A QSAR–ICE–SSD Model Prediction of the PNECs for Per- and Polyfluoroalkyl Substances and Their Ecological Risks in an Area of Electroplating Factories
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of QSAR–ICE Models
2.2. Sample Treatment and Analysis of PFASs
2.3. Ecological Risk Characterization
3. Results and Discussion
3.1. Predicted Toxicity Data by QSAR–ICE Models
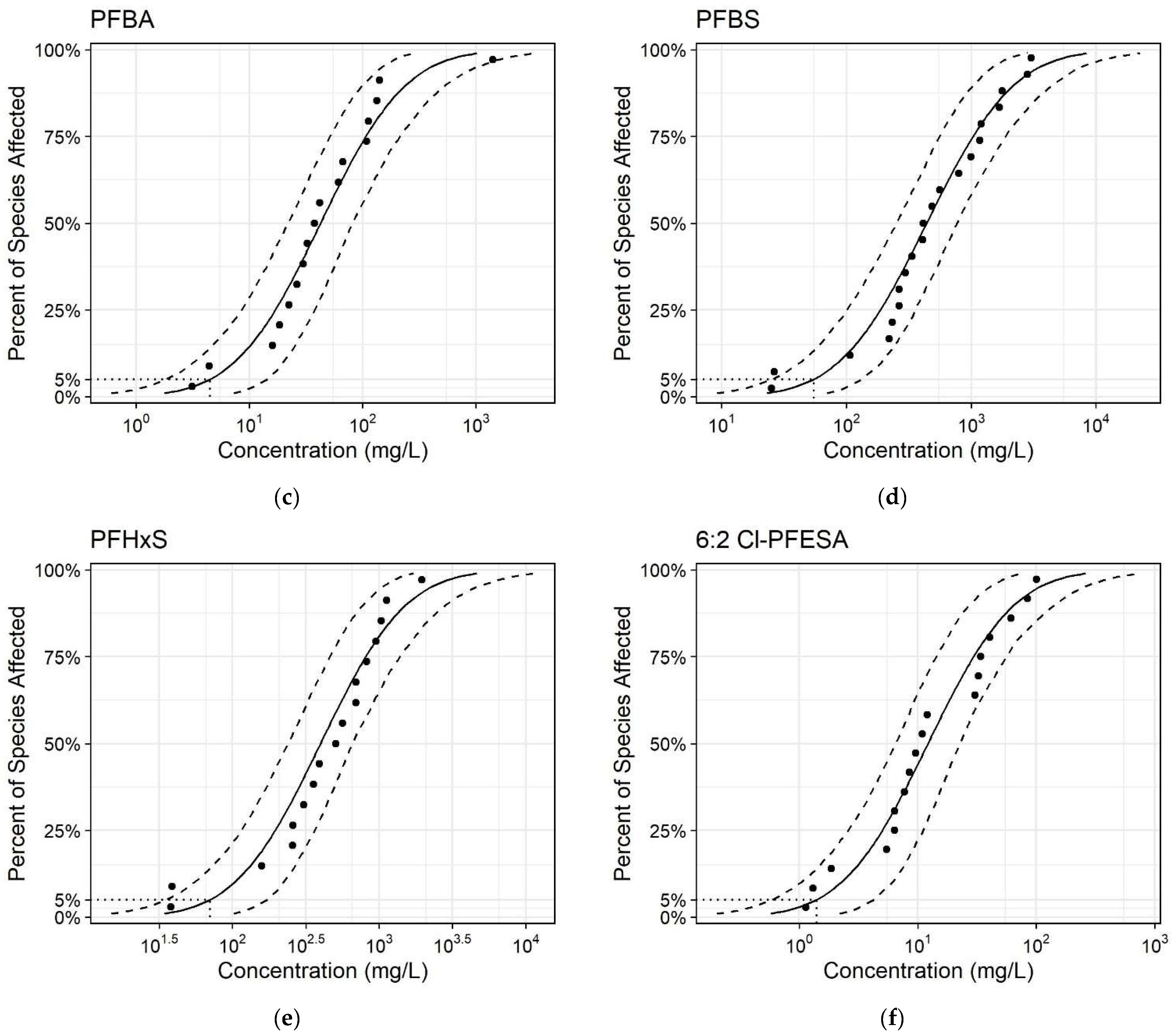
3.2. Calculation and Comparison of the PNEC Values of SSDs Produced Using Predicted and Measured Data
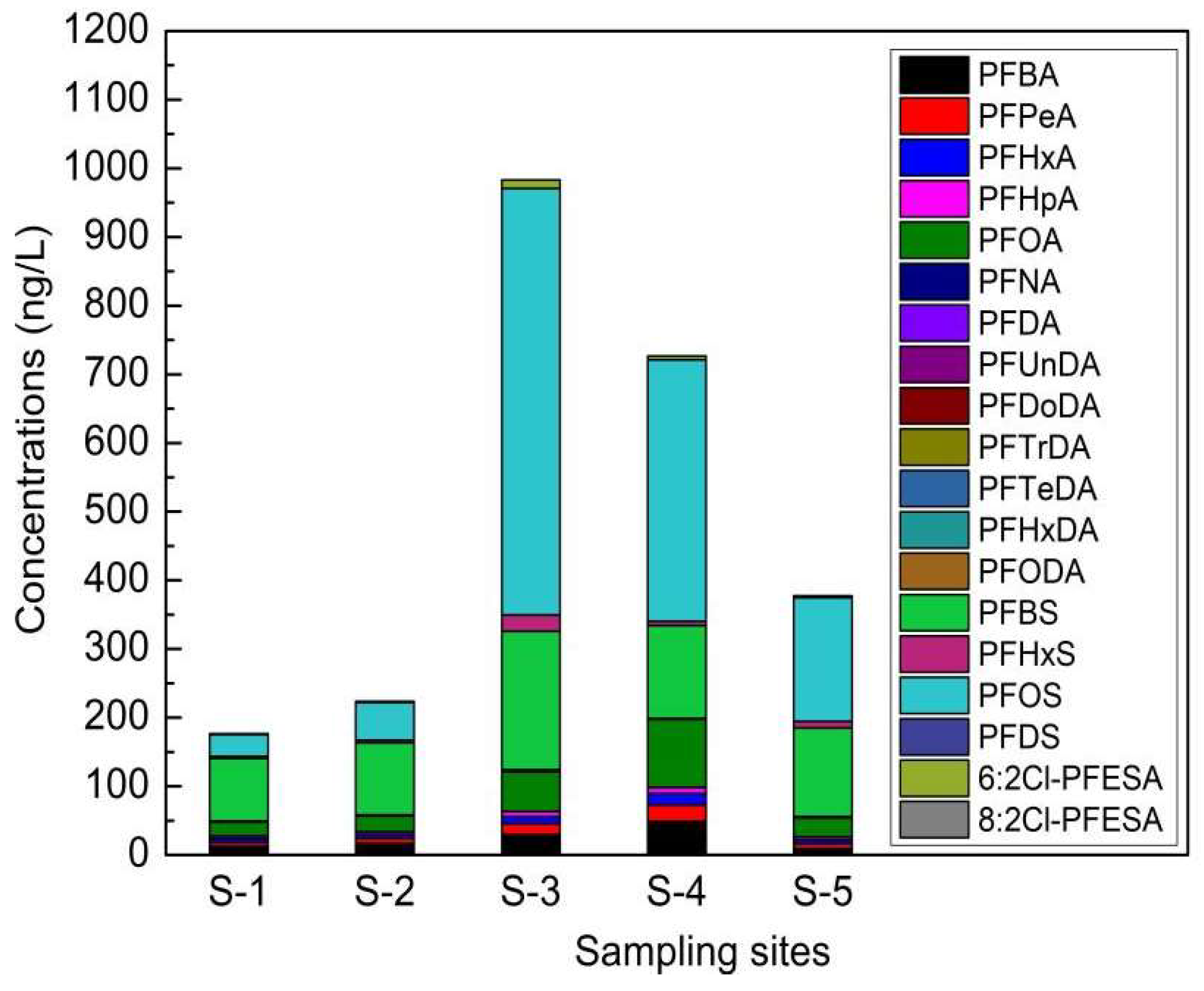
3.3. Concentrations of PFASs in the River near the Electroplating Factories
3.4. Ecological Risks of PFASs
3.5. Implications and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Buck, R.C.; Hungerbühler, K. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: Production and emissions from quantifiable sources. Environ. Int. 2014, 70, 62–75. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Eighth Meeting of the Conference of the Parties to the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ConferenceoftheParties/Meetings/COP8/tabid/5309/Default.aspx (accessed on 1 September 2021).
- United States Environmental Protection Agency. Guidelines for Ecological Risk Assessment. Available online: https://www.epa.gov/sites/production/files/2014-11/documents/eco_risk_assessment1998.pdf (accessed on 3 January 2020).
- Salice, C.J.; Anderson, T.A.; Anderson, R.H.; Olson, A.D. Ecological risk assessment of perfluooroctane sulfonate to aquatic fauna from a bayou adjacent to former fire training areas at a US Air Force installation. Environ. Toxicol. Chem. 2018, 37, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.I.; Lee, T.-Y.; Seo, H.; Kim, D.; Kim, D.; Cui, R.; An, Y.-J. Ecological risk assessment for perfluorooctanoic acid in soil using a species sensitivity approach. J. Hazard. Mater. 2020, 382, 121150. [Google Scholar] [CrossRef]
- European Chemicals Bureau. Technical Guidance Document on Risk Assessment. Available online: https://echa.europa.eu/documents/10162/16960216/tgdpart2_2ed_en.pdf (accessed on 3 January 2020).
- Grist, E.P.M.; O’Hagan, A.; Crane, M.; Sorokin, N.; Sims, I.; Whitehouse, P. Bayesian and Time-Independent Species Sensitivity Distributions for Risk Assessment of Chemicals. Environ. Sci. Technol. 2006, 40, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, D.J.; Hutchinson, T.H.; Heijerick, D.; Anderson, P.D.; Sumpter, J.P. Derivation of an Aquatic Predicted No-Effect Concentration for the Synthetic Hormone, 17α-Ethinyl Estradiol. Environ. Sci. Technol. 2008, 42, 7046–7054. [Google Scholar] [CrossRef] [PubMed]
- Garner, K.L.; Suh, S.; Lenihan, H.S.; Keller, A.A. Species Sensitivity Distributions for Engineered Nanomaterials. Environ. Sci. Technol. 2015, 49, 5753–5759. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.R.; Grist, E.P.M.; Leung, K.M.Y.; Morritt, D.; Crane, M. Species sensitivity distributions: Data and model choice. Mar. Pollut. Bull. 2002, 45, 192–202. [Google Scholar] [CrossRef]
- Maltby, L.; Blake, N.; Brock, T.C.M.; Van den Brink, P.J. Insecticide species sensitivity distributions: Importance of test species selection and relevance to aquatic ecosystems. Environ. Toxicol. Chem. 2005, 24, 379–388. [Google Scholar] [CrossRef]
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Salice, C.J.; et al. Assessing the Ecological Risks of Per- and Polyfluoroalkyl Substances: Current State-of-the Science and a Proposed Path Forward. Environ. Toxicol. Chem. 2020, 40, 564–605. [Google Scholar] [CrossRef]
- He, J.; Tang, Z.; Zhao, Y.; Fan, M.; Dyer, S.D.; Belanger, S.E.; Wu, F. The Combined QSAR-ICE Models: Practical Application in Ecological Risk Assessment and Water Quality Criteria. Environ. Sci. Technol. 2017, 51, 8877–8878. [Google Scholar] [CrossRef]
- Douziech, M.; Ragas, A.M.J.; van Zelm, R.; Oldenkamp, R.; Jan Hendriks, A.; King, H.; Oktivaningrum, R.; Huijbregts, M.A.J. Reliable and representative in silico predictions of freshwater ecotoxicological hazardous concentrations. Environ. Int. 2020, 134, 105334. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Wang, Z.; Fan, D.; Shi, L.; Liu, J. Derivation of freshwater water quality criteria for dibutyltin dilaurate from measured data and data predicted using interspecies correlation estimate models. Chemosphere 2017, 171, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Barron, M.G. Application of Interspecies Correlation Estimation (ICE) models and QSAR in estimating species sensitivity to pesticides. SAR QSAR Environ. Res. 2020, 31, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Hermens, J.L.M. Modes of action in ecotoxicology: Their role in body burdens, species sensitivity, QSARs, and mixture effects. Environ. Sci. Technol. 2002, 36, 4201–4217. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.D.; Versteeg, D.J.; Belanger, S.E.; Chaney, J.G.; Raimondo, S.; Barron, M.G. Comparison of species sensitivity distributions derived from interspecies correlation models to distributions used to derive water quality criteria. Environ. Sci. Technol. 2008, 42, 3076–3083. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, P.; Meng, J.; Liu, S.; Lu, Y.; Khim, J.S.; Giesy, J.P. A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China. Chemosphere 2015, 129, 87–99. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, J.; Higashiguchi, T.; Bo, T.; Niu, J. Quantitative determination and mass flow analysis of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) during reversed A2O wastewater treatment process. Acta Sci. Circumstantiae 2014, 34, 872–880. [Google Scholar]
- Higashiguchi, T.; Shi, J.; Zhang, H.; Liu, X. Distribution of Perfluorooctanesulfonate and Perfluorooctanoate in Water and the Sediment in Fenhe River, Shanxi Provice. Environ. Sci. 2013, 34, 4211–4217. [Google Scholar]
- Yamazaki, E.; Falandysz, J.; Taniyasu, S.; Hui, G.; Jurkiewicz, G.; Yamashita, N.; Yang, Y.-L.; Lam, P.K.S. Perfluorinated carboxylic and sulphonic acids in surface water media from the regions of Tibetan Plateau: Indirect evidence on photochemical degradation? J. Environ. Sci. Health Part A 2016, 51, 63–69. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Y.; Jin, W.; Rao, K.; Giesy, J.P.; Hollert, H.; Richardson, K.L.; Wang, Z. Ecological Risk of Nonylphenol in China Surface Waters Based on Reproductive Fitness. Environ. Sci. Technol. 2014, 48, 1256–1262. [Google Scholar] [CrossRef]
- Wang, X.-N.; Liu, Z.-T.; Yan, Z.-G.; Zhang, C.; Wang, W.-L.; Zhou, J.-L.; Pei, S.-W. Development of aquatic life criteria for triclosan and comparison of the sensitivity between native and non-native species. J. Hazard. Mater. 2013, 260, 1017–1022. [Google Scholar] [CrossRef]
- Feng, C.L.; Wu, F.C.; Dyer, S.D.; Chang, H.; Zhao, X.L. Derivation of freshwater quality criteria for zinc using interspecies correlation estimation models to protect aquatic life in China. Chemosphere 2013, 90, 1177–1183. [Google Scholar] [CrossRef]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for grouping per- and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Klimisch, H.J.; Andreae, M.; Tillmann, U. A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data. Regul. Toxicol. Pharm. 1997, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, B.; Fan, M.; Belanger, S.; Li, J.; Chen, J.; Gao, X.; Liu, Z. Development and use of interspecies correlation estimation models in China for potential application in water quality criteria. Chemosphere 2020, 240. [Google Scholar] [CrossRef] [PubMed]
- Taniyasu, S.; Kannan, K.; So, M.K.; Gulkowska, A.; Sinclair, E.; Okazawa, T.; Yamashita, N. Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J. Chromatogr. A 2005, 1093, 89–97. [Google Scholar] [CrossRef]
- Bhandari, G.; Atreya, K.; Vašíčková, J.; Yang, X.; Geissen, V. Ecological risk assessment of pesticide residues in soils from vegetable production areas: A case study in S-Nepal. Sci. Total Environ. 2021, 788, 147921. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wu, Y.; Ren, C.; Zhou, X.; Jiang, G. 3D-QSAR Studies on biarhibitors for the bromodomains of CBP/P300. Chemistry 2018, 81, 548–554. [Google Scholar]
- Yan, W.; Lin, G.; Zhang, R.; Liang, Z.; Wu, L.; Wu, W. Studies on molecular mechanism between ACE and inhibitory peptides in different bioactivities by 3D-QSAR and MD simulations. J. Mol. Liq. 2020, 304, 112702. [Google Scholar] [CrossRef]
- Tuppurainen, K. Frontier orbital energies, hydrophobicity and steric factors as physical qsar descriptors of molecular mutagenicity. A review with a case study: MX compounds. Chemosphere 1999, 38, 3015–3030. [Google Scholar] [CrossRef]
- Ferrari, T.; Lombardo, A.; Benfenati, E. QSARpy: A new flexible algorithm to generate QSAR models based on dissimilarities. The log Kow case study. Sci. Total Environ. 2018, 637–638, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, B.; Ra, J.-S.; Kim, K.-T. Predicting PBT and CMR properties of substances of very high concern (SVHCs) using QSAR models, and application for K-REACH. Toxicol. Rep. 2020, 7, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Ding, J.-J.; Pan, L.; Fu, L.; Tian, J.-H.; Cao, D.-S.; Jiang, H.; Ding, X.-Q. Quantitative structure-toxicity relationship model for acute toxicity of organophosphates via multiple administration routes in rats and mice. J. Hazard. Mater. 2021, 401, 123724. [Google Scholar] [CrossRef] [PubMed]
- Conroy-Ben, O.; Garcia, I.; Teske, S.S. In silico binding of 4,4’-bisphenols predicts in vitro estrogenic and antiandrogenic activity. Environ. Toxicol. 2018, 33, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Kropf, C.; Begnaud, F.; Gimeno, S.; Berthaud, F.; Debonneville, C.; Segner, H. In Vitro Biotransformation Assays Using Liver S9 Fractions and Hepatocytes from Rainbow Trout (Oncorhynchus mykiss): Overcoming Challenges with Difficult to Test Fragrance Chemicals. Environ. Toxicol. Chem. 2020, 39, 2396–2408. [Google Scholar] [CrossRef]
- Sheng, N.; Cui, R.; Wang, J.; Guo, Y.; Wang, J.; Dai, J. Cytotoxicity of novel fluorinated alternatives to long-chain perfluoroalkyl substances to human liver cell line and their binding capacity to human liver fatty acid binding protein. Arch. Toxicol. 2018, 92, 359–369. [Google Scholar] [CrossRef]
- Lu, Z.; Song, L.; Zhao, Z.; Ma, Y.; Wang, J.; Yang, H.; Ma, H.; Cai, M.; Codling, G.; Ebinghaus, R.; et al. Occurrence and trends in concentrations of perfluoroalkyl substances (PFASs) in surface waters of eastern China. Chemosphere 2015, 119, 820–827. [Google Scholar] [CrossRef]
- Wang, P.; Lu, Y.; Wang, T.; Fu, Y.; Zhu, Z.; Liu, S.; Xie, S.; Xiao, Y.; Giesy, J.P. Occurrence and transport of 17 perfluoroalkyl acids in 12 coastal rivers in south Bohai coastal region of China with concentrated fluoropolymer facilities. Environ. Pollut. 2014, 190, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013, 60, 242–248. [Google Scholar] [CrossRef]
- Li, C. Contamination Characteristics of F-53B, OBS and other Poly- and per Fluoroalky Substances in Typical Areas. Master’s Dissertation, Qingdao Technological University, Qingdao, China, 2016. [Google Scholar]
- Hoondert, R.P.J.; Oldenkamp, R.; de Zwart, D.; van de Meent, D.; Posthuma, L. QSAR-Based Estimation of Species Sensitivity Distribution Parameters: An Exploratory Investigation. Environ. Toxicol. Chem. 2019, 38, 2764–2770. [Google Scholar] [CrossRef]
- Kostal, J.; Plugge, H.; Raderman, W. Quantifying Uncertainty in Ecotoxicological Risk Assessment: MUST, a Modular Uncertainty Scoring Tool. Environ. Sci. Technol. 2020, 54, 12262–12270. [Google Scholar] [CrossRef] [PubMed]
- de Knecht, J.; Rorije, E.; Maslankiewicz, L.; Dang, Z. Feasibility of using interspecies relationships for integration of human and environmental hazard assessment. Hum. Ecol. Risk Assess. An. Int. J. 2021, 27, 1715–1731. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Hong, H. Developing QSAR Models with Defined Applicability Domains on PPARγ Binding Affinity Using Large Data Sets and Machine Learning Algorithms. Environ. Sci. Technol. 2021, 55, 6857–6866. [Google Scholar] [CrossRef] [PubMed]
- Perlich, C.; Provost, F.; Simonoff, J.S. Tree induction vs. logistic regression: A learning-curve analysis. J. Mach. Learn. Res. 2003, 4, 211–255. [Google Scholar] [CrossRef][Green Version]
- Nendza, M.; Müller, M.; Wenzel, A. Discriminating toxicant classes by mode of action: 4. Baseline and excess toxicity. SAR QSAR Environ. Res. 2014, 25, 393–405. [Google Scholar] [CrossRef]
- Kienzler, A.; Halder, M.; Worth, A. Waiving chronic fish tests: Possible use of acute-to-chronic relationships and interspecies correlations. Toxicol. Environ. Chem. 2017, 99, 1129–1151. [Google Scholar] [CrossRef]


| No. | Molecular Descriptors | Abbreviations | Units |
|---|---|---|---|
| 1 | Heat of formation | HOF | kcal/mol |
| 2 | Total energy | TE | EV |
| 3 | Electronic energy | EE | EV |
| 4 | Core–core repulsion energy | ECCR | EV |
| 5 | COSMO area | CA | Å2 |
| 6 | COSMO volume | CV | Å3 |
| 7 | Gradient norm | GN | - |
| 8 | Gradient norm per atom | GN p A | - |
| 9 | Ionization potential | IP | EV |
| 10 | Lowest unoccupied molecule orbital energy | ELUMO | EV |
| 11 | Highest occupied molecular orbital energy | EHOMO | EV |
| 12 | Molecular weight | MW | - |
| 13 | Octanol–water partition coefficient | Kow | - |
| Species | Models | Equations | n a | R2 b | r2 c | q2 d | p e |
|---|---|---|---|---|---|---|---|
| Pseudokirchneriella subcapitata | log EC50 = −log Kow × 8.82 + TE × 47.8 + log ELUMO × 1.47 − ECCR × 39.7 + 50.3 | (4) | 14 | 0.770 | 0.742 | 0.701 | 0.006 |
| Chlorella vulgaris | log EC50 = −4.18 × Kow − 0.332 × ECCR − 4.29 | (5) | 10 | 0.592 | 0.751 | 0.673 | 0.043 |
| Daphnia magna | log LC50 = −Kow × 4.09 + log TE × 9.75 − ECCR × 7.03 + log ELUMO × 1.63 + 1.95 | (6) | 10 | 0.370 | 0.605 | 0.580 | 0.045 |
| Danio rerio | log LC50 = −Kow × 1.03 − ECCR × 1.04 + ELUMO × 0.318 + 2.94 | (7) | 12 | 0.558 | 0.722 | 0.630 | 0.046 |
| PFASs | CAS No. | Pseudokirchneriella subcapitata | Chlorella vulgaris | Daphnia magna | Danio rerio |
|---|---|---|---|---|---|
| PFBA | 375-22-4 | 67.1 | 112 | 37.4 | 1410 |
| PFOA | 335-67-1 | 478 | 150 | 570 | 98.5 |
| PFBS | 375-73-5 | 2840 | 222 | 487 | 1000 |
| PFHxS | 355-46-4 | 1030 | 258 | 821 | 256 |
| PFOS | 1763-23-1 | 53 | 309 | 173 | 61.3 |
| 6:2 Cl-PFESA | 73606-19-6 | 1.3 | 84.9 | 10.9 | 32.7 |
| PFBA | PFOA | PFOA (Measured) | PFBS | PFHxS | PFOS | PFOS (Measured) | 6:2 Cl-PFESA | |
|---|---|---|---|---|---|---|---|---|
| HC5 (mg/L) | 4.02 | 31.4 | 27 | 50.5 | 64.5 | 10.5 | 8.72 | 1.27 |
| PNEC (mg/L) | 0.804 | 6.27 | - | 10.1 | 12.9 | 2.09 | - | 0.254 |
| Sample Sites | RQ Values | PFBA | PFOA | PFBS | PFHxS | PFOS | 6:2 Cl-PFESA |
|---|---|---|---|---|---|---|---|
| This Study | Range | 11.5–60.9 | 3.25–15.8 | 9–20 | 0.23–1.83 | 15.3–297 | 5.1–49.8 |
| Mean | 29.1 | 7.26 | 13.1 | 0.71 | 121 | 19.1 | |
| Gaoping [43] | Range | 2.44–24.1 | 0.18–2.81 | 0–0.6 | 0.04–0.48 | 0–7.66 | 0–1.38 |
| Mean | 11.1 | 0.57 | 0.21 | 0.23 | 1.07 | 0.12 | |
| Humen [43] | Range | 2.44–42.3 | 0.53–3.24 | 0.13–1.24 | 0–27,000 | 0–4.41 | 0–3.82 |
| Mean | 24.7 | 1.42 | 0.64 | 9290 | 1.29 | 0.39 | |
| Boluo [43] | Range | 16.2–54.3 | 0.064–2.99 | 0–14.9 | 0–1.87 | 0–15.6 | 0–1.26 |
| Mean | 24.3 | 0.76 | 4.17 | 0.31 | 2.03 | 0.16 | |
| Shatian [43] | Range | 22–105 | 0.99–6.98 | 0.23–4.7 | 0–0.3 | 0–6.44 | 0 |
| Mean | 38.9 | 3.4 | 1.7 | 0.2 | 1.57 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, M.; Tao, H.; Qi, G.; Guo, W.; Ge, H.; Shi, J. A QSAR–ICE–SSD Model Prediction of the PNECs for Per- and Polyfluoroalkyl Substances and Their Ecological Risks in an Area of Electroplating Factories. Molecules 2021, 26, 6574. https://doi.org/10.3390/molecules26216574
Zhang J, Zhang M, Tao H, Qi G, Guo W, Ge H, Shi J. A QSAR–ICE–SSD Model Prediction of the PNECs for Per- and Polyfluoroalkyl Substances and Their Ecological Risks in an Area of Electroplating Factories. Molecules. 2021; 26(21):6574. https://doi.org/10.3390/molecules26216574
Chicago/Turabian StyleZhang, Jiawei, Mengtao Zhang, Huanyu Tao, Guanjing Qi, Wei Guo, Hui Ge, and Jianghong Shi. 2021. "A QSAR–ICE–SSD Model Prediction of the PNECs for Per- and Polyfluoroalkyl Substances and Their Ecological Risks in an Area of Electroplating Factories" Molecules 26, no. 21: 6574. https://doi.org/10.3390/molecules26216574
APA StyleZhang, J., Zhang, M., Tao, H., Qi, G., Guo, W., Ge, H., & Shi, J. (2021). A QSAR–ICE–SSD Model Prediction of the PNECs for Per- and Polyfluoroalkyl Substances and Their Ecological Risks in an Area of Electroplating Factories. Molecules, 26(21), 6574. https://doi.org/10.3390/molecules26216574






