Evaluation of the Potential Release Tendency of Metals and Metalloids from the Estuarine Sediments: Case Study of Raša Bay
Abstract
:1. Introduction
2. Results
2.1. Grain Size Distribution in Sediments
2.2. Element Levels in Estuarine Water and Sediment
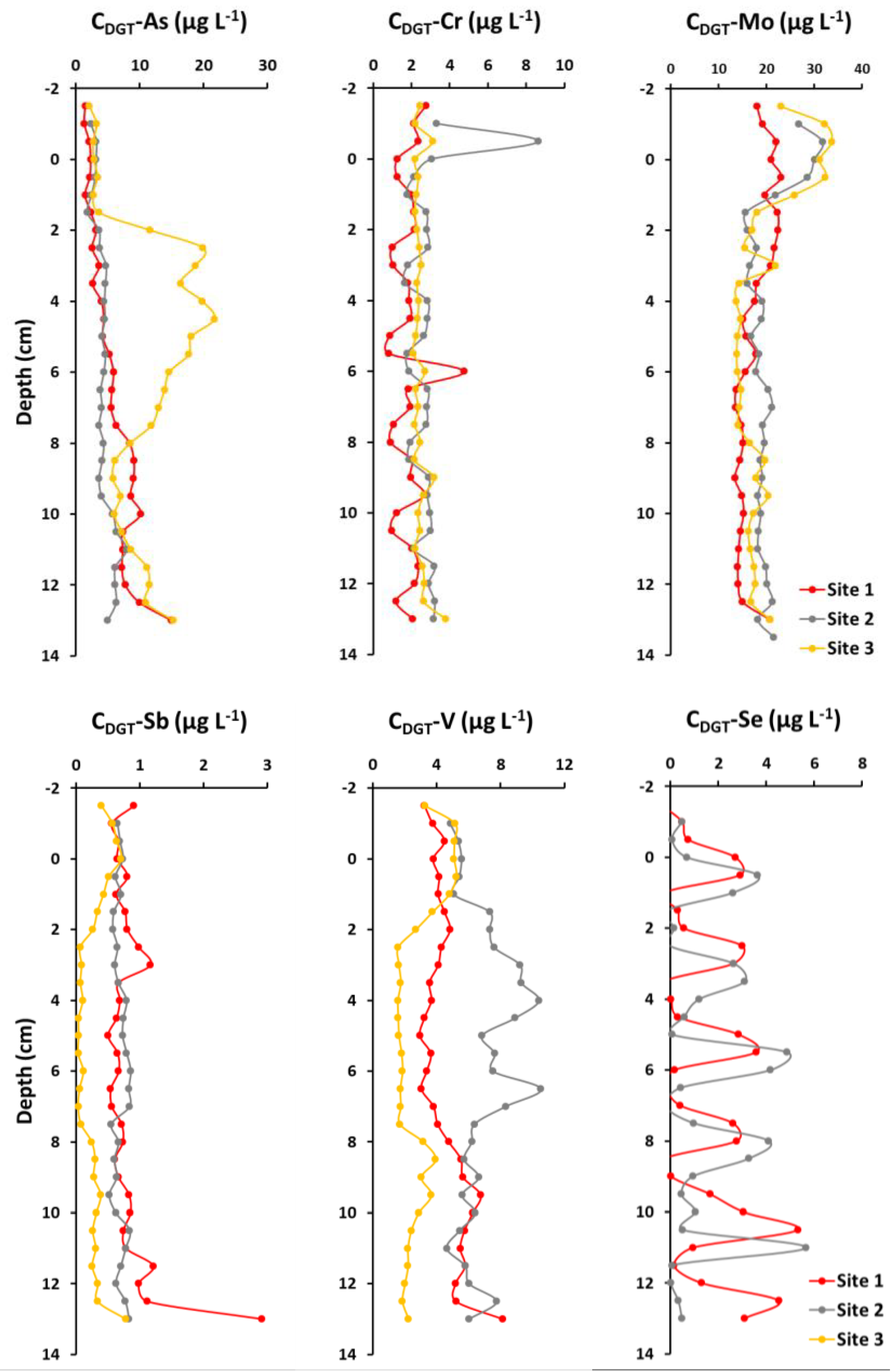
2.3. Element Concentrations in Sediment Pore Water
3. Discussion
3.1. Granulometric Characteristics of Sediments
3.2. Chemical Composition of Estuarine Water
3.3. Element Mass Fractions in Estuarine Sediments
3.4. Sediment Pore Water Geochemistry
3.5. Potential Release Tendency of Metals and Metalloids from the Sediments
4. Materials and Methods
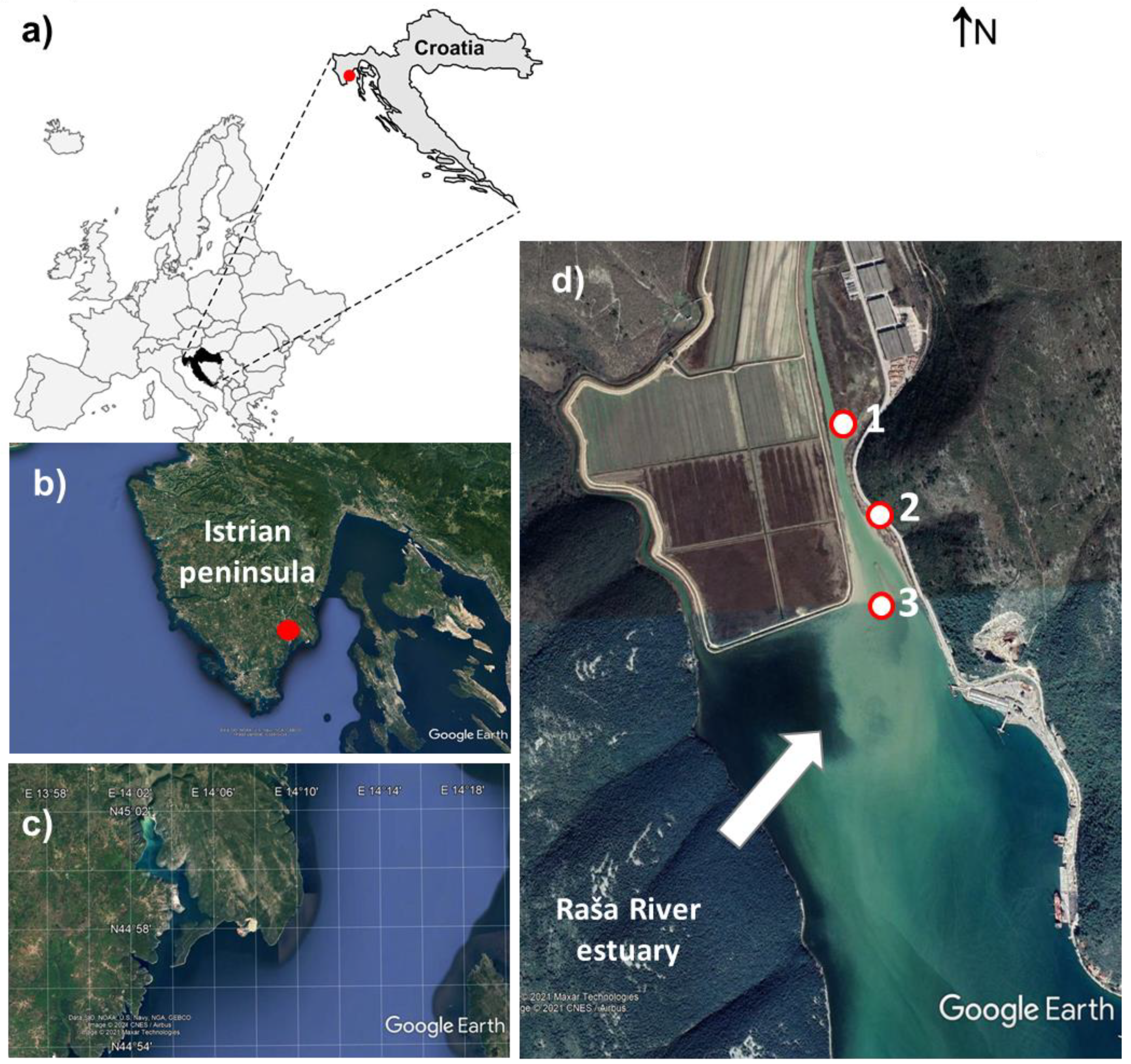
4.1. Study Area
4.2. Sampling and Sample Preparation
4.3. Analysis of Sediment Particle Size Distribution
4.4. DGT Preparation and Deployment
4.5. Multielement Analysis
4.6. Calculation of DGT Measured Concentration
4.7. The Assessment of Sediment Pollution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Samples Availability
References
- Tsikoti, C.; Genitsaris, S. Review of Harmful Algal Blooms in the Coastal Mediterranean Sea, with a Focus on Greek Waters. Diversity 2021, 13, 396. [Google Scholar] [CrossRef]
- Medunić, G.; Ahel, M.; Božičević Mihalić, I.; Gaurina Srček, V.; Kopjar, N.; Fiket, Ž.; Bituh, T.; Mikac, I. Toxic airborne S, PAH, and trace element legacy of the superhigh-organic-sulphur Raša coal combustion: Cytotoxicity and genotoxicity assessment of soil and ash. Sci. Total Environ. 2016, 566, 306–319. [Google Scholar] [CrossRef]
- Fiket, Ž.; Medunić, G.; Vidaković-Cifrek, Ž.; Jezidžić, P.; Cvjetko, P. Effect of coal mining activities and related industry on composition, cytotoxicity and genotoxicity of surrounding soils. Environ. Sci. Pollut. Res. 2020, 27, 6613–6627. [Google Scholar] [CrossRef]
- Vdović, N.; Lučić, M.; Mikac, N.; Bačić, N. Partitioning of Metal Contaminants between Bulk and Fine-Grained Fraction in Freshwater Sediments: A Critical Appraisal. Minerals 2021, 11, 603. [Google Scholar] [CrossRef]
- Wang, N.; Sun, X.; Zhao, Q.; Yang, Y.; Wang, P. Leachability and Adverse Effects of Coal Fly Ash: A Review. J. Hazard. Mater. 2020, 396, 122725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Coyte, R.; Cowan, E.; Stapleton, H.; Dwyer, G.; Vengosh, A. Evaluation and Integration of Geochemical Indicators for Detecting Trace Levels of Coal Fly Ash in Soils. Environ. Sci. Technol. 2021, 55/15, 10387–10397. [Google Scholar] [CrossRef]
- Liu, J.J.; Diao, Z.H.; Xu, X.R.; Xie, Q.; Ni, Z.X. In situ arsenic speciation and the release kinetics in coastal sediments: A case study in Daya Bay, South China Sea. Sci. Total Environ. 2019, 650, 2221–2230. [Google Scholar] [CrossRef]
- Gao, L.; Gao, B.; Zhou, H.; Xu, D.; Wang, Q.; Yin, S. Assessing the remobilization of Antimony in sediments by DGT: A case study in a tributary of the Three Gorges Reservoir. Environ. Pollut. 2016, 214, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Kong, L.; Liu, E.; Wang, L.; Zhu, J. Spatial distribution. ecological risk assessment and source identification for heavy metals in surface sediments from Dongping Lake. Shandong. East China. CATENA 2015, 125, 200–205. [Google Scholar] [CrossRef]
- Guan, D.X.; Williams, P.N.; Luo, J.; Zheng, J.L.; Xu, H.C.; Cai, C.; Ma, L.Q. Novel precipitated zirconia-based DGT technique for high-resolution imaging of oxyanions in waters and sediments. Environ. Sci. Technol. 2015, 49, 3653–3661. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.X.; Williams, P.N.; Xu, H.C.; Li, G.; Luo, J.; Ma, L.Q. High-resolution measurement and mapping of tungstate in waters. soils and sediments using the lowdisturbance DGT sampling technique. J. Hazard. Mater. 2016, 316, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zhou, C.; Gaulier, C.; Bratkič, A.; Galceran, J.; Puy, J.; Zhang, H.; Leermakers, M.; Baeyens, W. Labile trace metal concentration measurements in marine environments: From coastal to open ocean areas. Trends Analyt. Chem. 2019, 116, 92–101. [Google Scholar] [CrossRef]
- Österlund, H.; Chlot, S.; Faarinen, M.; Widerlund, A.; Rodushkin, I.; Ingri, J.; Baxter, D.C. Simultaneous measurements of As, Mo, Sb, V and W using a ferrihydrite diffusive gradients in thin films (DGT) device. Anal. Chim. Acta 2010, 682, 59–65. [Google Scholar] [CrossRef]
- Amato, E.D.; Simpson, S.L.; Remaili, T.M.; Spadaro, D.A.; Jarolimek, C.V.; Jolley, D.F. Assessing the effects of bioturbation on metal bioavailability in contaminated sediments by diffusive gradients in thin films (DGT). Environ. Sci. Technol. 2016, 50, 3055–3064. [Google Scholar] [CrossRef]
- Sun, H.; Gao, B.; Gao, L.; Xu, D.; Sun, K. Using diffusive gradients in thin films (DGT) and DGT-induced fluxes in sediments model to assess the dynamic release of copper in sediment cores from the Three Gorges Reservoir. China. Sci. Total Environ. 2019, 672, 192–200. [Google Scholar] [CrossRef]
- Medunić, G.; Bucković, D.; Prevendar Crnić, A.; Bituh, T.; Gaurina Srček, V.; Radošević, K.; Bajramović, M.; Zgorelec, Ž. Sulfur, metal(loid)s, radioactivity, and cytotoxicity in abandoned karstic Raša coal-mine discharges (the north Adriatic Sea). Min. Geol. Petrol. Eng. Bull. 2020, 35, 1–16. [Google Scholar] [CrossRef]
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Duris, M.; Gilucis, A.; Gregorauskiene, V.; Halamić, J.; et al. FOREGS Geochemical Atlas of Europe, Part 1: Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005; p. 526. [Google Scholar]
- Fiket, Ž.; Ivanić, M.; Furdek Turk, M.; Mikac, N.; Kniewald, G. Distribution of trace elements in waters of the Zrmanja River estuary (eastern Adriatic coast, Croatia). Croat. Chem. Acta 2018, 91, 29–41. [Google Scholar] [CrossRef]
- Fiket, Ž.; Mikac, N.; Kniewald, G. Sedimentary records of the Zrmanja River Estuary, eastern Adriatic coast—Natural vs. anthropogenic impacts. J. Soils Sediments 2017, 17, 1905–1916. [Google Scholar] [CrossRef]
- Lazzari, A.D.; Rampazzo, G.; Pavoni, B. Geochemistry of sediments in the Northern and Central Adriatic Sea. Estuar. Coast. Shelf Sci. 2004, 59, 429–440. [Google Scholar] [CrossRef]
- Cukrov, N.; Frančišković-Bilinski, S.; Hlača, B.; Barišić, D. A recent history of metal accumulation in the sediments of Rijeka harbour, Adriatic Sea, Croatia. Mar. Pollut. Bull. 2011, 62, 154–167. [Google Scholar] [CrossRef]
- Obhođaš, J.; Valković, V.; Kutle, A. Atlas of Sediments in Coastal Areas and Islands of the Croatian Adriatic Sea; Udruga Lijepa naša: Zagreb, Croatian, 2010; pp. 1–231. [Google Scholar]
- Arbanas, Ž.; Benac, Č.; Rubinić, J. The Geotechnical Properties of the Sediment Body in the Estuary of the Raša River, Croatia; Coastal Environment V, incorporating Oil Spill Studies; Brebbia, C.A., Saval Perez, J.M., Garcia Andion, L., Villacampa Esteve, Y., Eds.; WIT Press: Ashurst, UK, 2004; Available online: www.witpress.com (accessed on 15 July 2021).
- Angel, B.M.; Apte, S.C.; Batley, G.E.; Goldin, L.A. Geochemical controls on aluminium concentrations in coastal waters. Environ. Chem. 2015, 13, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Cindrić, A.-M.; Garnier, C.; Oursel, B.; Pižeta, I.; Omanović, D. Evidencing the natural and anthropogenic processes controlling trace metals dynamic in a highly stratified estuary: The Krka River estuary (Adriatic, Croatia). Mar. Pollut. Bull. 2015, 94, 199–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, S.; Laws, E.A.; Gambrell, R. Trace element remobilization following the resuspension of sediments under controlled redox conditions: City Park Lake, Baton Rouge, LA. Appl. Geochem. 2013, 28, 91–99. [Google Scholar] [CrossRef]
- Smrzka, D.; Zwicker, J.; Bach, W.; Feng, D.; Himmler, T.; Chen, D.; Peckmann, J. The behavior of trace elements in seawater, sedimentary pore water, and their incorporation into carbonate minerals: A review. Facies 2019, 65, 41. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Luek, J.L.; McIntosh, H.; Beck, A.J. Geochemistry of redox-sensitive trace elements in a shallow subterranean estuary. Mar. Chem. 2015, 172, 70–81. [Google Scholar] [CrossRef]
- Fiket, Ž.; Fiket, T.; Ivanić, M.; Mikac, N.; Kniewald, G. Pore water geochemistry and diagenesis of estuary sediments—An example of the Zrmanja River estuary (Adriatic coast, Croatia). J. Soils Sediments 2019, 19, 2048–2060. [Google Scholar] [CrossRef]
- Gao, L.; Gao, B.; Peng, W.; Xu, D.; Yin, S. Assessing potential release tendency of As, Mo and W in the tributary sediments of the Three Gorges Reservoir. China. Ecotoxicol. Environ. Saf. 2018, 147, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Gao, L.; Xu, D.; Zhou, Y.; Lu, J. Assessment of Cr pollution in tributary sediment cores in the Three Gorges Reservoir combining geochemical baseline and in situ DGT. Sci. Total Environ. 2018, 628–629, 241–248. [Google Scholar] [CrossRef]
- Gao, L.; Gao, B.; Xu, D.; Peng, W.; Lu, J.; Gao, J. Assessing remobilization characteristics of arsenic (As) in tributary sediment cores in the largest reservoir. China. Ecotoxicol. Environ. Saf. 2017, 140, 48–54. [Google Scholar] [CrossRef]
- Durn, G.; Ottner, F.; Slovenec, D. Mineralogical and geochemical indicators of the polygenetic nature of terra rossa in Istria, Croatia. Geoderma 1999, 91, 125–150. [Google Scholar] [CrossRef]
- Vlahović, I.; Tišljar, J.; Velić, I.; Matičec, D. Evolution of the Adriatic Carbonate Platform: Palaeogeography, main events and depositional dynamics. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 220, 333–360. [Google Scholar] [CrossRef]
- Medunić, G.; Rađenović, A.; Bajramović, M.; Švec, M.; Tomac, M. Once grand, now forgotten: What do we know about the superhigh-organic-sulphur Raša coal? Min. Geol. Petrol. Eng. Bull. 2016, 34, 27–45. [Google Scholar] [CrossRef] [Green Version]
- Halamić, J.; Peh, Z.; Miko, S.; Galović, L.; Šorša, A. Geochemical atlas of Croatia: Environmental implications and geodynamical thread. J. Geochem. Explor. 2012, 115, 36–46. [Google Scholar] [CrossRef]
- Medunić, G.; Grigore, M.; Dai, S.; Berti, D.; Hochella, M.F.; Mastalerz, M.; Valentim, B.; Guedes, A.; Hower, J.C. Characterization of superhigh-organic-sulfur Raša coal, Istria, Croatia, and its environmental implication. Int. J. Coal Geol. 2020, 217, 103344. [Google Scholar] [CrossRef]
- Folk, R.L. The distinction between grain size and mineral composition in sedimentary rock nomenclature. J. Geol. 1954, 62, 344–359. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. GRADISTAT: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Shepard, F.P. Nomenclature based on sand-silt-clay ratios. J. Sediment. Res. 1954, 24, 151–158. [Google Scholar]
- Fiket, Ž.; Mikac, N.; Kniewald, G. Mass Fractions of Forty-Six Major and Trace Elements, Including Rare Earth Elements, in Sediment and Soil Reference Materials Used in Environmental Studies. Geostand. Geoanalytical. Res. 2017, 41, 123–135. [Google Scholar] [CrossRef]
- Li, Y.-H.; Gregory, S. Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta 1974, 38, 703–714. [Google Scholar]
- Ullman, W.J.; Aller, R.C. Diffusion coefficients in nearshore marine sediments. Limnol. Oceanogr. 1982, 27, 552–556. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ. Sci. Technol. 2000, 34, 5084–5091. [Google Scholar] [CrossRef]
- Loska, K.; Wiechula, D.; Korus, I. Metal contamination of farming soils affected by industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Müller, G. Die Schwermetallbelastung der Sedimente des Neckars und seiner Nebenflüsse: Eine Bestandsaufnahme. Chem. Ztg. 1981, 6, 157–164. [Google Scholar]
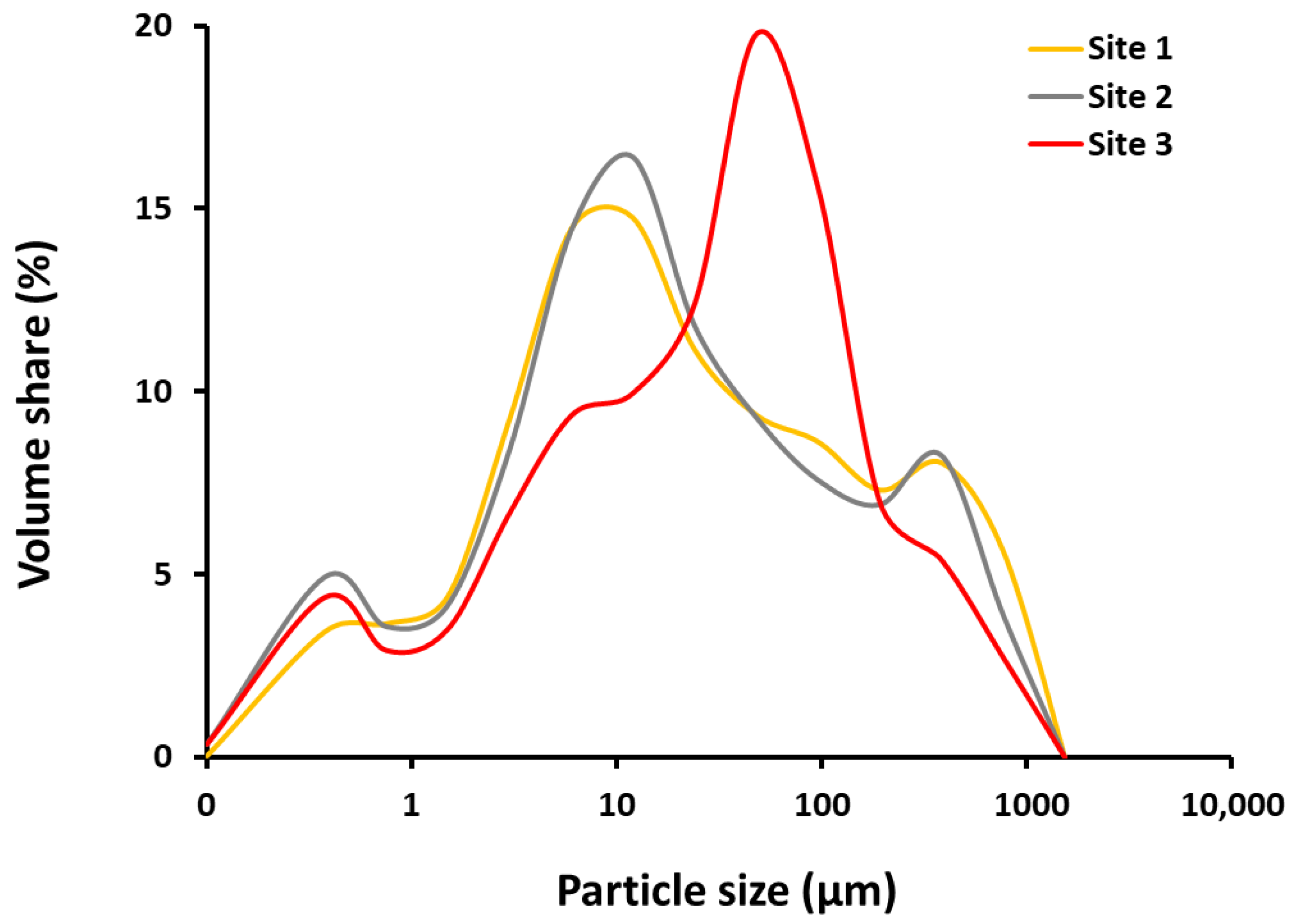

| (%) | Site 1 | Site 2 | Site 3 |
|---|---|---|---|
| clay | 20.8 | 21.5 | 17.8 |
| silt | 49.6 | 51.9 | 51.5 |
| sand | 29.6 | 26.6 | 30.7 |
| Element | Raša River Estuary (This Study) | Raša River Estuary [16] | Fonte Gaja (Natural Freshwater Spring) [16] | Stream Water of the Drainage Area [17] | Karin Sea [18] | Velebit Channel (Surface) [18] | Velebit Channel (Bottom) [18] | ||
|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | |||||||
| Al | 31.3 | 29.2 | 13.1 | 6.5 | 3–37 | 2.10–4.10 | 4.94 | 6.15 | 10.2 |
| As | 0.62 | 0.51 | 0.61 | 0.40–1.40 | 0.18–0.30 | 0.82 | 1.17 | 2.50 | |
| Ba | 40.2 | 37.0 | 16.5 | 31.3 | 14.6 | 10.1–14.9 | 10.5 | 11.3 | 7.84 |
| Be | 0.06 | 0.06 | 0.11 | 0.08 | 0.007 | <0.009 | |||
| Cd | 0.21 | 0.03–0.2 | 0.016–0.022 | ||||||
| Co | 0.05 | 0.04 | 0.03 | 0.06 | 0.04 | 0.11–0.16 | 0.38 | 0.21 | 0.040 |
| Cr | 0.40 | 0.28 | 0.26 | 0.60 | 0.5–5 | 0.28–0.38 | 0.72 | 0.58 | 1.18 |
| Cs | 0.06 | 0.07 | 0.10 | 0.04 | 0.004 | 0.002–0.003 | 0.12 | 0.13 | 0.320 |
| Cu | 0.55 | 0.33 | 1.81 | 0.60 | 0.43–10 | 0.88–1.20 | 0.83 | 1.16 | 1.72 |
| Fe | 33.0 | 29.8 | 12.6 | 2.1 | 1–390 | 30–67 | 5.52 | 4.71 | 10.0 |
| Li | 45.4 | 50.5 | 81.4 | 27.5 | 0.46 | 0.70–2.10 | 68.4 | 75.2 | 175 |
| Mn | 8.68 | 8.84 | 9.49 | 2.2 | 0.5–25 | 3.90–6.70 | 0.26 | 0.44 | 3.34 |
| Mo | 3.91 | 4.26 | 6.06 | 33.1 | 2.04 | 0.22–0.37 | 4.07 | 4.78 | 12.2 |
| Ni | 1.30 | 0.35–20 | 3.93–6.37 | 2.20 | 2.98 | 4.04 | |||
| Pb | 0.49 | 0.39 | 2.12 | 0.20 | 0.09–1 | 0.093–0.160 | |||
| Rb | 28.7 | 31.7 | 51.0 | 19.5 | 1.04 | 0.69–0.94 | 48.5 | 54.3 | 127 |
| S * | 219 | 243 | 396 | 153 | 5100 | ||||
| Sb | 0.09 | 0.07 | 0.07 | 0.37 | 0.07 | 0.02–0.04 | |||
| Se | 0.48 | 0.34 | 0.37 | 3.50 | 1.09 | 0.13–0.19 | |||
| Sn | 0.34 | 0.17 | 0.12 | 0.35 | 0.35 | ||||
| Sr | 2316 | 2508 | 3691 | 1797 | 191 | 50–190 | 3337 | 3691 | 8415 |
| Ti | 3.43 | 1.01 | 0.34 | 0.10 | 0.37 | 0.3–0.4 | |||
| Tl | 0.012 | 0.012 | 0.014 | 0.04 | 0.01 | 0.003–0.005 | |||
| U | 1.27 | 1.32 | 1.77 | 10.8 | 0.75 | 0.32–0.63 | 1.68 | 1.72 | 3.62 |
| V | 1.02 | 1.05 | 0.77 | 1.70 | 1.37 | 0.16–0.32 | 1.58 | 1.74 | 1.80 |
| Zn | 4.15 | 2.17 | 5.04 | 4.20 | 2.5–267 | 2.68–4.00 | |||
| Element | Raša River Estuary (This Study) | Stream Sediments of the Drainage Area [17] | Zrmanja River Estuary [19] | Northern and Central Adriatic [20] | Rijeka Harbour [21] | Adriatic [22] | ||
|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | ||||||
| Al * | 43.8 | 34.3 | 35.4 | 44.5–54.5 | 2.1–5.2 | 52.4 | 1.77 | |
| As | 6.52 | 6.37 | 6.12 | 6–9 | 5.66–16.7 | 21.4 | 19.7 | |
| Ba | 168 | 148 | 156 | 118–205 | 98.7–186 | 86.3 | ||
| Be | 1.17 | 0.96 | 0.91 | 0.78–1.00 | 0.693–1.81 | 0.92 | ||
| Cd | 0.44 | 0.32 | 0.29 | 0.40–0.51 | 0.32–0.41 | 0.11 | 1.07 | |
| Co | 13.8 | 11.2 | 11.1 | 6.0–8.0 | 4.55–9.86 | 9.9 | 12.9 | |
| Cr | 125 | 106 | 119 | 50–63 | 34.4–98.2 | 85.8 | 71.6 | 57.3 |
| Cs | 5.74 | 3.98 | 3.89 | 5–6 | 2.84–7.32 | 1.77 | ||
| Cu | 32.0 | 22.4 | 22.0 | 13–22 | 6.11–18.4 | 8.0 | 145 | |
| Fe * | 24.7 | 20.2 | 19.0 | 25.0–31.1 | 1.06–2.68 | 23.8 | 2.74 | 0.9 |
| Li | 46.2 | 35.3 | 34.7 | 30.5–39.3 | 26.8–61.4 | 38.9 | ||
| Mn | 507 | 504 | 511 | 310–387 | 170–351 | 542 | 343 | 130 |
| Mo | 2.53 | 1.04 | 0.79 | 0.63–0.81 | 0.64–2.27 | 3.53 | ||
| Ni | 72.3 | 53.6 | 54.6 | 15.0–21.0 | 12.2–45.4 | 37.9 | 86.1 | 23.8 |
| Pb | 18.4 | 17.7 | 18.5 | 17.0–20.5 | 18–33.8 | 29.7 | 227 | 52.7 |
| Rb | 93.7 | 68.3 | 67.2 | 57.6–70.0 | 39.7–96.4 | 31.3 | 34.3 | |
| Sb | 0.91 | 0.53 | 0.47 | 1.15–1.62 | 0.40–0.89 | 1.62 | ||
| Se | 0.48 | 0.34 | 0.37 | 1.05 | ||||
| Sn | 0.34 | 0.17 | 0.12 | 1.80–2.25 | 1.56–2.94 | 6.66 | ||
| Sr | 313 | 317 | 312 | 71.0–99.0 | 136–247 | 213 | ||
| Ti * | 3.0 | 2.5 | 2.6 | 3.1–3.7 | 1.5–3.3 | 2.4 | 0.995 | |
| Tl | 0.46 | 0.34 | 0.33 | 0.48–0.59 | 0.36–0.79 | 0.39 | ||
| U | 2.57 | 2.91 | 1.97 | 1.0–3.0 | 1.57–2.62 | 2.61 | ||
| V | 101 | 84.2 | 74.2 | 62–78 | 37.1–97 | 53.1 | 72.4 | |
| Zn | 92.9 | 66.2 | 95.8 | 45–71 | 76.6–112 | 76.2 | 369 | 206 |
| Element | Diffusion Coefficient (D/10−6 cm2 s−1) | Diffusive Flux (J/10−6 ng cm−2 s−1) | ||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | ||
| As | 6.3 | −2.4 | −2.1 | −2.4 |
| Cr | 7.8 | 0.08 | 0.3 | −0.08 |
| Mo | 6.9 | 3.4 | 6.5 | 8.2 |
| Sb | 5.8 | −0.05 | −0.01 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiket, Ž.; Petrović, M.; Medunić, G.; Ivošević, T.; Fiket, T.; Xu, L.Z.; Wang, Y.; Ding, S. Evaluation of the Potential Release Tendency of Metals and Metalloids from the Estuarine Sediments: Case Study of Raša Bay. Molecules 2021, 26, 6656. https://doi.org/10.3390/molecules26216656
Fiket Ž, Petrović M, Medunić G, Ivošević T, Fiket T, Xu LZ, Wang Y, Ding S. Evaluation of the Potential Release Tendency of Metals and Metalloids from the Estuarine Sediments: Case Study of Raša Bay. Molecules. 2021; 26(21):6656. https://doi.org/10.3390/molecules26216656
Chicago/Turabian StyleFiket, Željka, Marija Petrović, Gordana Medunić, Tatjana Ivošević, Tomislav Fiket, Lizzy Zhang Xu, Yan Wang, and Shiming Ding. 2021. "Evaluation of the Potential Release Tendency of Metals and Metalloids from the Estuarine Sediments: Case Study of Raša Bay" Molecules 26, no. 21: 6656. https://doi.org/10.3390/molecules26216656
APA StyleFiket, Ž., Petrović, M., Medunić, G., Ivošević, T., Fiket, T., Xu, L. Z., Wang, Y., & Ding, S. (2021). Evaluation of the Potential Release Tendency of Metals and Metalloids from the Estuarine Sediments: Case Study of Raša Bay. Molecules, 26(21), 6656. https://doi.org/10.3390/molecules26216656







