Abstract
Many Fusarium species are pathogenic, causing crop diseases during crop production and spoilage of agricultural products in both commercial and smallholder farming. Fusarium attack often results into food contamination, yield loss and increases in food insecurity and food prices. Synthetic fungicides have been used as a control strategy for the management of crop diseases caused by Fusarium pathogens. The negative effects associated with application of many synthetic pesticides has necessitated the need to search for alternative control strategies that are affordable and environmentally safe. Research on medicinal plants as control agents for Fusarium pathogens has received attention since plants are readily available and they contain wide variety of secondary metabolites that are biodegradable. The activities of solvent extracts, essential oils and compounds from medicinal plants have been tested against Fusarium phytopathogenic species. A summary of recent information on antifungal activity of plants against Fusarium species is valuable for the development of biopesticides. This paper reviews the antifungal research conducted on medicinal plants against Fusarium pathogens, over a 10-year period, from January 2012 to May 2021. We also highlight the challenges and opportunities of using natural products from medicinal plants in crop protection. Several databases (Science Direct and Web of Science) were used to obtain information on botanical products used to control Fusarium diseases on crops. Keywords search used included natural products, antifungal, Fusarium, crops diseases, phytopathogenic, natural compounds and essential oil.
Keywords:
Fusarium; medicinal plants; antifungal; isolated compounds; extracts; essential oils; crop diseases 1. Introduction
The genus Fusarium is among the largest fungal genera consisting of pathogenic and non-pathogenic species [1]. Although discovered over more than 200 years ago, the genus remains taxonomically complex [2]. The pathogenic Fusarium species are well known to consist of agriculturally important crop pathogens, mycotoxin producers and opportunistic human pathogens [3]. The members of this genus have been isolated from plant materials and soil as pathogens, ascomycetes, endophytes and saprobes [4,5]. Various members of Fusarium genus are known to cause diseases in crops, including maize, wheat, rice, potatoes, tomatoes, beans, sorghum, banana, sugar cane, mangoes and other economically important crops [6]. The growth and development of Fusarium pathogens depend on factors such as seasons, climatic conditions (temperature and humidity) and geographical locations [7,8].
Fusarium fungal pathogens such as F. graminearum, F. moniliforme, F. oxysporum and F. verticillioides are known to infect cereal crops, fruits and vegetables (Table 1). They cause diseases that include head or seed blights, vascular wilts, pokkah boeng, bakanae, panama disease, stem, ear, crown and root rots [9,10,11,12,13,14]. The diseases can cause devastating economic yield loss in the field and during post-harvest storage, and result in a greater impact on food insecurity. Fusarium species are also more prevalent and major causes of quality deterioration of fruit and vegetables. Fusarium diseases may initiate in the roots from soil-borne spores/inoculum or on the above-ground parts of the crop, introduced through air, water or agricultural equipment [15,16]. The pathogens can also infect crops via injuries made by emerging roots, insects, nematodes and other environmental factors, resulting in disease symptoms such as wilting, necrosis and chlorosis [17,18].
The economic damage caused by Fusarium species is through their direct attack of crops in the fields and by the production of allergenic compounds and mycotoxins, which contaminate commodities during post-harvest storage. They produce fungal secondary metabolites such as deoxynivalenol, nivalenol, diacetoxyscirpenol, zearalenone, fusaric acid and fumonisins, all of which are harmful to humans and livestock [19,20,21,22,23]. Mycotoxin contamination is a major food safety concern in many parts of the world, with an estimate of almost 25% of the world’s crops being affected [24,25]. Consumption of food products contaminated with mycotoxins is associated with health risks such as oesophageal cancer, carcinogenesis, mutagenicity and neural tube defects [26,27]. The world’s population is estimated to increase to more than 10 billion by 2050, and this will put more pressure on farmers to produce more nutritious and safe food [28]. On the other hand, climate change, drought, pests and diseases remain major factors affecting current food production systems [28,29].
Due to the economic impact of crop diseases in agriculture and the effect of mycotoxins on food safety and international trade, the Fusarium genus remains the focus of many studies [24]. It is clear that Fusarium crop diseases cause deleterious effect on crop production and quality; therefore, effective and safe control measures that are sustainable must be developed and implemented. An estimated 2 billion people amounting to a quarter of the global population were affected by moderate-to-severe food insecurity in 2019 [30], a condition that has been exacerbated by the recent global coronavirus pandemic. Thus, discovering sustainable, safe and effective control strategies for controlling crop diseases remains imperative towards achieving the second goal, amongst others, of the Sustainable Development Goals (SDGs), which is ‘’to end hunger, achieve food security and improved nutrition and promote sustainable agriculture’’. This review provides an overview of current research activities from 2012, as well as the challenges and prospects of developing natural products from medicinal plants as a source of biopesticides to control phytopathogenic Fusarium species against the backdrop of using synthetic chemicals.

Table 1.
The most common Fusarium species known to infect cereal crops, fruits and vegetables.
Table 1.
The most common Fusarium species known to infect cereal crops, fruits and vegetables.
| Pathogen | Crop | Common Disease | Reference |
|---|---|---|---|
| Fusarium acuminatum | kiwifruit | post-harvest rot | [31] |
| Fusarium asiaticum | soybean | head blight or ear rot | [32] |
| Fusarium avenaceum | wheat, beans, maize | head blight or ear rot | [33,34,35] |
| Fusarium boothii | wheat, maize | head blight or ear rot | [36] |
| Fusarium crookwellense | wheat, potatoes | ear rot, head blight, dry rot | [37,38] |
| Fusarium culmorum | wheat | seedling blight, ear blight, stalk rot | [35] |
| Fusarium equiseti | wheat, barley | crown rot, damping-off | [39] |
| Fusarium falciforme | bean | wilt disease, necrosis | [40] |
| Fusarium fujikuroi | rice | bakanae disease | [41] |
| Fusarium graminearum | wheat, corn | Fusarium head blight | [35] |
| Fusarium kuroshium | avocado tree | Fusarium dieback | [42] |
| Fusarium kyushuense | tobacco | Fusarium wilt | [43] |
| Fusarium langsethiae | oats, wheat, barley | Fusarium head blight | [44] |
| Fusarium nivale | wheat, rye | seedling blight, Fusarium head blight | [45] |
| Fusarium nygamai | corn, rice, sorghum, bean, cotton | seedling blight, foot rot | [46] |
| Fusarium oxysporum | Tomato, cucumber, watermelon | vascular wilt | [47] |
| Fusarium poae | wheat | Fusarium head blight | [33,34,35] |
| Fusarium proliferatum | wheat, maize, onion, soybean | necrotic leaf, bulb rot, root rot, ear rot diseases | [48,49,50] |
| Fusarium sambucinum | potato | sprout rot, dry rot | [51] |
| Fusarium semitectum | pineapple, okra, bitter gourd, cucumber, green chill | fusariosis, fruit rot | [52,53] |
| Fusarium solani | peas, soybean, beans, potatoes | stem rot, stem rot, dry rot | [54] |
| Fusarium sporotrichioides | wheat, cereals | Fusarium head blight | [55] |
| Fusarium subglutinans | maize, mango, pineapple, pine, sorghum | pitch canker, | [56,57] |
| Fusarium sulphureum | potato | dry rot | [58,59] |
| Fusarium thapsinum | sorghum, banana, maize, peanut, soybean | stalk rot | [60] |
| Fusarium tricinctum | cereal | root rot disease, Fusarium head blight | [61,62] |
| Fusarium verticillioides | maize, wheat, corn | ear and stalk rot | [63,64,65,66,67] |
2. Environmental and Health Implications of Fusarium Control in Crop Production Using Synthetic Chemicals
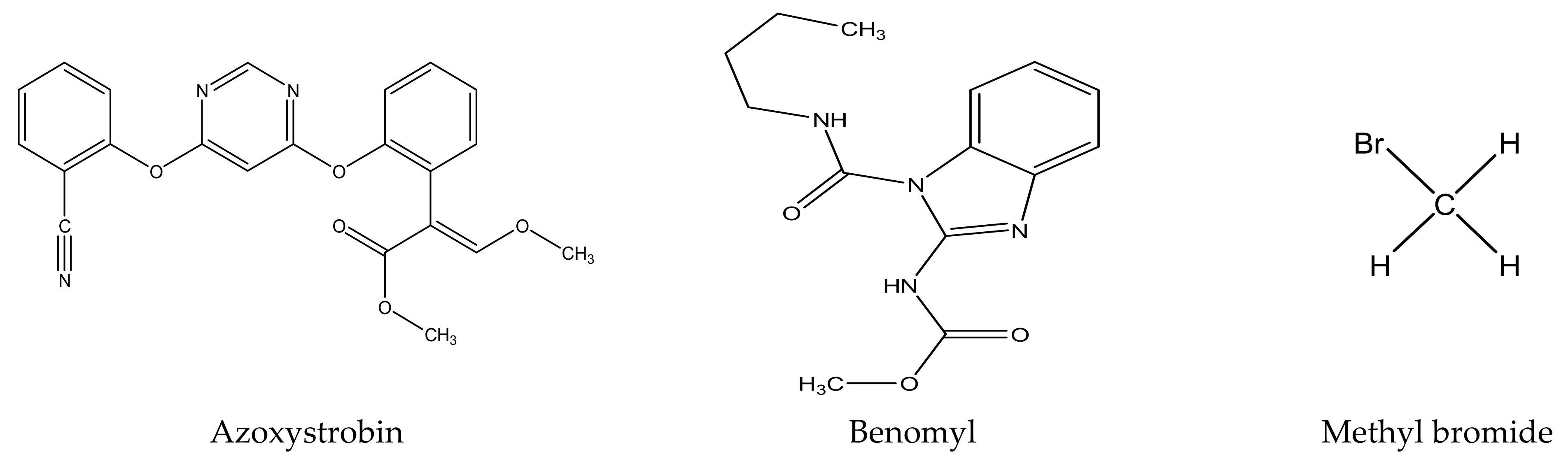
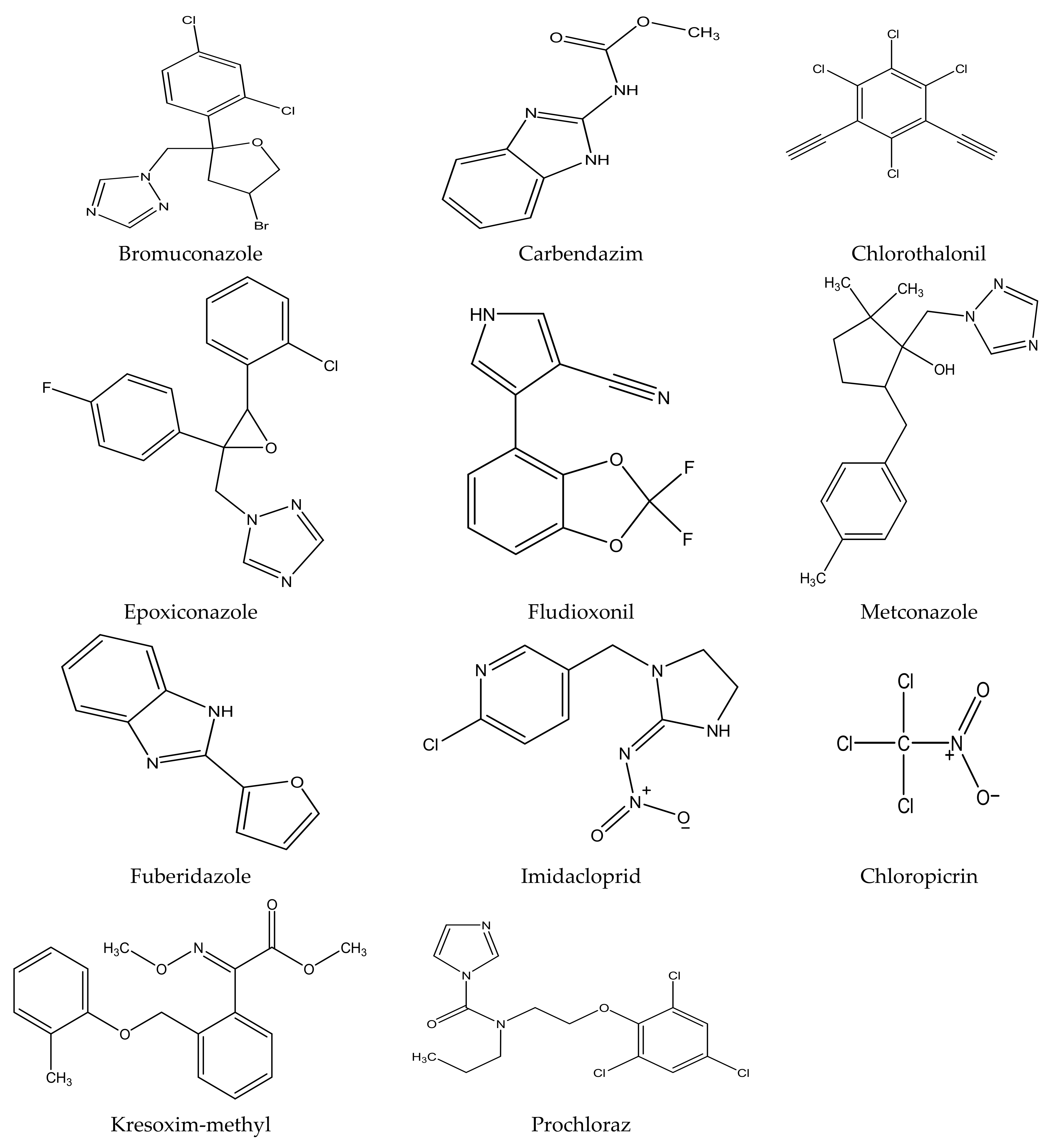
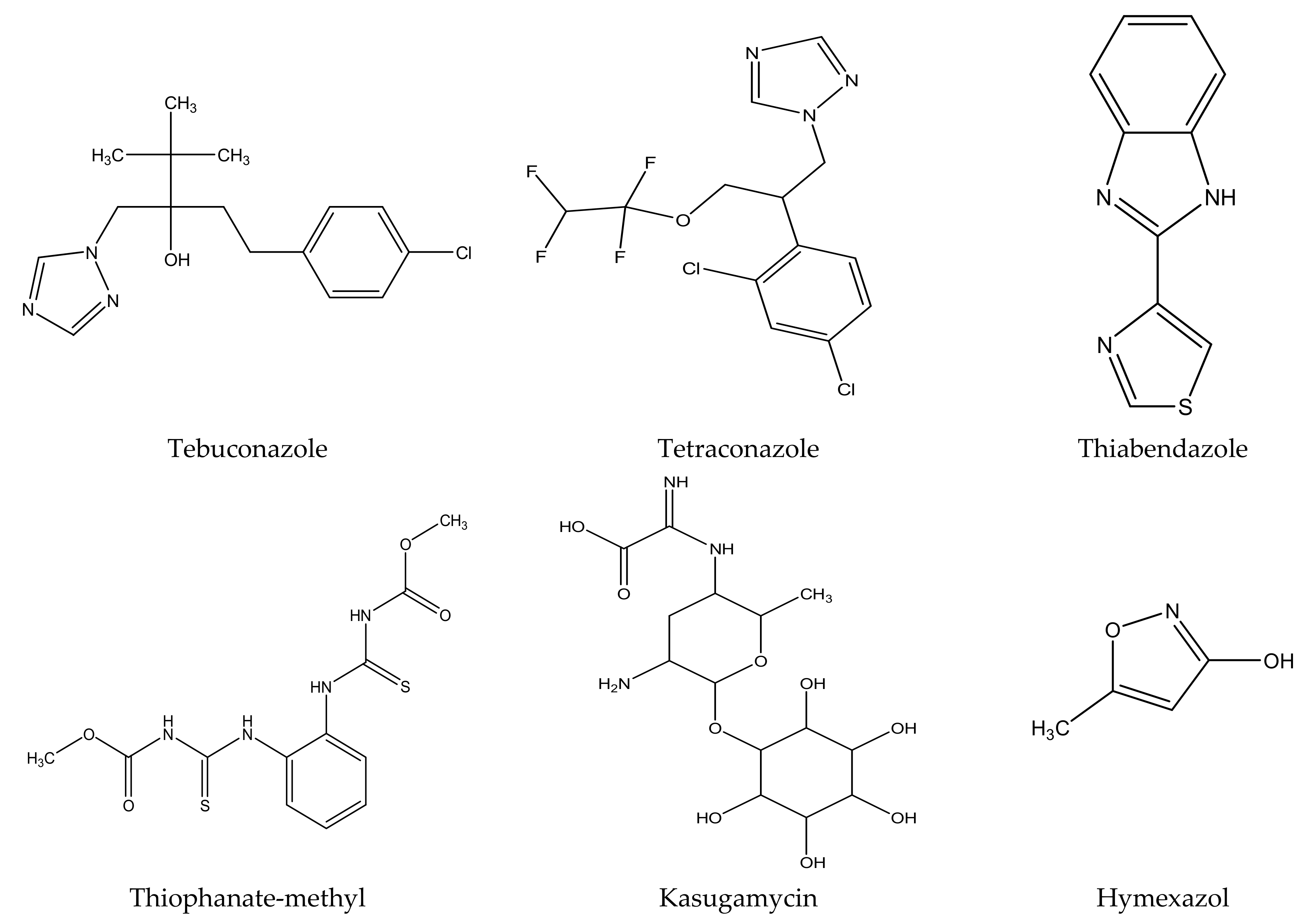
There are several strategies already used in crop production to control crop diseases caused by Fusarium species [68,69]. Historically, the application of synthetic pesticides remains the primary strategy to control diseases, which have benefited commercial farmers since the first fungicides were introduced in the 1800s. Random chemical synthesis and evaluation of the activity against phytopathogenic species has resulted in many agrochemicals in different parts of the world. The introduction of synthetic pesticides has reduced the effect of many crop diseases in agricultural production including those that are caused by Fusarium pathogens, and it remains a key component of disease management worldwide, particularly in developing countries [69,70]. Chemical control methods are preferred in commercial crop production due to their effectiveness to also control soil-borne crop pathogens and the availability of spraying technology for easy application. Figure 1 presents the structures of few synthetic fungicides used to control Fusarium pathogens [35,47,71,72,73,74,75,76]. The chemicals were formulated to be applied as fruit and seed treatments, fumigants or in foliar applications.
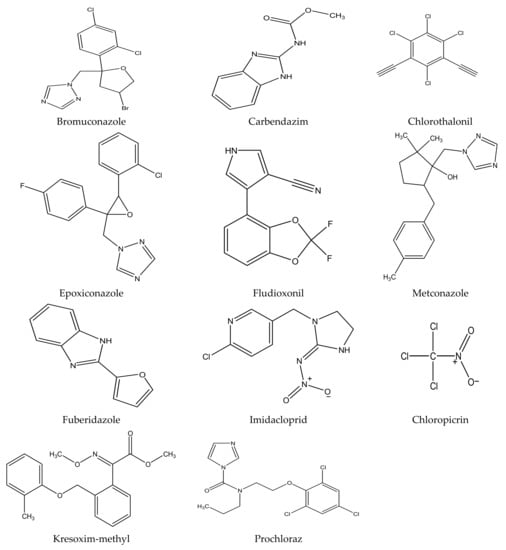
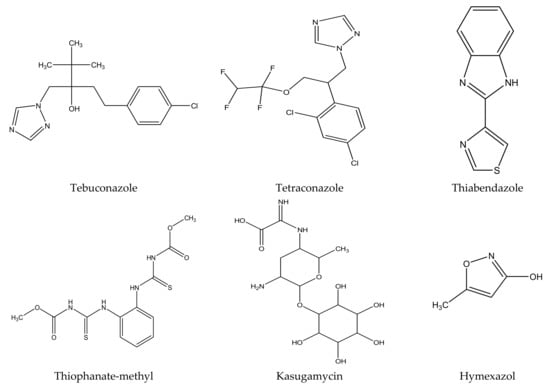
Figure 1.
Conventional synthetic fungicides used to control crop diseases caused by phytopathogenic Fusarium species.
Although synthetic fungicides have benefited crop production for decades, nowadays, the use of such chemicals is restricted or discouraged for several reasons. The overapplication or misuse of synthetic fungicides has raised serious concerns including their impact on the environment, contamination of drinking water and the effect on human health and livestock [77,78,79,80,81]. Generally, pesticides are known to affect soil microorganisms (often the untargeted species), and sometimes lead to an imbalance in the ecosystem [82,83,84]. The application of methyl bromide in the soil was a common sterilization practice in agriculture to control Fusarium species and other soil pests [85]. Methyl bromide was used as a versatile, single treatment and long-lasting soil fumigant with relatively no soil residue to sterilize soil before planting, as it controlled weeds, nematodes and almost all living organisms in the soil [86,87]. Being a very volatile gas, it usually ends up in the air causing smog as well as thinning of the protective ozone layer in the stratosphere [86,87]. Methyl bromide is categorized as a substance that causes ozone layer damage [85] and its use is banned under the Montreal Protocol international treaty to protect the ozone layer [86,87] Methyl bromide is also toxic and several studies have indicated its neurological effects in humans and resultant severe lung injuries [85].
Apart from environmental and human health challenges as a result of synthetic fungicides, farmers have been struggling with emergence of resistance against some commonly known fungicides since the 1970s [88,89]. As an example, thiabendazole, which was one of the most effective fungicides against a wide variety of pathogens, is no longer an effective treatment. However, some farmers are still using it in combination with other chemicals to control dry rot diseases. Carbendazim is another kind of fungicide that is no longer readily available on the market due to resistance concerns, and this fungicide is believed to be banned in some countries including in the European Union (EU) countries [90,91,92]. Fungicide poisoning to farmers is a common problem in many countries, especially in developing countries [93,94,95]. Although the World Health Organization (WHO) has regarded fludioxonil as a pesticide that does not cause hazard in normal use, its manufacturer specified that fludioxonil is moderately toxic against Oncorhynchus mykiss (Rainbow trout), daphnia and other aquatic invertebrates [96,97,98].
Other fungicides such as chloropicrin do not persist in the environment for a long period of time; however, vapour or toxic gases produced during decomposition of chloropicrin can cause severe headaches, pulmonary oedema and may have adverse effects on the nervous system [99]. Fungicides in the azole chemical class such as benzimidazoles are very successful in the treatment of many crop diseases worldwide [100]; however, they are predisposed to the emergence of resistance by crop pathogens. Nowadays, in order to minimize or delay resistance, azole fungicides are usually applied as a mixture with other fungicides such as benomyl [101]. However, it is noteworthy that the use of benomyl has been restricted in Sweden and New Zealand since 1982 [99]. On the other hand, the WHO justified benomyl as a moderately safe fungicide against mammals, whilst other international institutions in the United States of America have categorized benomyl as a teratogenic and carcinogenic chemical [99]. All these challenges have negatively affected the market and availability of fungicides used to control crop diseases, mostly in commercial farming. Additionally, synthetic fungicides are not recommended for application in organic farming system, and consumers are willing to pay more for food or crops that are produced organically [102]. This already demarcates the society and puts more financial pressure on the poorest; hence, there has been an increase in food insecurity. Furthermore, synthetic fungicides are not readily available and/or affordable to small-holder farmers. This kind of farming is largely practiced in poor resourced communities; however, it is still a source of food and income generation for many households [103,104].
In small-holder farming, crops and vegetables are in most cases collected and consumed upon harvest. After harvest, the surplus grains and vegetables are stored and consumed during the off-season. This practice makes it impractical to apply synthetic fungicides both in the field and during post-harvest storage. To make matters worse, synthetic fungicides may be adulterated by unscrupulous traders and their incorrect use by illiterate farmers might result in poisoning and increase in pathogen resistance [105,106,107,108,109]. In the light of the highlighted challenges, there is a pressing need to search for alternative, less expensive/affordable, safer and environmentally friendly fungicides to control Fusarium pathogens and other pests in crop production. The search for applicable medicinal and aromatic plant species has attracted increasing attention in an effort towards the development of safer biopesticides.
3. The Potential of Natural Products from Medicinal Plants for Controlling Fusarium Pathogens
The control of pests using plant products was practiced as part of indigenous knowledge systems until technology took over and synthetic pesticides were developed and embraced quickly, because they were able to control many crop diseases successfully [110]. As a result, indigenous applications of plant products faded until researchers became aware of the harmful effect of synthetic pesticides on human health and the environment. Medicinal plant species have a long history of use by many ethnic groups for the treatment of various diseases in both humans and domestic animals [111,112]. Nevertheless, medicinal plant species have demonstrated the potential to be used as fungicides in the agricultural sector to protect crops against pathogens [112,113,114,115]. The idea behind the discovery of fungicides from plant species is based on their ability to synthesize diverse arrays of secondary metabolites or compounds, which function to defend the plant against microbes, insects and herbivores [116,117].
The use of plant products against fungal pathogens may inhibit the development of resistance due to the presence of different constituent antimicrobial compounds and their synergisms [118,119]. Products from medicinal plant species are arguably relatively safe, show low human toxicity and are eco-friendly [120]. They are easily biodegradable because natural products particularly from plants are inherently unstable with elevated temperatures and, consequently, they do not persist in the environment for a long time compared to conventional synthetic fungicides [80]. Nonetheless, it is important to evaluate the safety or toxicity and environmental fates of every alternative fungicide including biopesticides from medicinal plants. Biopesticides may produce residues and become toxic; hence, their maximum residue level in crops and animal products need to be established during the registration process [121]. Plant-based fungicides may be developed as products from the leaves or any part of the plant and used as essential oils, extracts or isolated compounds formulated into standardised products.
Reducing the use of conventional synthetic fungicides in the presence of effective natural products is a vital step towards sustainable crop production. In the following subsections, we review some studies conducted in the past 10 years on antifungal activity of plant extracts, essential oils and compounds isolated from plants against phytopathogenic Fusarium species.
Plant Extracts, Essential Oils and Compounds with Antifungal Activity
Medicinal plant extracts have attracted attention in the pesticide industry as potential agents to control crop diseases in the field and during post-harvest storage. This is based on their antimicrobial properties due to spectrum of their constituent secondary metabolites such as phenols, polyphenols, flavonoids, glycosides, tannins, alkaloids and other compounds [122,123]. Table 2 shows the activity of extracts from some plant species evaluated for antifungal activity against phytopathogenic Fusarium species. Different solvent extracts obtained from 47 plant species belonging to 30 families were documented. The families with high frequencies of evaluated species against Fusarium pathogens were Solanaceae (with six species), followed by Combretaceae and Fabaceae (with four species each), and Euphorbiaceae (with three species). Plants in the Solanaceae family that were evaluated include Nicotiana glauca, Solanum aculeastrum, Solanum mauritianum and Solanum seaforthianum. Leaf extracts from these plants demonstrated potent in vitro activities (minimum inhibitory concentrations <1.0 mg/mL) against nine Fusarium species (Table 2). The Solanum species are regarded as invasive weeds, for which renewed biological control research has been advocated [124]. Their alternative use in the control of Fusarium pathogens could be beneficial for controlling their invasiveness. Extracts from species belonging to the Combretaceae and Fabaceae families similarly demonstrated potent activities against Fusarium species. While extracts could be prepared from different plant parts including roots, stems and leaves, most of the documented studies focused on leaf extracts. The use of leaves is particularly sustainable from a conservation point of view, as leaves are a renewable part that can be sustainably harvested without threatening plant growth and survival.
An important parameter to be considered is the choice of extraction solvents. In general, acetone, ethyl acetate, petroleum ether, chloroform, ethanol, methanol and water are commonly used for the extraction of various secondary metabolites from plants. Organic solvents such as acetone, ethyl acetate and petroleum ether demonstrated stronger antifungal activity against some Fusarium pathogens when compared to water extract obtained from the same plant species [125]. This observation correlated with the findings from several authors who reported that aqueous extract generally exhibited little or no antimicrobial activity compared to non-polar extracts [126,127,128]. This might be due to lower solubility of medicinal plant antifungal compounds in polar solvents as compared to non-polar solvents [129]. The polarity of constituent metabolites differs significantly and has influence on their solubility during extraction and thereafter in the antifungal activity of the extracts. On the other hand, the use of water extract would be applicable to resource-poor farmers since water is readily available; therefore, small-holder farmers can prepare crude plant extracts themselves. Bioactive water extracts are also particularly applicable in organic farming. Following the individual evaluation of plant extracts, a combination of bioactive plant extracts could result in stronger in vitro and in vivo antifungal activities due to possible synergistic antifungal activities of their constituent metabolites [130,131]. Solvents of different polarities may also be combined at varied ratios for improving extraction efficiency of bioactive constituents that may act synergistically. However, there remains a paucity of information on the combinational activity of plant extracts against plant pathogens as well as in vivo evaluation of bioactive extracts, which are important steps in developing plant-based biopesticides.
Several studies evaluated plant extracts against different Fusarium pathogens such as F. verticilloides, F. proliferatum, F. oxysporum and F. solani, all of which are known to infect cereals, fruits and vegetables. Fusarium oxysporum was the most frequently used pathogen (43 times) followed by F. graminearum and F. verticilloides, which were each used 23 times in the reported studies (Table 2). The least used pathogen was F. semitectum. Although the selection of Fusarium pathogen(s) for screening against plant extracts depends on many factors including the availability of pathogens and the target diseases to be controlled, the inclusion of multiple pathogenic strains in the screening process is more advantageous. The use of Fusarium pathogens with different morphological structures and defence mechanisms can help to discover active plant extracts against a wide spectrum of Fusarium pathogens. This approach could be beneficial for developing a biopesticide to manage different crop diseases caused by Fusarium pathogens.
The choice of assays used in evaluating medicinal plant extracts remains important to ensure the validity of extract potential. There are different screening methods or assays used to evaluate antifungal activity of plant extracts. The most common ones include microplate dilution and disk diffusion assays, with the microplate dilution assay being the most frequently used to evaluate antifungal activity of plant extracts against Fusarium pathogens (Table 2). The use of the agar diffusion method in determining antimicrobial activity of plant extracts is discouraged due to its pitfalls, including reproducibility issues between different laboratories and diffusion challenges with extracts of different polarities (particularly non-polar extracts) [132]. The measurement of the zone of inhibition depends on different factors such as the concentration and volume of test extracts, inoculum size and agar medium volume, amongst others, all of which make it difficult, if not impossible, to effectively compare antimicrobial activities reported as the inhibition zone of different extracts tested in different laboratories [132]. The use of an appropriate positive control is well known as a critical factor in validating antimicrobial assays [132]. Although other fungicides such as nystatin and ketoconazole may be used as a positive control, amphotericin B was used in most studies (Table 2). Of the studies consulted during the compilation of this review, at least 39 out of 51 studies included amphotericin B as a positive control. Compared to other fungicides, amphotericin B is easy to handle and store. Nevertheless, a number of studies evaluating the activity of plant extracts were conducted without including any positive control required to validate the experiment. In some other cases where a positive control was included in the experiments, the antifungal activity of the positive control was not reported. Antifungal activity studies without the use of any positive control raise validity concerns. The inclusion of the antifungal activity of standard positive controls can help to benchmark the antifungal potency of extracts and be used for inter-laboratory comparisons.
As presented in Table 2, the antifungal activity of plant extracts was expressed in terms of minimum inhibitory concentration (MIC), half-maximal inhibitory concentration (IC50) or percentage inhibition. Plant extract activities are usually dose dependent. Therefore, studies reporting percentage inhibition without specifying the concentration of the extract corresponding to such activity are of little value. Stating the antimicrobial activities of plant extracts in terms of their minimum inhibitory concentrations (MICs) is generally accepted as a minimum standard for reporting antimicrobial activity results [132]. Crude solvent extracts exhibiting MICs that are less than 1 mg/mL are generally regarded as having active/potential antimicrobial activity [133]. As shown in Table 2, extracts obtained from plant species such as Milletia grandis, Solanum panduriforme and Ziziphus mucronata demonstrated antifungal activity with a MIC value equal to or less than 0.01 mg/mL. Various extracts from Combretum caffrum, C. erythrophyllum, C. molle, Harpephyllum caffrum, Lantana camara, Melia azedarach, Nicotiana glauca, Olea europaea, Passiflora suberosa, Quercus acutissima, Senna didymobotrya, Solanum aculeastrum, Solanum mauritianum, Vangueria infausta, Waburgia salutaris and Withania somnifera demonstrated potent activities (with a MIC less than 1.0 mg/mL) against a number of Fusarium pathogens (Table 2). These plant extracts should be investigated further in vivo as part of efforts geared towards finding potential plant extracts to be developed into biopesticide products.
Few products developed from plants for application in crop protection are available on the market. Products such as Vertigo® made from the seeds of Cassia obtusifolia, Milsana® from Reynoutria sachlinesis and Owel® made from an extract of Macleaya cordata are among good examples of natural products developed from botanicals and registered for application in crop protection [134,135]. Other botanical products available on the market for the treatment of plant diseases, particularly during post-harvest storage, include NeemPro® and NeemAzal®. These products were reported to be successful as maize seed treatment agents [136,137]. The availability of such products indicates the possibility for formulating plant-based extracts against plant diseases caused by pathogenic Fusarium species.
Essential oils contain a mixture of different compounds such as monoterpenes, diterpenes, sesquiterpenes, aliphatic and other aromatic compounds that are volatile in nature [138,139,140]. Naturally, essential oils are usually obtained from medicinal plants, herbs, spices and aromatic plant species [141]. Different plant materials or parts including the flowers, leaves, barks, roots, seeds, fruits and whole plants can be utilized, depending on the plant species, for the extraction of essential oils [142,143]. They are commonly extracted by steam distillation or hydrodistillation process [144]. Essential oils are reputably used in traditional medicine, pharmaceutical, cosmetic and food industries [145,146]. Some oils are widely used as food preservatives, food flavours, appetizer promoters and perfumes [145,146].
The interest in the use of essential oils is due to their unique and excellent properties. Many studies have demonstrated antimicrobial activities, antioxidant activities, antiparasitic and insecticidal activities of essential oils [147,148,149,150,151]. Furthermore, essential oils have been investigated as control agents against growth of moulds and aflatoxin production [152,153,154,155]. Essential oils of some medicinal plant species were shown to be potential eco-friendly biocontrol agents [151,156]. These metabolites or substances can lead to new and different classes of botanical pesticides that may be used to control crop diseases including those caused by phytopathogenic Fusarium species. The application of essential oils against crop diseases is considered as a safe strategy to protect crops against pathogens. Because of their safety, the Federal Drug Administration (FDA) and Environmental Protection Agency (EPA) have allowed the use of certain essential oils in food [142,157]. Essential oils may be applicable in controlling post-harvest storage diseases. In addition to human safety, essential oils are fast or easily degraded in the environment and have low toxicity to non-target animals [158]. Thus, several studies have evaluated antifungal activity of essential oils obtained from different medicinal plant species against several Fusarium pathogens (Table 3). As indicated in Table 3, the essential oils from species belonging to the Lamiaceae, followed by the Apiaceae, Asteraceae and Myrtaceae plant families, were the most frequently evaluated against different Fusarium species. Essential oils from 26 species within the Lamiaceae family demonstrated various levels of activity against Fusarium pathogens. Essential oils from the genera Origanum and Thymus were the most utilized, followed by Zataria multiflora, Melaleuca alternifolia and Cymbopogon citratus. The very potent activities, based on the MIC values, reported in essential oils from Artemissia sieberi (MIC of 20 µg/mL against F. solani) and Thymus kotschyanus (MIC of 0.5 µg/mL against F. oxysporum) are particularly noteworthy. In Table 3, different methods used to evaluate activity of the essential oils were noted. Agar dilution, disc diffusion and microplate dilution methods were the most frequently used methods. The antifungal activity of essential oils was reported in a similar fashion as crude extracts (MIC values, IC50 values or percentage inhibition). Although there is no clear specified value used for classification to define whether an essential oil is highly active against Fusarium pathogens, the lower the MIC value, the higher the potency. The lack of a standardised assay method and reporting of results presents a challenge for effective comparison of the reported activities. Some of the assays were done without the use of appropriate controls, making it difficult to establish the validity of the assays used. Reporting of antifungal data without the use of positive control remains a challenge. About 40 experimental studies conducted to evaluate the activity of essential oils against Fusarium species were reported without a positive control (Table 3). Synthetic fungicide (fluconazole) was the mostly used positive control. Nonetheless, the recorded potent antifungal activity at low concentrations against some Fusarium species demonstrates the potential of developing biopesticides of plant origins. Further studies evaluating their in vivo potency against pathogenic Fusarium species are warranted. The plausible effectiveness of combining essential oils in developing suitable plant-based formulations merits scientific attention.
Medicinal plants are sources of bioactive secondary metabolites. These compounds belong to different chemical classes and have different structures. Of the plant families studied for the isolation of active compounds against Fusarium pathogens, Asteraceae was the most common, followed by Combretaceae and Zygophyllaceae. Compounds isolated from Artemisia annua were the most studied secondary metabolites against Fusarium pathogens (Table 4). These compounds were isolated from the leafy part of the plant. Bioactive compounds from medicinal plants are often present in very low amounts and may be difficult to purify on a large scale. However, they can be isolated, purified and characterized. The structures of isolated bioactive compounds may be used as a template during commercial production of biopesticides. Table 4 presents examples of isolated compounds from medicinal plants that demonstrated antifungal activity against several Fusarium pathogens. A number of isolated compounds showed strong potency (with minimum inhibitory concentration <20 µg/mL). Compounds isolated from medicinal plants are considered noteworthy when their reported minimum inhibitory concentration is less than 1 mg/mL [159]. Therefore, the isolated compounds reported in Table 4 demonstrated remarkable antifungal activity against a number of Fusarium pathogens.

Table 2.
Medicinal plants evaluated for antifungal activity against Fusarium phytopathogenic species. The plant extracts were evaluated using different screening methods/assays, and their antifungal activities were reported in terms of minimum inhibitory concentration (MIC) or percentage inhibition values.
Table 2.
Medicinal plants evaluated for antifungal activity against Fusarium phytopathogenic species. The plant extracts were evaluated using different screening methods/assays, and their antifungal activities were reported in terms of minimum inhibitory concentration (MIC) or percentage inhibition values.
| Plant Species (Family) | Solvents/Plant Parts Used | Method | Organism Tested | Positive Control | Activity of Positive Control | Results | References |
|---|---|---|---|---|---|---|---|
| Aconitum laeve Royle (Ranunculaceae) | Chloroform/tubers | poisoned food technique | F. oxysporum | Not stated | Not stated | Inhibition of 58.73 at 300 mg/mL | [160] |
| Annona squamosa L. (Annonaceae) | Methanol; Chloroform; Aqueous/leaf | broth dilution method | F. solani | 100 mg/mL ketoconazole | Not stated | MIC value of 600; 300; 800 µg/mL | [161] |
| Aristolochia elegans Mast (Aristolochiaceae) | Acetone/leaf | serial microdilution assay | F. oxysporum | amphotericin B | 7.5 µg/mL | MIC value of 0.08 mg/mL | [162,163] |
| Artemisia absinthium L. (Compositae) | Ethanol; Water/flowers | disk diffusion method | F. oxysporum | carbendazim | inhibition of 100% at 1% of the total volume | Inhibition of 65.69; 53.43 at 500 mg/L | [164] |
| Ethanol; Water/leaf | Inhibition of 62.69; 51.33 at 500 mg/L | ||||||
| Ethyl acetate; Ethanol/roots | Inhibition of 72.45; 64.63 at 500 mg/L | ||||||
| Asparagus officinalis L. (Asparagaceae) | Water | amended plate technique | F. oxysporum | Not stated | Not stated | Inhibition of 53.9 to 85.7 | [165] |
| Bauhinia galpinii N.E.Br. (Fabaceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.20 mg/mL | [166] |
| Hot water; Methanol: Dichloromethane (1:1)/leaf | microplate dilution method | F. graminearum | 0.004 mg/mL | MIC value of 0.30; 0.20 mg/mL | [167,168] | ||
| F. verticillioides | 0.006 mg/mL | MIC value of 3.13; 0.20 mg/mL | |||||
| F. oxysporum | 0.004 mg/mL | MIC value of 3.13; 1.56 mg/mL | |||||
| Breonadia salicina (Vahl) Hepper and J.R.I Wood (Rubiaceae) | Acetone; Hexane; Dichloromethane; Methanol/leaf | microplate method | F. oxysporum | amphotericin B | <0.02 mg/mL | MIC value of 0.32; 0.08; 0.16; 0.16 mg/mL | [115,169] |
| Bucida buceras L. (Combretaceae) | Acetone; Hexane; Dichloromethane; Methanol/leaf | microplate method | F. oxysporum | amphotericin B | MIC value of 0.02; 0.63; 0.32; 0.04 mg/mL | [115,169] | |
| Carpobrotus edulis (L.) N.E.Br. (Aizoaceae) | Hot water; Methanol: Dichloromethane (1:1)/leaf | microplate dilution method | F. graminearum | amphotericin B | 0.004 mg/mL | MIC value of 0.39; 3.13 mg/mL | [167,168] |
| F. verticillioides | 0.006 mg/mL | MIC value of 3.13; 0.10 mg/mL | |||||
| F. oxysporum | 0.004 mg/mL | MIC value of 3.13; 0.65 mg/mL | |||||
| Chromolaena odorata (L.) R.M.King & H.Rob. (Compositae) | Acetone/leaf | serial micro dilution assay | F. oxysporum | amphotericin B | 7.5 µg/mL | MIC value of 0.08 mg/mL | [162,163] |
| Combretum caffrum (Eckl. & Zeyh.) Kuntze (Combretaceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.31 mg/mL | [166] |
| Combretum erythrophyllum (Burch.) Sond. (Combretaceae) | Ethyl acetate; Acetone/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.04; 0.04 mg/mL | [131] |
| Water; Ethyl acetate; Acetone/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.31; 0.04; 0.04 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.16; 0.08; 0.04 mg/mL | ||||
| Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.16; 0.08 mg/mL | ||||
| Petroleum ether; Ethyl acetate; Acetone/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.04; 0.16; 0.04 mg/mL | [125] | |||
| Petroleum ether; Ethyl acetate; Acetone/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.63; 0.31; 0.31 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.63; 0.63; 0.04; 0.04 mg/mL | ||||
| Petroleum ether; Ethyl acetate; Acetone/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.04; 0.04; 0.08 mg/mL | ||||
| Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.04; 0.04; 0.08 mg/mL | ||||
| Combretum molle R. Br. ex G. Don (Combretaceae) | Ethyl acetate/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.61 mg/mL | [131] |
| Water; Ethyl acetate; Acetone/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04; 0.04 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.04; 0.04; 0.04 mg/mL | ||||
| Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.63; 0.63 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.63; 0.31; 0.16; 0.31 mg/mL | [125] | |||
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.31; 0.16; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.63; 0.04; 0.08; 0.04 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.63; 0.04; 0.04 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.63; 0.16; 0.63; 0.27 mg/ml | ||||
| Acetone; Ethyl acetate; Dichloromethane/leaf | serial microplate dilution method | F. oxysporum | Not stated | MIC value of 0.19; 0.21; 0.16 mg/mL | [170] | ||
| Euphorbia hirta L. (Euphorbiaceae) | Water; Ethanol/leaf | agar plate dilution method | F. oxysporum vasinfectum | Not stated | Not stated | IC50 of 12.38 mg/mL; MIC value of 0.31 mg/mL and IC50 of 2.93 mg/mL | [171] |
| Harpephyllum caffrum Bernh. (Anacardiaceae) | Water; Ethyl acetate/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.08; 0.08 mg/mL | [131] |
| Water; Ethyl acetate; Acetone/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04; 0.04 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.08; 0.04; 0.63 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.16; 0.08; 0.31 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.31; 0.16; 0.16 mg/mL | [125] | |||
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.31; 0.16; 0.31 mg/mL | ||||
| Water; Ethyl acetate/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.16; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 23.44 µg/mL | MIC value of 0.31; 0.08; 0.31; 0.78 mg/mL | ||||
| Acetone/leaf | microplate dilution method | F. verticilloides | 1.56 mg/mL | MIC value of 0.02 mg/mL | [166] | ||
| Acetone; Hexane; Dichloromethane; Methanol/leaf | microplate method | F. oxysporum | <0.02 mg/mL | MIC value of 0.32; 0.16; 0.04; 0.39 mg/mL | [115,169] | ||
| Hot water; Methanol: Dichloromethane (1:1)/leaf | microplate dilution method | F. graminearum | 0.004 mg/mL | MIC value of 0.20; 0.78 mg/mL | [167,168] | ||
| F. verticillioides | 0.006 mg/mL | MIC value of 0.20; 0.39 mg/mL | |||||
| F. oxysporum | 0.004 mg/mL | MIC value of 0.52; 0.24 mg/mL | |||||
| Ipomoea alba L. (Convolvulaceae) | Acetone/leaf | serial micro dilution assay | F. oxysporum | amphotericin B | 7.5 µg/mL | MIC value of 0.04 mg/mL | [162,163] |
| Lantana camara L. (Verbenaceae) | Water; Ethyl acetate; Acetone/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.16; 0.16; 0.04 mg/mL | [131] |
| Ethyl acetate; Acetone/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.16 mg/mL | ||||
| Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.04; 0.63 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.08; 0.63; 0.63 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.63; 0.31; 0.16 mg/mL | [125] | |||
| Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.31; 0.63 mg/mL | ||||
| Petroleum ether; Ethyl acetate/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.08; 0.04 mg/mL | ||||
| Water; Acetone/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.16; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.04; 0.04; 0.04; 0.39 mg/mL | ||||
| Maesa lanceolata Forsk (Primulaceae) | Hot water: Methanol: Dichloromethane (1:1)/leaf | microplate dilution method | F. graminearum | amphotericin B | 0.004 mg/mL | MIC value of 0.20; 0.78 mg/mL | [167,168] |
| F. verticillioides | 0.006 mg/mL | MIC value of 0.20; 0.78 mg/mL | |||||
| F. oxysporum | 0.004 mg/mL | MIC value of 0.26; 0.08 mg/mL | |||||
| Markhamia obtusifolia (Baker) Sprague (Bignoniaceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.31 mg/mL | [166] |
| Melia azedarach L. (Meliaceae) | Water; Ethyl acetate/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.16; 0.08 mg/mL | [131] |
| Water; Ethyl acetate/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.08 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.08; 0.04; 0.63 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.08; 0.16; 0.63 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.31; 0.16; 0.16 mg/mL | [125] | |||
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.16; 0.08; 0.16 mg/mL | ||||
| Petroleum ether; Ethyl acetate/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.31; 0.63 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.31; 0.63; 0.04; 0.08 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.16; 0.16; 0.08; 0.63 mg/mL | ||||
| Melianthus comosus Vahl. (Melianthaceae) | Carbon tetrachloride; Diethyl ether; Dichloromethane; Chloroform; Acetone; Ethanol; Ethyl acetate/leaf | serial microdilution assay | F. oxysporum | Not stated | Not stated | MIC value of 0.63; 0.63; 0.16; 0.16; 0.04; 0.08; 0.78 mg/mL | [172,173] |
| Milletia grandis (E. Mey) Skeels (Fabaceae) | Hot water; Methanol: Dichloromethane (1:1)/leaf | microplate dilution method | F. graminearum | amphotericin B | 0.004 mg/mL | MIC value of 0.01; 0.78; mg/mL | [167,168] |
| F. verticillioides | 0.006 mg/mL | MIC value of 0.10; 0.65 mg/mL | |||||
| F. oxysporum | 0.004 mg/mL | MIC value of 0.01; 0.01 mg/mL | |||||
| Methanol: Dichloromethane (1:1)/leaf | Not stated | F. graminarium | Not stated | Not stated | MIC value of 0.01 mg/mL | [174] | |
| Not stated | F. oxysporum | Not stated | Not stated | MIC value of 0.39 mg/mL | [174] | ||
| Momordica charantia Linn. (Cucurbitaceae) | Seed | Not stated | F. solani | Not stated | Not stated | MIC value of 0.08 mg/mL and Inhibition of 57.216 at 125 µg/mL | [175,176] |
| Mystroxylon aethiopicum (Thunb.) Loes (Celastraceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.16 mg/mL | [166] |
| Nicotiana glauca Graham (Solanaceae) | Water; Ethyl acetate/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.04; 0.16 mg/mL | [131] |
| Water; Ethyl acetate/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.16; 0.08; 0.63 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.16; 0.16; 0.08 mg/mL | ||||
| Olea europaea L. (Oleaceae) | Water; Ethyl acetate; Acetone/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.16; 0.16; 0.04 mg/mL | [131] |
| Water; Ethyl acetate/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04 mg/mL | ||||
| Water; Ethyl acetate/leaf | F. solani | 0.37 µg/mL | MIC value of 0.04; 0.04 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.02; 0.02; 0.63 mg/mL | ||||
| Petroleum ether; Ethyl acetate/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.31; 0.31 mg/mL | [125] | |||
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.63; 0.31; 0.31 mg/mL | ||||
| Acetone/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.04 mg/mL | ||||
| Water; Acetone/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.04; 0.3l mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.31; 0.31; 0.31; 0.08 mg/mL | ||||
| Olinia ventosa (L.) Cufod (Penaeaceae) | Acetone; Hexane; Dichloromethane; Methanol/leaf | microplate method | F. oxysporum | amphotericin B | <0.02 mg/mL | MIC value of 0.63; 0.31; 0.16; 0.16 mg/mL | [115,169] |
| Passiflora suberosa L. (Passifloraceae) | Acetone/leaf | serial microdilution assay | F. oxysporum | amphotericin B | 7.5 μg/mL | MIC value of 0.04 mg/mL | [162,163] |
| Quercus acutissima Carruth. (Fagaceae) | Water; Ethyl acetate/leaf | Microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.08; 0.08 mg/mL | [131] |
| Water; Ethyl acetate/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.04; 0.04; 0.31 mg/mL | ||||
| Water; Ethyl acetate/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.02; 0.02 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.31; 0.16; 0.08 mg/mL | [125] | |||
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.16; 0.08; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.63; 0.31; 0.31; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.04; 0.16; 0.04 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.16; 0.08; 0.63 mg/mL | ||||
| Rhus muelleri Standl. & F.A.Barkley (Anacardiaceae) | Ethanol/leaf | agar dilution method | F. oxysporum f. sp. lycopersici | Not stated | Not stated | MIC value of 0.39 mg/mL and inhibition of 56.8% at 4500 ppm | [177,178] |
| Ricinus communis L (Euphorbiaceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.39 mg/mL | [166] |
| Hot water/leaf | F. graminearum | 0.004 mg/mL | MIC value of 0.20 mg/mL | [167,168] | |||
| Hot water; Methanol: Dichloromethane (1:1)/leaf | F. verticillioides | 0.006 mg/mL | MIC value of 0.02; 0.78 mg/mL | ||||
| Hot water/leaf | F. oxysporum | 0.004 mg/mL | MIC value of 0.16 mg/mL | ||||
| Rumex vesicarius L. (Polygonaceae) | Aqueous extract or Water/shoot | agar dilution method | F. oxysporum | Not stated | Not stated | MIC value of 0.625 mg/mL and Inhibition of 50.97 at 25 mg/mL | [179,180] |
| Salacia macrosperma Wight. (Celastraceae) | Ethyl acetate; Methanol/leaf | disc diffusion | F. moniliforme | nystatin | 0.078 mg/mL | MIC value of 0.312; 0.312 mg/mL | [181] |
| Methanol/leaf | F. oxysporum | 0.156 mg/mL | MIC value of 0.625 mg/mL | ||||
| Schotia brachypetala Sond. (Fabaceae) | Water; Ethyl acetate/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.31; 0.16 mg/mL | [131] |
| Water; Ethyl acetate/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04 mg/mL | ||||
| Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.63; 0.04 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.16; 0.16; 0.31 mg/mL | ||||
| Senna didymobotrya (Fresen.) H.S. Irwin & Barneby (Fabaceae) | Water; Ethyl acetate; Acetone/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.16; 0.08; 0.04 mg/mL | [131] |
| Ethyl acetate/leaf | microplate dilution method | F. proliferetum | 0.37 µg/mL | MIC value of 0.04 mg/mL | |||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.08; 0.08; 0.63 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.16; 0.63; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.16; 0.31; 0.31 mg/mL | [125] | |||
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.31; 0.16; 0.16 mg/mL | ||||
| Water; Acetone/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.63; 0.04 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 23.44 µg/mL | MIC value of 0.08; 0.04; 0.08; 0.26 mg/mL | ||||
| Solanum aculeastrum Dunal (Solanaceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.39 mg/mL | [166] |
| Hot water; Methanol: Dichloromethane (1:1)/leaf | microplate dilution method | F. graminearum | 0.004 mg/mL | MIC value of 0.78; 0.39 mg/mL | |||
| F. verticillioides | 0.006 mg/mL | MIC value of 0.40; 0.20 mg/mL | |||||
| Hot water/leaf | F. oxysporum | 0.004 mg/mL | MIC value of 0.78 mg/mL | ||||
| Solanum mauritianum Scop. (Solanaceae) | Water; Ethyl acetate/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.04; 0.16 mg/mL | [131] |
| Water; Ethyl acetate/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.04; 0.04; 0.63 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.16; 0.04; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.31; 0.08; 0.31 mg/mL | [125] | |||
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.31; 0.08; 0.04 mg/mL | ||||
| Water/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.63 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.31; 0.31; 0.31; 0.08 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.16; 0.04; 0.04 mg/mL | ||||
| Solanum panduriforme E. Mey. (Solanaceae) | Hot water; Methanol: Dichloromethane (1:1)/leaf | microplate dilution method | F. graminearum | amphotericin B | 0.004 mg/mL | MIC value of 0.10; 0.78 mg/mL | [167,168] |
| F. verticillioides | 0.006 mg/mL | MIC value of 0.20; 0.39 mg/mL | |||||
| F. oxysporum | 0.004 mg/mL | MIC value of 0.01; 0.08 mg/mL | |||||
| Solanum seaforthianum Andrews (Solanaceae) | Acetone/leaf | serial microdilution assay | F. oxysporum | amphotericin B | 7.5 μg/mL | MIC value of 0.31 mg/mL | [162,163] |
| Spirostachys africana Sond. (Euphorbiaceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.63 mg/mL | [166] |
| Strychnos mitis S.Moore (Loganiaceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.24 mg/mL | [166] |
| Vangueria infausta Burch (Rubiaceae) | Water; Ethyl acetate/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.08; 0.04 mg/mL | [131] |
| Water; Ethyl acetate; Acetone/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04; 0.63 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. solani | 0.37 µg/mL | MIC value of 0.04; 0.04; 0.31 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. graminearum | 187.50 µg/mL | MIC value of 0.31; 0.16; 0.32 mg/mL | ||||
| Acetone; Hexane; Dichloromethane/leaf | F. oxysporum | < 0.02 mg/mL | MIC value of 0.63; 0.32; 0.32 mg/mL | [115,169] | |||
| Vangueria infausta Burch (Rubiaceae) | Water; Petroleum ether; Ethyl acetate; Acetone/leaf | microplate dilution method | F. equisite | amphotericin B | 187.50 µg/mL | MIC value of 0.63; 0.31; 0.16; 0.63 mg/mL | [125] |
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.31; 0.16; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.63; 0.08; 0.16; 0.04 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.63; 0.31; 0.08; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.31; 0.31; 0.31; 0.78 mg/mL | ||||
| Warburgia salutaris (G. Bertol) Chiov. (Canellaceae) | Hot water/leaf | microplate dilution method | F. graminearum | amphotericin B | 0.004 mg/mL | MIC value of 0.10 mg/mL | [167,168] |
| Hot water; Methanol: Dichloromethane (1:1)/leaf | F. verticillioides | 0.006 mg/mL | MIC value of 0.10; 0.78 mg/mL | ||||
| F. oxysporum | 0.004 mg/mL | MIC value of 0.10; 0.10 mg/mL | |||||
| Acetone/leaf | F. verticilloides | 1.56 mg/mL | MIC value of 0.63 mg/mL | [166] | |||
| Withania somnifera (L.) Dunal (Solanaceae) | Water; Ethyl acetate; Acetone/leaf | microplate dilution method | F. verticillioides | amphotericin B | 2.93 µg/mL | MIC value of 0.08; 0.08; 0.04 mg/mL | [131] |
| Water; Ethyl acetate; Acetone/leaf | F. proliferetum | 0.37 µg/mL | MIC value of 0.04; 0.04; 0.63 mg/mL | ||||
| Water; Ethyl acetate/leaf | F. solani | 0.37 µg/mL | MIC value of 0.08; 0.04 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. equisite | 187.50 µg/mL | MIC value of 0.63; 0.16; 0.31 mg/mL | [125] | |||
| Water; Petroleum ether; Ethyl acetate/leaf | F. oxysporum | 11.72 µg/mL | MIC value of 0.16; 0.08; 0.08 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate/leaf | F. semitectum | 23.44 µg/mL | MIC value of 0.63; 0.04; 0.08 mg/mL | ||||
| Water; Ethyl acetate; Acetone/leaf | F. chlamydosporum | 23.44 µg/mL | MIC value of 0.63; 0.63; 0.16 mg/mL | ||||
| Water; Petroleum ether; Ethyl acetate; Acetone/leaf | F. subglutinans | 93.75 µg/mL | MIC value of 0.08; 0.63; 0.31; 0.63 mg/mL | ||||
| Xylotheca kraussiana Hochst. (Achariaceae) | Acetone/leaf | microplate dilution method | F. verticilloides | amphotericin B | 1.56 mg/mL | MIC value of 0.63 mg/mL | [166] |
| Acetone; Hexane; Dichloromethane/leaf | F. oxysporum | <0.02 mg/mL | MIC value of 0.32; 0.32; 0.32 mg/mL | [115,169] | |||
| Methanol/leaf | F. oxysporum | MIC value of 0.08 mg/mL | |||||
| Ziziphus mucronata Wild. (Rhamnaceae) | Hot water; Methanol: Dichloromethane (1:1)/leaf | microplate dilution method | F. graminearum | amphotericin B | 0.006 mg/mL | MIC value of 0.01; 0.78 mg/mL | [167,168] |
| F. oxysporum | 0.004 mg/mL | MIC value of 0.39; 0.39 mg/mL | [167,168] |

Table 3.
Antifungal activity of essential oils obtained from plants used in traditional medicine. The oil samples were evaluated against Fusarium phytopathogenic species using different methods and their activities were reported as minimum inhibitory concentration, half-maximal inhibitory concentration (IC50) or percentage inhibition values.
Table 3.
Antifungal activity of essential oils obtained from plants used in traditional medicine. The oil samples were evaluated against Fusarium phytopathogenic species using different methods and their activities were reported as minimum inhibitory concentration, half-maximal inhibitory concentration (IC50) or percentage inhibition values.
| Plant Species (Family) Source of Essential Oil | Method | Organism Tested | Positive Control | Activity of Positive Control | Results | Reference |
|---|---|---|---|---|---|---|
| Achillea biebersteinii Afan. ex Hub.-Mor. (Asteraceae) | disc diffusion method | F. verticilloides | Not stated | Not stated | Inhibition of 92.9% at 25 µL | [182] |
| Aconitum laeve Royle (Ranunculaceae) | disc diffusion method | F. oxysporum | amphotericin B; clotrimazole | 200; 300 µg/mL | MIC value of 300 µg/mL | [157] |
| Aloysia polystachya (Griseb.) Moldenke Biurrum 8755 (Verbenaceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 1082.43 µg/mL | [158] |
| Artemisia sieberi Besser. (Asteraceae) | broth microdilution method | F. solani | Itraconazole; Fluconazole; Ketoconazole | 7; 18; 12 µg/mL | MIC value of 20 µg/mL | [183] |
| F. oxysporum | 9; 10; 9 µg/mL | MIC value of 60 µg/mL | ||||
| Asarum heterotropoides var. mandshuricum (Aristolochiaceae) | disc diffusion method | F. avenaceum | nystatin | Not stated | MIC50 of 0.61 mg/mL | [184] |
| F. trichothecioides | MIC50 of 0.72 mg/mL | |||||
| F. sporotrioides | MIC50 of 0.83 mg/mL | |||||
| Bupleurum falcatum L. (Apiaceae) | broth microdilution method | F. oxysporum | amphotericin B | 0.5 µg/mL | MIC of 2 µg/mL | [185] |
| Chenopodium ambrosioides L. (Chenopodiaceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 243.12 µg/mL | [158] |
| Cannabis sativa L. (Cannabidaceae) | agar dilution method | F. oxysporum | Not stated | Not stated | Inhibition of 93.58% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 88.17% at 1 µL/mL | |||||
| Cinnamomum camphora (Lauraceae) | toxic medium assay | F. oxysporum isolate S-1187. | ICA-Thiabendazole® 500SC | Not stated | Inhibition of 49% at 3000 µL/L | [186] |
| Cinnamon zeylanicum (Lauraceae) | F. oxysporum isolate S-1187. | Inhibition of 92% at 500 µL/L | ||||
| Citrus aurantium (Rutaceae) | agar dilution method. | F. oxysporum | Not stated | Not stated | Inhibition of 57.75% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 57.40% at 1 µL/mL | |||||
| Citrus reticulata L. (Rutaceae) | poisoned food technique | F. oxysporum | Not stated | Not stated | Inhibition of 70% at 0.15 mL/100 mL | [187] |
| Citrus sinensis L. (Rutaceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 1604.82 µL/L | [158] |
| Coriandrum sativum L. (Apiaceae) | microdilution technique | F. solani | fluconazole | Not stated | MIC value of 0.97 mg/mL | [188] |
| Cuminum cyminum (Apiaceae) | broth dilution method | F. solani isolates | Not stated | Not stated | MIC value of 69 µg/mL | [189] |
| F. oxysporum isolates | Not stated | Not stated | MIC value of 72 µg/mL | [189] | ||
| F. verticillioides isolates | MIC value of 73 µg/mL | |||||
| F. poae isolates | MIC value of 130 µg/mL | |||||
| F. equiseti isolates | MIC value of 75 µg/mL | |||||
| Curcuma longa L. (Zingiberaceae) | microwell dilution method | F. graminearum | Nystatin; Amphotericin B | 2200; 1400 µg/mL | MIC value of 2450 µg/mL | [190] |
| Cymbopogon citratus, Stapf. (Poaceae) | toxic medium assay | F. oxysporum isolate S-1187. | ICA-Thiabendazole® 500SC | Not stated | Inhibition of 100% at 2500 µL/L | [186] |
| Cymbopogon nardus (L.) Rendle (Poaceae) | agar dilution method | F. oxysporum | Not stated | Not stated | Inhibition of 85.56% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 75.74% at 1 µL/mL | |||||
| Daucus carota L. var. Chantenay (Apiaceae) | agar dilution method | F. verticillioides | Not stated | Not stated | Inhibition of 56.80% at 1 µL/mL | [155] |
| Echinophora platyloba DC. (Apiaceae) | agar dilution and disk diffusion methods | F. oxysporum | Not stated | Not stated | Inhibition of 51.8% at 1 µL/L | [191] |
| Eucalyptus sp. (Myrtaceae) | disk diffusion method | F. graminearum | Not stated | Not stated | Inhibition of 56% at 1000 µL/L | [192] |
| F. asiaticum | Inhibition of 67% at 1500 µL/L | |||||
| F. redolens f. sp. dianthus | Inhibition of 55.11% at 1000 µL/L | |||||
| F. verticillioides | Inhibition of 72.44% at 1500 µL/L | |||||
| F. oxysporum f. sp. lentis | Inhibition of 55.11% at 1500 µL/L | |||||
| Foeniculum vulgare Mill. (Apiaceae) | broth dilution method | F. solani isolates | Not stated | Not stated | MIC value of 77 µg/mL | [189] |
| F. oxysporum isolates | MIC value of 72 µg/mL | |||||
| F. verticillioides isolates | MIC value of 77 µg/mL | |||||
| F. poae isolates | MIC value of 96 µg/mL | |||||
| F. equiseti isolates | MIC value of 63 µg/mL | |||||
| Foeniculum vulgare Mill. (Apiaceae) fruits | agar disk diffusion | F. fujikuroi | Not stated | Not stated | MIC value of 2.0 µL/mL | [193] |
| Helichrysum splendidum (Thunb.) Less. (Asteraceae) | toxic medium assay | F. oxysporum isolate S-1187. | ICA-Thiabendazole® 500SC | Not stated | Inhibition of 58% at 3000 µL/L | [186] |
| Heracleum persicum Desf. Ex Fischer. (Apiaceae) | broth dilution method | F. solani isolates | Not stated | Not stated | MIC value of 675 µg/mL | [189] |
| F. oxysporum isolates | Not stated | Not stated | MIC value of 70 µg/mL | [189] | ||
| F. verticillioides isolates | MIC value of 225 µg/mL | |||||
| F. poae isolates | MIC value of 952 µg/mL | |||||
| F. equiseti isolates | MIC value of 1062 µg/mL | |||||
| F. solani | Itraconazole; Fluconazole; Ketoconazole | 7; 18; 12 µg/mL | MIC value of 480 µg/mL | [183] | ||
| F. oxysporum | 9; 10; 9 µg/mL | MIC value of 530 µg/mL | ||||
| Illicium verum Hook.f. (Schisandraceae) | microdilution technique | F. solani | fluconazole | Not stated | MIC value of 0.93 mg/mL | [188] |
| F. verticillioides | MIC value of 0.70 mg/mL | |||||
| Laurus nobilis L. (Lauraceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 1846.87 µL/L | [158] |
| Lavandula angustifolia Mill. (Lamiaceae) | agar dilution method | F. verticillioides | Not stated | Not stated | Inhibition of 68.64% at 1 µL/mL | [155] |
| Cymbopogon citratus, mycorrhizal lemongrass. (Poaceae) | food poisoning method | F. solani | Ridomil plus 44 WP | 100% at 250 ppm | Inhibition of 89% at 250 ppm | [194] |
| Cymbopogon citratus, non-mycorrhizal lemongrass. (Poaceae) | Inhibition of 71% at 250 ppm | |||||
| Lippia rehmannii H.Pearson (Verbenaceae) | toxic medium assay | F. oxysporum isolate S-1187. | ICA-Thiabendazole® 500SC | Not stated | Inhibition of 72% at 500 µL/L | [186] |
| Lippia scaberrima Sond. (Verbenaceae) | Inhibition of 87% at 3000 µL/L | |||||
| Matricaria recutita (L.) syn. (Asteraceae) | microbioassay technique | F. oxysporum | ketoconazole | 29.7% at 10 mg/disk | Inhibition of 56.0% at 62.5 µg/mL | [195] |
| Melaleuca alternifolia (Myrtaceae) | microdilution technique | F. verticillioides | fluconazole | Not stated | MIC value of 0.86 mg/mL | [188] |
| F. oxysporum | MIC value of 0.91 mg/mL | |||||
| Melaleuca alternifolia L. (Maiden and Betche) Cheel. (Myrtacea) | agar dilution method | F. oxysporum | Not stated | Not stated | Inhibition of 58.29% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 56.80% at 1 µL/mL | |||||
| Mentha spicata L. (spearmint) (Lamiaceae) | toxic medium assay | F. oxysporum isolate S-1187. | ICA-Thiabendazole® 500SC | Not stated | Inhibition of 79% at 2000 µL/L | [186] |
| Minthostachys verticillata Griseb. (Lamiaceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 1552.43 µL/L | [158] |
| Myrcia ovata Cambesse (Myrtaceae) | contact | F. solani | Viper 700 (0.07% w/v) | Not stated | Inhibition of 53.9% at 100 µL/mL | [54] |
| Nepeta cataria L. (Lamiaceae) | agar dilution method, | F. verticillioides | Not stated | Not stated | Inhibition of 91.72% at 1µL/mL | [155] |
| F. oxysporum | Inhibition of 97.86% at 1 µL/mL | |||||
| Ocimum basilicum L. (Lamiaceae) | agar dilution method. | F. oxysporum | Not stated | Not stated | Inhibition of 74.87% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 77.51% at 1 µL/mL | |||||
| Origanum heracleoticum L. (Lamiaceae) | microdilution technique | F. solani | fluconazole | Not stated | MIC value of 0.14 mg/mL | [188] |
| F. tricinctum | MIC value of 0.14 mg/mL | |||||
| F. sporotrichioides | MIC value of 0.28 mg/mL | |||||
| F. verticillioides | MIC value of 0.14 mg/mL | |||||
| F. oxysporum | MIC value of 0.07 mg/mL | |||||
| F. semitectum | MIC value of 0.28 mg/mL | |||||
| F. equiseti | MIC value of 0.28 mg/mL | |||||
| Origanum majorana L. (Lamiaceae) | agar dilution method | F. oxysporum | Not stated | Not stated | Inhibition of 59.36% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 75.74% at 1 µL/mL | |||||
| Origanum vulgare L. (Lamiaceae) | broth microdilution method | F. solani | Itraconazole; Fluconazole; Ketoconazole | 7; 18; 12 µg/mL | MIC value of 50 µg/mL | [183] |
| F. oxysporum | 9; 10; 9 µg/mL | MIC value of 50 µg/mL | ||||
| Origanum vulgare L. spp. virens (Lamiaceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 101.71 µL/L | [158] |
| Origanum vulgare L. spp. vulgare (Lamiaceae) | F. verticillioides | IC50 of 108.27 µL/L | ||||
| Origanum x applii (Domin Boros) (Lamiaceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 66.79 µL/L | [158] |
| Pelargonium graveolens L’Heritier. (Geraniaceae) | microdilution technique | F. equiseti | fluconazole | Not stated | MIC value of 0.66 mg/mL | [188] |
| Pelargonium odoratissimum (Geraniaceae) | agar dilution method | F. culmorum | Not stated | Not stated | Inhibition of 65.45% at 1 µL/L | [196] |
| Pelargonium roseum L. (Geraniaceae) | agar dilution method | F. verticillioides | Not stated | Not stated | Inhibition of 73.96% at 1 µL/mL | [117] |
| F. oxysporum | Inhibition of 85.56% at 1 µL/mL | |||||
| Mentha piperita L. (Lamiaceae) | microbroth dilution assay | F. oxyporum (MNHN 963917) | Amphotericin | MIC value of 1.50 µg/mL | MIC value of 1.50 µg/mL | [197] |
| F. acuminatum | MIC value of 1.50 µg/mL | MIC value of 2.50 µg/mL | ||||
| F. solani | MIC value of 1.25 µg/mL | MIC value of 10.0 µg/mL | ||||
| F. tabacinum | MIC value of 1.35 µg/mL | MIC value of 1.50 µg/mL | ||||
| Pimenta dioica (L.) Merr. (Myrtaceae) | agar dilution method | F. oxysporum | Not stated | Not stated | Inhibition of 100% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 100% at 1 µL/mL | |||||
| Pimpinella anisum L. (Apiaceae) | broth microdilution method | F. solani | Itraconazole; Fluconazole; Ketoconazole | 7; 18; 12 µg/mL | MIC value of 85 µg/mL | [183] |
| F. oxysporum | 9; 10; 9 µg/mL | MIC value of 120 µg/mL | ||||
| Rosa damascena P. Mill. (Rosaceae) | microdilution technique | F. subglutinans | fluconazole | Not stated | MIC value of 0.62 mg/mL | [188] |
| F. solani | MIC value of 0.29 mg/mL | |||||
| F. tricinctum | MIC value of 0.14 mg/mL | |||||
| F. sporotrichioides | MIC value of 0.29 mg/mL | |||||
| F. verticillioides | MIC value of 0.14 mg/mL | |||||
| F. oxysporum | MIC value of 0.29 mg/mL | |||||
| F. semitectum | MIC value of 0.64 mg/mL | |||||
| F. equiseti | MIC value of 0.30 mg/mL | |||||
| Rosmarinus officinalis (rosemary) (Lamiaceae) | broth microdilution method | F. solani | Itraconazole; Fluconazole; Ketoconazole | 7; 18; 12 µg/mL | MIC value of 320 µg/mL | [183] |
| F. oxysporum | 9; 10; 9 µg/mL | MIC value of 410 µg/mL | ||||
| Salvia sclarea L. (Lamiaceae) | agar dilution method | F. oxysporum | Not stated | Not stated | Inhibition of 58.82% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 65.09% at 1 µL/mL | |||||
| Satureja hortensis L. (Lamiaceae) | microdilution technique | F. subglutinans | fluconazole | Not stated | MIC value of 0.95 mg/mL | [188] |
| F. solani | MIC value of 0.14 mg/mL | |||||
| F. tricinctum | MIC value of 0.14 mg/mL | |||||
| F. sporotrichioides | MIC value of 0.27 mg/mL | |||||
| F. verticillioides | MIC value of 0.14 mg/mL | |||||
| F. oxysporum | MIC value of 0.14 mg/mL | |||||
| F. semitectum | MIC value of 0.14 mg/mL | |||||
| F. equiseti | MIC value of 0.62 mg/mL | |||||
| Schinus molle L. (Anacardiaceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 1226.76 µL/L | [158] |
| Silene armeria L. (Caryophyllaceae) | disc diffusion method | F. oxysporum KACC 41083 | Not stated | Not stated | MIC value of 500 µg/mL | [198] |
| F. solani KACC 41092 | MIC value of 125 µg/mL | |||||
| Stachys pubescens Ten. (Lamiaceae) | broth microdilution method | F. oxysporum | amphotericin B | 0.5 µg/mL | MIC value of 1 µg/mL | [185] |
| Syzigium aromaticum L. (Myrtaceae) | toxic medium assay | F. oxysporum isolate S-1187. | ICA-Thiabendazole® 500SC | Not stated | Inhibition of 83% at 250 µL/L | [186] |
| Tagetes riojana M. Ferraro Biurrum 8753 (Asteraceae) | disc diffusion method | F. verticillioides | Not stated | Not stated | IC50 of 764.75 µL/L | [158] |
| Thymus daenensis Celak. (Lamiaceae) | broth microdilution method | F. oxysporum | amphotericin B | 0.5 µg/mL | MIC value of 4 µg/mL | [185] |
| Thymus kotschyanus Boiss. & Hohen. (Lamiaceae) | broth microdilution method | F. oxysporum | amphotericin B | 0.5 µg/mL | MIC value of 0.5 µg/mL | [185] |
| F. solani | Itraconazole; Fluconazole; Ketoconazole | 7; 18; 12 µg/mL | MIC value of 40 µg/mL | [183] | ||
| F. oxysporum | 9; 10; 9 µg/mL | MIC value of 75 µg/mL | ||||
| Thymus mastichina L. (Lamiaceae) | agar dilution method. | F. verticillioides | Not stated | Not stated | Inhibition of 51.48% at 1 µL/mL | [155] |
| Thymus vulgaris L. (Lamiaceae) | microdilution technique | F. solani | fluconazole | Not stated | MIC value of 0.16 mg/mL | [188] |
| F. tricinctum | MIC value of 0.19 mg/mL | |||||
| F. sporotrichioides | MIC value of 0.61 mg/mL | |||||
| F. verticillioides | MIC value of 0.14 mg/mL | |||||
| F. oxysporum | MIC value of 0.14 mg/mL | |||||
| F. semitectum | MIC value of 0.19 mg/mL | |||||
| F. equiseti | MIC value of 0.98 mg/mL | |||||
| Thymus vulgaris L. (Lamiaceae) | toxic medium assay | F. oxysporum isolate S-1187. | ICA-Thiabendazole® 500SC | Not stated | Inhibition of 61% at 250 µL/L | [186] |
| Thymus vulgaris L. (Lamiaceae) | agar dilution method | F. culmorum | Not stated | Not stated | Inhibition of 99.71% at 1 µL/L | [196] |
| Thymus vulgaris L. (Lamiaceae) | agar dilution method | F. oxysporum | Not stated | Not stated | Inhibition of 98.41% at 1 µL/mL | [155] |
| F. verticillioides | Inhibition of 98.22% at 1 µL/mL | |||||
| Xylopia aethiopica (Dunal) A. Rich. (Annonaceae) | incorporation method | F. oxysporum | Not stated | Not stated | MIC value of 3000 ppm | [199] |
| Zataria multiflora Boiss. (Lamiaceae) | broth dilution method | F. solani isolates | Not stated | Not stated | MIC value of 76 µg/mL | [189] |
| F. oxysporum isolates | MIC value of 66 µg/mL | |||||
| F. verticillioides isolates | MIC value of 77 µg/mL | |||||
| F. poae isolates | MIC value of 99 µg/mL | |||||
| F. equiseti isolates | MIC value of 99 µg/mL | |||||
| Zataria multiflora Boiss. (Lamiaceae) | broth microdilution method | F. solani | Itraconazole; Fluconazole; Ketoconazole | 7; 18; 12 µg/mL | MIC value of 40 µg/mL | [183] |
| F. oxysporum | 9; 10; 9 µg/mL | MIC value of 20 µg/mL | ||||
| Zhumeria majdae Rech. f. & Wendelbo (Lamiaceae) | disk diffusion method | F. graminearum | Not stated | Not stated | Inhibition of 75.11% at 1000 µL/L | [192] |
| F. asiaticum | Inhibition of 100% at 1500 µL/L | |||||
| F. redolens fsp. dianthus | Inhibition of 100% at 1500 µL/L | |||||
| F. verticillioides | Inhibition of 70.66% at 1500 µL/L | |||||
| F. oxysporum f. sp. lentis | Inhibition of 60.44% at 1500 µL/L | |||||
| Zingiber cassumunar Roxb. (Zingiberaceae) | agar dilution method | F. verticillioides | Not stated | Not stated | Inhibition of 67.46% at 1 µL/mL | [155] |

Table 4.
Antifungal activity of compounds isolated from plants used in traditional medicine. The compounds were evaluated against different Fusarium pathogens and their antifungal activities were reported as minimum inhibitory concentration, percentage inhibition or half-maximal effective concentration.
Table 4.
Antifungal activity of compounds isolated from plants used in traditional medicine. The compounds were evaluated against different Fusarium pathogens and their antifungal activities were reported as minimum inhibitory concentration, percentage inhibition or half-maximal effective concentration.
| Compound | Chemical Structure | Plant Species (Family) | Plant Part | Organism Tested | Positive Control | Activity of Positive Control | Results | Reference |
|---|---|---|---|---|---|---|---|---|
| (±)-Qinghaocoumarin A | 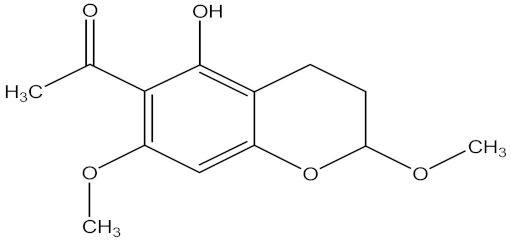 | Artemisia annua L. (Asteraceae) | leaves | F. oxysporum | Hymexazol | 13.02 µg/mL | MIC value of 18.75 µg/mL | [200] |
| F. solani | 41.67 µg/mL | MIC value of 18.75 µg/mL | ||||||
| (3R,3aS,6R,6aS,7aR,8aS,9aS,9bR)-decahydro-9b-hydroxy-3,6,8a-trimethyl-oxireno[c]pyrano [4,3,2-jk] benzoxepin-2(3H)-one | 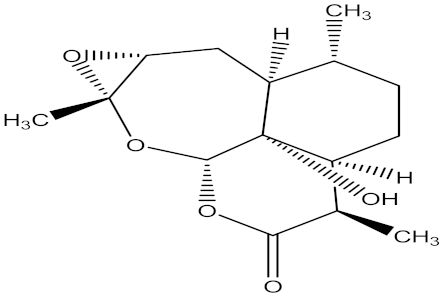 | F. oxysporum | Hymexazol | 13.02 µg/mL | MIC value of 62.50 µg/mL | |||
| F. solani | 41.67 µg/mL | MIC value of 21.79 µg/mL | ||||||
| 1,2-dimethoxy-4(2-propenyl) benzene | 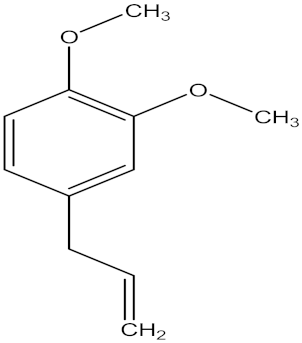 | Acorus tatarinowii Schott (Acoraceae) | whole plant | F. oxysporum f. sp. niveum | Not stated | Not stated | Inhibition of 100% at 0.4 g/L | [201] |
| 3,4-dihydroxy-3,4-dimethoxy-6,7- cyclolignan | 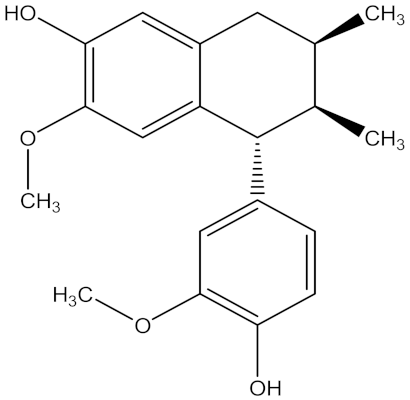 | Larrea divaricata Cav. (Zygophyllaceae) | leaves and stem | F. verticillioides | Not stated | Not stated | MIC value of 250 µg/mL | [202] |
| F. graminearum | MIC value of 15.6 µg/mL | |||||||
| F. solani | MIC value of 125 µg/mL | |||||||
| 5-hydroxy-7,40-dimethoxyflavone | 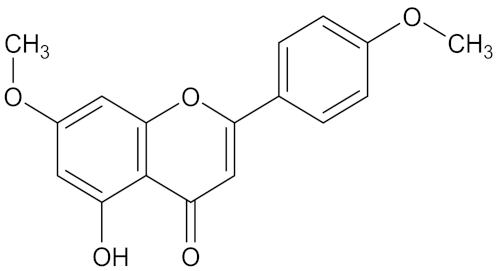 | Combretum erythrophyllum (Burch.) Sond. (Combretaceae) | leaves | F. verticilloides | amphotericin B | 0.003 mg/mL | 0.31 mg/mL | [203] |
| F. proliferatum | 0.0004 mg/mL | 0.01 mg/mL | ||||||
| F. solani | 1.2 mg/mL | 0.31 mg/mL | ||||||
| F. graminearum | 2.3 mg/mL | 0.63 mg/mL | ||||||
| F. chlamydosporum | 2.3 mg/mL | 0.63 mg/mL | ||||||
| 3′, 4′-de- O-methylenehinokinin | 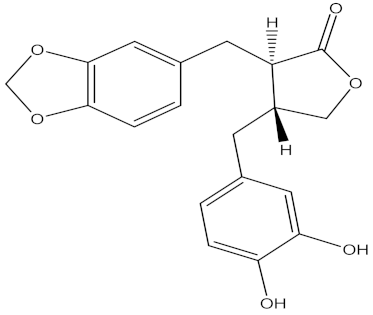 | Artemisia annua L. (Asteraceae) | leaves | F. oxysporum | Hymexazol | 13.02 µg/mL | MIC value of 31.25 µg/mL | [200] |
| F. solani | 41.67 µg/mL | MIC value of 75.00 µg/mL | ||||||
| 3α,7α-dihydroxy amorph-4-ene 3-acetate | 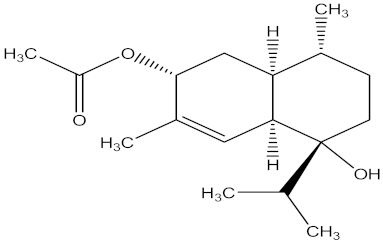 | leaves | F. oxysporum | Hymexazol | 13.02 µg/mL | MIC value of 50.00 µg/mL | ||
| F. solani | 41.67 µg/mL | MIC value of 43.75 µg/mL | ||||||
| artemetin |  | F. oxysporum | 13.02 µg/mL | MIC value of >150.00 µg/mL | ||||
| F. solani | 41.67 µg/mL | MIC value of >150.00 µg/mL | ||||||
| dehydrodiconiferyl alcohol |  | F. oxysporum | 13.02 µg/mL | MIC value of 150.00 µg/mL | ||||
| F. solani | 41.67 µg/mL | MIC value of 37.50 µg/mL | ||||||
| denudatin A |  | F. oxysporum | 13.02 µg/mL | MIC value of 150.00 µg/mL | ||||
| F. solani | 41.67 µg/mL | MIC value of 37.5 µg/mL | ||||||
| denudatin B | 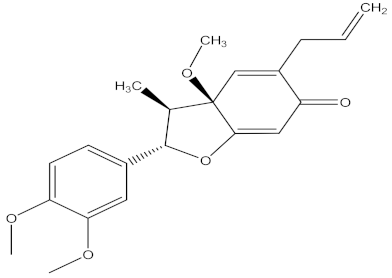 | F. oxysporum | 13.02 µg/mL | MIC value of 37.50 µg/mL | ||||
| F. solani | 41.67 µg/mL | MIC value of 87.5 µg/mL | ||||||
| futokadsurin B | 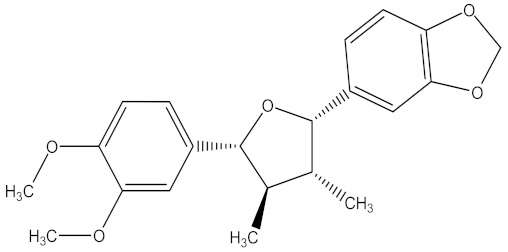 | F. oxysporum | 13.02 µg/mL | MIC value of 150.00 µg/mL | ||||
| F. solani | 41.67 µg/mL | MIC value of 75.00 µg/mL | ||||||
| futokadsurin C | 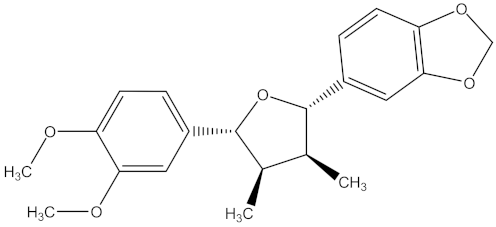 | F. oxysporum | 13.02 µg/mL | MIC value of 125.00 µg/mL | ||||
| F. solani | 41.67 µg/mL | MIC value of 100.00 µg/mL | ||||||
| Gallic acid | 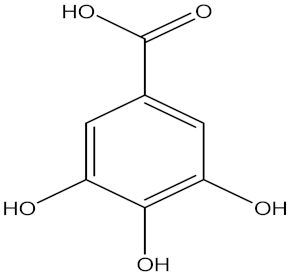 | Terminalia nigrovenulosa Pierre (Combretaceae) | bark | F. solani | Not stated | Not stated | Inhibition of 75% at 500 ppm | [204] |
| Maslinic acid | 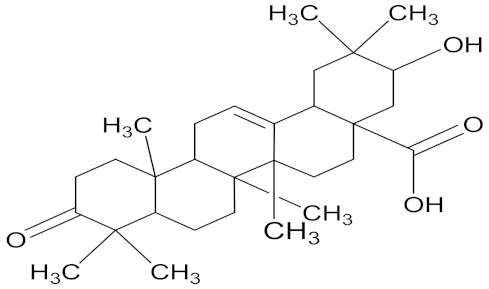 | Combretum erythrophyllum (Combretaceae) | leaves | F. oxysporum | amphotericin B | 1.2 mg/mL | 0.31 mg/mL | [203] |
| F. verticilloides | 0.003 mg/mL | 0.08 mg/mL | ||||||
| F. subglutinans | 9.4 mg/mL | 0.63 mg/mL | ||||||
| F. proliferatum | 0.0004 mg/mL | 0.31 mg/mL | ||||||
| F. solani | 1.2 mg/mL | 0.63 mg/mL | ||||||
| F. graminearum | 2.3 mg/mL | 0.63 mg/mL | ||||||
| N1-decarbomethoxy chanofruticosinic acid | 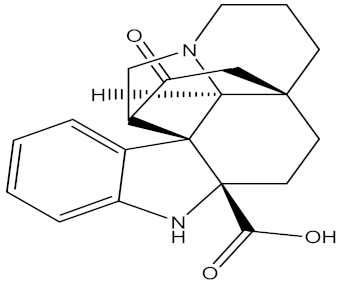 | Kopsia hainanensis Tsiang (Apocynaceae) | leaves and stem | F. oxysporum f. sp. Cubense | mildothane | EC50 value of 57.0 µg/mL | EC50 value of 15.2 µg/mL | [205] |
| Fusarium oxysporum f. sp. Niveum | EC50 value of 101.0 µg/mL | EC50 value of 43.8 µg/mL | ||||||
| EC50 value of 31.8 µg/mL | ||||||||
| nordihydroguaiaretic acid |  | Larrea divaricata Cav. (Zygophyllaceae) | leaves and stem | F. graminearum | Not stated | Not stated | MIC value of 62.5 µg/mL | [202] |
| F. solani | MIC value of 250 µg/mL | |||||||
| F. verticillioides | MIC value of 125 µg/mL | |||||||
| penduletin |  | Artemisia annua L. (Asteraceae) | leaves | F. oxysporum | Hymexazol | 13.02 µg/mL | MIC value of 100.00 µg/mL | [200] |
| F. solani | 41.67 µg/mL | MIC value of 100.00 µg/mL | ||||||
| Phloroglucinol derivative | 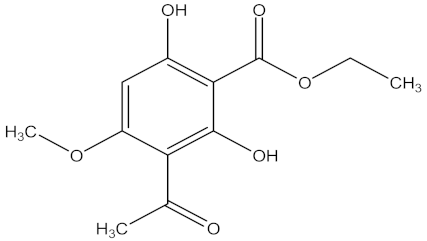 | F. oxysporum | 13.02 µg/mL | MIC value of 62.50 µg/mL | ||||
| F. solani | 41.67 µg/mL | MIC value of 87.50 µg/mL | ||||||
| Qinghaocoumarin B | 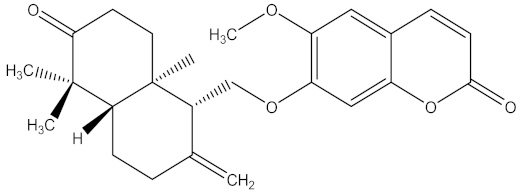 | F. oxysporum | 13.02 µg/mL | MIC value of 62.50 µg/mL | ||||
| F. solani | 41.67 µg/mL | MIC value of 43.75 µg/mL | ||||||
| Withaferin A | 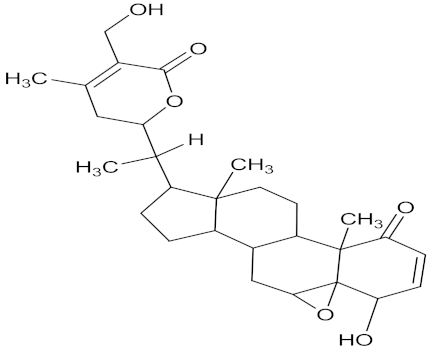 | Withania somnifera (L.) Dunal. (Solanaceae) | leaves | F. verticilloides | amphotericin B | 0.003 mg/mL | 0.16 mg/mL | [203] |
| Qinghaolignan B | 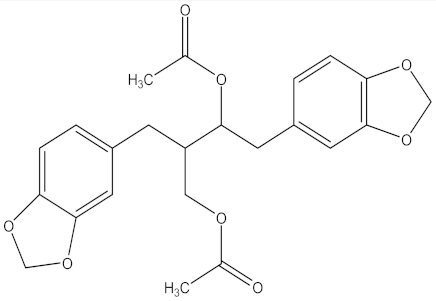 | Artemisia annua L. (Asteraceae) | leaves | F. oxysporum | Hymexazol | 13.02 µg/mL | MIC value of 150.00 µg/mL | [200] |
| F. solani | 41.67 µg/mL | MIC value of 37.50 µg/mL |
4. Mechanisms of Action
Understanding the mechanisms of action of natural products from medicinal plants or synthetic chemicals against Fusarium pathogens is an important approach towards crop disease control. Pesticides inhibit the growth of pathogens by interfering with numerous useful metabolic processes of the pathogens. As an example, benzimidazole fungicides were reported to inhibit fungi by binding protein subunits of spindle and disrupting their functions [101]. Additionally, the application of pesticides may activate morphological and biochemical defence mechanisms of the crop against diseases. Although no mechanism of actions was proposed, studies have reported treatment of tomato plants with chemicals such as K- and Na-benzthiazolylthiocycloate, 4-chloro-3,5-dimethylphenoxyethanol, dinitroaniline and DL-3-aminobutyric acid, which induce the plant defence mechanism against Fusarium wilt disease [206]. The different mechanisms of action of fungicides acting against Fusarium pathogens are summarized in Table 5.
Generally, antifungal chemicals inhibit pathogen growth by interfering with the biosynthesis of the major components of the cell wall and cell membrane or through the formation of ion channels on the cellular membrane [207,208]. Antifungal agents can act by inhibiting normal functions of the topoisomerase enzymes, increasing permeability of fungal cell wall and by targeting the plasma membrane in most pathogens [209]. With regard to plant products (extracts and essential oils), their main mechanisms of action can include the following: disruption of the fungal cell wall integrity through the inhibition of chitin and β-glucans synthesis; disruption of the cell membrane, such as by binding to or inhibiting ergosterol biosynthesis; mitochondria dysfunction arising from inhibition of electron transport and respiratory chain proton pumps; cell division inhibition via interference with microtubule polymerization; inhibition of ribonucleic acid, deoxyribonucleic acid or protein synthesis; and efflux pump inhibition [210]. Disruption of the fungal membrane may lead to membrane permeability and eventually prevent normal biochemical functions [211]. Nonetheless, more studies are required in order to fully understand the different mechanisms of actions and their dynamics, particularly of medicinal plant products (extracts, essential oils and isolated compounds).

Table 5.
Possible mechanisms of action of pesticides against Fusarium phytopathogenic species.
Table 5.
Possible mechanisms of action of pesticides against Fusarium phytopathogenic species.
| Extracts/Fungicides | Target Site | Possible Mechanism of Action | Reference |
|---|---|---|---|
| 95% ethanol extract of Curcuma longa (Zingiberaceae) | Protein synthesis and enzymatic pathways | Inhibition of GAPDH, tRNA synthetase family II and Zinc binuclear structural-containing fungal protein | [212] |
| Cell membrane synthesis | Inhibition of ergosterol synthesis | ||
| Respiratory system | Suppression of the activity of NADH oxidase and SDH | ||
| 2,5-dicyclopentylidene cyclopentanone | Cell membrane and cell wall | Inhibition of sterol biosynthesis | [213] |
| Amoxicillin, Chloramphenicol, Erythromycin and Raficillin | Cell wall enzymatic pathways | Inhibit the polygalacturonase and pectinmethylgalacturonase enzyme activities | [209] |
| Rifampin and Rifabutin, members of the Rifamycin class and Azithromycin | Protein synthesis | Inhibition of RNA and protein synthesis | [214,215,216] |
| Benzimidazole | Protein synthesis | Binding to fungal β-tubulin and disrupt microtubule dynamic including interference with monomeric tubulin polymerization | [217] |
| Peptide Fengycins | Cell membrane | Formation of ion channels on cellular membrane by interfering with synthesis of ergosterol | [208] |
| Azole fungicides | Fungal cell membrane | Inhibition of the heme protein and 14α-demethylation of lanosterol | [218] |
5. Challenges and Future Perspectives
There is an abundance of medicinal plant species that can be screened for antifungal activity of their extracts, essential oils and isolated compounds as potential biocontrol agents for possible application in crop production. The number of in vitro antifungal activity studies of medicinal plant materials against human and crop pathogens is increasing every year [219,220,221]. On the other hand, the number of formulated products developed from these natural resources remains very few in comparison. Many researchers in academic and research institutions are very interested in evaluating medicinal plant materials for application as safe and biodegradable pesticides. As shown in Table 2, Table 3 and Table 4, these natural products have exhibited very good antifungal activity against different Fusarium pathogens; however, there are challenges and limitations that must be addressed in order to develop these natural resources into beneficial final products or biopesticides.
It is critical that appropriate valid test assays incorporating suitable positive and negative controls be used for in vitro screening. The results should include the minimum inhibitory concentration that allows for effective inter-laboratory comparisons of the results. Biological activities of crude extracts, essential oils and isolated compounds are generally dose-dependent activities. Hence, while stating the inhibition percentage at a concentration may indicate potency at that concentration, it does not allow for an effective comparison at dose-dependent levels. It is desirable that the assays also determine the potential fungicidal effect of the extracts and/or compounds. Many plant extracts have demonstrated potent antifungal activity (with MIC values below 1.0 mg/mL) using in vitro assays (Table 2), but only a few were tested in vivo [130,222,223,224,225,226,227]. The potent in vitro antifungal activity of Melia azedarach, Combretum erythrophyllum and Quercus acutissima leaf extracts [130] were confirmed in vivo. The leaf extract of Melia azedarach showed strong antifungal activity against F. proliferatum inoculated on maize seeds, while combined leaf extracts from Combretum erythrophyllum and Quercus acutissima exhibited potent inhibitory activity against F. verticilloides in vivo without any phytotoxic effect [130]. One of the limiting factors is the unavailability of resources and skills required to conduct relevant in vivo experiments either in the greenhouse or in the field. This gap can, however, be bridged through collaborative research. The frustrating and time-consuming process and regulations involved during registration of biopesticides is also a challenge. The amount of plant extracts, essential oils or isolated compounds required to conduct in vivo field experiments can be a limiting factor, especially if these are obtained from non-renewable plant parts. Thus, we recommend that the use of renewable plant parts such as the leaves be given more attention in designing appropriate experiments. Medicinal plants with very promising antifungal activity against crop pathogens may need to be cultivated in order to guarantee a regular supply of quality raw materials required for product development. Quality control protocols and the standardization of cultivation practices for selected plants are important to ensure consistent high-quality raw materials [228]. On the other hand, the use of invasive species such as those in the Solanaceae family that demonstrate potent in vitro activity, if confirmed in vivo, may be a relatively cheap alternative.
Several studies have focused on individual plant extracts (Table 2), essential oils (Table 3) or isolated compounds (Table 4) against some specific pathogens. In some cases, the antifungal activity demonstrated by an isolated compound may be disappointing when compared to the originating plant extracts or fractions [229]. Although pathogen and plant species specific, it was noticed that combinations of extracts from different plant species may improve antifungal activity [131]. In a study evaluating the antifungal effect of combining plant extracts against Fusarium species, 150 extract out of 204 extract combinations exhibited either a synergistic or additive effect [131]. In particular, a combination of Harpephyllum caffrum and Combretum erythrophyllum leaf acetone extracts demonstrated very strong synergistic inhibitory activity in comparison to their individual extracts against F. graminearum, F. proliferatum and F. verticillioides [131]. Plants contain several metabolites that could interact in various ways to produce desired activities against a panel of microorganisms. The desired activity may therefore be lost when isolated compounds acting together in a synergistic manner in an extract are tested individually [230]. It may be worthwhile to evaluate the potentiating effect of different combinations of plant extracts or isolated compounds in vitro and in vivo as part of the screening process for formulating plant-based products. The phytotoxicity determination and potential biostimulant effect of promising extracts and/or compounds on plant growth as well as their biochemical mode of action need to be established.
Ordinarily, plant extracts, essential oils and isolated compounds obtained from medicinal plants are poorly soluble in water. Products or formulations prepared from these plant materials are usually dissolved in organic solvents and that itself poses a toxicity challenge. Such organic solvents may be phytotoxic to the crops and can also evaporate during storage period, thus affecting the concentration of the constituents. Furthermore, the formulation or product may not persist in the environment to deliver desired effect and may lead to frequent biopesticide applications [80]. Some of these challenges may be addressed through application and implementation of nanotechnology strategies, which can improve the stability and efficacy of natural products (extracts, essential oils and isolated compounds) developed from medicinal plants.
There must be robust analytical techniques and quality control procedures to determine chemical composition and quantity of active ingredients in both raw materials and finished products. Agronomical practices and post-treatment processes, including drying, processing and storage, have a negative impact on the activity and phytochemical content of plant extracts. These practices were reported to be plant species specific and may affect the quality of plant products [231,232,233]. In addition, the chemical structures of isolated compounds that exhibited good antifungal activity against Fusarium may be used as scaffold molecules or in computational studies for designing synthetic approaches that will result in more yield during industrial production. Different derivatives for those active compounds may also be developed.
The use of nanotechnology is an important step towards development of biopesticides from natural products. The combination of nanoparticles into a delivery system of natural plant products was used in several studies to increase therapeutic activity, bioavailability and target a specific action site of the product. This application is well known and has been successful in the treatment of human diseases [234,235]. A similar approach may be applied in crop protection to increase stability and activity of plant extracts. Currently there is a paucity of information on the incorporation of nanotechnology strategies in order to improve stability and efficacy of natural products from plants with potential for controlling crop diseases in the agricultural sector. Although formulation development may add cost to the overall process, this field of research is worth investigating.
With regard to essential oils, which are a mixture of different volatile compounds, their screening process should include their chemical profiles. Thereafter, the structure-activity of the oils can be computed to establish which chemical constituent(s) demonstrated stronger antifungal activity. That information can be utilized to specifically synthesize such active compounds. The constituents or compounds may be combined into different ratios and re-evaluated for antifungal activity and further developed into a product. The phenomenon of combining different constituents from essential oils may also be done with isolated active compounds. This approach may help to delay development of fungal resistance.
Regardless of the time-consuming procedures required to develop and register biopesticide products, it is important to carefully study and evaluate efficacy, safety and stability of natural plant products. This will help to have a better understanding of their toxicity towards non-target organisms and their long-term impact on the environment. In vivo cytotoxicity determination and mechanisms of action of these natural products against tested Fusarium pathogens are other areas of study to be explored. In conjunction with stability studies, the knowledge of their cytotoxicity, phytotoxicity and mechanisms of action would make it easy to also understand their frequency of application in the field when combating crop disease outbreaks.
6. Conclusions
To address the challenges of pesticide resistance development, as evidenced by most Fusarium pathogens against conventional synthetic pesticides, natural products from medicinal plant species are considered as alternative control agents. Extracts from plant species in the families Solanaceae, Combretaceae and Fabaceae are among the most commonly used agents against Fusarium pathogens. Other families with a high potential include the Euphorbiaceae, Rubiaceae, Asteraceae and Celastraceae families. The majority of studies have focused attention on the use of leaves, a renewable plant part, as the source of secondary metabolites with antifungal activity against Fusarium pathogens. While different organic solvents have been used for extraction of bioactive compounds as crude extracts, water extract demonstrated relatively good antifungal activity in some cases. Water is readily available and may be used by resource-poor farmers for extraction. On the other hand, the extraction of plant materials with organic solvents, such as acetone and ethyl acetate, enhances the possibility of extracting a wide range of antifungals. Essential oils derived from species belonging to the Lamiaceae, Apiaceae, Asteraceae and Myrtaceae families demonstrated potent activity against Fusarium pathogens. Particularly noteworthy are the essential oils from Thymus vulgaris, Cymbopogon citratus and Melaleuca alternifolia. Medicinal plant products (extracts, essential oils and isolated compounds) are perceived to be safer, are biodegradable and are environmentally friendly. They are also expected to have less side effects since they have been used in many countries to treat different aliments affecting animals and human. Plant products are inherently unstable to higher temperatures and sunlight; therefore, they may not persist in the environment for a very long period of time. Incorporation of nanotechnology approaches may be used to improve stability and efficiency of natural products developed from medicinal plants. Medicinal plants are abundant sources of different bioactive metabolites or chemicals. Therefore, investment in the development of medicinal plant products to control crop diseases including those caused by Fusarium pathogens is a growing sector to be closely considered. Regardless of the challenges, plant natural products remain potential alternative sources of environmentally friendly biopesticides to control Fusarium pathogens known to cause diseases in crop production.
Author Contributions
Conceptualization, H.A.S., S.O.A. and H.A.S.; writing—original draft preparation; review and editing, W.N. and S.O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation, South Africa (NRF Grant No. 98670 and 129370), and was supported by the Agricultural Research Council.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We are grateful to the Agricultural Research Council and the University of Limpopo.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohamed Zubi, W.S.; Mohd, M.H.; Mohamed Nor, N.M.I.; Zakaria, L. Fusarium species in mangrove soil in northern peninsular Malaysia and the soil physico-chemical properties. Microorganisms 2021, 9, 497. [Google Scholar] [CrossRef]
- Babadoost, M. Fusarium: Historical and continued importance. In Fusarium—Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers; Askun, T., Ed.; Intech Open: London, UK, 2018. [Google Scholar]
- Segal, B.H.; Walsh, T.J.; Liu, J.M.; Wilson, J.D.; Kwon-Chung, K.J. Invasive infection with Fusarium chlamydosporum in a patient with aplastic Anemia. J. Clin. Microbiol. 1998, 36, 1772–1776. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 1996, 74, 1195–1202. [Google Scholar] [CrossRef]
- Summerell, B.A.; Laurence, M.H.; Liew, E.C.Y.; Leslie, J.F. Biogeography and phylogeography of Fusarium: A review. Fungal Divers. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Summerell, B.A.; Leslie, J.F.; Liew, E.C.Y.; Laurence, M.H.; Bullock, S.; Petrovic, T.; Bentley, A.R.; Howard, C.G.; Peterson, S.A.; Walsh, J.L.; et al. Fusarium species associated with plants in Australia. Fungal Divers. 2011, 46, 1–27. [Google Scholar] [CrossRef]
- Thiel, P.G.; Marasas, W.F.O.; Sydenham, E.W.; Shephard, G.S.; Gelderblom, W.C.A. The implications of naturally occurring levels of fumonisins in corn for human and animal health. Mycopathologia 1992, 117, 3–9. [Google Scholar] [CrossRef]
- Cahagnier, B.; Melcion, D.; Richard-Molard, D. Growth of Fusarium moniliforme and its biosynthesis of fumonisin B1 on maize grain as a function of different water activities. Lett. Appl. Microbiol. 1995, 20, 247–251. [Google Scholar] [CrossRef]
- Lew, H.; Adler, A.; Ediner, W. Moniliformin and the European corn borer (Ostrnia nubilalis). Mycotoxin Res. 1991, 79, 727–731. [Google Scholar]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Logrieco, A.; Mule, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Windels, C.E. Economic and social impacts of Fusarium head blight: Changing farms and rural communities in the Northern Great Plains. Phytopathology 2000, 90, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fkikm usarium wilt of banana. Phytopathology 1990, 105, 1512–1521. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 2006, 96, 653–656. [Google Scholar] [CrossRef]
- Headrick, J.; Pataky, J. Maternal influence on the resistance of sweet corn lines to kernel infection by Fusarium moniliforme. Phytopathology 1991, 81, 268–274. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Carlton, M.W. Influence of inoculation method on systemic Fusarium moniliforme infection of maize plants grown from infected seeds. Plant Dis. 1997, 81, 211–216. [Google Scholar] [CrossRef]
- Lewandowski, S.M.; Bushnell, W.R.; Evans, C.K. Distribution of mycelial colonies and lesions in field-grown barley inoculated with Fusarium graminearum. Phytopathology 2006, 96, 567–581. [Google Scholar] [CrossRef]
- Incremona, M.E.; Gonzalez, M.; Pioli, R.N.; Salinas, A. Infection of maize silks by a native Fusarium (Fusarium graminearum) isolate in Argentina. Chil. J. Agric. Anim. Sci. 2014, 30, 203–211. [Google Scholar]
- Voss, K.A.; Plattner, R.D.; Riley, R.T.; Meredith, F.I.; Norred, W.P. In vivo effects of fumonisin B1-producing and fumonisin B1 nonproducing Fusarium moniliforme isolates are similar: Fumonisins B2 and B3 cause hepato- and nephrotoxicity in rats. Mycopathologia 1998, 141, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Cartwright, R.D.; Xie, W.; Shier, W.T. Aflatoxin and fumonisin contamination of corn (maize, Zea mays) hybrids in Arkansas. Crop. Prot. 2006, 25, 1–9. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing Professional: Ames, IA, USA, 2006. [Google Scholar]
- Marasas, W.F.O.; Nelson, P.E.; Toussoun, T.A. Toxigenic Fusarium Species Identity and Mycotoxicology; The Pennsylvania State University Press: Pennsylvania, PA, USA, 1984. [Google Scholar]
- Desjardin, A.E. Fusarium Mycotoxins: Chemistry, Genetics and Biology; American Phytopathological Society Press: Saint Paul, MN, USA, 2006; pp. 335–336. [Google Scholar]
- Garcia, D.; Barros, G.; Chulze, S.; Ramos, A.J.; Sanchis, V.; Marín, S. Impact of cycling temperatures on Fusarium verticillioides and Fusarium graminearum growth and mycotoxins production in soybean. J. Sci. Food Agric. 2012, 92, 2952–2959. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Wehner, F.C.; Van Rensburg, S.J.; Van Schlkwyk, D.J. Mycoflora of corn produced in human oesophageal cancer areas in Transkei. Phytopathology 1981, 71, 792–796. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Nurdijati, S.B.; Salleh, B. An overview of plant-derived products on control of mycotoxigenic fungi and mycotoxins. Asian J. Plant. Sci. 2010, 9, 126. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- United Nations Economic and Social Council. Progress towards the Sustainable Development Goals—E/2021/58. 2021. Available online: https://undocs.org/en/E/2021/58 (accessed on 17 October 2021).
- Wang, C.W.; Ai, J.; Fan, S.T.; Lv, H.Y.; Qin, H.Y.; Yang, Y.M.; Liu, Y.X. Fusarium acuminatum: A new pathogen causing postharvest rot on stored kiwifruit in China. Am. Phytopathol. Soc. 2015, 99, 1644. [Google Scholar] [CrossRef]
- Zhang, J.B.; Li, H.P.; Dang, F.J.; Qu, B.; Xu, Y.B.; Zhao, C.S.; Liao, Y.C. Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol. Res. 2007, 111, 967–997. [Google Scholar] [CrossRef]
- Casulli, F.; Pancaldi, D.; De Lillo, E.; Alberti, I. Observations on wheat crown rot and head blight caused by Fusarium spp. in Italy. In Proceedings of the Abstracts of International Seminar on Fusarium Mycotoxins, Taxonomy and Pathogenicity, Martina Franca, Italy, 9–13 May 1995; pp. 139–140. [Google Scholar]
- Pancaldi, D.; Casulli, F.; Grazzi, G.; Grifoni, F. Indagine sulla fusariosi della spiga del frumento duro in Emilia Romagna. Inf. Fitopatol. 1997, 47, 43–48. [Google Scholar]
- Menniti, A.M.; Pancaldi, D.; Maccaferri, M.; Casalini, L. Effect of fungicides on Fusarium head blight and deoxynivalenol content in durum wheat grain. Eur. J. Plant Pathol. 2003, 109, 109–115. [Google Scholar] [CrossRef]
- Boutigny, A.L.; Ward, T.J.; Van Coller, G.J.; Flett, B.; Lamprecht, S.C.; O’Donnell, K.; Viljoen, A. Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genet. Biol. 2011, 48, 914–920. [Google Scholar] [CrossRef]
- Chelkowski, J. Formation of mycotoxins produced by Fusaria in head of wheat, triticale, and rye. In Fusarium, Mycotoxins, Taxonomy and Pathogenicity; Chelkowski, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Scott, D.B.; De Jager, E.J.H.; van Wyk, P.S. Head blight of irrigated wheat in South Africa. Phytophylactica 1988, 20, 317–319. [Google Scholar]
- Bencheikh, A.; Rouag, N.; Mamache, W.; Belabed, I. First report of Fusarium equiseti causing crown rot and damping-off on durum wheat in Algeria. Arch. Phytopathol. Plant Prot. 2020, 53, 915–931. [Google Scholar] [CrossRef]
- Díaz-Nájera, J.F.; Ayvar-Serna, S.; Mena-Bahena, A.; Baranda-Cruz, E.; Vargas-Hernández, M.; Alvarado-Gómez, O.G.; Fuentes-Aragón, D. First report of Fusarium falciforme (FSSC 3+4) causing wilt disease of Phaseolus vulgaris in Mexico. Plant Dis. 2021, 105, 710. [Google Scholar] [CrossRef]
- Cen, Y.K.; Lin, J.G.; Wang, Y.L.; Wang, J.Y.; Liu, Z.Q.; Zheng, Y.G. The gibberellin producer Fusarium fujikuroi: Methods and technologies in the current toolkit. Front. Bioeng. Biotechnol. 2020, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Na, F.; Carrillo, J.D.; Mayorquin, J.S.; Ndinga-Muniania, C.; Stajich, J.E.; Stouthamer, R.; Huang, Y.T.; Lin, Y.T.; Chen, C.Y.; Eskalen, A. Two novel fungal symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of kuroshio shot hole borer (Euwallacea sp. nr. fornicatus) cause Fusarium dieback on woody host species in California. Plant Dis. 2018, 102, 1154–1164. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Chen, Q.; Wang, M.; Hsiang, T.; Shang, S.; Yu, Z. Metabolic effects of azoxystrobin and kresoxim-methyl against Fusarium kyushuense examined using the Biolog FF MicroPlate. Pestic. Biochem. Physiol. 2016, 130, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Divon, H.H.; Bøe, L.; Tveit, M.M.N.; Klemsdal, S.S. Infection pathways and penetration modes of Fusarium langsethiae. Eur. J. Plant Pathol. 2019, 154, 259–271. [Google Scholar] [CrossRef]
- Chełkowski, J.; Goliński, P.; Perkowski, J.; Visconti, A.; Rakowska, M.; Wakuliński, W. Toxinogenicity of Microdochium nivale (Fusarium nivale) isolates from cereals in Poland. Mycotoxin Res. 1991, 7, 140–145. [Google Scholar] [CrossRef]
- Balmas, V.; Corda, P.; Marcello, A.; Bottalico, A. Fusarium nygamai associated with Fusarium foot rot of rice in Sardinia. Plant Dis. 2000, 84, 807. [Google Scholar] [CrossRef]
- Martyn, R.D.; Hartz, T.K. Use of soil solarization to control Fusarium wilt of watermelon. Plant Dis. 1986, 79, 762–766. [Google Scholar] [CrossRef]
- Beck, K.D.; Reyes-Corral, C.; Rodriguez-Rodriguez, M.; May, C.; Barnett, R.; Thornton, M.K.; Bates, A.A.; Woodhall, J.W.; Schroeder, B.K. First report of Fusarium proliferatum causing necrotic leaf lesions and bulb rot on storage onion (Allium cepa) in Southwestern Idaho. Plant Dis. 2020, 105, 494. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.F.; Hwang, S.F.; Conner, R.L.; Ahmed, H.U.; Zhou, Q.; Turnbull, G.D.; Strelkov, S.E.; McLaren, D.L.; Gossen, B.D. First report of Fusarium proliferatum causing root rot in soybean (Glycine max L.) in Canada. Crop. Prot. 2015, 67, 52–58. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Manandhar, H.K.; Plattner, R.D.; Manandhar, G.G.; Poling, S.M.; Maragos, C.M. Fusarium species from nepalese rice and production of mycotoxins and gibberellic acid by selected species. Appl. Environ. Microbiol. 2000, 66, 1020–1102. [Google Scholar] [CrossRef]
- Wharton, P.S.; Tumbalam, P.; Kirk, W.W. First report of potato tuber sprout rot caused by Fusarium sambucinum in Michigan. disease notes. Am. Phytophalogical Soc. 2006, 90, 1460. [Google Scholar]
- Ibrahim, N.F.; Masratul, H.M.; Nor, N.M.I.M.; Latiffah, Z. Pathogenicity of Fusarium semitectum and Fusarium chlamydosporum associated with pineapple fusariosis. Malays. J. Microbiol. 2016, 12, 164–170. [Google Scholar]
- Latiffah, Z.; Nurul Huda, M.S.; Akram, T.M.A.T.A. Characterization of Fusarium semitectum from isolates vegetable fruits. Sains Malays. 2013, 42, 629–633. [Google Scholar]
- Sampaio, T.S.; de Castro Nizio, D.A.; White, L.A.S.; de Oliveira Melo, J.; Almeida, C.S.; Alves, M.F.; Gagliardi, P.R.; de Fátima Arrigoni-Blank, M.; Junior, A.W.; Sobral, M.E.G.; et al. Chemical diversity of a wild population of Myrcia ovata Cambessedes and antifungal activity against Fusarium solani. Ind. Crop. Prod. 2016, 86, 196–209. [Google Scholar] [CrossRef]
- Leslie, J.; Bandyopadhyay, R.; Visconti, A. (Eds.) Mycotoxins: Detection Method, Management, Public Health and Agricultural Trade; Centre for Agriculture and Biosciences International (CABI): Wallingford, UK, 2008. [Google Scholar]
- Booth, C. Fusarium: Laboratory Guide to the Identification of the Major Species; The Common Wealth Mycological Institute: Kew, UK, 1971; p. 237. [Google Scholar]
- Viljoen, A.; Marasas, W.F.O.; Wingfield, M.J.; Viljoen, C.D. Characterization of Fusarium subglutinans f. sp. pini causing root disease of Pinus patula seedlings in South Africa. Mycol. Res. 1997, 101, 437–445. [Google Scholar] [CrossRef]
- Sun, X.; Yang, B.I.; Li, Y.; Han, R.; Ge, Y. Postharvest chitosan treatment induces resistance in potato against Fusarium sulphureum. Agric. Sci. China 2008, 7, 615–621. [Google Scholar] [CrossRef]
- Li, Y.C.; Bi, Y.; Ge, Y.H.; Sun, X.J.; Wang, Y. Antifungal activity of sodium silicate on Fusarium sulphureum and its effect on dry rot of potato tubers. J. Food Sci. 2009, 74, M213–M218. [Google Scholar] [CrossRef]
- Klittich, C.J.R.; Leslie, J.F.; Nelson, P.E.; Marasas, W.F.O. Fusarium thapsinum (Gibberella thapsina): A new species in section liseola from Sorghum. Mycologia 1997, 89, 643–652. [Google Scholar] [CrossRef]
- Castañares, E.; Stenglein, S.A.; Dinolfo, M.I.; Moreno, M.V. Fusarium tricinctum associated with head blight on wheat in Argentina. Plant Dis. 2011, 95, 496. [Google Scholar] [CrossRef]
- Moreira, G.M.; Machado, F.J.; Pereira, C.B.; Neves, D.L.; Tessmann, D.J.; Ward, T.J.; Del Ponte, E.M. First report of the Fusarium tricinctum species complex causing Fusarium head blight of wheat in Brazil. Plant Dis. 2019, 104, 586. [Google Scholar] [CrossRef]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Desjardins, A.E. Fumonisins in maize: Can we reduce their occurrence? Plant Dis. 1997, 81, 556–565. [Google Scholar] [CrossRef] [PubMed]
- De León, C.; Pandey, S. Improvement of resistance to ear and stalk rots and agronomic traits in tropical maize gene pools. Crop Sci. 1989, 29, 12–17. [Google Scholar] [CrossRef]
- King, S.B.; Scott, G.E. Genotypic differences in maize to kernel infection by Fusarium moniliforme. Phytopathology 1981, 71, 1245–1247. [Google Scholar] [CrossRef]
- Ochor, T.E.; Trevathan, L.E.; King, S.B. Relationship of harvest date and host genotype to infection of maize kernels by Fusarium moniliforme. Plant Dis. 1987, 71, 311–313. [Google Scholar] [CrossRef]
- Smith, I.M. (Ed.) Fungicides for Crop Protection: 100 Years of Progress. Proceedings; British Crop Protection Council: London, UK, 1986. [Google Scholar]
- Sumner, D.A. Imperfect information and intervention in farm credit: Discussion. Am. J. Agric. Econ. 1990, 72, 780–781. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Colleate, A.; Baxter, E.S. Relationship between growth and mycotoxin production by Fusarium species, biocides and environment. Eur. J. Plant Pathol. 2002, 108, 685–690. [Google Scholar] [CrossRef]
- Freije, A.N.; Wise, K.A. Impact of Fusarium graminearum inoculum availability and fungicide application timing on Fusarium head blight in wheat. Crop. Prot. 2015, 77, 139–147. [Google Scholar] [CrossRef]
- Zhang, M.; Jeyakumar, J.M.J. Fusarium species complex causing Pokkah Boeng in China. In Fusarium-Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers; Intech Open: London, UK, 2018. [Google Scholar]
- Shi, X.; Qiao, K.; Li, B.; Zhang, S. Integrated management of Meloidogyne incognita and Fusarium oxysporum in cucumber by combined application of abamectin and fludioxonil. Crop. Prot. 2019, 126, 104922. [Google Scholar] [CrossRef]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, D.; Visconti, A. Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J. Sci. Food Agric. 2005, 85, 191–198. [Google Scholar] [CrossRef]
- Cuypers, E.; Vanhove, W.; Gotink, J.; Bonneure, A.; Van Damme, P.; Tytgat, J. The use of pesticides in Belgian illicit indoor cannabis plantations. Forensic Sci. Int. 2017, 277, 59–65. [Google Scholar] [CrossRef]
- Igbedioh, S.O. Effects of agricultural pesticides on humans, animals and higher plants in developing countries. Arch. Environ. Health 1991, 46, 218. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Renfrew, M.J.; Woolridge, M.W. Assessing the risk of pesticide residues to consumers: Recent and future developments. Food Addit. Contam. 2001, 18, 1124–1129. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- MartÍnez, J.A. Natural fungicides obtained from plants, fungicides for plant and animal diseases. In Fungicides for Plant and Animal Diseases; Dhanasekaran, D., Ed.; InTech Open: Shanghai, China, 2012. [Google Scholar]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Oyeleke, S.B.; Oyewole, O.A.; Dagunduro, J.N. Effect of herbicide (pendimethalin) on soil microbial population. J. Sci. Food Agric. 2011, 1, 40–43. [Google Scholar]
- Wang, X.; Song, M.; Gao, C.; Dong, B.; Zhang, Q.; Fang, H.; Yu, Y. Carbendazim induces a temporary change in soil bacterial community structure. J. Environ. Sci. 2009, 21, 1679–1683. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G., Jr. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616, 255–268. [Google Scholar] [CrossRef]
- Molina, R. Methyl bromide, brief description of its toxicology as a basis for occupational health surveillance. Cienc. Trab. 2007, 26, 182–185. [Google Scholar]
- Meadows, R. Researchers develop alternatives to methyl bromide fumigation. Calif. Agric. 2013, 67, 125–127. [Google Scholar] [CrossRef]
- Backstrom, M.J. Methyl bromide: The problem, the phase out, and the alternatives. Drake J. Agric. L. 2002, 7, 213–239. [Google Scholar]
- Possiede, Y.M.; Gabardo, J.; Kava, V.; Galli-Terasawa, L.V.; Azevedo, J.L.; Glienke, C. Fungicide resistance and genetic variability in plant pathogenic strains of Guignardia citricarpa. Braz. J. Microbiol. 2009, 40, 308–313. [Google Scholar] [CrossRef]
- Ishii, H. Impact of fungicide resistance in plant pathogens on crop disease control and agricultural environment. Jpn. Agric. Res. Q. 2006, 40, 205–211. [Google Scholar] [CrossRef]
- Gea, F.J.; Navarro, M.J.; Tello, J.C. Reduced sensitivity of the mushroom pathogen Verticillium fungicola to prochloraz-manganese in vitro. Mycol Res. 2005, 109, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Gea, F.J.; Tello, J.C.; Navarro, M.J. Efficacy and effects on yield of different fungicides for control of wet bubble disease of mushroom caused by the mycoparasite Mycogone perniciosa. Crop. Prot. 2010, 29, 1021–1025. [Google Scholar] [CrossRef]
- Potočnik, I.S.; Stepanović, M.; Rekanović, E.; Todorović, B.; Milijašević-Marčić, S. Disease control by chemical and biological fungicides in cultivated mushrooms: Button mushroom, oyster mushroom and shiitake. Pestic. Fitomed. 2015, 30, 201–208. [Google Scholar] [CrossRef]
- Francis, P. Targeting cell death in Dementia. Alzheimer Dis. Assoc. Disord. 2006, 20, S3–S7. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Anticholinesterase insecticide retrospective. Chem. Biol. Interact. 2013, 203, 221–225. [Google Scholar] [CrossRef]
- Fournier, D. Mutations of acetylcholinesterase which confer insecticide resistance in insect populations. Chem. Biol. Interact. 2005, 157, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Syngenta. Safety Data Sheet, According to Regulation (EC) No. 1907/2006. Available online: https://www.syngenta.co.za/sites/g/files/zhg436/f/media/2019/09/25/scholar_sds-_12072017.pdf?token=1569406952 (accessed on 31 March 2021).
- Al-Mughrabi, K. Biological control of Fusarium dry rot and other potato tuber diseases using Pseudomonas fluorens and Enterobacter cloacae. Biol. Control 2010, 53, 280–284. [Google Scholar] [CrossRef]
- WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020; p. 52. [Google Scholar]
- Prieto, J.A.; Patiño, O.J.; Plazas, E.A.; Pabón, L.C.; Ávila, M.C.; Guzmán, J.D.; Delgado, W.A.; Cuca, L.E. Natural products from plants as potential source agents for controlling Fusarium. In Fungicides, Showcases of Intergrated Plant Disease Management from Around the World; Nita, M., Ed.; Intech Open: London, UK, 2013. [Google Scholar]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? 2nd ed.; FRAC: Brussels, Belgium, 2007; p. 37. [Google Scholar]
- Delp, C.J. Coping with resistance to plant disease control agents. Plant Dis. 1980, 64, 652–657. [Google Scholar] [CrossRef]
- Owusu, A.M.; Owusu, A.M. Consumer willingness to pay a premium for organic fruit and vegetable in Ghana. Int. Food Agribus. Manag. Rev. 2013, 16, 67–86. [Google Scholar]
- Thembo, K.M.; Vismer, H.F.; Nyazema, N.Z.; Gelderblom, W.C.A.; Katerere, D.R. Antifungal activity of four weedy plant extracts against selected mycotoxigenic fungi. J. Appl. Microbiol. 2010, 109, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Mabagala, R.B.; Mamiro, D.P. Efficacy of selected plant extracts against Pyricularia grisea, causal agent of rice blast disease. Am. J. Plant Sci. 2015, 6, 602–611. [Google Scholar] [CrossRef]
- Ntow, W.J. The Use and Fate of Pesticide in Vegetable-Based Agroecosystems in Ghana; Taylor & Francis: Leiden, The Netherlands, 2008; pp. 10–24. [Google Scholar]
- Coulibaly, O.; Cherry, A.J.; Nouhoheflin, T.; Aitchedji, C.C.; Al-Hassan, R. Vegetable producer perceptions and willingness to pay for biopesticides. J. Veg. Sci. 2007, 12, 27–42. [Google Scholar] [CrossRef]
- Rathi, M.; Gopalakrishnan, S. Insecticidal activity of aerial parts of Synedrella nodiflora Gaertn (Compisitae) on Spodoptera litura (Fab). J. Cent. Eur. Agric. 2006, 6, 223–228. [Google Scholar]
- Wei, S.J.; Shi, B.C.; Gong, Y.J.; Jin, G.H.; Chen, X.X.; Meng, X.F. Genetic structure and demographic history reveal migration of the diamond back moth Plutella xylostella (Lepidoptera: Plutellide) from the Southern to Northern Regions of China. PLoS ONE 2013, 8, e59654. [Google Scholar]
- Kumar, R.; Mishra, A.K.; Dubey, N.K.; Tripathi, Y.B. Evaluation Chenopodium ambrosoides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int. J. Food Microbiol. 2007, 115, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mahmood., I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- Masika, P.J.; Afolayan, A.J. Antimicrobial activity of some plants used for the treatment of livestock disease in the Eastern Cape, South Africa. J. Ethnopharmacol. 2002, 83, 129–134. [Google Scholar] [CrossRef]
- Ribeiro, A.; Romeiras, M.M.; Tavares, J.; Faria, M.T. Ethnobotanical survey in Canhane village, district of Massingir, Mozambique: Medicinal plants and traditional knowledge. J. Ethnobiol. Ethnomed. 2010, 6, 33. [Google Scholar] [CrossRef]
- Mdee, L.K.; Masoko, P.; Eloff, J.N. The activity of extracts of seven common invasive plant species on fungal phytopathogens. S. Afr. J. Bot. 2009, 75, 375–379. [Google Scholar] [CrossRef]
- Amadioha, A.C. Controlling Rice Blast in vitro and in vivo with extracts of Azadirachta indica. J. Crop. Prot. 2000, 19, 287–290. [Google Scholar] [CrossRef]
- Mahlo, S.M.; McGaw, L.J.; Eloff, J.N. Antifungal activity of leaf extracts from South African trees against plant pathogens. Crop. Prot. 2010, 29, 1529–1533. [Google Scholar] [CrossRef]
- Harborne, J.B.; Baxter, H.; Moss, G.P. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; Taylor & Francis: London, UK, 1995. [Google Scholar]
- Ahmad, I.; Beg, A.Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J. Ethnopharmacol. 2001, 74, 113–123. [Google Scholar] [CrossRef]
- Fandohan, P.; Gbenou, J.D.; Gnonlonfin, B.; Hell, K.; Marasas, W.F.; Wingfield, M.J. Effect of essential oils on the growth of Fusarium verticillioides and fumonisin contamination in corn. J. Agric. Food Chem. 2004, 52, 6824–6829. [Google Scholar] [CrossRef]
- Calvo, M.A.; Arosemena, E.L.; Shiva, C.; Adelantado, C. Antimicrobial activity of plant natural extracts and essential oils. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; FORMATEX: Barcelona, Spain, 2011; pp. 1179–1185. [Google Scholar]
- Brinker, F. Herb Contraindications and Drug Interactions, 2nd ed.; Eclectic Medical Publications: Sandy, Oregon, 1998. [Google Scholar]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.J. Challenges of biopesticides under the European regulation (EC) No. 1107/2009: An overview of new trends in residue analysis. Stud. Nat. Prod. Chem. 2014, 43, 437–482. [Google Scholar]
- Rishi, K.; Singh, R. Chemical components and insecticidal properties of Bakain (Melia azedarach L.)—A review. Agric. Revolut. 2003, 24, 101–115. [Google Scholar]
- Sultana, S.; Akhtar, N.; Asif, H.M. Phytochemical screening and antipyretic effects of hydro-methanol extract of Melia azedarach leaves in rabbits. Bangladesh J. Pharmacol. 2013, 8, 214–217. [Google Scholar] [CrossRef]
- Cowie, B.W.; Venter, N.; Witkowski, E.T.F.; Bryne, M.J.; Olckers, T. A review of Solanum mauritianum biocontrol: Prospects, promise and problems: A way forward for South Africa and globally. BioControl 2018, 63, 475–491. [Google Scholar] [CrossRef]
- Seepe, H.A.; Amoo, S.O.; Nxumalo, W.; Adeleke, R.A. Antifungal activity of medicinal plant extracts for potential management of Fusarium pathogens. Res. Crop. 2019, 20, 399–406. [Google Scholar]
- Parekh, J.; Chanda, S.V. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 2007, 3, 53–58. [Google Scholar]
- Van Vuuren, S.F.; Naidoo, D. An antibacterial investigation of plants used traditionally in South Africa to treat sexually transmitted infections. J. Ethnopharmacol. 2010, 130, 552–558. [Google Scholar] [CrossRef]
- Kitonde, C.K.; Fidahusein, D.S.; Lukhoba, C.W.; Jumba, M.M. Antimicrobial activity and phytochemical screening of Senna didymobotrya used to treat bacterial and fungal infections in Kenya. Int. J. Educ. Res. 2014, 2, 1–12. [Google Scholar]
- Bhattacharjee, I.; Chatterjee, S.K.; Ghosh, A.; Chandra, G. Antibacterial activities of some plant extracts used in Indian traditional folk medicine. Asian Pac. J. Trop. Biomed. 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Seepe, H.A.; Lodama, K.E.; Sutherland, R.; Nxumalo, W.; Amoo, S.O. In vivo antifungal activity of South African medicinal plant extracts against Fusarium pathogens and their phytotoxicity evaluation. Plants 2020, 9, 1668. [Google Scholar] [CrossRef]
- Seepe, H.A.; Amoo, S.O.; Nxumalo, W.; Adeleke, R.A. Sustainable use of thirteen South African medicinal plants for the management of crop diseases caused by Fusarium species–An in vitro study. S. Afr. J. Bot. 2020, 130, 456–464. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complementary Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou-Doltsinis, S.; Markellou, E.; Kasselaki, A.M.; Fanouraki, M.N.; Koumaki, C.M.; Schmitt, A.; Liopa-Tsakalidis, A. Efficacy of Milsana®, a formulated plant extract from Reynoutria sachalinensis, against powdery mildew of tomato (Leveillula taurica). BioControl 2006, 51, 375–392. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest. Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef] [PubMed]
- Nukenine, E.N.; Tofel, H.K.; Adler, C. Comparative efficacy of NeemAzal® and local botanicals derived from Azadirachta indica and Plectranthus glandulosus against Sitophilus zeamais on maize. J. Pest. Sci. 2011, 84, 479–486. [Google Scholar] [CrossRef]
- Danga, S.P.Y.; Nukenine, E.N.; Fotso, G.T.; Adler, C. Use of NeemPro®, a neem product to control maize weevil Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae) on three maize varieties in Cameroon. Agric. Food Secur. 2015, 4, 18. [Google Scholar] [CrossRef]
- Nuzhat, T.; Vidyasagar, G.M. Antifungal investigations on plant essential oils. A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 19–28. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Koul, O.; Walial, S.; Dhaliwal, G.S. Essential Oils as Green Pesticides: Potential and Constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- UNIDO; FAO. Herbs, Spices and Essential Oils Post-Harvest Operations in Developing Countries; United Nation Industrial Development Organisation: Vienna, Austria, 2005; p. 9. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Van de Braak, S.A.A.J.; Leijten, G.C.J.J. Essential Oils and Oleoresins: A Survey in the Netherlands and other Major Markets in the European Union; CBI, Centre for the Promotion of Imports from Developing Countries: Rotterdam, The Netherlands, 1999; p. 116. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Zaika, L.L. Spices and herbs: Their antimicrobial activity and its determination. J. Food Saf. 1988, 9, 97–118. [Google Scholar] [CrossRef]
- Yu, J.; Su, J.; Li, F.; Gao, J.; Li, B.; Pang, M.; Lv, G.; Chen, S. Identification and quantification of pine needle essential oil from different habitats and species of China by GC-MS and GC method. Afr. J. Tradit. Complementary Altern. Med. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Celikel, N.; Kavas, G. Antimicrobial properties of some essential oils against some pathogenic microorganisms. Czech. J. Food Sci. 2008, 26, 174–181. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Sokovic’, M.; van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post-harvest fungal infestation of food commodities. Innov. Food Sci. Emerg. Technol. 2008, 9, 575–580. [Google Scholar] [CrossRef]
- Mahboubi, M.; Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 2008, 119, 325–327. [Google Scholar] [CrossRef]
- Mishra, P.K.; Shukla, R.; Singh, P.; Prakash, B.; Kedia, A.; Dubey, N.K. Antifungal, antiaflatoxigenic and antioxidant efficacy of Jamrosa essential oil for preservation of herbal raw materials. Int. Biodeterior. Biodegrad. 2012, 74, 11–16. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crop. Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Chutia, M.; Mahanta, J.J.; Saikia, R.C.; Baruah, A.K.S.; Sarma, T.C. Influence of leaf blight disease on yield of oil and its constituents of Java citronella and in-vitro control of the pathogen using essential oils. World J. Agric. Res. 2006, 2, 319–321. [Google Scholar]
- Da Cruz Cabral, L.; Pinto, V.F.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pizzolitto, R.P.; Jacquat, A.G.; Usseglio, V.L.; Achimón, F.; Cuello, A.E.; Zygadlo, J.A.; Dambolena, J.S. Quantitative-structure-activity relationship study to predict the antifungal activity of essential oils against Fusarium verticillioides. Food Control 2020, 108, 106836. [Google Scholar] [CrossRef]
- Gibbons, S. Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem. Rev. 2005, 4, 63–78. [Google Scholar] [CrossRef]
- Kumar, S.; Javed, M.S.; Kumar, P.; Gupta, S.; Kumar, R.; Singh, P.K. In-vitro antifungal and anti-bacterial activity of chloroform extract from tubers of Aconitum laeve Royle: Endangered species, India. Mater. Today Proc. 2021, 34, 563–568. [Google Scholar] [CrossRef]
- Kalidindi, N.; Thimmaiah, N.V.; Jagadeesh, N.V.; Nandeep, R.; Swetha, S.; Kalidindi, B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. leaves. J. Food Drug Anal. 2015, 23, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Meela, M.M.; Mdee, L.K.; Masoko, P.; Eloff, J.N. Acetone leaf extracts of seven invasive weeds have promising activity against eight important plant fungal pathogens. S. Afr. J. Bot. 2019, 121, 442–446. [Google Scholar] [CrossRef]
- Grollman, A.P.; Marcus, D.M. Global hazards of herbal remedies: Lessons from Aristolochia. EMBO Rep. 2016, 17, 619–625. [Google Scholar] [CrossRef]
- Liu, T.T.; Wu, H.B.; Wu, H.B.; Zhang, J. Wormwood (Artemisia absinthium L.) as a promising nematicidal and antifungal agent: Chemical composition, comparison of extraction techniques and bioassay-guided isolation. Ind. Crop. Prod. 2019, 133, 295–303. [Google Scholar] [CrossRef]
- Rosado-Álvarez, C.; Molinero-Ruiz, L.; Rodríguez-Arcos, R.; Basallote-Ureba, M.J. Antifungal activity of asparagus extracts against phytopathogenic Fusarium oxysporum. Sci. Hortic. 2014, 171, 51–57. [Google Scholar] [CrossRef]
- Dikhoba, P.M.; Mongalo, N.I.; Elgorashi, E.E.; Makhafola, T.J. Antifungal and anti-mycotoxigenic activity of selected South African medicinal plants species. Heliyon 2019, 5, e02668. [Google Scholar] [CrossRef]
- Mongalo, N.I.; Dikhoba, P.M.; Soyingbe, S.O.; Makhafola, T.J. Antifungal, anti-oxidant activity and cytotoxicity of South African medicinal plants against mycotoxigenic fungi. Heliyon 2018, 4, e00973. [Google Scholar] [CrossRef] [PubMed]
- Mahwasane, S.; Middleton, L.; Baoduo, N. An ethnobotanical survey of indigenous knowledge on medicinal plants used by the traditional healers of the Lwamondo area, Limpopo province, South Africa. S. Afr. J. Bot. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Al-Qurainy, F.; Abdel-Rhman, Z.G.; Khan, S.; Nadeem, M.; Tarroum, M.; Alaklabi, A.; Thomas, J. Antibacterial activity of leaf extract of Breonadia salicina (Rubeaceae), an endangered medicinal plant of Saudi Arabia. Genet. Mol. Res. 2013, 12, 3212–3219. [Google Scholar] [CrossRef] [PubMed]
- Mogashoa, M.M.; Masoko, P.; Eloff, J.N. Different Combretum molle (Combretaceae) leaf extracts contain several different antifungal and antibacterial compounds. S. Afr. J. Bot. 2019, 126, 322–327. [Google Scholar] [CrossRef]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, D.; Stefani, E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. S. Afr. J. Bot. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Eloff, J.N.; Angeh, I.E.; McGaw, L.J. Solvent-solvent fractionation can increase the antifungal activity of a Melianthus comosus (Melianthaceae) acetone leaf extract to yield a potentially useful commercial antifungal product. Ind. Crop. Prod. 2017, 110, 103–112. [Google Scholar] [CrossRef]
- Maroyi, A. A review of the ethnomedicinal uses, phytochemistry and pharmacological properties of Melianthus comosus Vahl. J. Pharm. Sci. Res. 2019, 11, 3655–3660. [Google Scholar]
- Molele, P.K.; Mongalo, N.I.; Dikhoba, P.M.; Makhafola, T.J. Antifungal and antioxidant properties of ten medicinal plants collected from KwaDlangezwa area, KwaZulu-Natal Province. S. Afr. J. Bot 2016, 2, 115. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Xiang, F.; Li, S.; Yang, G. Antifungal activity of Momordica charantia seed extracts toward the pathogenic fungus Fusarium solani L. J. Food Drug Anal. 2016, 24, 881–887. [Google Scholar] [CrossRef]
- Krawinkel, M.B.; Keding, G.B. Bitter gourd (Momordica charantia): A dietary approach to hyperglycemia. Nutr. Rev. 2006, 64, 331–337. [Google Scholar] [CrossRef]
- De Rodríguez, D.J.; Trejo-González, A.F.; Rodríguez-García, R.; Díaz-Jimenez, M.L.V.; Sáenz-Galindo, A.; Hernández-Castillo, F.D.; Villarreal-Quintanilla, J.A.; Pena-Ramos, F.M. Antifungal activity in vitro of Rhus muelleri against Fusarium oxysporum f. sp. lycopersici. Ind. Crop. Prod. 2015, 75, 150–158. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Alotibi, F.O.; Ashour, E.H.; Al-Basher, G. Evaluation of the antifungal activity of Rumex vesicarius L. and Ziziphus spina-christi (L.) Desf. Aqueous extracts and assessment of the morphological changes induced to certain myco-phytopathogens. Saudi J. Biol. Sci. 2020, 27, 2818–2828. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.A.M.; EL Bakry, A.A.; Eman, A.A. Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicarius L. (Polygonaceae). Int. J. Pharm. Pharm. Sci. 2011, 3, 109–118. [Google Scholar]
- Mahendra, C.; Murali, M.; Manasa, G.; Sudarshana, M.S. Biopotentiality of leaf and leaf derived callus extracts of Salacia macrosperma Wight—An endangered medicinal plant of Western Ghats. Ind. Crop. Prod. 2020, 143, 111921. [Google Scholar]
- Mirahmadi, S.F.; Norouzi, R. Chemical composition, phenolic content, free radical scavenging and antifungal activities of Achillea biebersteinii. Food Biosci. 2017, 18, 53–59. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Saffarian, Z. Anti-fungal activity of some native essential oils against emerging multidrug resistant human non dermatophytic moulds. J. Herb. Med. 2020, 23, 100370. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Z.; Gu, H.; Yang, F.; Zhang, L.; Yang, L. Improved method to obtain essential oil, Asarinin and Sesamin from Asarum heterotropoides var. mandshuricum using microwave-assisted steam distillation followed by solvent extraction and antifungal activity of essential oil against Fusarium spp. Ind. Crop. Prod. 2021, 162, 113295. [Google Scholar] [CrossRef]
- Mohammadi, A.; Nazari, H.; Imani, S.; Amrollahi, H. Antifungal activities and chemical composition of some medicinal plants. J. Mycol. Med. 2014, 24, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.C.; Regnier, T.; Olivier, E.I. Antimicrobial activities of selected essential oils against Fusarium oxysporum isolates and their biofilms. S. Afr. J. Bot. 2015, 99, 115–121. [Google Scholar] [CrossRef]
- Chutia, M.; Bhuyan, P.D.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India, LWT. Food Sci. Technol. 2009, 42, 777–780. [Google Scholar]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Gođevac, D.; Dimkić, I.; Stanković, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crop. Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Naeini, A.; Ziglari, T.; Shokri, H.; Khosravi, A.R. Assessment of growth-inhibiting effect of some plant essential oils on different Fusarium isolates. J. Mycol Med. 2010, 20, 174–178. [Google Scholar] [CrossRef]
- Kumar, K.N.; Venkataramana, M.; Allen, J.A.; Chandranayaka, S.; Murali, H.S.; Batra, H.V. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum, LWT. Food Sci. Technol. 2016, 69, 522–528. [Google Scholar]
- Moghaddam, M.; Taheri, P.; Pirbalouti, A.G.; Mehdizadeh, L. Chemical composition and antifungal activity of essential oil from the seed of Echinophora platyloba DC. Against phytopathogens fungi by two different screening methods, LWT. Food Sci. Technol. 2015, 61, 536–542. [Google Scholar] [CrossRef]
- Davari, M.; Ezazi, R. Chemical composition and antifungal activity of the essential oil of Zhumeria majdae, Heracleum persicum and Eucalyptus sp. against some important phytopathogenic fungi. J. Mycol. Med. 2017, 27, 463–468. [Google Scholar] [CrossRef]
- Chen, F.; Guo, Y.; Kang, J.; Yang, X.; Zhao, Z.; Liu, S.; Ma, Y.; Gao, W.; Luo, D. Insight into the essential oil isolation from Foeniculum vulgare Mill. Fruits using double-condensed microwave-assisted hydrodistillation and evaluation of its antioxidant, antifungal and cytotoxic activity. Ind. Crop. Prod. 2020, 144, 112052. [Google Scholar] [CrossRef]
- Eke, P.; Adamou, S.; Fokom, R.; Nya, V.D.; Fokou, P.V.T.; Wakam, L.N.; Nwaga, D.; Boyom, F.F. Arbuscular mycorrhizal fungi alter antifungal potential of lemongrass essential oil against Fusarium solani, causing root rot in common bean (Phaseolus vulgaris L.). Heliyon 2020, 6, e05737. [Google Scholar] [CrossRef]
- Jamalian, A.; Shams-Ghahfarokhi, M.; Jaimand, K.; Pashootan, N.; Amani, A.; Razzaghi-Abyaneh, M. Chemical composition and antifungal activity of Matricaria recutita flower essential oil against medically important dermatophytes and soil-borne pathogens. J. Mycol. Med. 2012, 22, 308–315. [Google Scholar] [CrossRef]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crop. Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha Piperita L. (peppermint) essential oils. J. King Saud Univ. Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.C. Chemical composition and antifungal activity of essential oil and various extract of Silene armeria L. Bioresour. Technol. 2008, 99, 8903–8908. [Google Scholar] [CrossRef]
- Tegang, A.S.; Beumo, T.M.N.; Dongmo, P.M.J.; Ngoune, L.T. Essential oil of Xylopia aethiopica from Cameroon: Chemical composition, antiradical and in vitro antifungal activity against some mycotoxigenic fungi. J. King Saud Univ. Sci. 2018, 30, 466–471. [Google Scholar] [CrossRef]
- Li, K.M.; Dong, X.; Ma, Y.N.; Wu, Z.N.; Yan, Y.M.; Cheng, Y.X. Antifungal coumarins and lignans from Artemisia annua. Fitoterapia 2019, 134, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, K.; Yu, Y.; Deng, Z.; Kong, Z. In vitro antifungal activity of the extract and compound from Acorus tatarinowii against seven plant pathogenic fungi. Agric. Sci. China 2010, 9, 71–76. [Google Scholar] [CrossRef]
- Vogt, V.; Cifuente, D.; Tonn, C.; Sabini, L.; Rosas, S. Antifungal activity in vitro and in vivo of extracts and lignans isolated from Larrea divaricata Cav. against phytopathogenic fungus. Ind. Crop. Prod. 2013, 42, 583–586. [Google Scholar] [CrossRef]
- Seepe, H.A.; Ramakadi, T.G.; Lebepe, C.M.; Amoo, S.O.; Nxumalo, W. Antifungal activity of isolated compounds from the leaves of Combretum erythrophyllum (Burch.) Sond. and Withania somnifera (L.) dunal against Fusarium pathogens. Molecules 2021, 26, 4732. [Google Scholar] [CrossRef]
- Nguyen, D.M.C.; Seo, D.J.; Lee, H.B.; Kim, I.S.; Kim, K.Y.; Park, R.D.; Jung, W.J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb. Pathog. 2013, 56, 8–15. [Google Scholar] [CrossRef]
- Chen, J.; Yang, M.L.; Zeng, J.; Gao, K. New broad-spectrum antibacterial and antifungal alkaloids from Kopsia hainanensis. Phytochem. Lett. 2014, 7, 156–160. [Google Scholar] [CrossRef]
- Chamsai, J.; Siegrist, J.; Buchenauer, H. Mode of action of the resistance-inducing 3-aminobutyric acid in tomato roots against Fusarium wilt. J. Plant. Dis. Prot. 2004, 111, 273–291. [Google Scholar]
- Meng, S. Studies on Antifungal Activity and Mechanism of Bio-Active Components from Allium Chinense; Hunan Normal University: Changsha, China, 2006. [Google Scholar]
- Hu, L.B.; Zhou, W.; Zhang, T.; Yang, Z.M.; Xu, J.H.; Shi, Z.Q. Mechanism of inhibition to Fusarium moniliforme by antimicrobial peptide Fengycins. Microbiol. China 2010, 37, 251–255. [Google Scholar]
- Kawakami, K.; Inuzuka, H.; Hori, N.; Takahashi, N.; Ishida, K.; Mochizuki, K.; Ohkusu, K.; Muraosa, Y.; Watanabe, A.; Kamei, K. Inhibitory effects of antimicrobial agents against Fusarium species. Med. Mycol. 2015, 53, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Sahraoui, A.L. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef]
- Du, R.; Liu, J.; Sun, P.; Li, H.; Wang, J. Inhibitory effect and mechanism of Tagetes erecta L. fungicide on Fusarium oxysporum f. sp. niveum. Sci. Rep. 2017, 7, 14442. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Chopra, S.; Mehta, P. Antibiotic inhibition of pectolytic and cellulolytic enzyme activity in two Fusarium species. Mycopathologia 1993, 124, 185–188. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. The combination of amphotericin B and azithromycin as a potential new therapeutic approach to fusariosis. J. Antimicrob. Chemother. 1998, 41, 127–130. [Google Scholar] [CrossRef][Green Version]
- Clancy, C.J.; Yu, Y.C.; Lewin, A.; Nguyen, M.H. Inhibition of RNA synthesis as a therapeutic strategy against Aspergillus and Fusarium: Demonstration of in vitro synergy between rifabutin and amphotericin B. Antimicrob. Agents Chemother. 1998, 42, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, J.; Zhu, Y.; Duan, Y.; Zhou, M. Mechnism of action of the benzimidazole fungicides of Fusarium graminearum: Interfering with polymerization of monomeric tubulin but not polymerized microbubes. Phytopathology 2016, 106, 807–813. [Google Scholar] [CrossRef]
- Hitchcock, A.C.; Dickinson, K.; Brown, S.B.; Evans, E.G.; Adams, D.J. Interaction of azole antifungal antibiotics with cytochrome P450-dependent 14 a-sterol demethylase purified from Candida albicans. J. Biochem. 1990, 266, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid.-Based Complement. Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef] [PubMed]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as alternative strategy to control post-harvest fungal rotting of fruits and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Castellanos, L.M.; Olivas, N.A.; Ayala-Soto, J.; De La O Contreras, C.M.; Ortega, M.Z.; Salas, F.S.; Hernández-Ochoa, L. In vitro and in vivo antifungal activity of clove (Eugenia caryophyllata) and pepper (Piper nigrum L.) essential oils and functional extracts against Fusarium oxysporum and Aspergillus niger in tomato (Solanum lycopersicum L.). Int. J. Microbiol. 2020, 2020, 1702037. [Google Scholar] [CrossRef]
- Mannai, S.; Benfradj, N.; Karoui, A.; Salem, I.B.; Fathallah, A.; M’Hamdi, M.; Boughalleb-M’Hamdi, N. Analysis of chemical composition and in vitro and in vivo antifungal activity of Raphanus raphanistrum extracts against Fusarium and Pythiaceae, affecting apple and peach seedlings. Molecules 2021, 26, 2479. [Google Scholar] [CrossRef]
- Gonçalves, D.C.; de Queiroz, V.T.; Costa, A.V.; Lima, W.P.; Belan, L.L.; Moraes, W.P.; Iorio, N.L.P.P.; Póvoa, H.C.C. Reduction of fusarium wilt symptoms in tomato seedlings following seed treatment with Origanum vulgare L. essential oil and carvacrol. Crop. Prot. 2021, 141, 105487. [Google Scholar] [CrossRef]
- Drakopoulos, D.; Kägi, A.; Gimeno, A.; Six, J.; Jenny, E.; Forrer, H.R.; Musa, T.; Meca, G.; Vogelgsang, S. Prevention of Fusarium head blight infection and mycotoxins in wheat with cut-and-carry biofumigation and botanicals. Field Crop. Res. 2020, 246, 107681. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.A.; Waheed, U.; Raheel, M.; Khan, Z.; Alrefaei, A.F.; Alkhamis, H.H. Morphological and genetic characterization of Fusarium oxysporum and its management using weed extracts in cotton. J. King Saud Univ. Sci. 2021, 33, 101299. [Google Scholar] [CrossRef]
- Tegegne, G.; Pretorius, J.C. In vitro and in vivo antifungal activity of crude extracts and powdered dry material from Ethiopian wild plants against economically important plant pathogens. BioControl 2007, 52, 877–888. [Google Scholar] [CrossRef]
- Isman, M.B. Bridging the gap: Moving botanical insecticides from the laboratory to the farm. Ind. Crop. Prod. 2017, 110, 10–14. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef]
- Mahlo, S.M.; Chauke, H.R.; McGaw, L.; Eloff, J. Antioxidant and antifungal activity of selected medicinal plant extracts against phytopathogenic fungi. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Laher, F.; Aremu, A.O.; Van Staden, J.; Finnie, J.F. Evaluating the effect of storage on the biological activity and chemical composition of three South African medicinal plants. S. Afr. J. Bot. 2013, 88, 414–418. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Badi, H.N.; Yazdani, D.; Mohammad Ali, S.; Nazari, F. Effects of spacing and harvesting time on herbage yield and quality/quantity of oil in thyme, Thymus vulgaris L. Ind. Crop. Prod. 2004, 19, 231–236. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Sanna, V. Impact of nanotechnology on the delivery of natural products for cancar prevention and therapy. Mol. Nutr. Food Res. 2016, 60, 1330–1341. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).