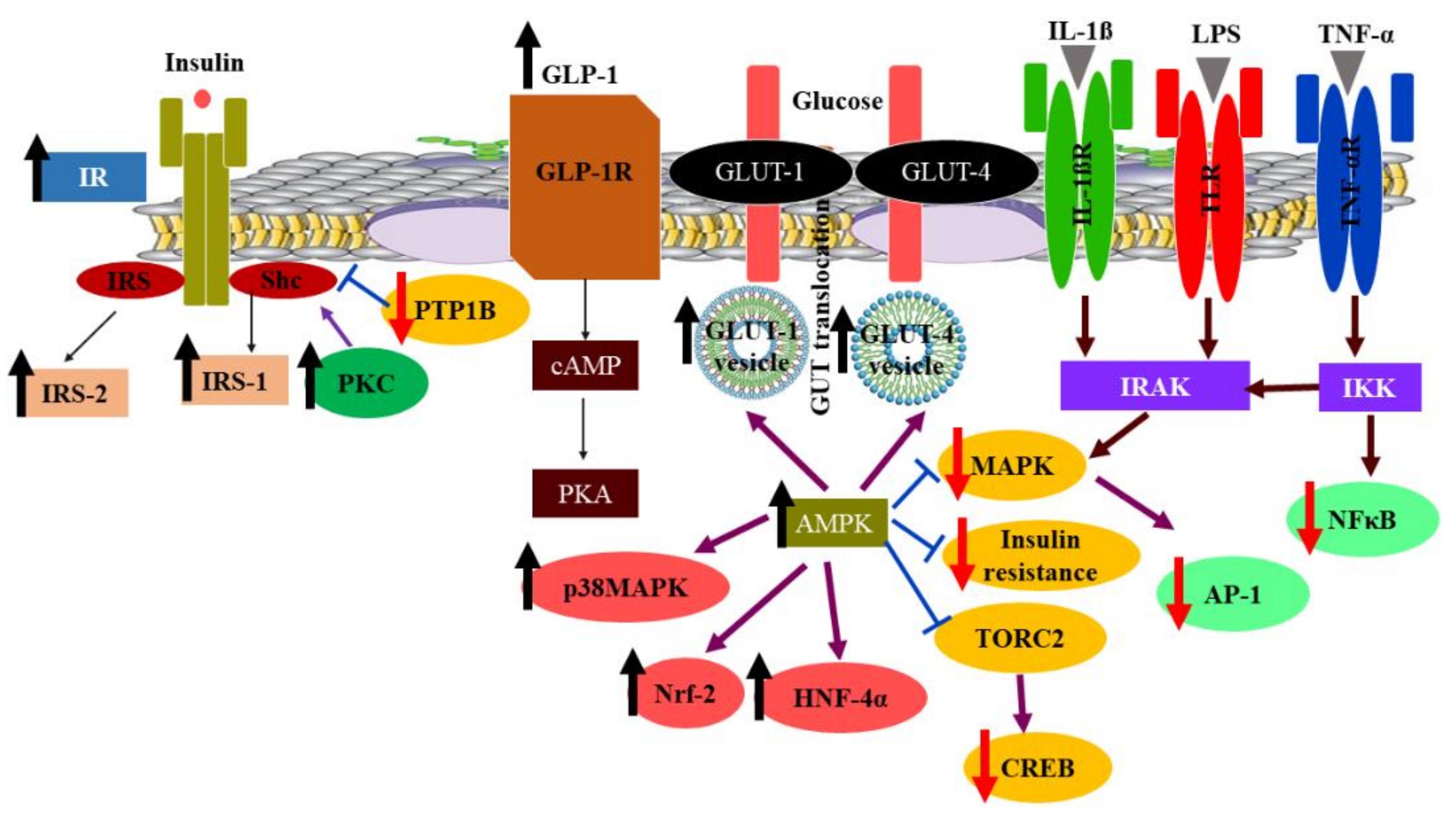
3.2. Berberine (BER)
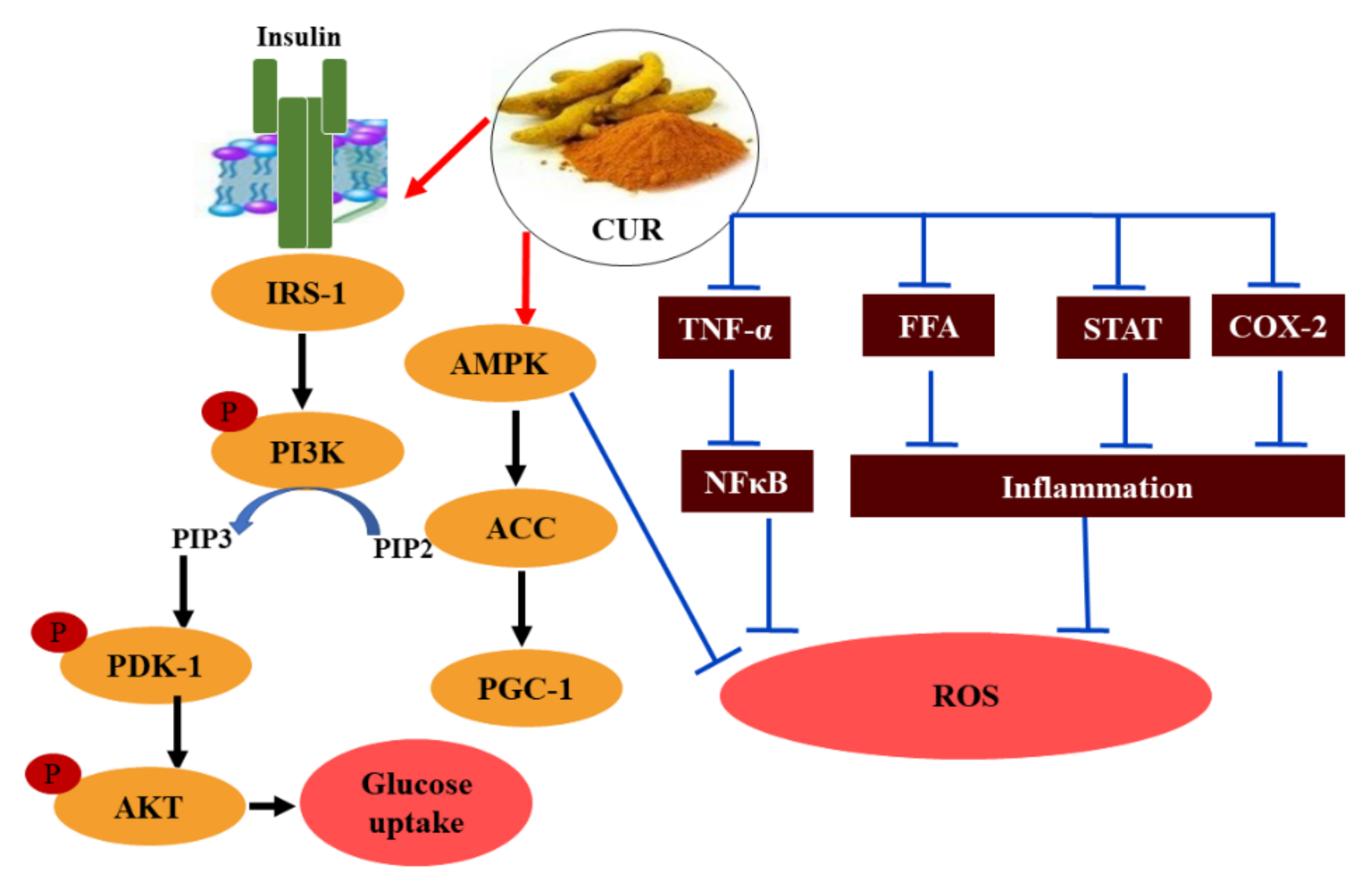
3.3. Curcumin (CUR)
3.4. Moringa Oleifera (MO)
3.5. Portulaca Oleracea (PO)
3.6. Punica Granatum (PG)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nouraei, H.; Jahromi, M.G.; Jahromi, L.R.; Zomorodian, K.; Pakshir, K. Potential Pathogenicity of Candida Species Isolated from Oral Cavity of Patients with Diabetes Mellitus. Biomed. Res. Int. 2021, 2021, 9982744. [Google Scholar] [CrossRef]
- Abdelalim, E.M. Modeling different types of diabetes using human pluripotent stem cells. Cell Mol. Life Sci. 2021, 78, 2459–2483. [Google Scholar] [CrossRef]
- Altamura, S.; Mudder, K.; Schlotterer, A.; Fleming, T.; Heidenreich, E.; Qiu, R.; Hammes, H.P.; Nawroth, P.; Muckenthaler, M.U. Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Mol. Metab. 2021, 51, 101235. [Google Scholar] [CrossRef]
- Aalaa, M.; Sanjari, M.; Esfahani, E.N.; Atlasi, R.; Larijani, B.; Mohajeri-Tehrani, M.R.; Mehrdad, N.; Amini, M.R. Diabetic Foot scientific activities in Endocrinology and Metabolism Research Institute. J. Diabetes Metab. Disord. 2021, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Z.; Wu, J.; Wang, H.; Gao, L.; Xiao, J. Effect of type II diabetes-induced osteoarthritis on articular cartilage aging in rats: A study in vivo and in vitro. Exp. Gerontol. 2021, 150, 111354. [Google Scholar] [CrossRef]
- Srivastava, B.; Sen, S.; Bhakta, S.; Sen, K. Effect of caffeine on the possible amelioration of diabetic neuropathy: A spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 264, 120322. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M. Are people with uncontrolled diabetes mellitus at high risk of reinfections with COVID-19? Prim. Care Diabetes 2021, 15, 18–20. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharm. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Ye, Y.; Yu, M.; Qu, X. Prospect of Sodium-Glucose Co-transporter 2 Inhibitors Combined With Insulin for the Treatment of Type 2 Diabetes. Front. Endocrinol. 2020, 11, 190. [Google Scholar] [CrossRef]
- Odawara, M.; Aoi, S.; Takeshima, T.; Iwasaki, K. Comparative Effects of Metformin and Dipeptidyl Peptidase-4 Inhibitors in Japanese Obese Patients with Type 2 Diabetes: A Claims Database Study. Diabetes 2021, 12, 2165–2177. [Google Scholar] [CrossRef]
- van Gorp, A.M.; Rolfes, L.; Harmark, L.; van der Horst, P.; Hendriks, J.; Vorstenbosch, S. Insight in the safety profile of antidiabetic agents glucagon-like peptide-1 agonists and dipeptidyl peptidase-4 inhibitors in daily practice from the patient perspective. Pharm. Drug Saf. 2020, 29, 1588–1595. [Google Scholar]
- Didari, E.; Sarhangi, N.; Afshari, M.; Meybodi, H.R.A.; Hasanzad, M.A. pharmacogenetic pilot study of CYP2C9 common genetic variant and sulfonylureas therapeutic response in type 2 diabetes mellitus patients. J. Diabetes Metab. Disord. 2021, 1–7. [Google Scholar] [CrossRef]
- Rocha, R.F.; Rodrigues, T.; Menegatti, A.C.O.; Bernardes, G.J.L.; Terenzi, H. The antidiabetic drug lobeglitazone has the potential to inhibit PTP1B activity. Bioorg. Chem. 2020, 100, 103927–103923. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, D.; Raul, A.D.; Wanjari, P.; Bharatam, P.V. Biguanides: Species with versatile therapeutic applications. Eur. J. Med. Chem. 2021, 219, 113378–113417. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, S.K.; Bhatt, R.; Kumar, A. Targeting type II diabetes with plant terpenes: The new and promising antidiabetic therapeutics. Biologia 2020, 76, 241–254. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25–38. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Hamid, Z.A.; Budin, S.B. The Potential Role of Flavonoids in Ameliorating Diabetic Cardiomyopathy via Alleviation of Cardiac Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 5094. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Chen, L.; Liu, R.; Chang, M.; Wang, X. Identification and in vitro anti-inflammatory activity of different forms of phenolic compounds in Camellia oleifera oil. Food Chem. 2021, 344, 128660. [Google Scholar] [CrossRef]
- Lodhi, S.; Kori, M.L. Structure—Activity Relationship and Therapeutic Benefits of Flavonoids in the Management of Diabetes and Associated Disorders. Pharm. Chem. J. 2021, 54, 1106–1125. [Google Scholar] [CrossRef]
- Martin, M.A.; Ramos, S. Dietary Flavonoids and Insulin Signaling in Diabetes and Obesity. Cells 2021, 10, 1474. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pr. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Moreno, A.; Quintanar, E.M.A.; Garcia Garza, R.; Hady, K.; Melendez, V.A.; Marszalek, J.E.; Sharara-Nunez, I.; Delgadillo-Guzman, D. All-trans retinoic acid improves pancreatic cell proliferation on induced type 1 diabetic rats. Fundam. Clin. Pharm. 2020, 34, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Grizzanti, J.; Corrigan, R.; Servizi, S.; Casadesus, G. Amylin signaling in diabetes and Alzheimer’s disease: Therapy or pathology? J. Neurol. Neuromed. 2019, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; Naya, I.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; Asúnsolo, Á.; de la Torre, B. Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients 2020, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- El-Zeftawy, M.; Ali, S.A.M.; Salah, S.; Hafez, H.S. The functional nutritional and regulatory activities of calcium supplementation from eggshell for obesity disorders management. J. Food Biochem. 2020, 44, e13313. [Google Scholar] [CrossRef]
- Rorbach-Dolata, A.; Piwowar, A. Neurometabolic Evidence Supporting the Hypothesis of Increased Incidence of Type 3 Diabetes Mellitus in the 21st Century. Biomed. Res. Int. 2019, 2019, 1435276. [Google Scholar] [CrossRef]
- Jia, J.-J.; Zeng, X.-S.; Song, X.-Q.; Zhang, P.-P.; Chen, L. Diabetes mellitus and Alzheimer’s disease: The protection of epigallocatechin-3-gallate in streptozotocin injection-induced models. Front. Pharmacol. 2017, 8, 834. [Google Scholar] [CrossRef]
- Keller, A.; Varela, V.C.; Dangol, R.; Damm, P.; Heitmann, B.L.; Händel, M.N. The Role of Vitamin D in the Development of Diabetes Post Gestational Diabetes Mellitus: A Systematic Literature Review. Nutrients 2020, 12, 1733. [Google Scholar] [CrossRef]
- Wu, L.; Nahm, C.B.; Jamieson, N.B.; Samra, J.; Clifton-Bligh, R.; Mittal, A.; Tsang, V. Risk factors for development of diabetes mellitus (Type 3c) after partial pancreatectomy: A systematic review. Clin. Endocrinol. 2020, 92, 396–406. [Google Scholar] [CrossRef]
- Rickels, M.R. Hypoglycemia-associated autonomic failure, counterregulatory responses, and therapeutic options in type 1 diabetes. Ann. N. Y. Acad. Sci. 2019, 1454, 68–79. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Balaji, R.; Duraisamy, R.; Kumar, M.P. Complications of diabetes mellitus: A review. Drug Invent. Today 2019, 12, 98–103. [Google Scholar]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pr. 2019, 148, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: A Review. Int. J. Mol. Sci. 2020, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef]
- Dariya, B.; Nagaraju, G.P. Advanced glycation end products in diabetes, cancer and phytochemical therapy. Drug Discov. Today 2020, 25, 1614–1623. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, N.; Jiang, M.; Liu, L.; Zhu, Y.; Wu, H.; Chen, J.; Fu, Y.; Du, Q.; Xu, H. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF—κB pathway. J. Cell. Mol. Med. 2020, 24, 6083–6095. [Google Scholar] [CrossRef]
- Tang, S.G.; Liu, X.Y.; Ye, J.M.; Hu, T.T.; Yang, Y.Y.; Han, T.; Tan, W. Isosteviol ameliorates diabetic cardiomyopathy in rats by inhibiting ERK and NF-kappaB signaling pathways. J. Endocrinol. 2018, 238, 47–60. [Google Scholar] [CrossRef]
- Sharma, D.; Gondaliya, P.; Tiwari, V.; Kalia, K. Kaempferol attenuates diabetic nephropathy by inhibiting RhoA/Rho-kinase mediated inflammatory signalling. Biomed. Pharm. 2019, 109, 1610–1619. [Google Scholar] [CrossRef]
- Abo El-Nasr, N.M.E.; Saleh, D.O.; Mahmoud, S.S.; Nofal, S.M.; Abdelsalam, R.M.; Safar, M.M.; El-Abhar, H.S. Olmesartan attenuates type 2 diabetes-associated liver injury: Cross-talk of AGE/RAGE/JNK, STAT3/SCOS3 and RAS signaling pathways. Eur. J. Pharm. 2020, 874, 173010. [Google Scholar] [CrossRef]
- Joshi, T.; Singh, A.K.; Haratipour, P.; Sah, A.N.; Pandey, A.K.; Naseri, R.; Juyal, V.; Farzaei, M.H. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell Physiol. 2019, 234, 17212–17231. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, N.; Liu, J.; Huang, J.; Shi, J.; Jin, F. AMPK: A bridge between diabetes mellitus and Alzheimer’s disease. Behav. Brain Res. 2021, 400, 113043. [Google Scholar] [CrossRef] [PubMed]
- Szrejder, M.; Piwkowska, A. AMPK signalling: Implications for podocyte biology in diabetic nephropathy. Biol. Cell 2019, 111, 109–120. [Google Scholar] [CrossRef]
- Shrikanth, C.B.; Nandini, C.D. AMPK in microvascular complications of diabetes and the beneficial effects of AMPK activators from plants. Phytomedicine 2020, 73, 152808. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small molecule NF-κB pathway inhibitors in clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tao, W.; Bu, D.; Zhao, Y.; Zhang, T.; Chong, D.; Xue, B.; Xing, Z.; Li, C. Egr-1 transcriptionally activates protein phosphatase PTP1B to facilitate hyperinsulinemia-induced insulin resistance in the liver in type 2 diabetes. FEBS Lett 2019, 593, 3054–3063. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Zhao, Z.; Chen, J.; Li, C.; Zhao, G. Paeoniflorin inhibited nod-like receptor protein-3 inflammasome and NF-kappaB-mediated inflammatory reactions in diabetic foot ulcer by inhibiting the chemokine receptor CXCR2. Drug Dev. Res. 2020, 82, 404–411. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Drzewoski, J.; Sliwinska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef]
- Majid, M.; Masood, A.; Kadla, S.A.; Hameed, I.; Ganai, B.A. Association of Pro12Ala Polymorphism of Peroxisome Proliferator-Activated Receptor gamma 2 (PPARgamma2) Gene with Type 2 Diabetes Mellitus in Ethnic Kashmiri Population. Biochem. Genet. 2017, 55, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Kokozidou, M.; Auffarth, A.; Schulze-Tanzil, G. The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review. Cells 2020, 9, 2208. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.; Deng, T.; Ge, H.-P.; Xiao, X.-C. Identification of C3 as a therapeutic target for diabetic nephropathy by bioinformatics analysis. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Grzybowski, A.; Brona, P.; Lim, G.; Ruamviboonsuk, P.; Tan, G.S.W.; Abramoff, M.; Ting, D.S.W. Artificial intelligence for diabetic retinopathy screening: A review. Eye 2019, 34, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Lauri, C.; Glaudemans, A.; Campagna, G.; Keidar, Z.; Muchnik Kurash, M.; Georga, S.; Arsos, G.; Noriega-Alvarez, E.; Argento, G.; Kwee, T.C.; et al. Comparison of White Blood Cell Scintigraphy, FDG PET/CT and MRI in Suspected Diabetic Foot Infection: Results of a Large Retrospective Multicenter Study. J. Clin. Med. 2020, 9, 1645. [Google Scholar] [CrossRef]
- Nashawi, M.; Sheikh, O.; Battisha, A.; Ghali, A.; Chilton, R. Neural tone and cardio-renal outcomes in patients with type 2 diabetes mellitus: A review of the literature with a focus on SGLT2 inhibitors. Heart Fail. Rev. 2020, 2020 26, 643–652. [Google Scholar] [CrossRef]
- Sözen, T.; Başaran, N.Ç.; Tınazlı, M.; Özışık, L. Musculoskeletal problems in diabetes mellitus. Eur. J. Rheumatol. 2018, 5, 258. [Google Scholar] [CrossRef]
- Luca, M.; Di Mauro, M.; Di Mauro, M.; Luca, A. Gut Microbiota in Alzheimer’s Disease, Depression, and Type 2 Diabetes Mellitus: The Role of Oxidative Stress. Oxid. Med. Cell Longev. 2019, 2019, 4730539. [Google Scholar] [CrossRef]
- Lega, I.C.; Lipscombe, L.L. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr. Rev. 2020, 41, 33–52. [Google Scholar] [CrossRef]
- Abo Baker, S.; Moawad, A. Anti-diabetic effect of Moringa oleifera extract on parotid gland of albino rats. Egypt. Dent. J. 2020, 66, 187–196. [Google Scholar] [CrossRef][Green Version]
- Reddy, P.K.; Kuchay, M.S.; Mehta, Y.; Mishra, S.K. Diabetic ketoacidosis precipitated by COVID-19: A report of two cases and review of literature. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Raju, B.; Santhanakumar, K.S.; Kesavachandran, U. Gastrointestinal involvement of unusual Mucormycete Syncephalastrum racemosum in a diabetic patient with adenocarcinoma: Rare case presentation with review of literature. Infection 2020, 48, 791–797. [Google Scholar] [CrossRef]
- Hussain, A.; Bhowmik, B.; do Vale Moreira, N.C. COVID-19 and diabetes: Knowledge in progress. Diabetes Res. Clin. Pract. 2020, 162, 108142. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M.; Yadav, U.; Bhattacharjee, S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: A systematic review of literature. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1563–1569. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Mohebbati, R.; Abbasnezhad, A. Effects of Nigella sativa on endothelial dysfunction in diabetes mellitus: A review. J. Ethnopharmacol. 2020, 252, 112585. [Google Scholar] [CrossRef]
- Lutterodt, H.; Luther, M.; Slavin, M.; Yin, J.-J.; Parry, J.; Gao, J.-M.; Yu, L. Fatty acid profile, thymoquinone content, oxidative stability, and antioxidant properties of cold-pressed black cumin seed oils. LWT Food Sci. Technol. 2010, 43, 1409–1413. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Cataudella, E.; Giordano, M.; Nunnari, G.; Chisari, G.; Malaguarnera, M. Toxic hepatitis in occupational exposure to solvents. World J. Gastroenterol. 2012, 18, 2756–2766. [Google Scholar] [CrossRef]
- Balbaa, M.; Abdulmalek, S.A.; Khalil, S. Oxidative stress and expression of insulin signaling proteins in the brain of diabetic rats: Role of Nigella sativa oil and antidiabetic drugs. PLoS ONE 2017, 12, e0172429. [Google Scholar] [CrossRef]
- Balbaa, M.; El-Zeftawy, M.; Ghareeb, D.; Taha, N.; Mandour, A.W. Nigella sativa relieves the altered insulin receptor signaling in streptozotocin-induced diabetic rats fed with a high-fat diet. Oxidative Med. Cell. Longev. 2016, 2016, 1–16. [Google Scholar] [CrossRef]
- Shahin, Y.R.; Elguindy, N.M.; Abdel Bary, A.; Balbaa, M. The protective mechanism of Nigella sativa against diethylnitrosamine-induced hepatocellular carcinoma through its antioxidant effect and EGFR/ERK1/2 signaling. Environ. Toxicol. 2018, 33, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Invally, M.; Khan, M.K.; Jadhav, P. A nutraceutical combination of Cinnamomum cassia &Nigella sativa for Type 1 diabetes mellitus. J. Ayurveda Integr. Med. 2018, 9, 27–37. [Google Scholar] [PubMed]
- Hamdan, A.; Haji Idrus, R.; Mokhtar, M.H. Effects of Nigella Sativa on Type-2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 4911. [Google Scholar] [CrossRef]
- Alli-oluwafuyi, A.-M.; Amin, A.; Abdulmajeed, W.I.; Imam, A.; Niyi-odumosu, F.; Abdulraheem, H.; Gwadabe, S.; Biliaminu, A.S. Nigella sativa L. oil ameliorates insulin resistance caused by dexamethasone treatment in male Wistar rats. Afr. J. Pharm. Pharmacol. 2017, 11, 144–151. [Google Scholar] [CrossRef]
- Balbaa, M.; El-Zeftawy, M.; Abdulmalek, S.A.; Shahin, Y.R. Health-Promoting Activities of Nigella sativa Fixed Oil. In Black Cumin (Nigella sativa) Seeds: Chemistry, Technology, Functionality, and Applications; Springer: Cham, Switzerland, 2021; pp. 361–379. [Google Scholar]
- Meddah, B.; Ducroc, R.; El Abbes Faouzi, M.; Eto, B.; Mahraoui, L.; Benhaddou-Andaloussi, A.; Martineau, L.C.; Cherrah, Y.; Haddad, P.S. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J. Ethnopharmacol. 2009, 121, 419–424. [Google Scholar] [CrossRef]
- Sobhi, W.; Stevigny, C.; Duez, P.; Calderon, B.B.; Atmani, D.; Benboubetra, M. Effect of lipid extracts of Nigella sativa L. seeds on the liver ATP reduction and alpha-glucosidase inhibition. Pak. J. Pharm. Sci. 2016, 29, 111–117. [Google Scholar]
- Elseweidy, M.M.; Amin, R.S.; Atteia, H.H.; Aly, M.A. Nigella sativa Oil and Chromium Picolinate Ameliorate Fructose-Induced Hyperinsulinemia by Enhancing Insulin Signaling and Suppressing Insulin-Degrading Enzyme in Male Rats. Biol. Trace Elem. Res. 2018, 184, 119–126. [Google Scholar] [CrossRef]
- Rashidmayvan, M.; Mohammadshahi, M.; Seyedian, S.S.; Haghighizadeh, M.H. The effect of Nigella sativa oil on serum levels of inflammatory markers, liver enzymes, lipid profile, insulin and fasting blood sugar in patients with non-alcoholic fatty liver. J. Diabetes Metab. Disord. 2019, 18, 453–459. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.J. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 2016, 9, 145–153. [Google Scholar]
- Karandrea, S.; Yin, H.; Liang, X.; Slitt, A.L.; Heart, E.A. Thymoquinone ameliorates diabetic phenotype in Diet-Induced Obesity mice via activation of SIRT-1-dependent pathways. PLoS ONE 2017, 12, e0185374. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes Metab. J. 2013, 37, 315–325. [Google Scholar] [CrossRef]
- Kooshki, A.; Tofighiyan, T.; Rastgoo, N.; Rakhshani, M.H.; Miri, M. Effect of Nigella sativa oil supplement on risk factors for cardiovascular diseases in patients with type 2 diabetes mellitus. Phytother. Res. 2020, 34, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.R.; Mohammadizadeh, M. Therapeutic potentials of Nigella sativa preparations and its constituents in the management of diabetes and its complications in experimental animals and patients with diabetes mellitus: A systematic review. Complement. Med. 2020, 50, 102391. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharm. 2021, 133, 110984. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Bisht, A.; Devkota, H.P.; Ullah, H.; Khan, H.; Pandey, A.; Bhatt, I.D.; Echeverria, J. Phytopharmacology and Clinical Updates of Berberis Species Against Diabetes and Other Metabolic Diseases. Front. Pharm. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, K.; Zhou, J.; Sullivan, M.A.; Liu, Y.; Gilbert, R.G.; Deng, B. Metformin and Berberine suppress glycogenolysis by inhibiting glycogen phosphorylase and stabilizing the molecular structure of glycogen in db/db mice. Carbohydr. Polym. 2020, 243, 116435. [Google Scholar] [CrossRef]
- Amin, A.R.; Kassab, R.B.; Abdel Moneim, A.E.; Amin, H.K. Comparison among garlic, berberine, resveratrol, Hibiscus sabdariffa, genus Zizyphus, hesperidin, red beetroot, Catha edulis, Portulaca oleracea, and mulberry leaves in the treatment of hypertension and type 2 DM: A comprehensive review. Nat. Prod. Commun. 2020, 15, 1934578X20921623. [Google Scholar]
- Meng, Z.; Yu, Y.; Zhang, Y.; Yang, X.; Lv, X.; Guan, F.; Hatch, G.M.; Zhang, M.; Chen, L. Highly bioavailable Berberine formulation improves Glucocorticoid Receptor-mediated Insulin Resistance via reduction in association of the Glucocorticoid Receptor with phosphatidylinositol-3-kinase. Int. J. Biol. Sci. 2020, 16, 2527–2541. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, C.; Ying, Y.; Luo, L.; Huang, D.; Luo, Z. Metformin and berberine, two versatile drugs in treatment of common metabolic diseases. Oncotarget 2018, 9, 10135. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xu, Z.; Li, C.; Zhu, Y.; Liu, R.; Zhang, F.; Chang, H.; Li, M.; Sheng, L.; Li, Z.; et al. Therapeutic inhibition of miR-802 protects against obesity through AMPK-mediated regulation of hepatic lipid metabolism. Theranostics 2021, 11, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- El-Zeftawy, M.; Ghareeb, D.; ElBealy, E.R.; Saad, R.; Mahmoud, S.; Elguindy, N.; El-Kott, A.F.; El-Sayed, M. Berberine chloride ameliorated PI3K/Akt-p/SIRT-1/PTEN signaling pathway in insulin resistance syndrome induced in rats. J. Food Biochem. 2019, 43, e13049. [Google Scholar] [CrossRef]
- Li, M.; Zhou, W.; Dang, Y.; Li, C.; Ji, G.; Zhang, L. Berberine compounds improve hyperglycemia via microbiome mediated colonic TGR5-GLP pathway in db/db mice. Biomed. Pharm. 2020, 132, 110953. [Google Scholar] [CrossRef]
- Cui, H.X.; Hu, Y.N.; Li, J.W.; Yuan, K. Hypoglycemic Mechanism of the Berberine Organic Acid Salt under the Synergistic Effect of Intestinal Flora and Oxidative Stress. Oxid. Med. Cell Longev. 2018, 2018, 8930374. [Google Scholar] [CrossRef]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharm. 2020, 127, 110137. [Google Scholar] [CrossRef]
- Yue, S.J.; Liu, J.; Wang, A.T.; Meng, X.T.; Yang, Z.R.; Peng, C.; Guan, H.S.; Wang, C.Y.; Yan, D. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E73–E85. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine protects against diabetic kidney disease via promoting PGC-1alpha-regulated mitochondrial energy homeostasis. Br. J. Pharm. 2020, 177, 3646–3661. [Google Scholar] [CrossRef]
- Liu, L.Z.; Cheung, S.C.; Lan, L.L.; Ho, S.K.; Xu, H.X.; Chan, J.C.; Tong, P.C. Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol. Cell Endocrinol. 2010, 317, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wang, M.Q.; Ni, S.H.; Wang, M.; Liu, L.Y.; You, H.Y.; Wu, X.H.; Wang, Y.J.; Lu, L.; Wei, L.B. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-kappaB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharm. 2020, 867, 172797. [Google Scholar] [CrossRef]
- Calvani, M.; Subbiani, A.; Bruno, G.; Favre, C. Beta-Blockers and Berberine: A Possible Dual Approach to Contrast Neuroblastoma Growth and Progression. Oxid. Med. Cell Longev. 2020, 2020, 7534693. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ai, G.; Wang, Y.; Lu, Q.; Luo, C.; Tan, L.; Lin, G.; Liu, Y.; Li, Y.; Zeng, H.; et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-kappaB pathway. Pharm. Res. 2020, 152, 104603. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.; Wen, B.; Zhang, Z. The Pathogenesis of Diabetes Mellitus by Oxidative Stress and Inflammation: Its Inhibition by Berberine. Front. Pharm. 2018, 9, 782. [Google Scholar] [CrossRef]
- Mahata, S.; Bharti, A.C.; Shukla, S.; Tyagi, A.; Husain, S.A.; Das, B.C. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer 2011, 10, 39. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Fekri, H.S.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Therapeutic and biological activities of berberine: The involvement of Nrf2 signaling pathway. J. Cell Biochem. 2020, 121, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Zych, M.; Wojnar, W.; Kielanowska, M.; Folwarczna, J.; Kaczmarczyk-Sedlak, I. Effect of Berberine on Glycation, Aldose Reductase Activity, and Oxidative Stress in the Lenses of Streptozotocin-Induced Diabetic Rats In Vivo-A Preliminary Study. Int. J. Mol. Sci. 2020, 21, 4278. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocinska, K.; Zielinska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharm. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Balbaa, M.; El-Zeftawy, M.; Taha, N.; Mandour, A.W. Zinc and curcumin lower arylsulfatses and some metabolic parameters in streptozotocin-induced diabetes. J. Diabetes Metab. Disord. 2017, 16, 11. [Google Scholar] [CrossRef]
- Ibanez, M.D.; Blazquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2020, 10, 44. [Google Scholar] [CrossRef]
- Panahi, Y.; Ahmadi, Y.; Teymouri, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a potential candidate for treating hyperlipidemia: A review of cellular and metabolic mechanisms. J. Cell Physiol. 2018, 233, 141–152. [Google Scholar] [CrossRef]
- Jin, T.R. Curcumin and dietary polyphenol research: Beyond drug discovery. Acta Pharm. Sin. 2018, 39, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Li, Y.C.; Kong, L.D.; Hu, Q.H. Curcumin inhibits hepatic protein-tyrosine phosphatase 1B and prevents hypertriglyceridemia and hepatic steatosis in fructose-fed rats. Hepatology 2010, 51, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Osorio, A.S.; Monroy, A.; Alavez, S. Curcumin and insulin resistance-Molecular targets and clinical evidences. Biofactors 2016, 42, 561–580. [Google Scholar] [CrossRef]
- Eshaghian, A.; Khodarahmi, A.; Safari, F.; Binesh, F.; Moradi, A. Curcumin attenuates hepatic fibrosis and insulin resistance induced by bile duct ligation in rats. Br. J. Nutr. 2018, 120, 393–403. [Google Scholar] [CrossRef]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular Mechanisms Underlying Curcumin-Mediated Therapeutic Effects in Type 2 Diabetes and Cancer. Oxid Med. Cell Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.H.; Zhang, S.Y.; Chen, Y.S.; Li, K.; Chen, W.B.; Liu, Y.Q. Curcumin anti-diabetic effect mainly correlates with its anti-apoptotic actions and PI3K/Akt signal pathway regulation in the liver. Food Chem. Toxicol. 2020, 146, 111803. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, D.C.; Kim, C.H.; Han, I.; Gil, E.Y.; Kim, K.T. Effect of curcumin on the inflammatory reaction and functional recovery after spinal cord injury in a hyperglycemic rat model. Spine J. 2019, 19, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3beta and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell Physiol. 2018, 233, 830–848. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.J.; Chang, H.H.; Tsai, T.H.; Lee, T.Y. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int. J. Mol. Med. 2012, 30, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Simmons, E.C.; Scholpa, N.E.; Schnellmann, R.G. Mitochondrial biogenesis as a therapeutic target for traumatic and neurodegenerative CNS diseases. Exp. Neurol. 2020, 329, 113309. [Google Scholar] [CrossRef]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef]
- Moulin, S.; Arnaud, C.; Bouyon, S.; Pepin, J.L.; Godin-Ribuot, D.; Belaidi, E. Curcumin prevents chronic intermittent hypoxia-induced myocardial injury. Adv. Chronic Dis. 2020, 11, 2040622320922104. [Google Scholar] [CrossRef]
- Bahrami, A.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Effects of curcumin on hypoxia-inducible factor as a new therapeutic target. Pharm. Res. 2018, 137, 159–169. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, H.; Wang, P.; Zhao, Y.; Li, Q.; Wei, X.; Cui, Y.; Sun, J.; Shang, Q.; Liu, D.; et al. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cell Physiol. Biochem. 2013, 32, 1167–1177. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Tiano, L.; Louw, J.; Jastroch, M.; Mazibuko-Mbeje, S.E. Uncoupling proteins as a therapeutic target to protect the diabetic heart. Pharm. Res. 2018, 137, 11–24. [Google Scholar] [CrossRef]
- Su, J.; Zhou, X.; Wang, L.; Yin, X.; Wang, Z. Curcumin inhibits cell growth and invasion and induces apoptosis through down-regulation of Skp2 in pancreatic cancer cells. Am. J. Cancer Res. 2016, 6, 1949–1962. [Google Scholar] [PubMed]
- Tsai, Y.C.; Kuo, P.L.; Kuo, M.C.; Hung, W.W.; Wu, L.Y.; Chang, W.A.; Wu, P.H.; Lee, S.C.; Chen, H.C.; Hsu, Y.L. The Interaction of miR-378i-Skp2 Regulates Cell Senescence in Diabetic Nephropathy. J. Clin. Med. 2018, 7, 468. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; El Rabey, H.A. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Biomed. Res. Int. 2015, 2015, 381040. [Google Scholar] [CrossRef]
- Nova, E.; Redondo-Useros, N.; Martinez-Garcia, R.M.; Gomez-Martinez, S.; Diaz-Prieto, L.E.; Marcos, A. Potential of Moringa oleifera to Improve Glucose Control for the Prevention of Diabetes and Related Metabolic Alterations: A Systematic Review of Animal and Human Studies. Nutrients 2020, 12, 2050. [Google Scholar] [CrossRef]
- Jaiswal, D.; Kumar Rai, P.; Kumar, A.; Mehta, S.; Watal, G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009, 123, 392–396. [Google Scholar] [CrossRef]
- Tshabalala, T.; Ndhlala, A.R.; Ncube, B.; Abdelgadir, H.A.; Van Staden, J. Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. South. Afr. J. Bot. 2020, 129, 106–112. [Google Scholar] [CrossRef]
- Fatoumata, B.A.; MamadouSaïdou, B.A.H.; Mohamet, S.; Joseph, K.S.; Modou, M.G.; El Hadji, M.B.A. Antidiabetic properties of Moringa oleifera: A review of the literature. J. Diabetes Endocrinol. 2020, 11, 18–29. [Google Scholar] [CrossRef]
- Ma, Z.F.; Ahmad, J.; Zhang, H.; Khan, I.; Muhammad, S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. South. Afr. J. Bot. 2020, 129, 40–46. [Google Scholar] [CrossRef]
- Attakpa, E.S.; Sangaré, M.M.; Béhanzin, G.J.; Ategbo, J.M.; Seri, B.; Khan, N.A. Moringa olifeira Lam. stimulates activation of the insulin-dependent akt pathway. Antidiabetic effect in a diet-induced obesity (DIO) mouse model. Folia Biol. 2017, 63, 42. [Google Scholar]
- Bao, Y.; Xiao, J.; Weng, Z.; Lu, X.; Shen, X.; Wang, F. A phenolic glycoside from Moringa oleifera Lam. improves the carbohydrate and lipid metabolisms through AMPK in db/db mice. Food Chem. 2020, 311, 125948. [Google Scholar] [CrossRef]
- Wang, F.; Bao, Y.; Shen, X.; Zengin, G.; Lyu, Y.; Xiao, J.; Weng, Z. Niazirin from Moringa oleifera Lam. attenuates high glucose-induced oxidative stress through PKCzeta/Nox4 pathway. Phytomedicine 2019, 153066. [Google Scholar] [CrossRef]
- Chen, G.L.; Xu, Y.B.; Wu, J.L.; Li, N.; Guo, M.Q. Hypoglycemic and hypolipidemic effects of Moringa oleifera leaves and their functional chemical constituents. Food Chem. 2020, 333, 127478. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, M.H.; Shin, H.J. Extracts of Moringa oleifera leaves from different cultivation regions show both antioxidant and antiobesity activities. J. Food Biochem. 2020, 44, e13282. [Google Scholar] [CrossRef] [PubMed]
- Melilli, M.G.; Pagliaro, A.; Scandurra, S.; Gentile, C.; Di Stefano, V. Omega-3 rich foods: Durum wheat spaghetti fortified with Portulaca oleracea. Food Biosci. 2020, 37, 100730. [Google Scholar] [CrossRef]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (Portulaca oleracea L.) genotypes. Food Chem. 2020, 320, 126621. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Cho, S.-K.; Ghodake, G.; Bharagava, R.N.; Park, Y.; Mulla, S.I.; Kim, D.-S.; Kadam, A.; Nair, S.; et al. Investigation of photocatalytic degradation of reactive textile dyes by Portulaca oleracea-functionalized silver nanocomposites and exploration of their antibacterial and antidiabetic potentials. J. Alloy. Compd. 2020, 833, 155083. [Google Scholar] [CrossRef]
- Hu, Q.; Niu, Q.; Song, H.; Wei, S.; Wang, S.; Yao, L.; Li, Y.P. Polysaccharides from Portulaca oleracea L. regulated insulin secretion in INS-1 cells through voltage-gated Na(+) channel. Biomed. Pharm. 2019, 109, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Mo, F.; Ling, C.; Peng, H.; Gu, W.; Li, M.; Chen, Z. Portulaca oleracea L. alleviates liver injury in streptozotocin-induced diabetic mice. Drug Des. Devel 2018, 12, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.E.; Han, J.S. Portulaca oleracea L. extract reduces hyperglycemia via PI3k/Akt and AMPK pathways in the skeletal muscles of C57BL/Ksj-db/db mice. J. Ethnopharmacol. 2020, 260, 112973. [Google Scholar] [CrossRef]
- Chen, D.; Yao, J.-N.; Liu, T.; Zhang, H.-Y.; Li, R.-R.; Zhang, Z.-J.; Gu, X.-Z. Research and application of Portulaca oleracea in pharmaceutical area. Chin. Herb. Med. 2019, 11, 150–159. [Google Scholar] [CrossRef]
- Park, J.E.; Park, J.Y.; Seo, Y.; Han, J.S. A new chromanone isolated from Portulaca oleracea L. increases glucose uptake by stimulating GLUT4 translocation to the plasma membrane in 3T3-L1 adipocytes. Int. J. Biol. Macromol. 2019, 123, 26–34. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Jimenez-Cabrera, T.; Urrutia-Hernandez, T.A.; Chehue-Romero, A.; Olvera-Hernandez, E.G.; Bautista, M. Punica protopunica Balf. the Forgotten Sister of the Common Pomegranate (Punica granatum L.): Features and Medicinal Properties-A Review. Plants 2020, 9, 1214. [Google Scholar] [CrossRef]
- Chaves, F.M.; Pavan, I.C.B.; da Silva, L.G.S.; de Freitas, L.B.; Rostagno, M.A.; Antunes, A.E.C.; Bezerra, R.M.N.; Simabuco, F.M. Pomegranate juice and peel extracts are able to inhibit proliferation, migration and colony formation of prostate cancer cell lines and modulate the Akt/mTOR/S6K signaling pathway. Plant. Foods Hum. Nutr. 2020, 75, 54–62. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 1–29. [Google Scholar] [CrossRef]
- Jandari, S.; Hatami, E.; Ziaei, R.; Ghavami, A.; Yamchi, A.M. The effect of pomegranate (Punica granatum) supplementation on metabolic status in patients with type 2 diabetes: A systematic review and meta-analysis. Complement. Med. 2020, 52, 102478. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Luna, D.; Martinez-Hinojosa, E.; Cancino-Diaz, J.C.; Belefant-Miller, H.; Lopez-Rodriguez, G.; Betanzos-Cabrera, G. Daily supplementation with fresh pomegranate juice increases paraoxonase 1 expression and activity in mice fed a high-fat diet. Eur. J. Nutr. 2018, 57, 383–389. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Jin, C.; Xiong, Y.; Cheng, Y.Y.; Chen, K. Pomegranate flower extract bidirectionally regulates the proliferation, differentiation and apoptosis of 3T3-L1 cells through regulation of PPARgamma expression mediated by PI3K-AKT signaling pathway. Biomed. Pharm. 2020, 131, 110769. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A.; Fashtaky, R.A.; Makahleh, S.M.; El-Akawi, Z.J.; Khabour, O.F.; Saadeh, N.A. Effect of fresh pomegranate juice on the level of melatonin, insulin, and fasting serum glucose in healthy individuals and people with impaired fasting glucose. Food Sci. Nutr. 2020, 8, 567–574. [Google Scholar] [CrossRef]
- Les, F.; Arbones-Mainar, J.M.; Valero, M.S.; Lopez, V. Pomegranate polyphenols and urolithin A inhibit alpha-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Tkacz, K.; Turkiewicz, I.P.; Nowicka, P.; Hernandez, F.; Lech, K.; Carbonell-Barrachina, A.A.; Wojdylo, A. Inhibition of enzymes associated with metabolic and neurological disorder by dried pomegranate sheets as a function of pomegranate cultivar and fruit puree. J. Sci. Food Agric. 2020, 101, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Ahmed, M.G.; Bhattacharjee, A. Potential pharmacodynamic and pharmacokinetic interaction of pomegranate juice and nateglinide against diabetis induced complications in rats. Synergy 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Alipour, B. Punicic acid: A potential compound of pomegranate seed oil in Type 2 diabetes mellitus management. J. Cell. Physiol. 2019, 234, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sharfaraz, A.; Dutta, A.; Ahsan, A.; Masud, M.A.; Ahmed, I.A.; Goh, B.H.; Urbi, Z.; Sarker, M.M.R.; Ming, L.C. A review of ethnobotany, phytochemistry, antimicrobial pharmacology and toxicology of Nigella sativa L. Biomed. Pharm. 2021, 143, 112182. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, B.; Kulkarni, G.T.; Dhiman, N.; Joshi, D.D.; Chander, S.; Kharkwal, A.; Sharma, A.K.; Kharkwal, H. Recent advances on Berberis aristata emphasizing berberine alkaloid including phytochemistry, pharmacology and drug delivery system. J. Herb. Med. 2021, 27, 100433. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Zhang, W. Berberine for bone regeneration: Therapeutic potential and molecular mechanisms. J. Ethnopharmacol. 2021, 277, 114249. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Attempts to enhance the anti-cancer activity of curcumin as a magical oncological agent using transdermal delivery. Adv. Tradit. Med. 2020, 21, 15–29. [Google Scholar] [CrossRef]
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid. Med. Cell Longev. 2021, 2021, 3149223. [Google Scholar] [CrossRef]
- Fernandes, A.; Bancessi, A.; Pinela, J.; Dias, M.I.; Liberal, A.; Calhelha, R.C.; Ciric, A.; Sokovic, M.; Catarino, L.; Ferreira, I.; et al. Nutritional and phytochemical profiles and biological activities of Moringa oleifera Lam. edible parts from Guinea-Bissau (West Africa). Food Chem. 2021, 341, 128229. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.; Mohanty, S.; Bhuyan, S.K.; Bhuyan, R. Phytoperspective of Moringa oleifera for oral health care: An innovative ethnomedicinal approach. Phytother. Res. 2021, 35, 1345–1357. [Google Scholar] [CrossRef]
- Duan, Y.; Ying, Z.; He, F.; Ying, X.; Jia, L.; Yang, G. A new skeleton flavonoid and a new lignan from Portulaca oleracea L. and their activities. Fitoterapia 2021, 153, 104993. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Gholamnezhad, Z.; Rezaee, R.; Boskabady, M.H. Immuno-modulatory and anti-inflammatory effects of Thymus vulgaris, Zataria multiflora, and Portulaca oleracea and their constituents. Pharmacol. Res. Mod. Chin. Med. 2021, 1, 100010. [Google Scholar] [CrossRef]
- Abdulmalek, S.; Eldala, A.; Awad, D.; Balbaa, M. Ameliorative effect of curcumin and zinc oxide nanoparticles on multiple mechanisms in obese rats with induced type 2 diabetes. Sci. Rep. 2021, 11, 20677. [Google Scholar] [CrossRef]
- Ezzat, S.M.; El Bishbishy, M.H.; Aborehab, N.M.; Salama, M.M.; Hasheesh, A.; Motaal, A.A.; Rashad, H.; Metwally, F.M. Upregulation of MC4R and PPAR-alpha expression mediates the anti-obesity activity of Moringa oleifera Lam. in high-fat diet-induced obesity in rats. J. Ethnopharmacol. 2020, 251, 112541. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, B.K.; Schaalan, M.F.; Tolba, A.M. Hypoglycemic and pancreatic protective effects of Portulaca oleracea extract in alloxan induced diabetic rats. BMC Complement. Altern Med. 2017, 17, 37. [Google Scholar] [CrossRef] [PubMed]
| Scientific Name | Plant Family | Common Name | Traditional Use | References |
|---|---|---|---|---|
| Nigella sativa | Ranunculaceae | Black cumin | Anti-inflammatory, antidiabetic, antiparasitic, and analgesic | [163,164] |
| Berberis vulgaris | Berberidaceae | Berberine | Antihyperlipidemic, anticancer, anti-inflammatory, antioxidant, hepatoprotective, and hypoglycemic agent | [165,166] |
| Curcuma longa | Zingiberaceae | Turmeric | Anticancer, antihyperglycemic, neuroprotective, antiapoptotic, antimicrobial, and cardioprotective | [167,168] |
| Moringa oleifera | Moringaceae | Moringa | Hypoglycemic, neuroprotective, hepatoprotective, hypolipidemic, and antiviral agent | [169,170] |
| Portulaca oleracea | Portulacaceae | Purslane | Anti-inflammatory, antidiabetic, anticancer, analgesic, immunostimulant, antimicrobial, and antiviral | [171,172] |
| Scientific Name | Treatment Form | Dose | Fasting Blood Glucose Level (mg/dL) | Fasting Insulin Level (µIU/mL) | References | ||
|---|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Pre- Treatment | Post- Treatment | ||||
| Nigella sativa | Oil | 100 mg in 10% DMSO/kg Bwt * | 581.31 ± 36.31 | 142.76 ± 16.94 | 101.59 ± 5.78 | 127.86 ± 1.27 | [73] |
| Berberis vulgaris | Berberine chloride | 100 mg/kg Bwt | 180.1 ± 4.38 | 97.7 ± 5.61 | 20.17 ± 2.93 | 15.67 ± 2.42 | [97] |
| Curcuma longa | Curcumin | 50 mg/kg Bwt | 481 ± 0.71 | 109.20 ± 0.86 | 180.44 ± 0.43 | 80.44 ± 0.15 | [173] |
| Moringa oleifera | Ethanolic extract of leaves | 200 mg/kg Bwt | 167.0 ± 1.96 | 94.0 ± 4.96 | 45.03 ± 13.8 | 36.4 ± 4.66 | [174] |
| Portulaca oleracea | Water extract | 250 mg/kg Bwt | 293.2 ± 2.4 | 125.0 ± 1.3 | 18.97 ± 0.09 | 33.50 ± 0.08 | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balbaa, M.; El-Zeftawy, M.; Abdulmalek, S.A. Therapeutic Screening of Herbal Remedies for the Management of Diabetes. Molecules 2021, 26, 6836. https://doi.org/10.3390/molecules26226836
Balbaa M, El-Zeftawy M, Abdulmalek SA. Therapeutic Screening of Herbal Remedies for the Management of Diabetes. Molecules. 2021; 26(22):6836. https://doi.org/10.3390/molecules26226836
Chicago/Turabian StyleBalbaa, Mahmoud, Marwa El-Zeftawy, and Shaymaa A. Abdulmalek. 2021. "Therapeutic Screening of Herbal Remedies for the Management of Diabetes" Molecules 26, no. 22: 6836. https://doi.org/10.3390/molecules26226836
APA StyleBalbaa, M., El-Zeftawy, M., & Abdulmalek, S. A. (2021). Therapeutic Screening of Herbal Remedies for the Management of Diabetes. Molecules, 26(22), 6836. https://doi.org/10.3390/molecules26226836






