Peanut Shell Derived Carbon Combined with Nano Cobalt: An Effective Flame Retardant for Epoxy Resin
Abstract
:1. Introduction
- (1)
- Developing three kinds of flame retardants based on carbon derived from peanut shells to improve the flame performance of EP through facile ways;
- (2)
- Evaluating the effectiveness of neat EP and its composites by conducting characterisation and benchmark fire safety tests such as cone calorimeter;
- (3)
- Analysing the influence of carbonisation degree and the incorporation of nano Cobalt on fire safety behaviour of EP;
- (4)
- Investigating flame retardant mechanisms from two aspects including condensed phase and gaseous phase with the help of material characterisation techniques.
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation
2.3. Measurements
3. Results and Discussion
3.1. Characterisation of Flame Retardants
3.2. Thermal Stability of EP and Its Composites
3.3. Fire Performance of EP and Its Composites
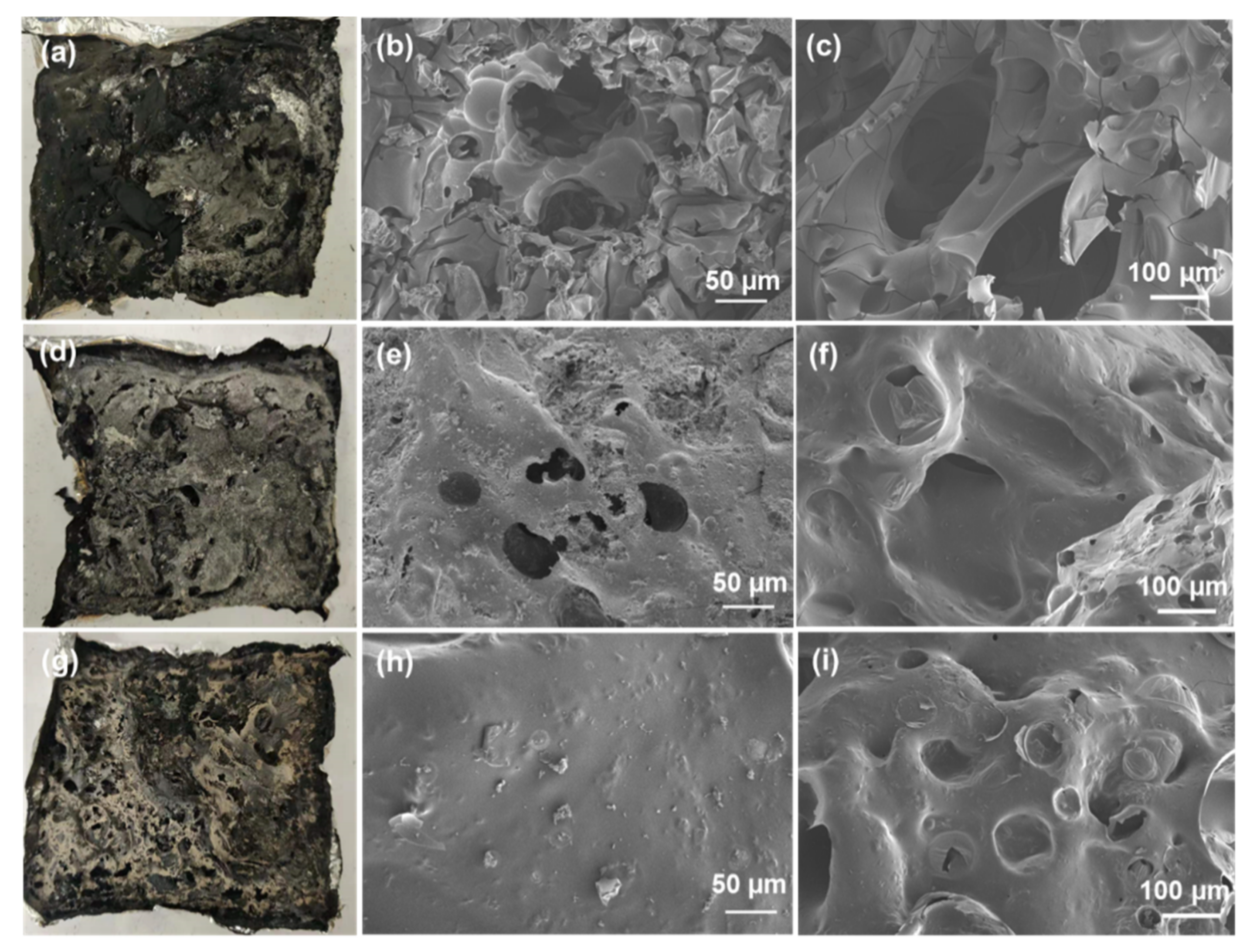
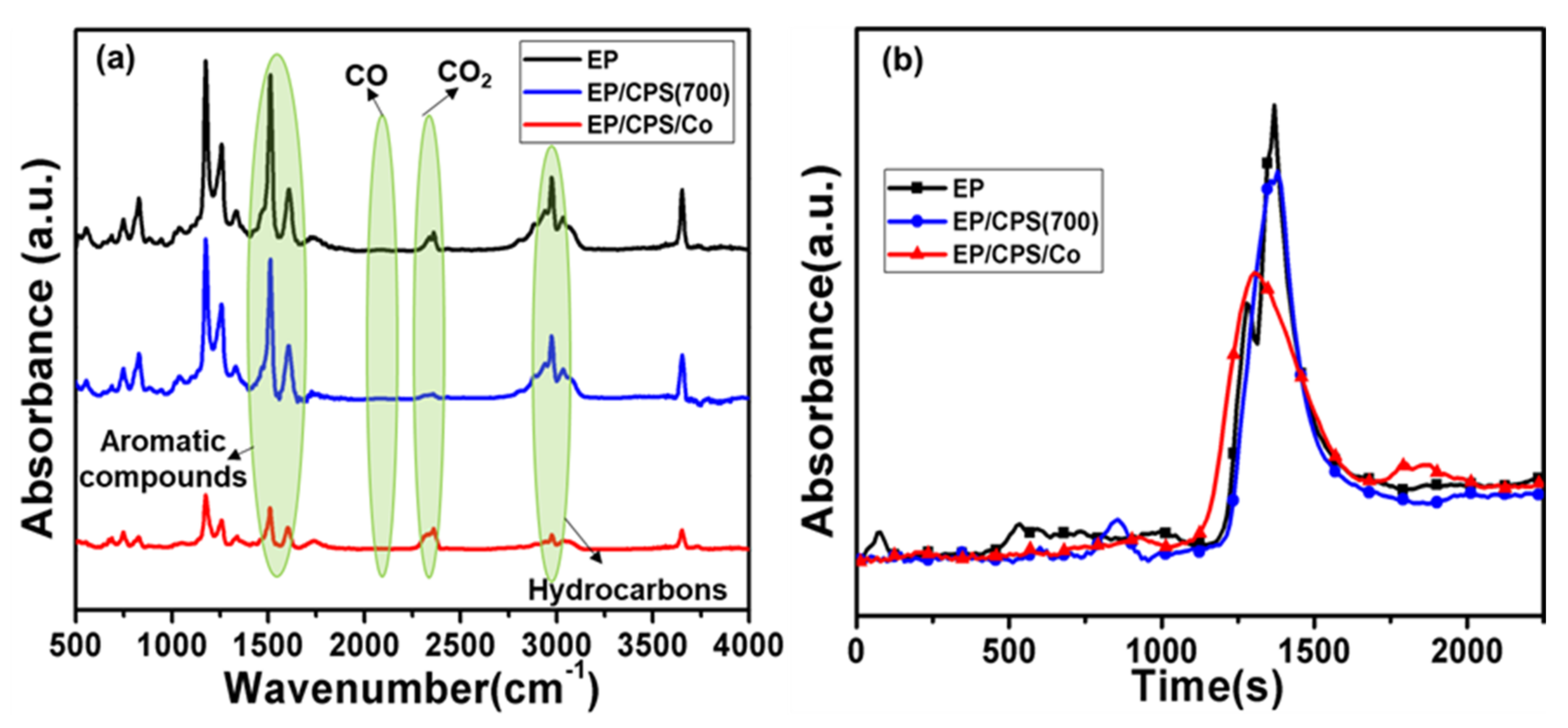
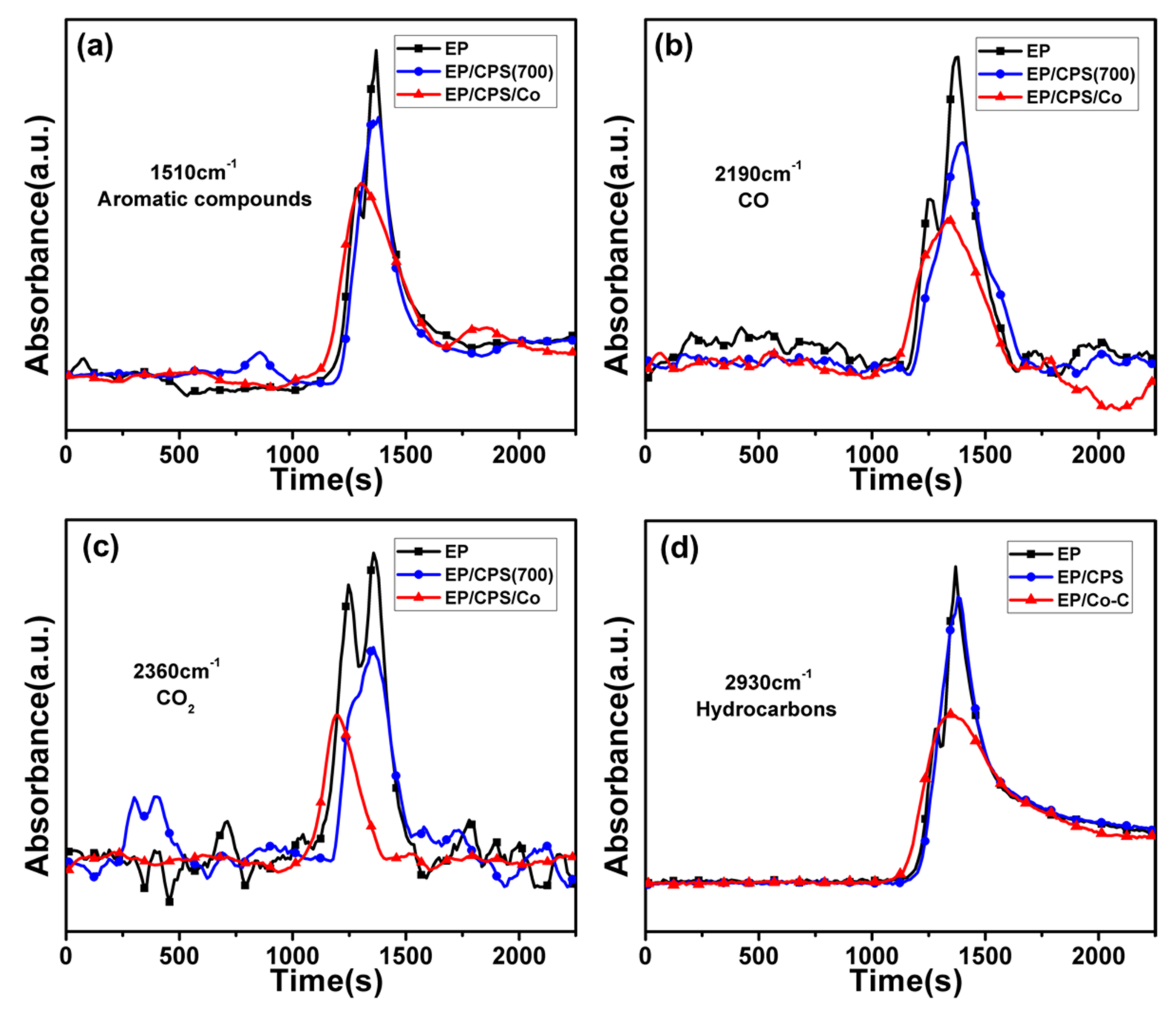
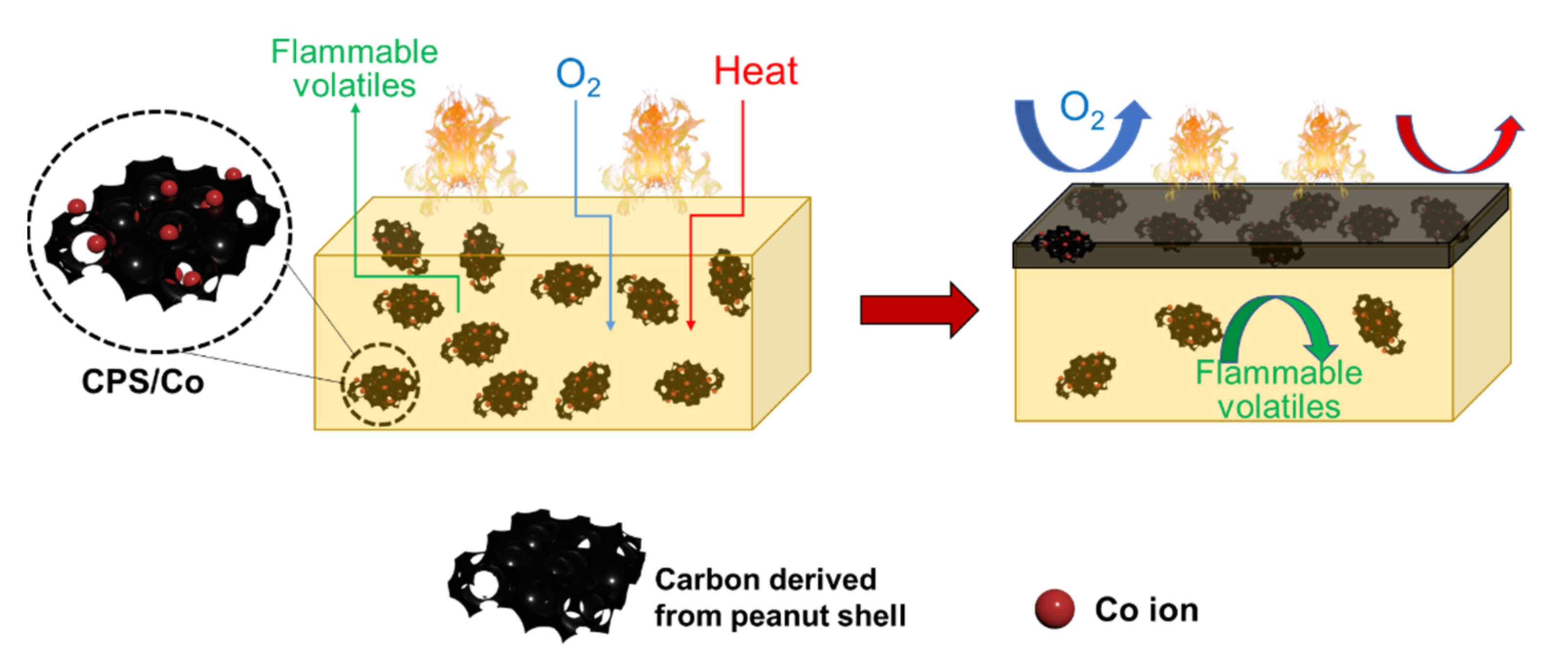
3.4. Flame Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef] [Green Version]
- Weil, E.D.; Levchik, S. A Review of Current Flame Retardant Systems for Epoxy Resins. J. Fire Sci. 2004, 22, 25–40. [Google Scholar] [CrossRef]
- Spontón, M.; Mercado, L.A.; Ronda, J.C.; Galià, M.; Cádiz, V. Preparation, thermal properties and flame retardancy of phosphorus- and silicon-containing epoxy resins. Polym. Degrad. Stab. 2008, 93, 2025–2031. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, D.; Li, Z.; Li, Z.; Peng, X.; Liu, C.; Zhang, Y.; Zheng, P. Recent developments in the flame-retardant system of epoxy resin. Materials 2020, 13, 2145. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, Y.L.; Chiu, Y.S. Epoxy resins possessing flame retardant elements from silicon incorporated epoxy compounds cured with phosphorus or nitrogen containing curing agents. Polymers 2002, 43, 4277–4284. [Google Scholar] [CrossRef]
- Huang, G.; Gao, J.; Wang, X.; Liang, H.; Ge, C. How can graphene reduce the flammability of polymer nanocomposites? Mater. Lett. 2012, 66, 187–189. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, S.C.; Lee, H.; Jeong, H.M.; Raghu, A.V.; Reddy, K.R.; Kim, B.K. Graphite oxides as effective fire retardants of epoxy resin. Macromol. Res. 2011, 19, 66–71. [Google Scholar] [CrossRef]
- Elbasuney, S. Surface engineering of layered double hydroxide (LDH) nanoparticles for polymer flame retardancy. Powder Technol. 2015, 277, 63–73. [Google Scholar] [CrossRef]
- Xu, W.; Li, A.; Liu, Y.; Chen, R.; Li, W. CuMoO4@hexagonal boron nitride hybrid: An ecofriendly flame retardant for polyurethane elastomer. J. Mater. Sci. 2018, 53, 11265–11279. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, W.; Pan, H.; Zhan, J.; Hu, Y. Fabrication of montmorillonite and titanate nanotube based coatings: Via layer-by-layer self-assembly method to enhance the thermal stability, flame retardancy and ultraviolet protection of polyethylene terephthalate (PET) fabric. RSC Adv. 2016, 6, 53625–53634. [Google Scholar] [CrossRef]
- Cai, W.; Hong, N.; Feng, X.; Zeng, W.; Shi, Y.; Zhang, Y.; Wang, B.; Hu, Y. A facile strategy to simultaneously exfoliate and functionalize boron nitride nanosheets via Lewis acid-base interaction. Chem. Eng. J. 2017, 330, 309–321. [Google Scholar] [CrossRef]
- Madakbaş, S.; Çakmakçi, E.; Kahraman, M.V. Preparation and thermal properties of polyacrylonitrile/hexagonal boron nitride composites. Thermochim. Acta 2013, 552, 1–4. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wang, D.-Y. Two-Dimensional Inorganic Nanomaterials: A Solution to Flame Retardant Polymers. Nano Adv. 2016, 1, 155. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Li, C.; Ni, Y.; Xu, Y.; Wang, J. Flame retardant nanocomposites based on 2D layered nanomaterials: A review. J. Mater. Sci. 2019, 54, 13070–13105. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. Biocomposites from waste derived biochars: Mechanical, thermal, chemical, and morphological properties. Waste Manag. 2016, 49, 560–570. [Google Scholar] [CrossRef]
- Babu, K.; Rendén, G.; Mensah, R.A.; Kim, N.K.; Jiang, L.; Xu, Q.; Restás, Á.; Neisiany, R.E.; Hedenqvist, M.S.; Försth, M.; et al. A review on the flammability properties of carbon-based polymeric composites: State-of-the-art and future trends. Polymers 2020, 12, 1518. [Google Scholar] [CrossRef] [PubMed]
- Nizamuddin, S.; Jadhav, A.; Qureshi, S.S.; Baloch, H.A.; Siddiqui, M.T.H.; Mubarak, N.M.; Griffin, G.; Madapusi, S.; Tanksale, A.; Ahamed, M.I. Synthesis and characterization of polylactide/rice husk hydrochar composite. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.; Bartoli, M.; Frache, A.; Malucelli, G. Investigation of different types of biochar on the thermal stability and fire retardance of ethylene-vinyl acetate copolymers. Polymers 2021, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Omega, A.C.S. Intumescent-grafted bamboo charcoal: A natural nontoxic fire-retardant filler for polylactic acid (PLA) composites. ACS Omega 2021, 41, 26990–27006. [Google Scholar]
- Wu, M.F.; Hsiao, C.H.; Lee, C.Y.; Tai, N.H. Flexible Supercapacitors Prepared Using the Peanut-Shell-Based Carbon. ACS Omega 2020, 5, 14417–14426. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy-power rivals lithium ion capacitors. Energy Environ. Sci. 2015, 8, 941–955. [Google Scholar] [CrossRef]
- Wang, H.; Yu, W.; Shi, J.; Mao, N.; Chen, S.; Liu, W. Biomass derived hierarchical porous carbons as high-performance anodes for sodium-ion batteries. Electrochim. Acta 2016, 188, 103–110. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, Y.; Zhou, K. A novel exploration of metal–organic frameworks in flame-retardant epoxy composites. J. Therm. Anal. Calorim. 2019, 138, 905–914. [Google Scholar] [CrossRef]
- Wu, N.; Yang, R. Effects of metal oxides on intumescent flame-retardant polypropylene. Polym. Adv. Technol. 2011, 22, 495–501. [Google Scholar] [CrossRef]
- Morgan, A.B. Chapter 19 A Review of Transition Metal-Based Flame Retardants: Transition Metal Oxide/Salts, and Complexes the Periodic Table of Flame Retardants. Transition 2010, 19, 312–328. [Google Scholar]
- Wang, B.; Zhou, K.; Jiang, S.; Hu, Y.; Gui, Z. The application of transition metal molybdates (AMoO4, A = Co, Ni, Cu) as additives in acrylonitrile-butadiene-styrene with improved flame retardant and smoke suppression properties. Polym. Adv. Technol. 2014, 25, 1419–1425. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, H.; Guo, J.; Sun, J.; Li, H.; Zhang, S.; Gu, X. Preparation of cobalt-based metal organic framework and its application as synergistic flame retardant in thermoplastic polyurethane (TPU). Compos. Part B Eng. 2020, 182, 107498. [Google Scholar] [CrossRef]
- López-Romero, S.; Chávez-Ramírez, J. Synthesis of TiC thin films by CVD from toluene and titanium tetrachloride with nickel as catalyst. Matéria 2007, 12, 487–493. [Google Scholar] [CrossRef]
- Qiu, T.; Yang, J.G.; Bai, X.J.; Wang, Y.L. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef] [Green Version]
- Sinkó, K.; Szabó, G.; Zrínyi, M. Liquid-phase synthesis of cobalt oxide nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 4127–4135. [Google Scholar] [CrossRef]
- Dang, N.M.; Zhao, W.W.; Yusa, S.I.; Noguchi, H.; Nakashima, K. Cobalt oxide hollow nanoparticles as synthesized by templating a tri-block copolymer micelle with a core-shell-corona structure: A promising anode material for lithium ion batteries. New J. Chem. 2015, 39, 4726–4730. [Google Scholar] [CrossRef]
- Ansari, S.M.; Bhor, R.D.; Pai, K.R.; Sen, D.; Mazumder, S.; Ghosh, K.; Kolekar, Y.D.; Ramana, C.V. Cobalt nanoparticles for biomedical applications: Facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Appl. Surf. Sci. 2017, 414, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Marton, M.; Vojs, M.; Zdravecká, E.; Himmerlich, M.; Haensel, T.; Krischok, S.; Kotlár, M.; Michniak, P.; Veselý, M.; Redhammer, R. Raman spectroscopy of amorphous carbon prepared by pulsed arc discharge in various gas mixtures. J. Spectrosc. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Pardanaud, C.; Martin, C.; Roubin, P.; Giacometti, G.; Hopf, C.; Schwarz-Selinger, T.; Jacob, W. Raman spectroscopy investigation of the H content of heated hard amorphous carbon layers. Diam. Relat. Mater. 2013, 34, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Carosio, F.; Maddalena, L.; Gomez, J.; Saracco, G.; Fina, A. Graphene Oxide Exoskeleton to Produce Self-Extinguishing, Nonignitable, and Flame Resistant Flexible Foams: A Mechanically Tough Alternative to Inorganic Aerogels. Adv. Mater. Interfaces 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.; Guo, W.; Qiu, S.; Wang, X.; Lo, S.; Hu, Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites: Via sol-gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon N. Y. 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, J.; Shi, Y.; Jiang, S.; Wang, D.; Hu, Y.; Gui, Z. MoS2 nanolayers grown on carbon nanotubes: An advanced reinforcement for epoxy composites. ACS Appl. Mater. Interfaces 2015, 7, 6070–6081. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yuen, A.C.Y.; Chen, T.B.Y.; Yu, B.; Yang, W.; Zhang, J.; Yao, Y.; Wu, S.; Wang, C.H.; Yeoh, G.H. Experimental and numerical perspective on the fire performance of MXene/Chitosan/Phytic acid coated flexible polyurethane foam. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Salasinska, K.; Celiński, M.; Mizera, K.; Kozikowski, P.; Leszczyński, M.K.; Gajek, A. Synergistic effect between histidine phosphate complex and hazelnut shell for flammability reduction of low-smoke emission epoxy resin. Polym. Degrad. Stab. 2020, 181, 10292. [Google Scholar] [CrossRef]
- Wang, W.; Pan, H.; Shi, Y.; Pan, Y.; Yang, W.; Liew, K.M.; Song, L.; Hu, Y. Fabrication of LDH nanosheets on β-FeOOH rods and applications for improving the fire safety of epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 80, 259–269. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Gengembre, L. XPS study of an intumescent coating. Appl. Surf. Sci. 1997, 120, 15–29. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Xing, W.; Yu, B.; Feng, X.; Song, L.; Hu, Y. Self-assembly of Ni-Fe layered double hydroxide/graphene hybrids for reducing fire hazard in epoxy composites. J. Mater. Chem. A 2013, 1, 4383–4390. [Google Scholar] [CrossRef]
- Wang, W.; Kan, Y.; Yu, B.; Pan, Y.; Liew, K.M.; Song, L.; Hu, Y. Synthesis of MnO2 nanoparticles with different morphologies and application for improving the fire safety of epoxy. Compos. Part A Appl. Sci. Manuf. 2017, 95, 173–182. [Google Scholar] [CrossRef]
- Qiu, S.; Zou, B.; Zhang, T.; Ren, X.; Yu, B.; Zhou, Y.; Kan, Y.; Hu, Y. Integrated effect of NH2-functionalized/triazine based covalent organic framework black phosphorus on reducing fire hazards of epoxy nanocomposites. Chem. Eng. J. 2020, 401, 126058. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, R.K.K.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. Small 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Zhou, K.; Yang, W.; Hu, Y.; Gong, X. The influence of manganese-cobalt oxide/graphene on reducing fire hazards of poly(butylene terephthalate). J. Hazard. Mater. 2014, 278, 391–400. [Google Scholar] [CrossRef]
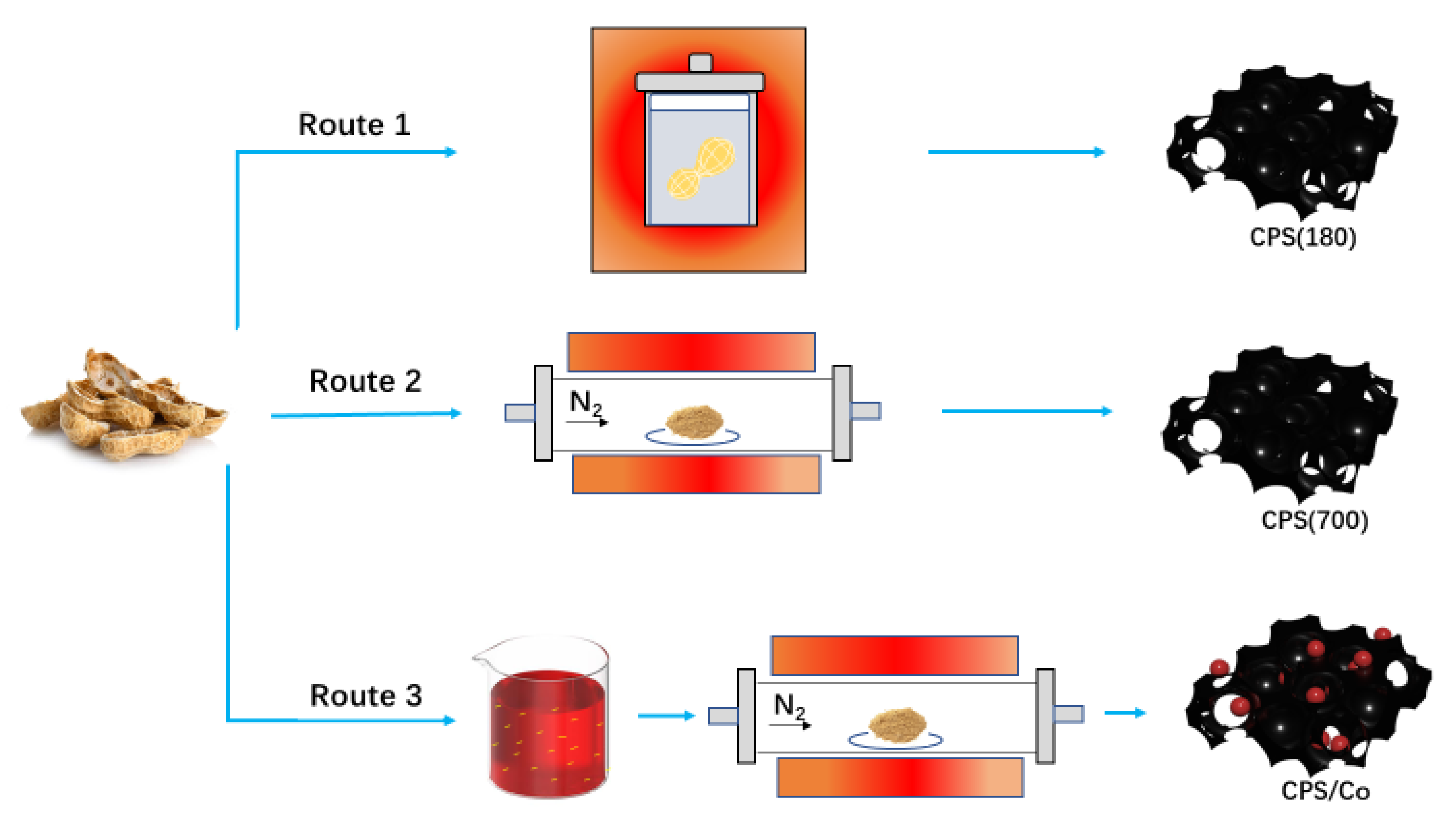
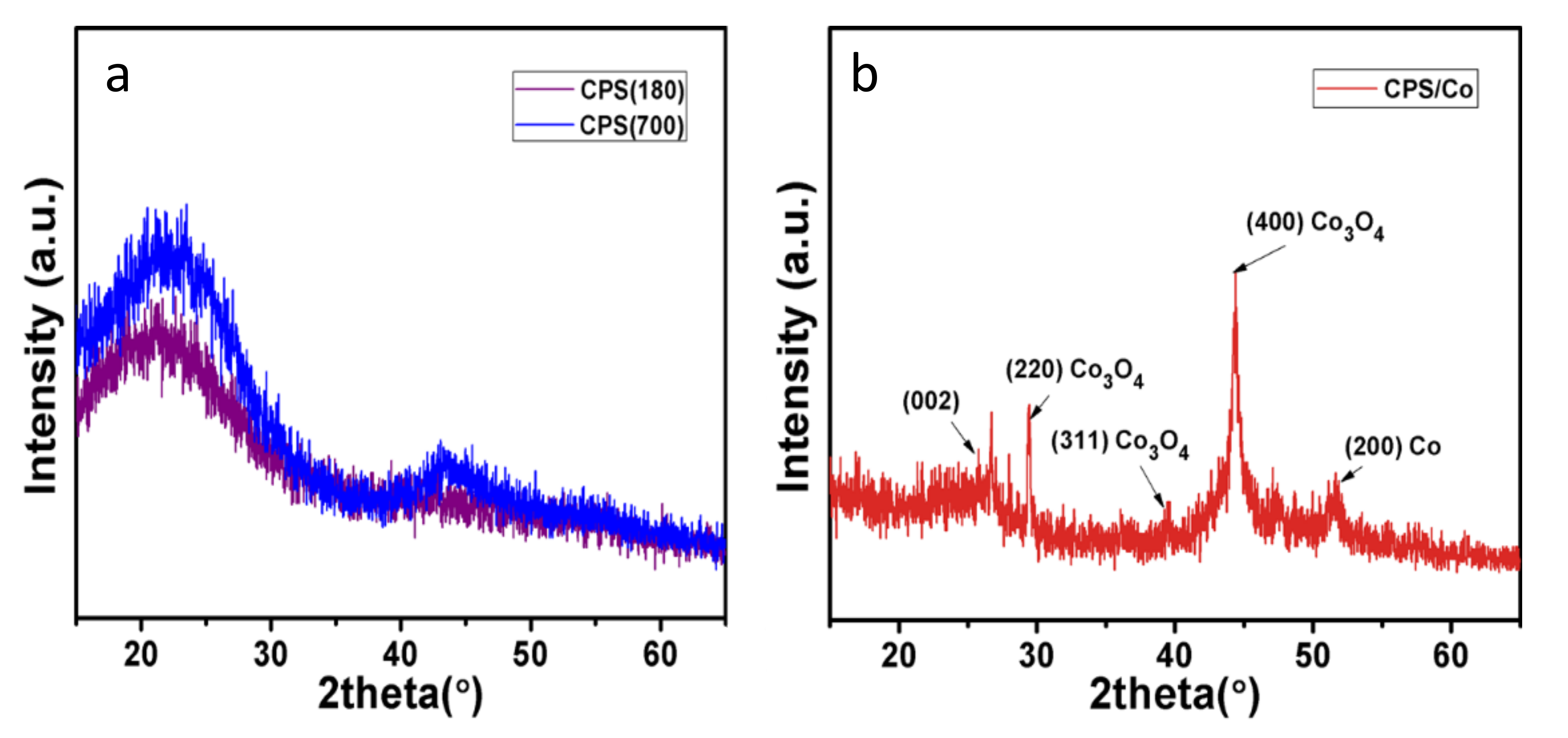

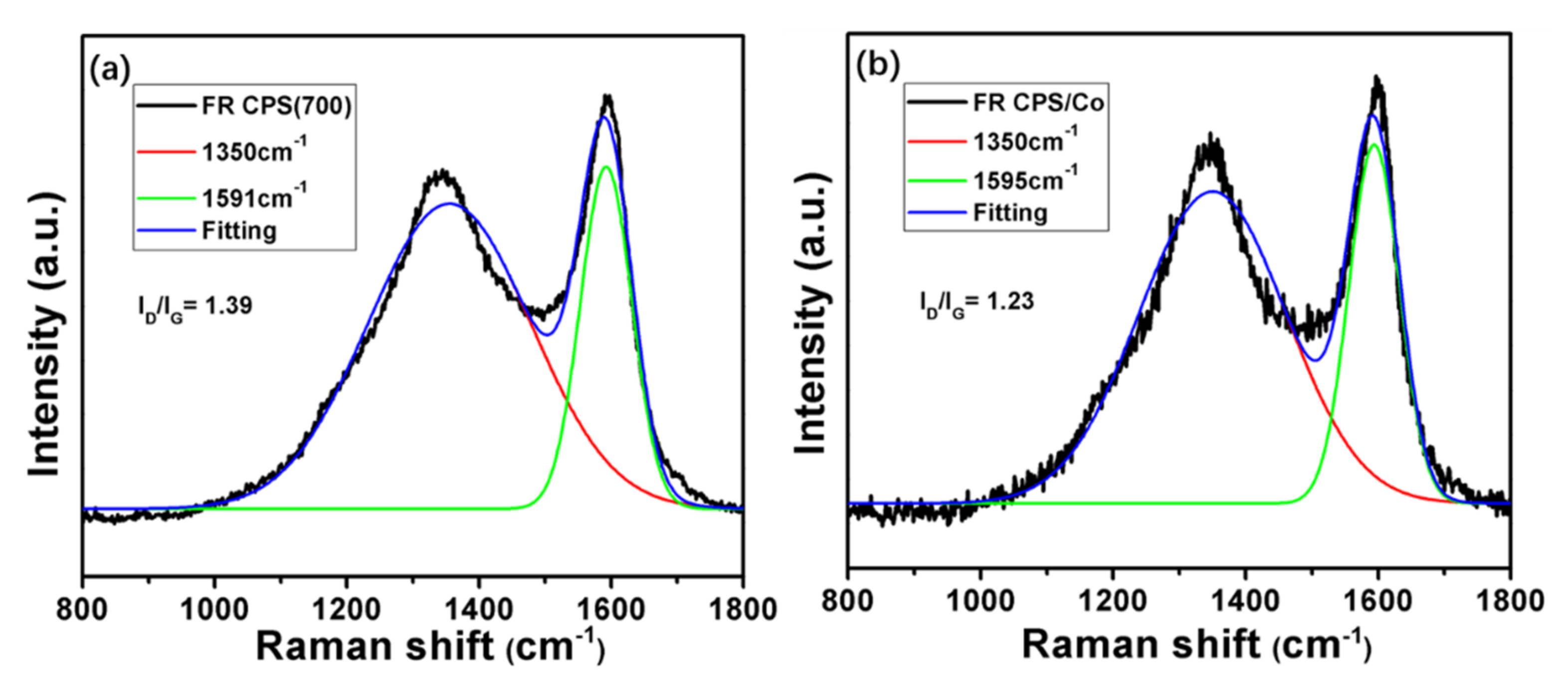
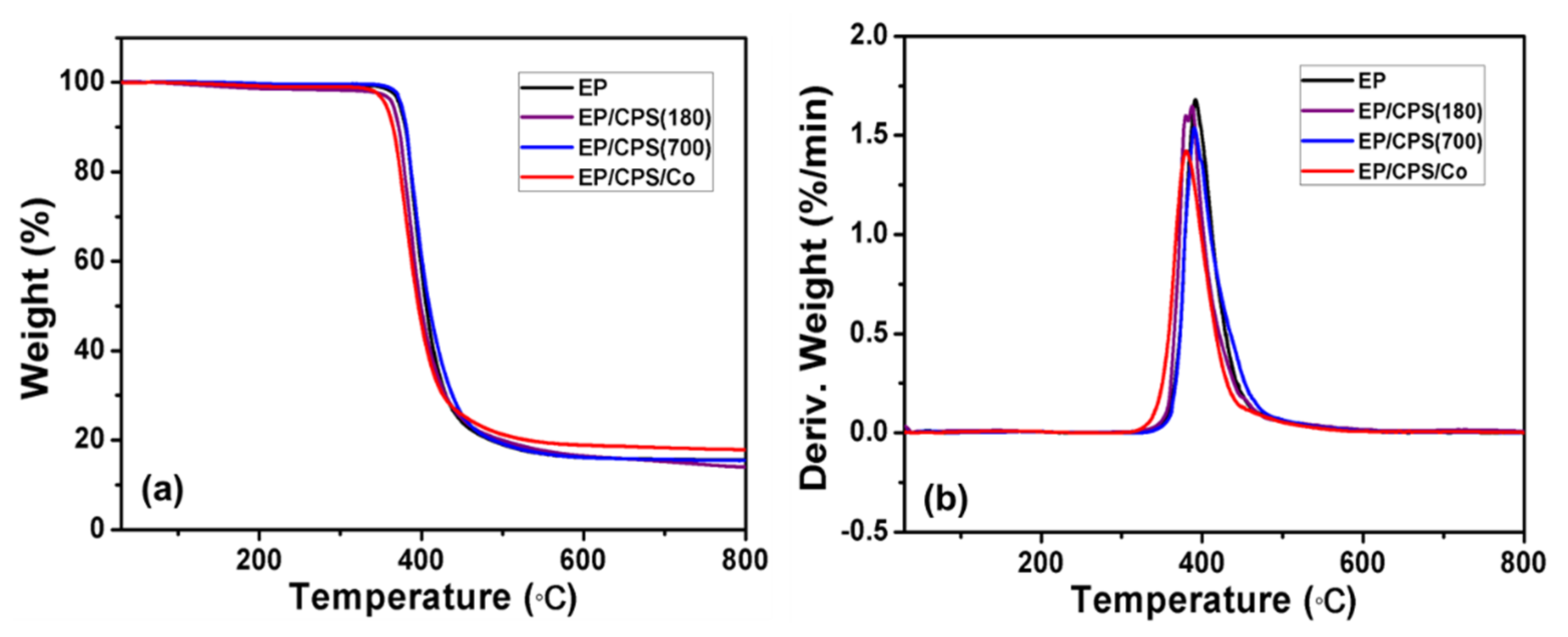
| Samples | Under Nitrogen | ||
|---|---|---|---|
| T−5% (°C) | Tmax (°C); %/min | Char Residue at 700 °C (%) | |
| EP | 372.92 | 391.7; 1.68 | 15.69 |
| EP/CPS (180) | 364.83 | 387.4; 1.65 | 15.25 |
| EP/CPS (700) | 375.03 | 390.3; 1.54 | 15.73 |
| EP/CPS/Co | 355.93 | 380.2; 1.42 | 18.31 |
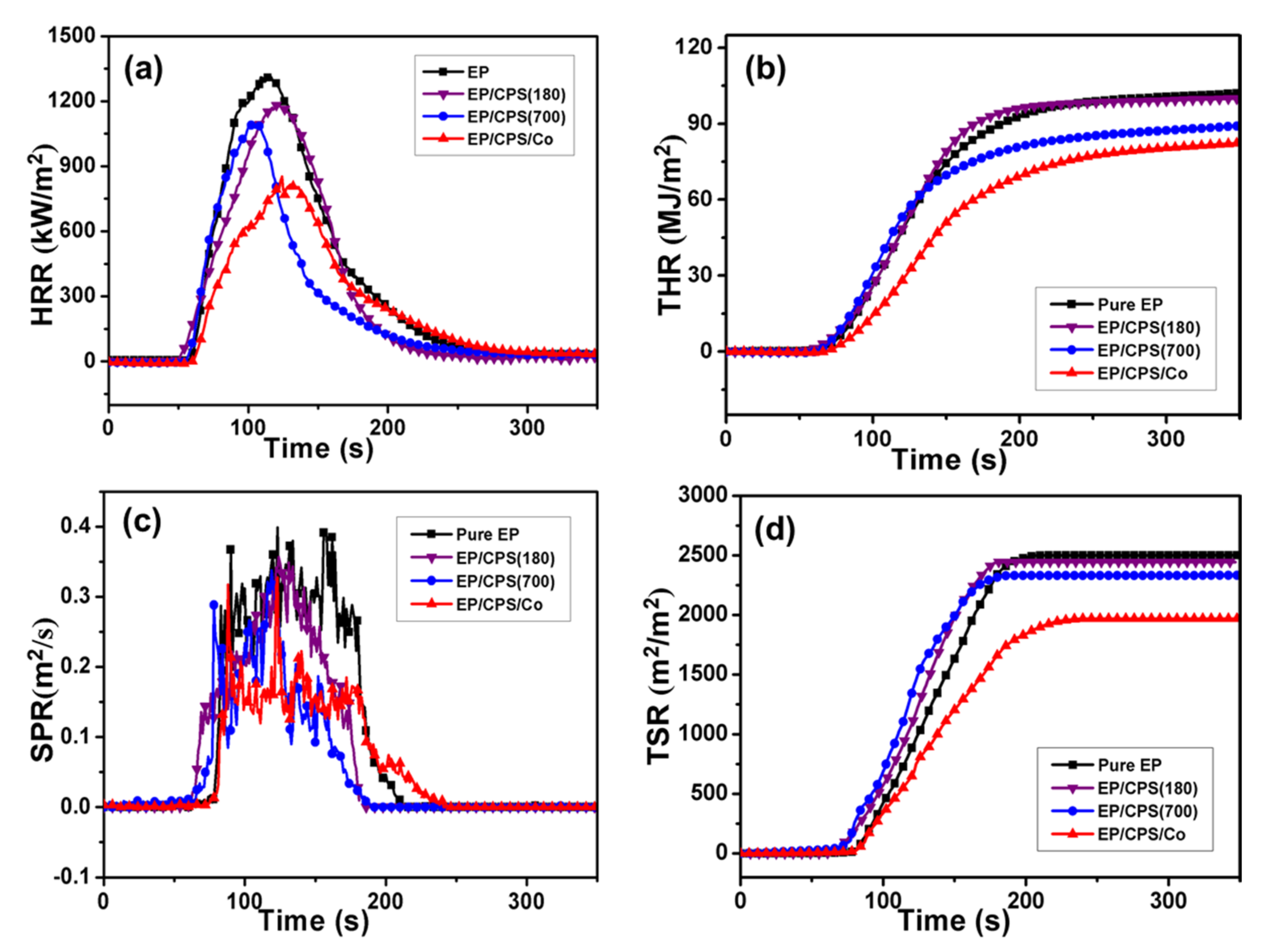
| Samples | TTI (s) | pHRR (kw/m2) | THR (MJ/m2) | PSPR (m2/s) | TSR (m2/m2) | MARHE (kw/m2) | Residue (%) |
|---|---|---|---|---|---|---|---|
| EP | 75 ± 1 | 1313 ± 32 | 102 ± 2 | 0.36 ± 0.02 | 2501 ± 45 | 471 ± 11 | 9.6 ± 0.3 |
| EP/CPS (180) | 73 ± 3 | 1180 ± 21 | 100 ± 3 | 0.31 ± 0.01 | 2444 ± 29 | 462 ± 7 | 8.2 ± 0.2 |
| EP/CPS (700) | 75 ± 1 | 1084 ± 25 | 89 ± 2 | 0.31 ± 0.02 | 2334 ± 37 | 455 ± 15 | 9.9 ± 0.2 |
| EP/CPS/Co | 77 ± 2 | 816 ± 29 | 82 ± 2 | 0.24 ± 0.01 | 1970 ± 17 | 426 ± 5 | 14.8 ± 0.3 |
| Samples | pHRR (%) | THR (%) | PSPR (%) | TSR (%) |
|---|---|---|---|---|
| EP | 0 | 0 | 0 | 0 |
| EP/CPS (180) | 10.1 | 2.0 | 13.9 | 2.3 |
| EP/CPS (700) | 17.4 | 12.7 | 13.9 | 6.7 |
| EP/CPS/Co | 37.9 | 19.6 | 30.7 | 21.2 |
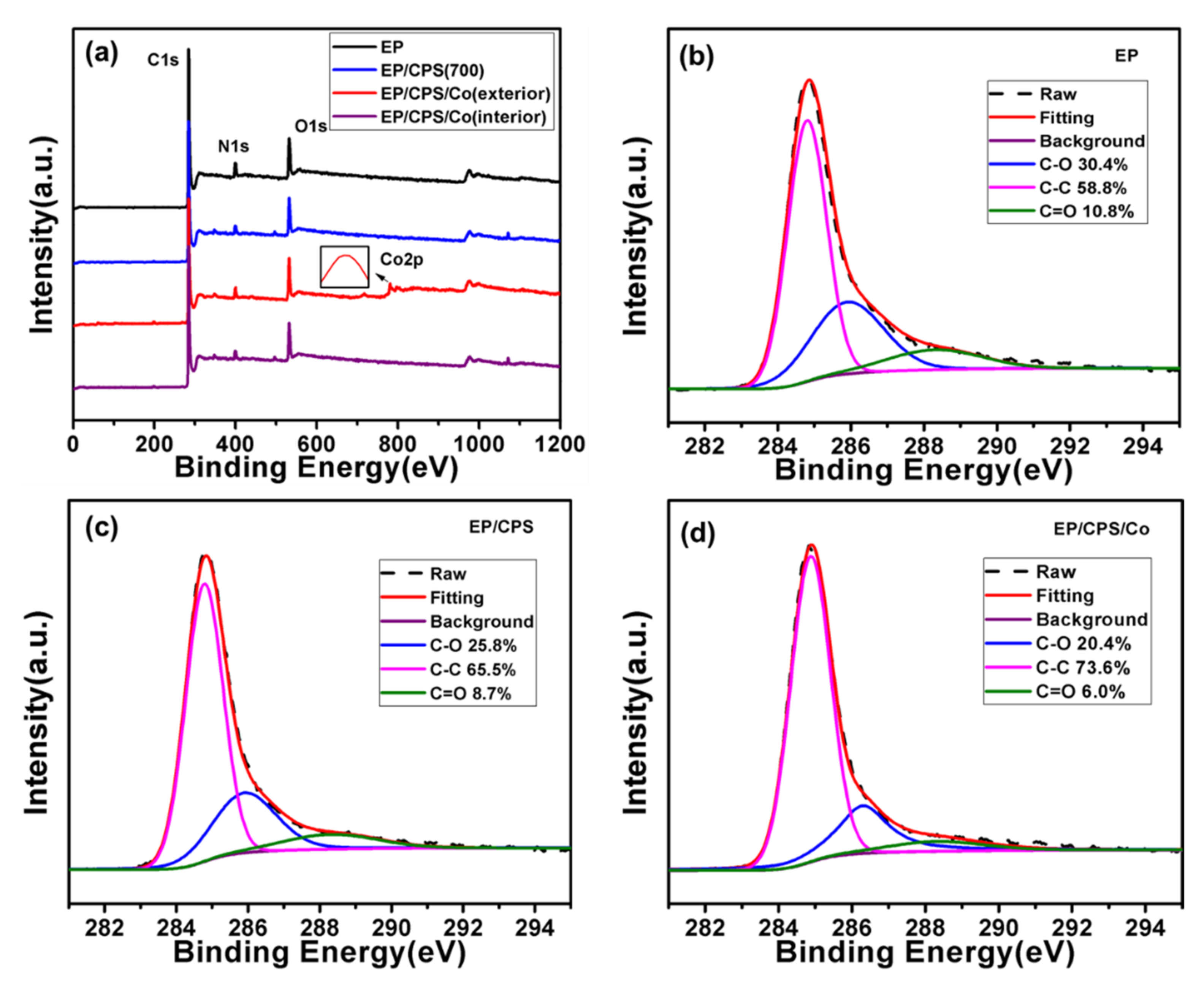
| Samples | Mass Percentage (%) | |||
|---|---|---|---|---|
| C | O | N | Co | |
| EP/CPS/Co | - | - | - | 0.2 |
| EP/CPS/Co char residue (interior) | 82.9 | 10.1 | 3.7 | 3.2 |
| EP/CPS/Co char residue (exterior) | 74.6 | 13.4 | 4.5 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Yang, W.; Yuen, A.C.Y.; Long, H.; Qiu, S.; De Cachinho Cordeiro, I.M.; Wang, W.; Chen, T.B.Y.; Hu, Y.; Yeoh, G.H. Peanut Shell Derived Carbon Combined with Nano Cobalt: An Effective Flame Retardant for Epoxy Resin. Molecules 2021, 26, 6662. https://doi.org/10.3390/molecules26216662
Liang J, Yang W, Yuen ACY, Long H, Qiu S, De Cachinho Cordeiro IM, Wang W, Chen TBY, Hu Y, Yeoh GH. Peanut Shell Derived Carbon Combined with Nano Cobalt: An Effective Flame Retardant for Epoxy Resin. Molecules. 2021; 26(21):6662. https://doi.org/10.3390/molecules26216662
Chicago/Turabian StyleLiang, Jing, Wenhao Yang, Anthony Chun Yin Yuen, Hu Long, Shuilai Qiu, Ivan Miguel De Cachinho Cordeiro, Wei Wang, Timothy Bo Yuan Chen, Yuan Hu, and Guan Heng Yeoh. 2021. "Peanut Shell Derived Carbon Combined with Nano Cobalt: An Effective Flame Retardant for Epoxy Resin" Molecules 26, no. 21: 6662. https://doi.org/10.3390/molecules26216662
APA StyleLiang, J., Yang, W., Yuen, A. C. Y., Long, H., Qiu, S., De Cachinho Cordeiro, I. M., Wang, W., Chen, T. B. Y., Hu, Y., & Yeoh, G. H. (2021). Peanut Shell Derived Carbon Combined with Nano Cobalt: An Effective Flame Retardant for Epoxy Resin. Molecules, 26(21), 6662. https://doi.org/10.3390/molecules26216662










