Harmine Hydrochloride Mediates the Induction of G2/M Cell Cycle Arrest in Breast Cancer Cells by Regulating the MAPKs and AKT/FOXO3a Signaling Pathways
Abstract
:1. Introduction
2. Results
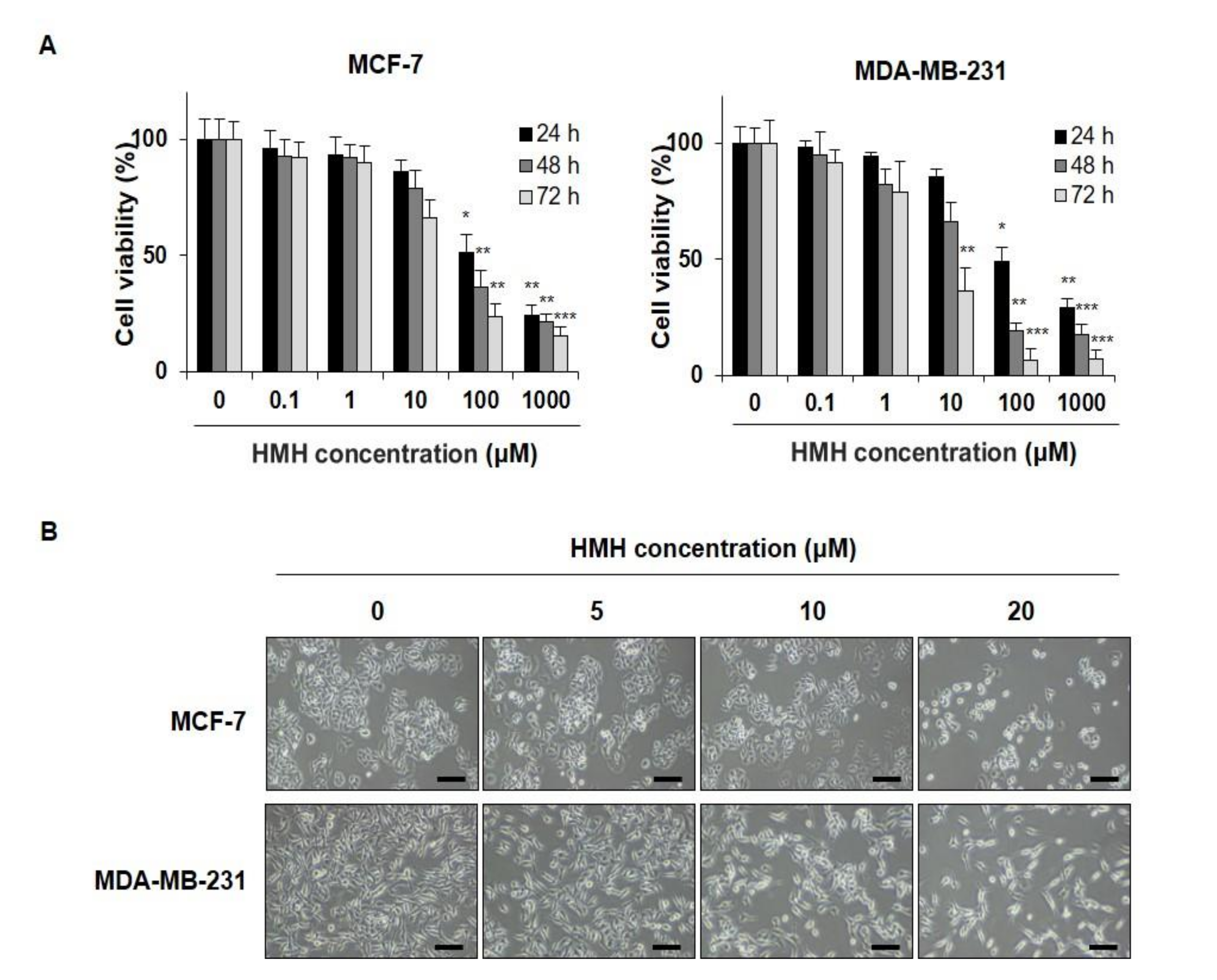
2.1. HMH Suppresses Cell Proliferation in BC Cells
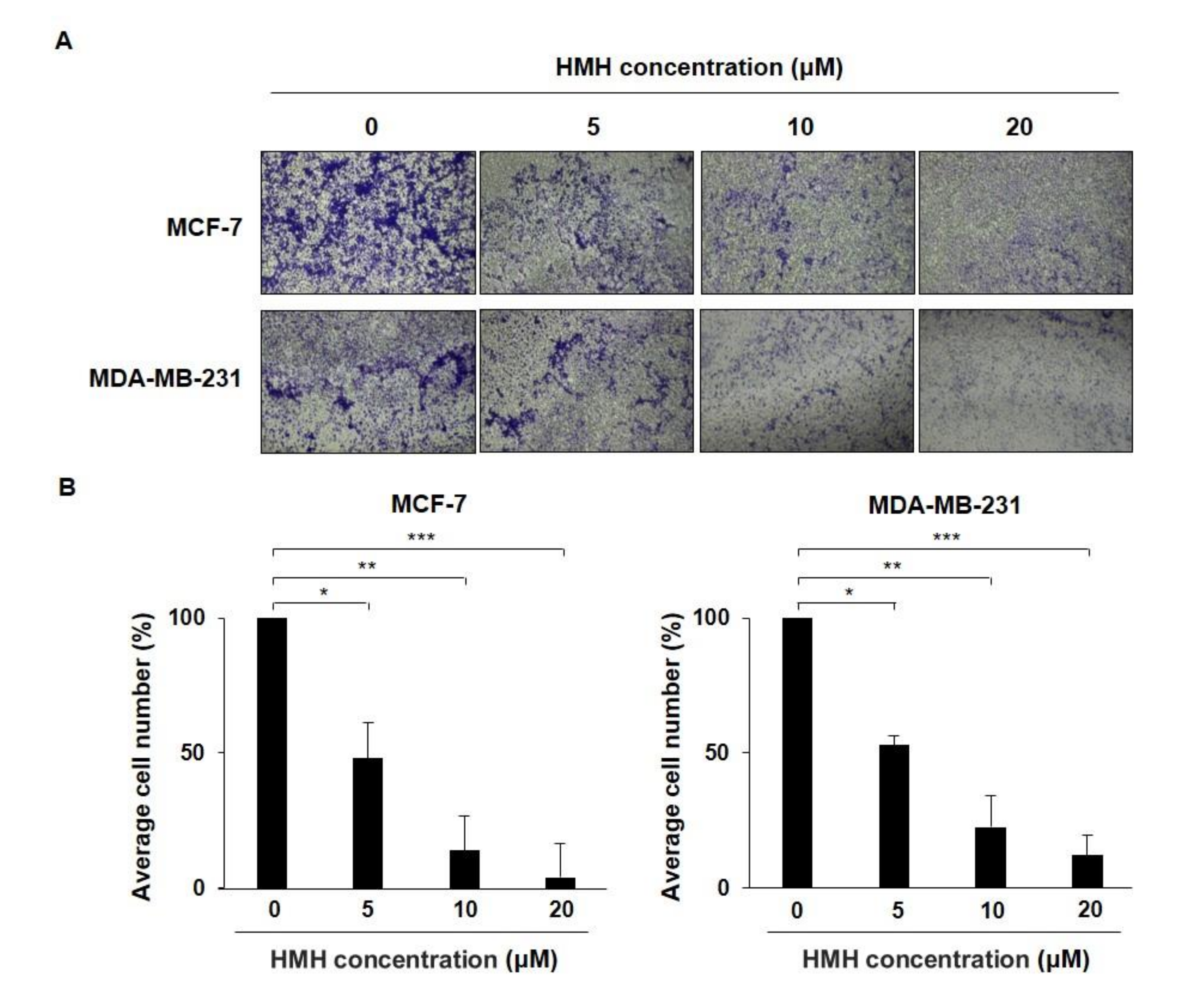
2.2. HMH Inhibits the Migration of BC Cells
2.3. HMH Inhibits the Invasion of BC Cells
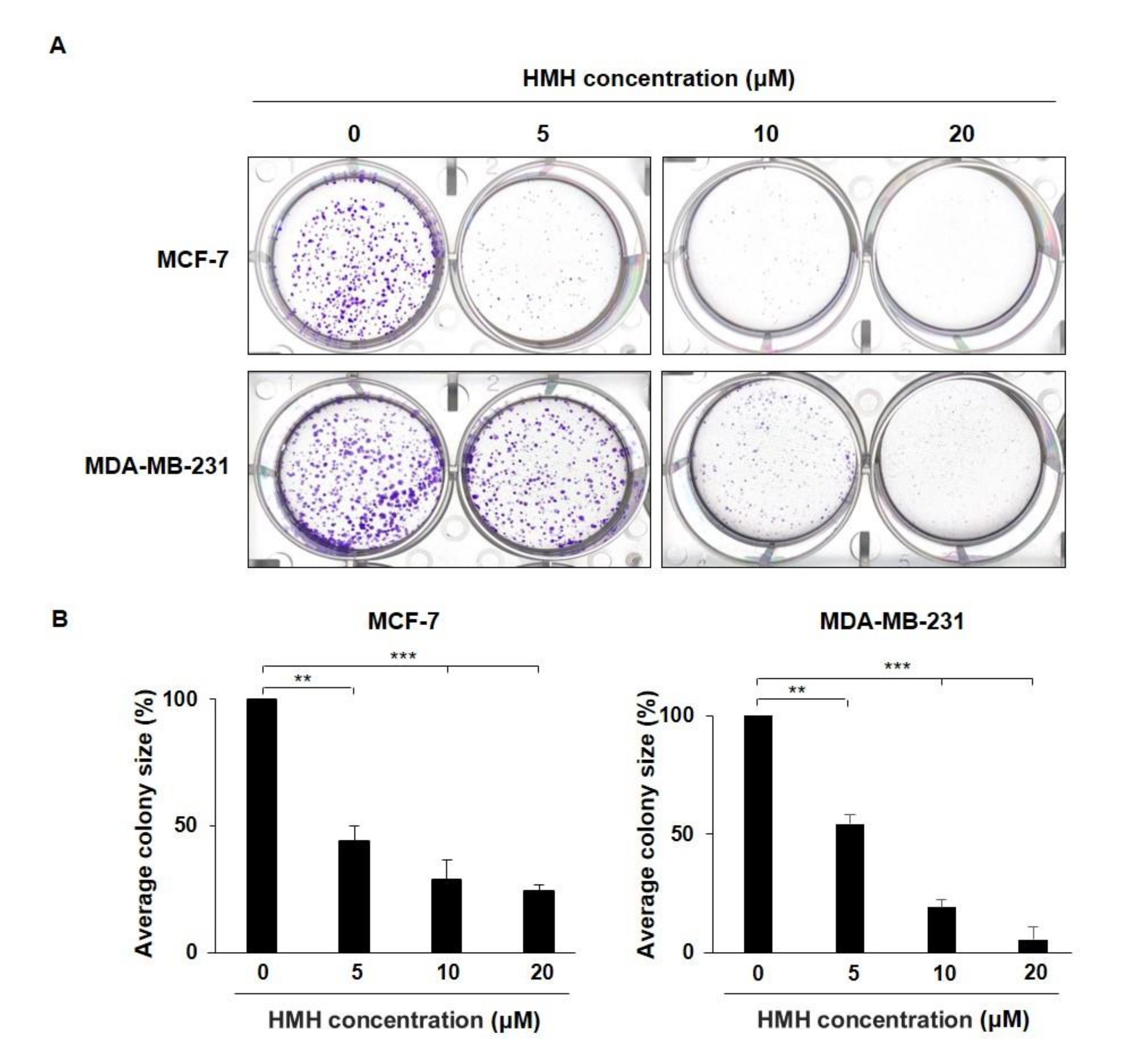
2.4. HMH Inhibits Colony Formation of BC Cells
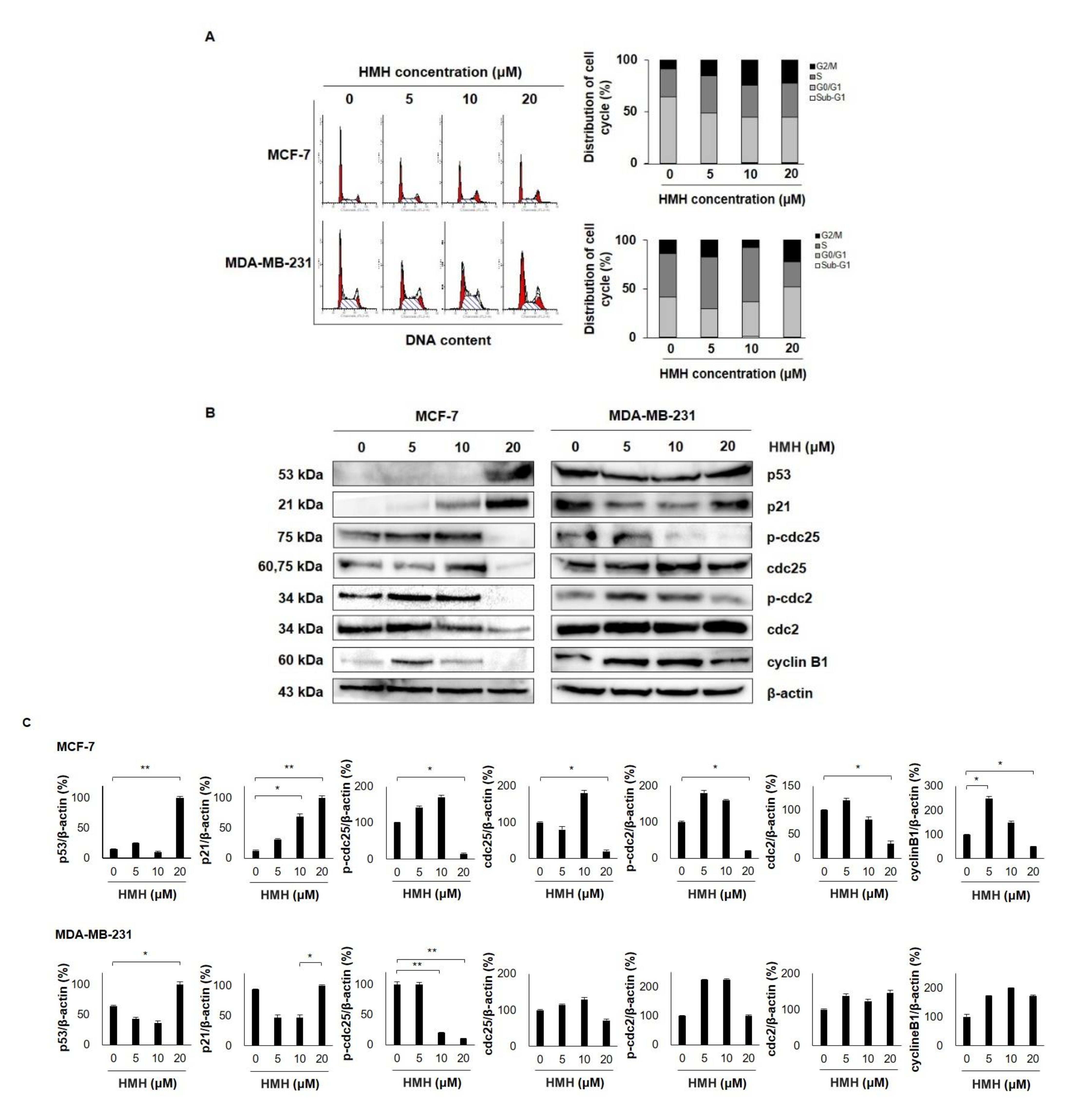
2.5. HMH Induces G2/M Cell Cycle Arrest in BC Cells
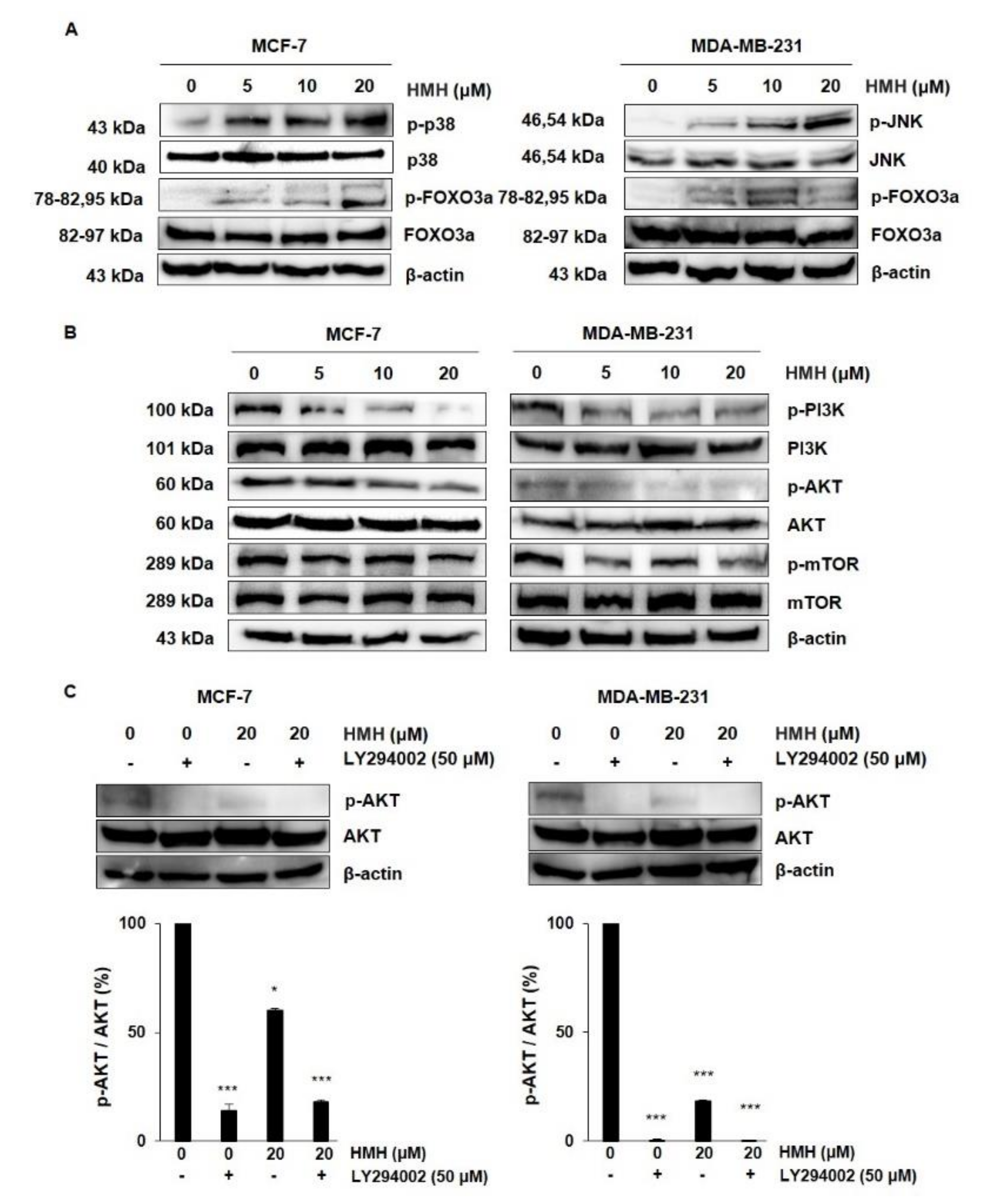
2.6. HMH Regulates the MAPKs and AKT/FOXO3a Signaling Pathways in BC Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. MTT Assay
4.4. Wound Healing Assay for the Migration Assay
4.5. Invasion Assay
4.6. Colony Formation Assay
4.7. Flow Cytometry Analysis for Cell Cycle Distribution
4.8. Determination of Protein Expression by Western Blotting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nagaraj, G.; Ma, C.X. Clinical Challenges in the Management of Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: A Literature Review. Adv. Ther. 2021, 38, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Santeufemia, D.A.; Basso, S.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Weiner, L.S.; Hartman, S.J.; Horvath, S.; Jeste, D.; Mischel, P.S.; Kado, D.M. Breast cancer treatment and its effects on aging. J. Geriatr. Oncol. 2019, 10, 346–355. [Google Scholar] [CrossRef]
- Nathanson, K.L.; Domchek, S.M. Therapeutic approaches for women predisposed to breast cancer. Annu. Rev. Med. 2011, 62, 295–306. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, S.; Gao, W.; Wei, C.; Liu, C. Anticancer drugs from traditional toxic Chinese medicines. Phytother. Res. 2012, 26, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.K.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Sun, K.; Ding, J.; Xu, H.; Zhu, L.; Zhang, K.; Li, X.; Sun, W. Harmine induces apoptosis and inhibits tumor cell proliferation, migration and invasion through down-regulation of cyclooxygenase-2 expression in gastric cancer. Phytomedicine 2014, 21, 348–355. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Qin, J.J.; Wang, M.H.; Sharma, H.; Buolamwini, J.K.; Wang, H.; Zhang, R. JKA97, a novel benzylidene analog of harmine, exerts anti-cancer effects by inducing G1 arrest, apoptosis, and p53-independent up-regulation of p21. PloS ONE 2012, 7, e34303. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Li, Y.; Zhao, Q.; Fan, L.; Zhang, M. The impact of Harmine hydrochloride on growth, apoptosis and migration, invasion of gastric cancer cells. Pathol. Res. Pract. 2020, 216, 152995. [Google Scholar] [CrossRef]
- Liu, H.; Han, D.; Liu, Y.; Hou, X.; Wu, J.; Li, H.; Yang, J.; Shen, C.; Yang, G.; Fu, C.; et al. Harmine hydrochloride inhibits Akt phosphorylation and depletes the pool of cancer stem-like cells of glioblastoma. J. Neurooncol. 2013, 112, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, C.R.; Wang, W.; Zhang, X.K.; Chen, J.J.; Wang, J.J.; Lin, C.; Jiang, J.W. Harmine Hydrochloride Triggers G2 Phase Arrest and Apoptosis in MGC-803 Cells and SMMC-7721 Cells by Upregulating p21, Activating Caspase-8/Bid, and Downregulating ERK/Bad Pathway. Phytother. Res. 2016, 30, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Peti, W.; Page, R. Molecular basis of MAP kinase regulation. Protein Sci. 2013, 22, 1698–1710. [Google Scholar] [CrossRef]
- Turjanski, A.G.; Vaqué, J.P.; Gutkind, J.S. MAP kinases and the control of nuclear events. Oncogene 2007, 26, 3240–3253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, A.; Raju, M.B.; Rahman, M.A. Current Insights of Inhibitors of p38 Mitogen-Activated Protein Kinase in Inflammation. Med. Chem. 2021, 17, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.; Ley, S. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Davis, R. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Miao, S.; Zhou, W.; Elnesr, S.S.; Dong, X.; Zou, X. MAPK, AKT/FoxO3a and mTOR pathways are involved in cadmium regulating the cell cycle, proliferation and apoptosis of chicken follicular granulosa cells. Ecotoxicol. Environ. Saf. 2021, 214, 112091. [Google Scholar] [CrossRef]
- Essers, M.; Weijzen, S.; Vries-Smits, A.; Saarloos, I.; Ruiter, N.; Bos, J.; Burgering, B. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO. J. 2004, 23, 4802–4812. [Google Scholar] [CrossRef] [Green Version]
- Sunayama, J.; Tsuruta, F.; Masuyama, N.; Gotoh, Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J. Cell. Biol. 2005, 170, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Papadatos-Pastos, D.; Rabbie, R.; Ross, P.; Sarker, D. The role of the PI3K pathway in colorectal cancer. Crit. Rev. Oncol. Hematol. 2015, 94, 18–30. [Google Scholar] [CrossRef]
- Qin, H.; Liu, L.; Sun, S.; Zhang, D.; Sheng, J.; Li, B.; Yang, W. The impact of PI3K inhibitors on breast cancer cell and its tumor microenvironment. PeerJ 2018, 6, e5092. [Google Scholar] [CrossRef] [Green Version]
- Golob-Schwarzl, N.; Krassnig, S.; Toeglhofer, A.M.; Park, Y.N.; Gogg-Kamerer, M.; Vierlinger, K.; Schröder, F.; Rhee, H.; Schicho, R.; Fickert, P.; et al. New liver cancer biomarkers: PI3K/AKT/mTOR pathway members and eukaryotic translation initiation factors. Eur. J. Cancer 2017, 83, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Murthy, D.; Attri, K.; Singh, P. Phosphoinositide 3-Kinase Signaling Pathway in Pancreatic Ductal Adenocarcinoma Progression, Pathogenesis, and Therapeutics. Front. Physiol. 2018, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Zhang, H.; Hu, M.; Liu, C.; Zhao, Y.; Zhang, S.; Liu, D. Sinomenine inhibits hypoxia induced breast cancer side population cells metastasis by PI3K/Akt/mTOR pathway. Bioorg. Med. Chem. 2021, 31, 115986. [Google Scholar] [CrossRef]
- Faridi, J.; Wang, L.; Endemann, G.; Roth, R. Expression of constitutively active Akt-3 in MCF-7 breast cancer cells reverses the estrogen and tamoxifen responsivity of these cells in vivo. Clin. Cancer Res. 2003, 9, 2933–2939. [Google Scholar] [PubMed]
- Hu, M.; Lee, D.F.; Xia, W.; Golfman, L.; Ou-Yang, F.; Yang, J.Y.; Zou, Y.; Bao, S.; Hanada, N.; Saso, H.; et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004, 117, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Fang, R.; Shao, J.; Cai, Z. Erianin induces triple-negative breast cancer cells apoptosis by activating PI3K/Akt pathway. Biosci. Rep. 2021, 41, BSR20210093. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Hung, M.C. A new fork for clinical application: Targeting forkhead transcription factors in cancer. Clin. Cancer Res. 2009, 15, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Nepal, S.; Kim, M.J.; Chang, J.H.; Kim, S.H.; Jeong, G.S.; Jeong, C.H.; Park, G.H.; Jung, S.; Lim, J.; et al. Critical Role of AMPK/FoxO3A Axis in Globular Adiponectin-Induced Cell Cycle Arrest and Apoptosis in Cancer Cells. J. Cell. Physiol. 2016, 231, 357–369. [Google Scholar] [CrossRef]
- Taylor, S.; Lam, M.; Pararasa, C.; Brown, J.; Carmichael, A.; Griffiths, H. Evaluating the evidence for targeting FOXO3a in breast cancer: A systematic review. Cancer Cell Int. 2015, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Berrougui, H.; Martín-Cordero, C.; Khalil, A.; Hmamouchi, M.; Ettaib, A.; Marhuenda, E.; Dolores Herrera, M.D. Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L. seeds in isolated rat aorta. Pharmacol. Res. 2006, 54, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chao, R.; Chen, H.; Hou, X.; Yan, H.; Zhou, S.; Peng, W.; Xu, A. Antitumor and neurotoxic effects of novel harmine derivatives and structure-activity relationship analysis. Int. J. Cancer. 2005, 114, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Chen, Y.; Song, Y.; Huang, L.; Zhai, D.; Dong, Y.; Lai, L.; Zhang, T.; Li, D.; Pang, X.; et al. A natural small molecule harmine inhibits angiogenesis and suppresses tumour growth through activation of p53 in endothelial cells. PloS ONE 2012, 7, e52162. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Yamada, H. Harmol induces apoptosis by caspase-8 activation independently of Fas/Fas ligand interaction in human lung carcinoma H596 cells. Anticancer Drugs 2009, 20, 373–381. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Wang, C.; Yi, X.; Li, M.; He, X. Anticancer activities of harmine by inducing a pro-death autophagy and apoptosis in human gastric cancer cells. Phytomedicine 2017, 28, 10–18. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Zhang, W.; Chen, L.; Gao, N.; Men, Y.; Xu, X.; Jiang, Y. Harmine suppresses homologous recombination repair and inhibits proliferation of hepatoma cells. Cancer Biol. Ther. 2015, 16, 1585–1595. [Google Scholar] [CrossRef] [Green Version]
- Filali, I.; Bouajila, J.; Znati, M.; Garah, F.; Jannet, H. Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. J. Enzyme Inhib. Med. Chem. 2015, 30, 371–376. [Google Scholar] [CrossRef]
- Abe, A.; Yamada, H.; Moriya, S.; Miyazawa, K. The β-carboline alkaloid harmol induces cell death via autophagy but not apoptosis in human non-small cell lung cancer A549 cells. Biol. Pharm. Bull. 2011, 34, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Madunić, I.V.; Madunić, J.; Antunović, M.; Paradžik, M.; Garaj-Vrhovac, V.; Breljak, D.; Marijanović, I.; Gajski, G. Apigenin, a dietary flavonoid, induces apoptosis, DNA damage, and oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Zhang, L.; Duan, Y.; Zhang, M.; Wang, G.; Zhang, J.; Zhao, Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer. Cell Int. 2014, 1491, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yu, X.H.; Yan, Y.G.; Wang, C.; Wang, W.J. PI3K/Akt signaling in osteosarcoma. Clin. Chim. Acta 2015, 444, 182–192. [Google Scholar] [CrossRef]
- Sunters, A.; Mattos, S.F.; Stahl, M.; Brosens, J.; Zoumpoulidou, G.; Saunders, C.; Coffer, P.; Medema, R.; Coombes, C.; Lam, E. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J. Biol. Chem. 2003, 278, 49795–49805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, K.; Ii, H.; Kageyama, S.; Takagi, H.; Chano, T.; Kawauchi, A.; Nakata, S. Depletion of gamma-glutamylcyclotransferase inhibits cancer cell growth by activating the AMPK-FOXO3a-p21 axis. Biochem. Biophys. Res. Commun. 2019, 517, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Nasimian, A.; Farzaneh, P.; Tamanoi, F.; Bathaie, Z. Cytosolic and mitochondrial ROS production resulted in apoptosis induction in breast cancer cells treated with Crocin: The role of FOXO3a, PTEN and AKT signaling. Biochem. Pharmacol. 2020, 177, 113999. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, I.; Dynlacht, B.D. New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 2005, 16, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Southgate, H.; Tweddle, D.; Curtin, N. DNA damage checkpoint kinases in cancer. Expert Rev. Mol. Med. 2020, 22, e2. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ock, C.W.; Kim, G.D. Harmine Hydrochloride Mediates the Induction of G2/M Cell Cycle Arrest in Breast Cancer Cells by Regulating the MAPKs and AKT/FOXO3a Signaling Pathways. Molecules 2021, 26, 6714. https://doi.org/10.3390/molecules26216714
Ock CW, Kim GD. Harmine Hydrochloride Mediates the Induction of G2/M Cell Cycle Arrest in Breast Cancer Cells by Regulating the MAPKs and AKT/FOXO3a Signaling Pathways. Molecules. 2021; 26(21):6714. https://doi.org/10.3390/molecules26216714
Chicago/Turabian StyleOck, Chae Won, and Gi Dae Kim. 2021. "Harmine Hydrochloride Mediates the Induction of G2/M Cell Cycle Arrest in Breast Cancer Cells by Regulating the MAPKs and AKT/FOXO3a Signaling Pathways" Molecules 26, no. 21: 6714. https://doi.org/10.3390/molecules26216714
APA StyleOck, C. W., & Kim, G. D. (2021). Harmine Hydrochloride Mediates the Induction of G2/M Cell Cycle Arrest in Breast Cancer Cells by Regulating the MAPKs and AKT/FOXO3a Signaling Pathways. Molecules, 26(21), 6714. https://doi.org/10.3390/molecules26216714





