Anti-Obesity Effects of Matoa (Pometia pinnata) Fruit Peel Powder in High-Fat Diet-Fed Rats
Abstract
:1. Introduction
2. Results
2.1. Biological Effects
2.1.1. Comparison of the Effects of MPP and SPP in HFD-Fed Rats (Animal Experiment 1)
2.1.2. Dose-Dependency of Anti-Obesity Effects of MPP in HFD-Fed Rats (Animal Experiment 2)
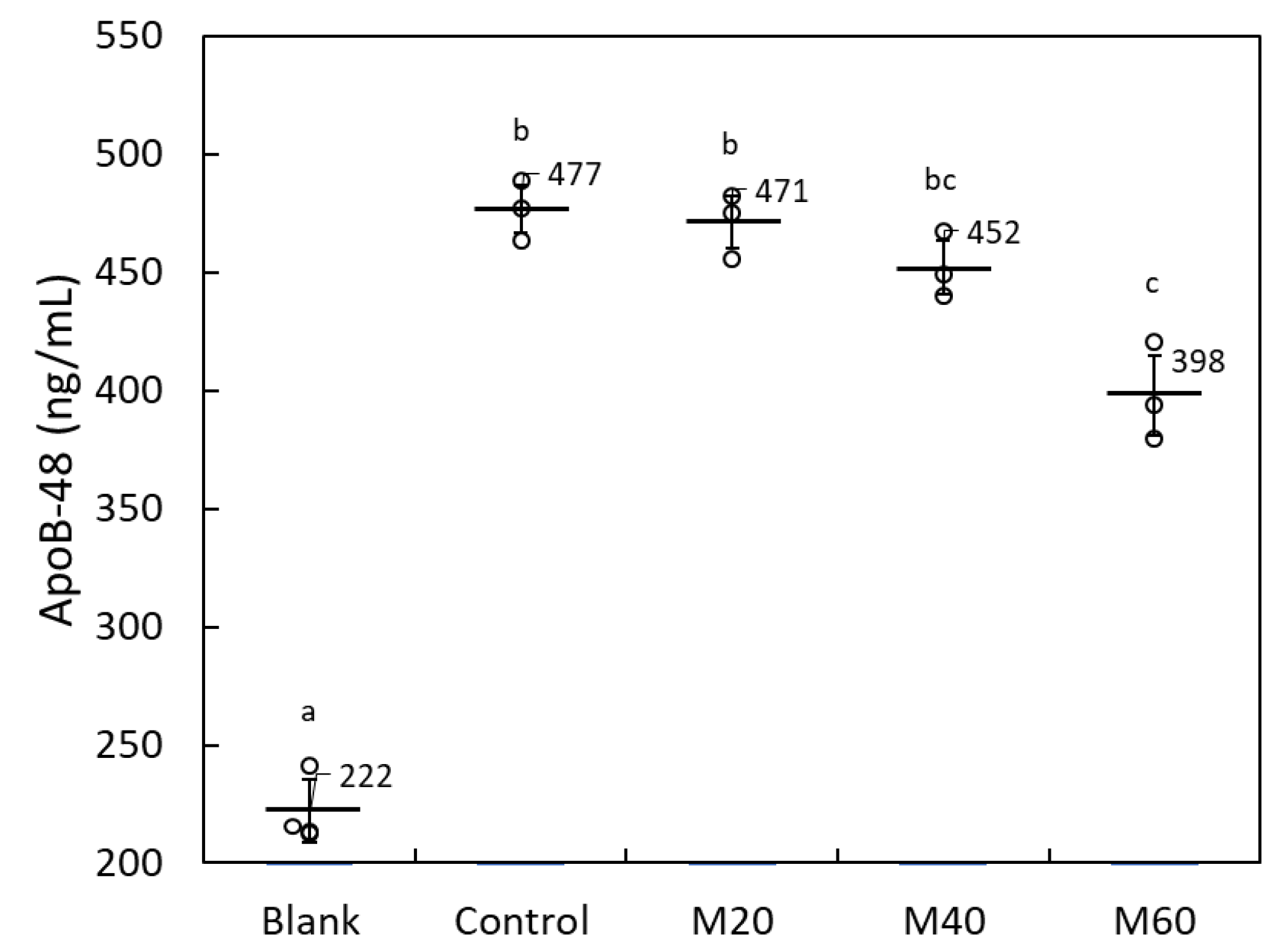
2.1.3. Matoa Peel Extract Suppressed Lipid Micelle-Dependent ApoB-48 Secretion in Caco-2 Monolayers
2.1.4. Matoa Peel Extract Did Not Suppress Oleic Acid-Dependent Lipid Accumulation in HuH-7 Hepatoma Cells
2.2. Chemical Analyses
2.2.1. Identification of Saponin in Matoa Peel
2.2.2. Hederagenin Saponin (HGS) Content in Matoa and Salak Peels
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.1.1. Fruit Peel Powders
4.1.2. Matoa Peel Extract (Ethanol Extraction)
4.2. Biological Effects
4.2.1. Animal Experiments
4.2.2. Collection of Serum and Organ Samples
4.2.3. Serum Biochemical Analyses
4.2.4. Determination of Hepatic Lipid Content
4.2.5. Determination of Fecal TG Content
4.2.6. Cell Culture
4.2.7. Cell Growth and Cytotoxicity Assay
4.2.8. Lipid Micelle-Dependent ApoB-48 Secretion in Caco-2 Monolayers
4.2.9. OA-Dependent Lipid Accumulation in HuH-7 Cells
4.2.10. Statistical Analysis
4.3. Chemical Analyses
4.3.1. Identification of Compound 1 in MPP
4.3.2. Hydrolysis of Saponins and Extraction of Sapogenin (Hederagenin)
4.3.3. HGS Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ritchie, H.; Roser, M. Obesity. Available online: https://ourworldindata.org/obesity (accessed on 6 May 2021).
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 May 2021).
- Arya, A.; Nahar, L.; Khan, H.U.; Sarker, S.D. Chapter Thirteen—Anti-obesity natural products. In Annual Reports in Medicinal Chemistry; Sarker, S.D., Nahar, L., Eds.; Academic Press: Cambridge MA, USA, 2020; Volume 55, pp. 411–433. [Google Scholar]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-Obesity Effects and Different Mechanisms of Action. J. Agric. Food. Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharm. 2019, 110, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; Chung, H.J.; Kim, H.J.; Hong, S.T. Paradoxical Effects of Fruit on Obesity. Nutrients 2016, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Dimou, C.; Karantonis, H.C.; Skalkos, D.; Koutelidakis, A.E. Valorization of Fruits by-Products to Unconventional Sources of Additives, Oil, Biomolecules and Innovative Functional Foods. Curr. Pharm. Biotechnol. 2019, 20, 776–786. [Google Scholar] [CrossRef]
- John, O.D.; Mouatt, P.; Panchal, S.K.; Brown, L. Rind from Purple Mangosteen (Garcinia mangostana) Attenuates Diet-Induced Physiological and Metabolic Changes in Obese Rats. Nutrients 2021, 13, 319. [Google Scholar] [CrossRef]
- John, O.D.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Physiological and Metabolic Effects of Yellow Mangosteen (Garcinia dulcis) Rind in Rats with Diet-Induced Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 272. [Google Scholar] [CrossRef] [Green Version]
- Irawan, C.; Hanafi; Sulistiawaty, L.; Rochaeni, H.; Lestari, P.S. Comparison of total phenolic content in seed, flesh fruit and peel of Pometia pinnata from Indonesia. J. Med. Plants Stud. 2017, 5, 163–165. [Google Scholar]
- Hanafi; Irawan, C.; Sirait, S.M.; Sulistiawaty, L.; Setyawati, S.R. Toxicity Test with BSLT (Brine Shrimp Lethality Test) Method on Methanol, Ethyl Acetate Extract, Hexane on Seeds and Rind of Matoa extract (Pometia pinnata). Orient. J. Chem. 2020, 36, 1143–1147. [Google Scholar] [CrossRef]
- Mataputun, S.P.; Rorong, J.A.; Pontoh, J. Aktivitas Inhibitor α-Glukosidase Ekstrak Kulit Batang Matoa (Pometia Pinnata. Spp.) sebagai Agen Antihiperglikemik. J. MIPA Unsrat. Online 2013, 2, 119–123. [Google Scholar] [CrossRef]
- Fitri, A.; Andriani, M.; Sudarman, A.; Toharmat, T.; Yonekura, L.; Tamura, H.; Ramli, N. Screening of antioxidant activities and their bioavailability of tropical fruit byproducts from Indonesia. Int. J. Pharm. Sci. 2016, 8, 96–100. [Google Scholar]
- Pal, S.; Ho, S.S.; Takechi, R. Red wine polyphenolics suppress the secretion of ApoB48 from human intestinal CaCo-2 cells. J. Agric. Food Chem. 2005, 53, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.F.; Meng, F.C.; Cao, L.J.; Jiang, C.H.; Zhao, M.G.; Shang, X.L.; Fang, S.Z.; Ye, W.C.; Zhang, Q.W.; Zhang, J.; et al. Triterpenoids from Cyclocarya paliurus and their inhibitory effect on the secretion of apolipoprotein B48 in Caco-2 cells. Phytochemistry 2017, 142, 76–84. [Google Scholar] [CrossRef]
- Kanuri, G.; Bergheim, I. In vitro and in vivo models of non-alcoholic fatty liver disease (NAFLD). Int. J. Mol. Sci. 2013, 14, 11963–11980. [Google Scholar] [CrossRef] [Green Version]
- Chateau, D.; Pauquai, T.; Delers, F.; Rousset, M.; Chambaz, J.; Demignot, S. Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells. J. Cell Physiol. 2005, 202, 767–776. [Google Scholar] [CrossRef]
- Suedee, A.; Tewtrakul, S.; Panichayupakaranant, P. Anti-HIV-1 integrase compound from Pometia pinnata leaves. Pharm. Biol. 2013, 51, 1256–1261. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Elkahoui, S.; Bartley, G.E.; Yokoyama, W.H.; Friedman, M. Dietary Supplementation of Potato Peel Powders Prepared from Conventional and Organic Russet and Non-Organic Gold and Red Potatoes Reduces Weight Gain in Mice on a High-Fat Diet. J. Agric. Food Chem. 2018, 66, 6064–6072. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio-Perez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef]
- Mohammad, F.V.; Noorwala, M.; Ahmad, V.U.; Zahoor, A.; Lajis, N.H.J. A New Monodesmosidic Triterpenoid Saponin from the Leaves of Pometia pinnata. Nat. Prod. Commun. 2012, 7, 1423–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.C.; Liao, S.C.; Chang, F.R.; Kuo, Y.H.; Wu, Y.C. Molluscicidal saponins from Sapindus mukorossi, inhibitory agents of golden apple snails, Pomacea canaliculata. J. Agric. Food Chem. 2003, 51, 4916–4919. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shi, L.; Wu, J.; Zhou, X.; Li, X.; Sun, X.; Zhu, L.; Xia, T.S.; Ding, Q. A hederagenin saponin isolated from Clematis ganpiniana induces apoptosis in breast cancer cells via the mitochondrial pathway. Oncol. Lett. 2018, 15, 1737–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, T.L.; Wang, C.C.; Kuo, Y.H.; Huang, H.C.; Wu, Y.C.; Kuo, L.M.; Wu, Y.H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.Z.; Qi, Q.; Tao, L.; Zhao, Q.; Mu, R.; Gu, H.Y.; Wang, M.; Feng, X.; Guo, Q.L. Macranthoside B, a hederagenin saponin extracted from Lonicera macranthoides and its anti-tumor activities in vitro and in vivo. Food Chem. Toxicol. 2009, 47, 1716–1721. [Google Scholar] [CrossRef]
- Jeepipalli, S.P.K.; Du, B.; Sabitaliyevich, U.Y.; Xu, B. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food. Chem. 2020, 318, 126474. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.T.; Nakamura, Y.; Akasaka, T.; Katakura, Y.; Tanaka, Y.; Shirouchi, B.; Jiang, Z.; Yuan, X.; Sato, M. Soyasaponin ameliorates obesity and reduces hepatic triacylglycerol accumulation by suppressing lipogenesis in high-fat diet-fed mice. J. Food. Sci. 2021, 86, 2103–2117. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, C.; Yao, N.; Li, Y.; Wang, Q.; Fang, S.; Shang, X.; Zhao, M.; Che, C.; Ni, Y.; et al. Antihyperlipidemic effect of Cyclocarya paliurus (Batal.) Iljinskaja extract and inhibition of apolipoprotein B48 overproduction in hyperlipidemic mice. J. Ethnopharmacol. 2015, 166, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Guan, J.H.; Huang, Y.L.; Pan, Y.W.; Yang, W.; Lan, H.; Huang, S.; Hu, J.; Zhao, G.P. Experimental Study of Antiatherosclerosis Effects with Hederagenin in Rats. Evid. Based. Complement. Alternat. Med. 2015, 2015, 456354. [Google Scholar] [CrossRef]
- Hitahata, R.; Kobayashi, M.; Egusa, S.; Sakakibara, R.; Fukuda, M. Effects of fermented soymilk on lipid metabolism in rats fed a high fat diet. Nippon. Shokuhin Kagaku Kogaku Kaishi 2012, 59, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Navarro del Hierro, J.; Herrera, T.; Fornari, T.; Reglero, G.; Martin, D. The gastrointestinal behavior of saponins and its significance for their bioavailability and bioactivities. J. Funct. Foods 2018, 40, 484–497. [Google Scholar] [CrossRef]
- Reeves, P.G.; Rossow, K.L.; Lindlauf, J. Development and testing of the AIN-93 purified diets for rodents: Results on growth, kidney calcification and bone mineralization in rats and mice. J. Nutr. 1993, 123, 1923–1931. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kraus, D.; Yang, Q.; Kahn, B.B. Lipid Extraction from Mouse Feces. Biol. Protoc. 2015, 5, e1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Van Puyvelde, L.; Maes, L.; Bosselaers, J.; De Kimpe, N. Antitrichomonas in vitro activity of Cussonia holstii. Engl. Nat. Prod. Res. 2003, 17, 127–133. [Google Scholar] [CrossRef]
- Kimata, H.; Nakashima, T.; Kokubun, S.; Nakayama, K.; Mitoma, Y.; Kitahara, T.; Yata, N.; Tanaka, O. Saponins of pericarps of Sapindus mukurossi Gaertn. and solubilization of monodesmosides by bisdesmosides. Chem. Pharm. Bull. 1983, 31, 1998–2005. [Google Scholar] [CrossRef] [Green Version]
- Kirmizigül, S.; Anil, H. Transsylvanoside A, an acidic triterpene glycoside from Cephalania transsylvanica. Phytochemistry 1994, 35, 1075–1076. [Google Scholar] [CrossRef]
- Rao, G.S.R.S.; Kondaiah, P.; Singh, S.K.; Ravanan, P.; Sporn, M.B. Chemical modifications of natural triterpenes-glycyrrhetinic and boswellic acids: Evaluation of their biological activity. Tetrahedron 2008, 64, 11541–11548. [Google Scholar] [CrossRef]
- Reddy, D.R.S.; Mallika, D.; Reddy, G.D.; Murthy, Y.L.N. Phytochemical and Antimicrobial Activities of the Soapnut Saponin and its Derivatives from the Sapindus mukurossi. Asian J. Chem. 2010, 22, 5399–5403. [Google Scholar]
- Tava, A.; Biazzi, E.; Mella, M.; Quadrelli, P.; Avato, P. Artefact formation during acid hydrolysis of saponins from Medicago spp. Phytochemistry 2017, 138, 116–127. [Google Scholar] [CrossRef]
- Katsumi, E.; Oshima, N.; Kagawa, N.; Ohara, H.; Hada, N. Changes in the extracted amounts and seasonally variable constituents of Diospyros kaki at different growth stages. J. Nat. Med. 2021, 75, 105–115. [Google Scholar] [CrossRef] [PubMed]
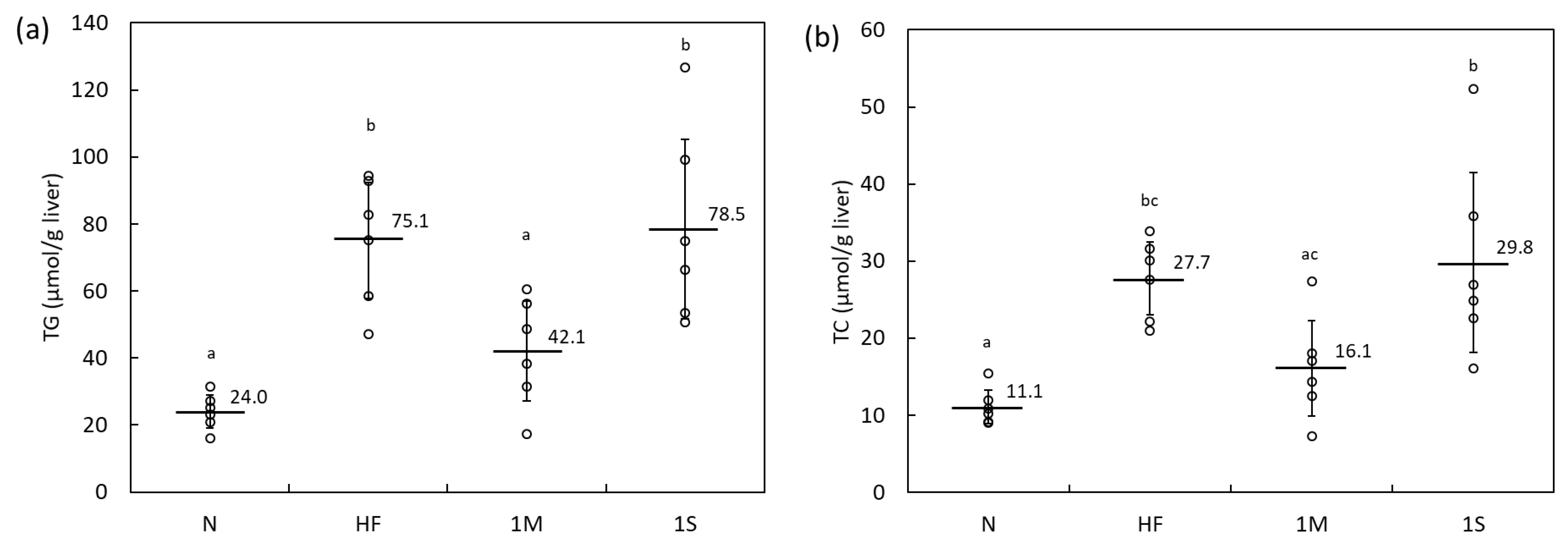
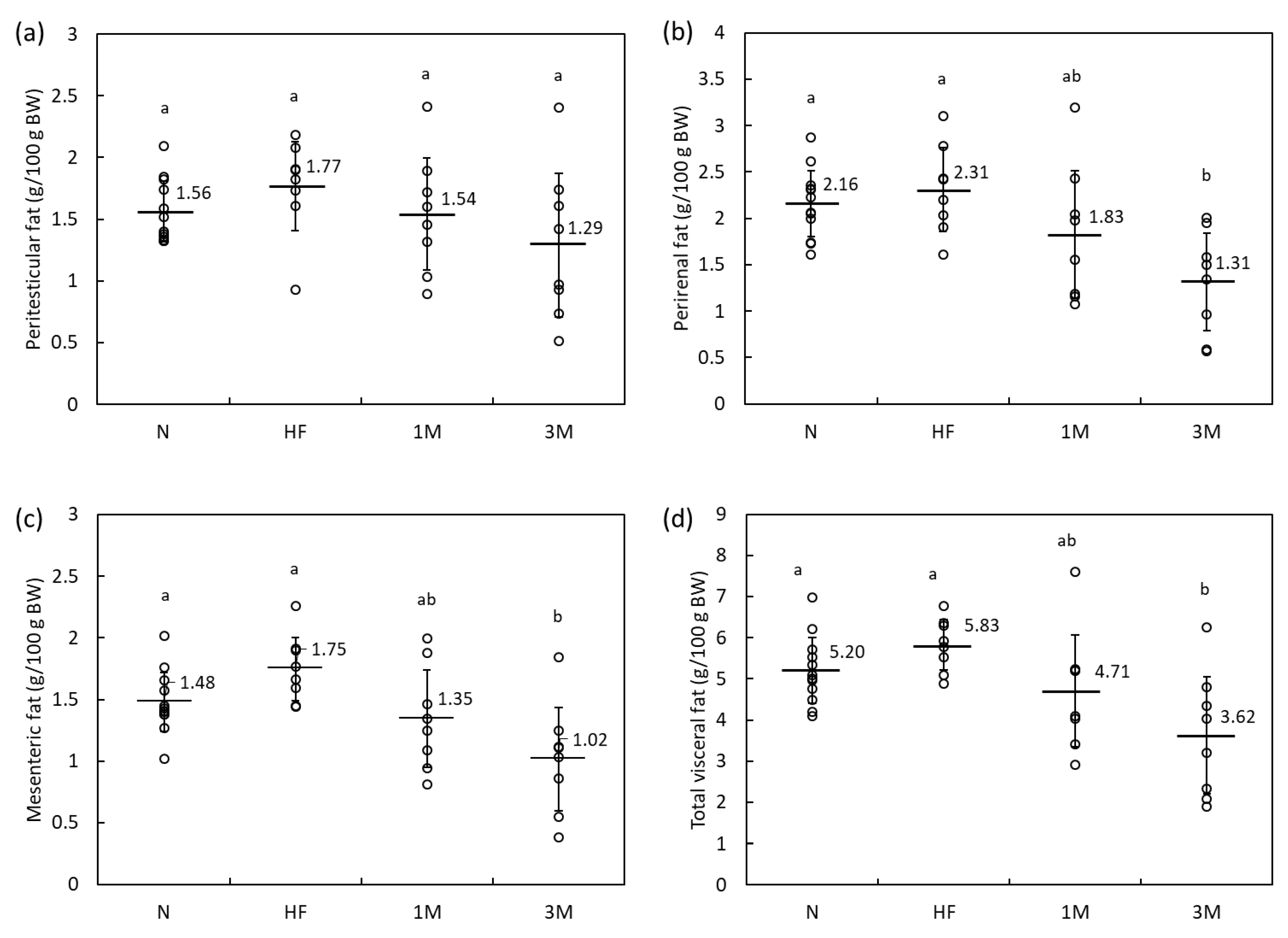
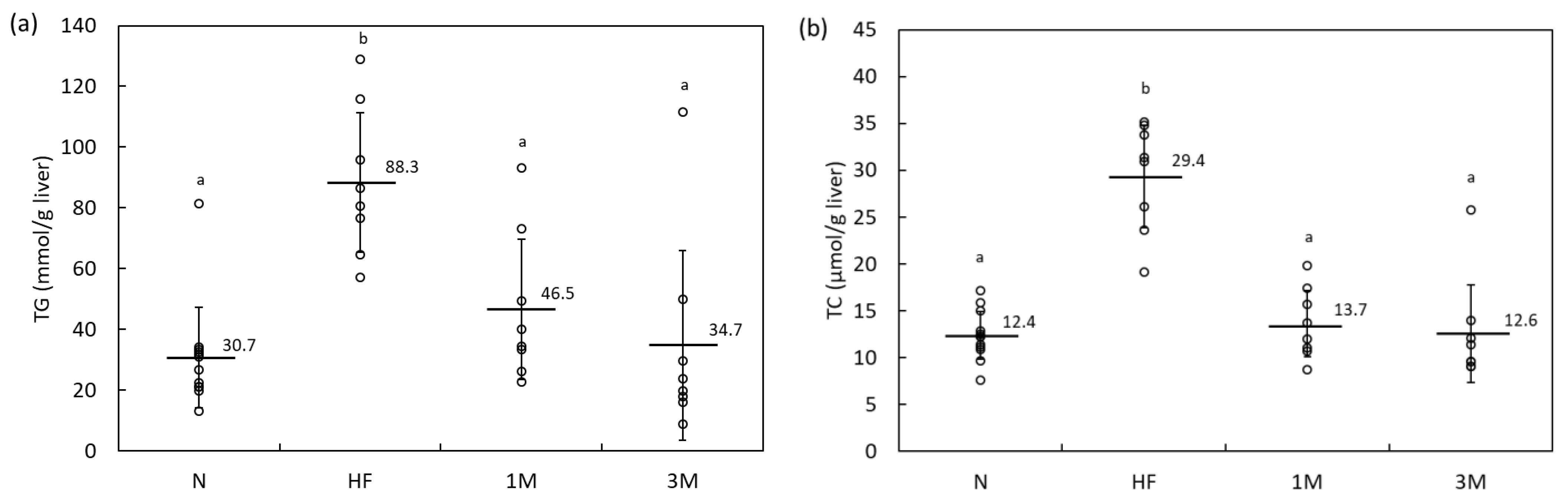
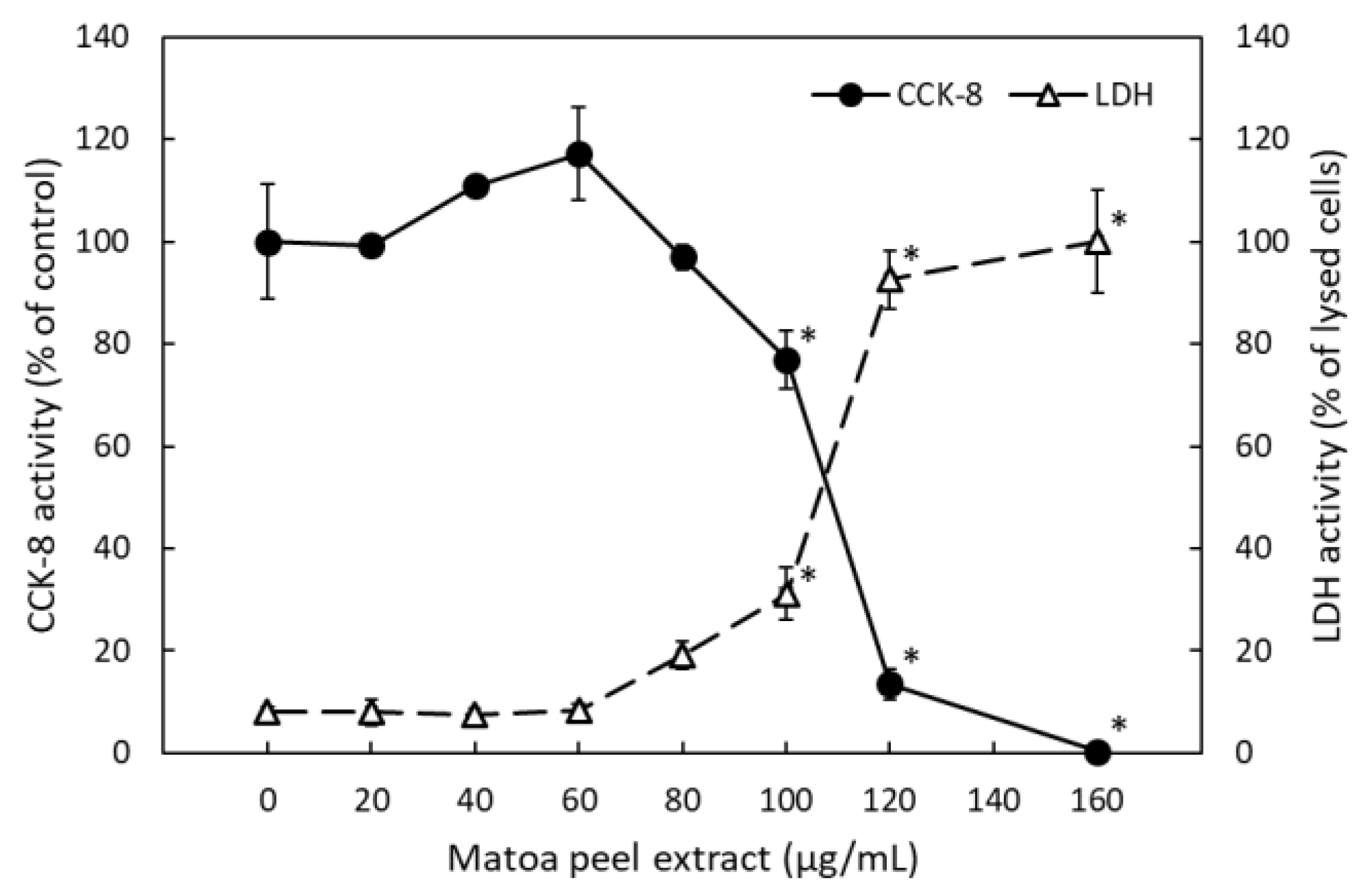
| Parameter | Experimental Group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HF | 1M | 1S | ||||||||||||
| Initial BW (g) | 250 | ± | 3 a | 251 | ± | 6 a | 249 | ± | 9 a | 247 | ± | 4 a | |||
| Final BW (g) | 390 | ± | 10 a | 442 | ± | 22 b | 436 | ± | 12 b | 442 | ± | 33 b | |||
| Dietary intake (g/day) | 21.1 | ± | 0.4 a | 20.4 | ± | 0.4 a | 20.6 | ± | 1.3 a | 19.8 | ± | 1.8 a | |||
| Energy intake (kJ/day) | 342 | ± | 6 a | 400 | ± | 8 b | 403 | ± | 25 b | 387 | ± | 35 b | |||
| Liver (g) | 10.3 | ± | 0.6 a | 12.3 | ± | 1.1 b | 12.0 | ± | 0.8 ab | 12.7 | ± | 1.4 b | |||
| Kidney (g) | 2.8 | ± | 0.1 a | 2.9 | ± | 0.2 a | 2.9 | ± | 0.4 a | 2.7 | ± | 0.4 a | |||
| Spleen (g) | 0.8 | ± | 0.1 a | 0.8 | ± | 0.1 a | 0.8 | ± | 0.1 a | 0.8 | ± | 0.1 a | |||
| Peritesticular fat (g) | 5.1 | ± | 1.2 a | 9.1 | ± | 1.8 b | 8.5 | ± | 1.0 b | 8.7 | ± | 1.5 b | |||
| Perirenal fat (g) | 7.4 | ± | 1.3 a | 11.9 | ± | 2.7 b | 9.5 | ± | 2.0 ab | 10.9 | ± | 2.1 ab | |||
| Mesenteric fat (g) | 5.5 | ± | 0.6 a | 8.9 | ± | 1.1 b | 7.9 | ± | 0.8 ab | 8.3 | ± | 1.4 b | |||
| Parameter | Experimental Group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HF | 1M | 1S | ||||||||||||
| Glucose (mmol/L) | 11.0 | ± | 2.7 | 10.6 | ± | 1.8 | 10.9 | ± | 3.8 | 13.6 | ± | 3.2 | |||
| TG (mmol/L) | 0.88 | ± | 0.13 | 1.04 | ± | 0.12 | 0.98 | ± | 0.17 | 0.98 | ± | 0.23 | |||
| TC (mmol/L) | 1.37 | ± | 0.15 | 1.23 | ± | 0.21 | 1.17 | ± | 0.19 | 1.25 | ± | 0.13 | |||
| HDL-C (mmol/L) | 0.85 | ± | 0.16 | 0.63 | ± | 0.16 | 0.69 | ± | 0.15 | 0.59 | ± | 0.15 | |||
| ALT (U/L) | 15.0 | ± | 0.8 | 14.7 | ± | 1.4 | 14.5 | ± | 1.8 | 15.7 | ± | 2.4 | |||
| AST (U/L) | 55.2 | ± | 5.7 | 55.5 | ± | 5.6 | 50.3 | ± | 7.1 | 54.2 | ± | 7.5 | |||
| γ-GTP (U/L) | <1 | <1 | <1 | <1 | |||||||||||
| Parameter | Experimental Group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HF | 1M | 3M | ||||||||||||
| Initial BW (g) | 225 | ± | 8 a | 223 | ± | 6 a | 226 | ± | 9 a | 225 | ± | 6 a | |||
| Final BW (g) | 391 | ± | 13 a | 424 | ± | 26 b | 401 | ± | 28 ab | 373 | ± | 31 a | |||
| Diet intake (g/day) | 21.8 | ± | 0.6 a | 19.8 | ± | 1.7 b | 18.6 | ± | 1.5 bc | 17.3 | ± | 1.5 c | |||
| Energy intake (kJ/day) | 353 | ± | 10 a | 387 | ± | 34 b | 364 | ± | 29 ab | 339 | ± | 29 a | |||
| Liver (g) | 10.8 | ± | 0.8 ab | 12.2 | ± | 1.1 a | 11.2 | ± | 1.5 ab | 10.1 | ± | 1.4 b | |||
| Kidney (g) | 2.7 | ± | 0.1 a | 2.8 | ± | 0.2 a | 2.8 | ± | 0.3 a | 2.6 | ± | 0.2 a | |||
| Spleen (g) | 0.8 | ± | 0.1 ab | 0.9 | ± | 0.1 a | 0.8 | ± | 0.1 b | 0.8 | ± | 0.1 ab | |||
| Peritesticular fat (g) | 6.1 | ± | 0.9 a | 7.5 | ± | 1.5 a | 6.2 | ± | 2.0 a | 5.0 | ± | 2.6 a | |||
| Perirenal fat (g) | 8.4 | ± | 1.3 a | 9.8 | ± | 2.1 a | 7.5 | ± | 3.3 ab | 5.0 | ± | 2.3 b | |||
| Mesenteric fat (g) | 5.8 | ± | 0.9 a | 7.5 | ± | 1.4 a | 5.5 | ± | 2.0 a | 3.9 | ± | 1.9 b | |||
| Feces, dry weight (g/day) | 2.0 | ± | 0.3 a | 2.0 | ± | 0.4 a | 2.1 | ± | 0.3 a | 2.0 | ± | 0.1 a | |||
| Fecal TG (mg/day) | 0.80 | ± | 0.6 a | 3.55 | ± | 1.8 b | 3.68 | ± | 1.8 b | 3.48 | ± | 1.3 b | |||
| Parameter | Experimental Group | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HF | 1M | 3M | ||||||||||||||
| Glucose (mmol/L) | 10.6 | ± | 2.6 a | 10.1 | ± | 2.9 a | 9.1 | ± | 2.4 a | 8.3 | ± | 1.7 a | |||||
| TG (mmol/L) | 1.27 | ± | 0.41 a | 1.04 | ± | 0.33 ab | 0.84 | ± | 0.13 bc | 0.50 | ± | 0.19 c | |||||
| TC (mmol/L) | 1.44 | ± | 0.21 a | 1.12 | ± | 0.11 b | 1.30 | ± | 0.23 ab | 1.32 | ± | 0.23 ab | |||||
| HDL-C (mmol/L) | 0.88 | ± | 0.17 a | 0.58 | ± | 0.08 b | 0.77 | ± | 0.19 ab | 0.71 | ± | 0.23 ab | |||||
| ALT (U/L) | 14.1 | ± | 2.6 a | 15.1 | ± | 1.5 a | 18.0 | ± | 5.0 a | 15.9 | ± | 1.3 a | |||||
| AST (U/L) | 57.1 | ± | 10.3 a | 57.9 | ± | 11.4 a | 66.4 | ± | 29.3 a | 53.5 | ± | 8.0 a | |||||
| γ-GTP (U/L) | <1 | <1 | <1 | <1 | |||||||||||||
| Peel | HGS Content [% (w/w)] | ||
|---|---|---|---|
| Matoa | 1.41 | ± | 0.039 a |
| Salak | 0.0154 | ± | 0.0026 b |
| Parameter | Diet Group | |||||
|---|---|---|---|---|---|---|
| Normal (N) | High-Fat (HF) | HF + 1% Matoa (1M) | HF + 3% Matoa (3M) | HF + 1% Salak (1S) | ||
| Energy (kJ/kg) | 16,200 | 19,500 | 19,500 | 19,500 | 19,500 | |
| Ratio of energy-producing nutrients (kJ %) | ||||||
| Protein | 21 | 17 | 17 | 17 | 17 | |
| Carbohydrate | 69 | 43 | 43 | 43 | 43 | |
| Fat | 10 | 40 | 40 | 40 | 40 | |
| Ingredient (g/kg diet) | ||||||
| Bovine milk casein | 200 | 200 | 200 | 200 | 200 | |
| L-cystine | 3 | 3 | 3 | 3 | 3 | |
| Corn starch | 422.5 | 262.5 | 262.5 | 262.5 | 262.5 | |
| α-Corn starch | 132 | 132 | 132 | 132 | 132 | |
| Sucrose | 102.5 | 102.5 | 102.5 | 102.5 | 102.5 | |
| Soybean oil | 25 | 25 | 25 | 25 | 25 | |
| Lard | 20 | 180 | 180 | 180 | 180 | |
| t-Butylhydroquinone | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | |
| Cellulose | 50 | 50 | 40 | 20 | 40 | |
| AIN-93G Mineral mix | 35 | 35 | 35 | 35 | 35 | |
| AIN-93 Vitamin mix with choline bitartrate | 10 | 10 | 10 | 10 | 10 | |
| Matoa peel powder | 0 | 0 | 10 | 30 | 0 | |
| Salak peel powder | 0 | 0 | 0 | 0 | 10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Nagata, M.; Kagawa, N.; Takano, S.; Nahrowi; Nomura, J. Anti-Obesity Effects of Matoa (Pometia pinnata) Fruit Peel Powder in High-Fat Diet-Fed Rats. Molecules 2021, 26, 6733. https://doi.org/10.3390/molecules26216733
Suzuki T, Nagata M, Kagawa N, Takano S, Nahrowi, Nomura J. Anti-Obesity Effects of Matoa (Pometia pinnata) Fruit Peel Powder in High-Fat Diet-Fed Rats. Molecules. 2021; 26(21):6733. https://doi.org/10.3390/molecules26216733
Chicago/Turabian StyleSuzuki, Toshikazu, Mayumi Nagata, Natsuko Kagawa, Shiori Takano, Nahrowi, and Jun Nomura. 2021. "Anti-Obesity Effects of Matoa (Pometia pinnata) Fruit Peel Powder in High-Fat Diet-Fed Rats" Molecules 26, no. 21: 6733. https://doi.org/10.3390/molecules26216733
APA StyleSuzuki, T., Nagata, M., Kagawa, N., Takano, S., Nahrowi, & Nomura, J. (2021). Anti-Obesity Effects of Matoa (Pometia pinnata) Fruit Peel Powder in High-Fat Diet-Fed Rats. Molecules, 26(21), 6733. https://doi.org/10.3390/molecules26216733







