Homocystamide Conjugates of Human Serum Albumin as a Platform to Prepare Bimodal Multidrug Delivery Systems for Boron Neutron Capture Therapy
Abstract
1. Introduction
2. Results
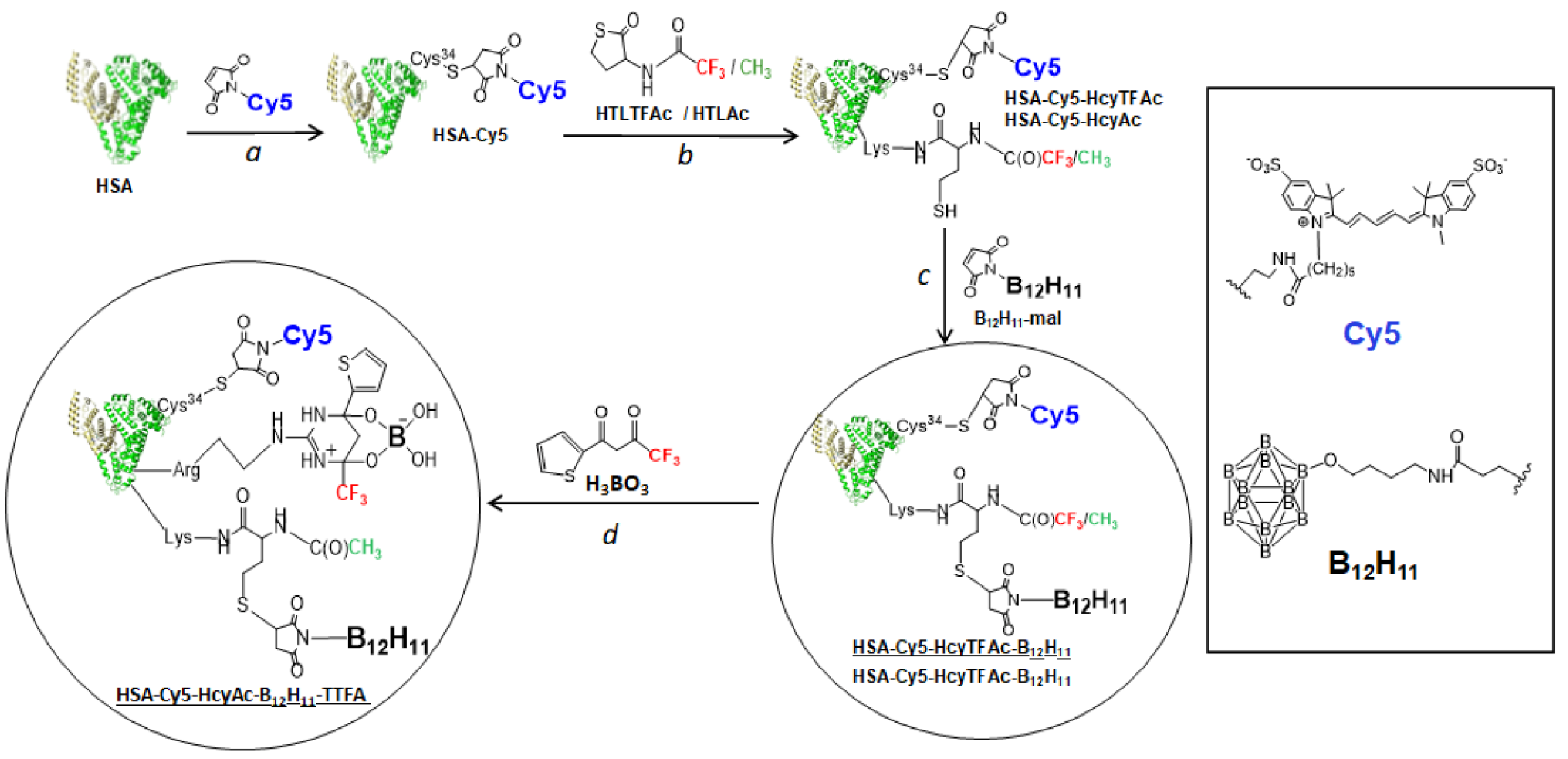
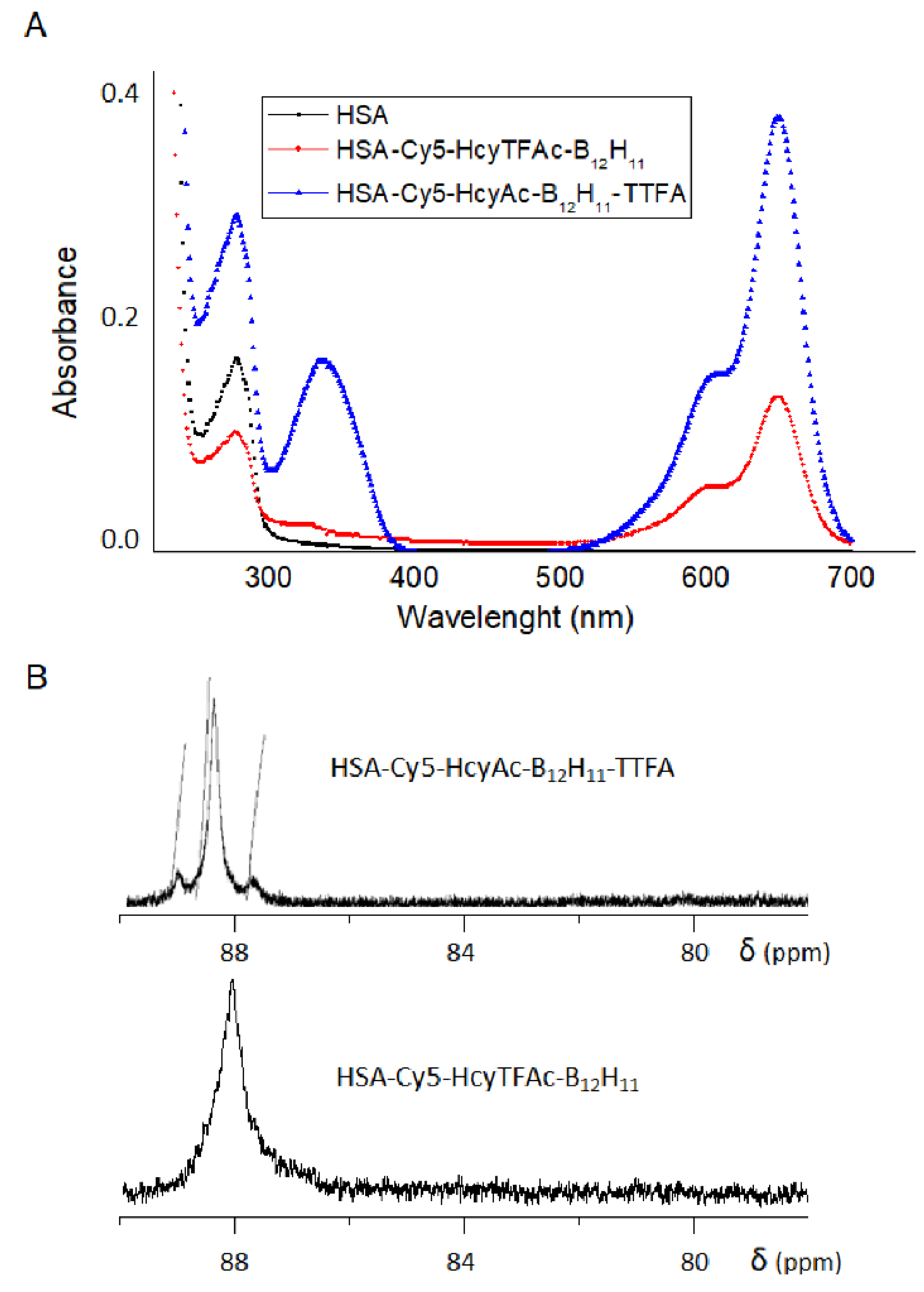
2.1. Conjugation to Human Serum Albumin
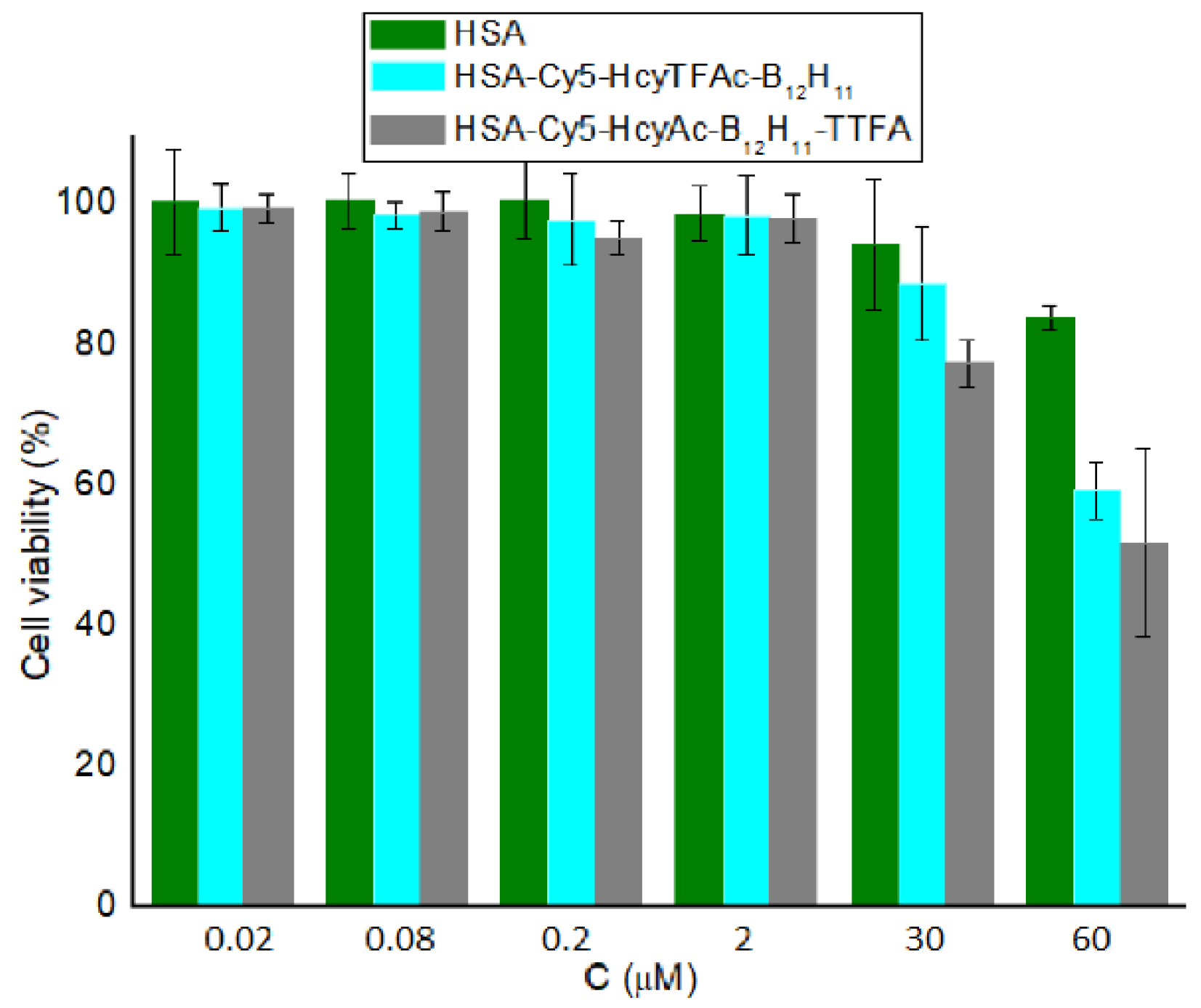
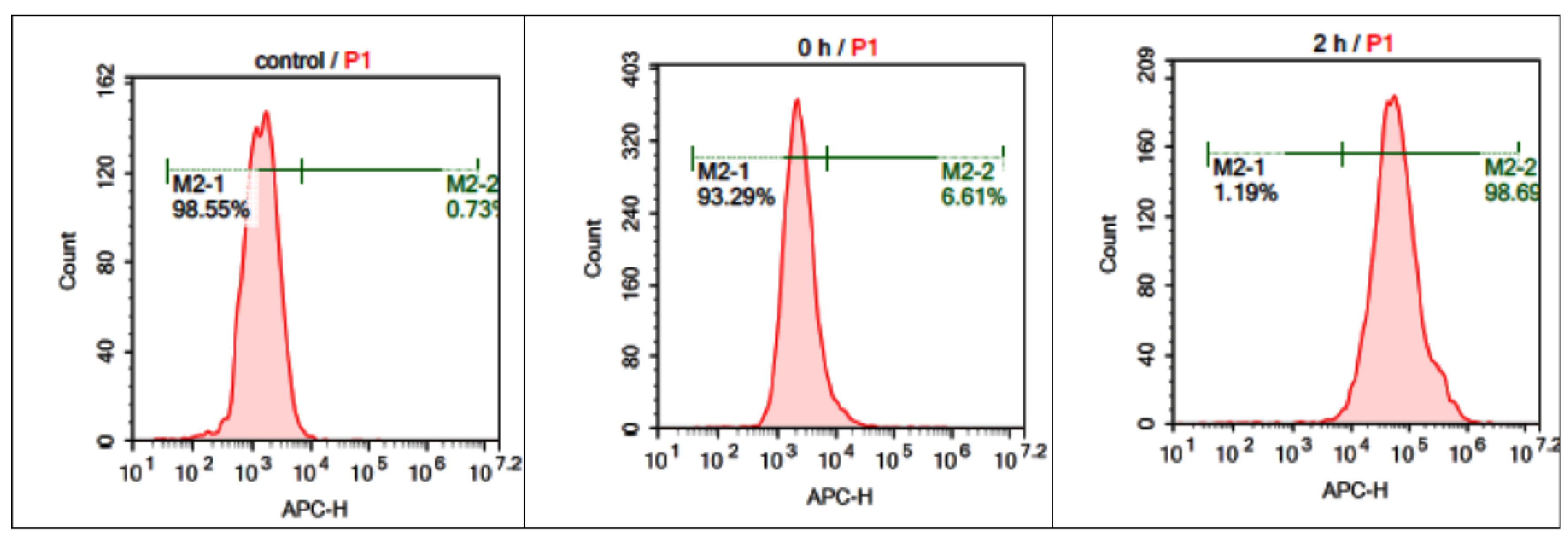
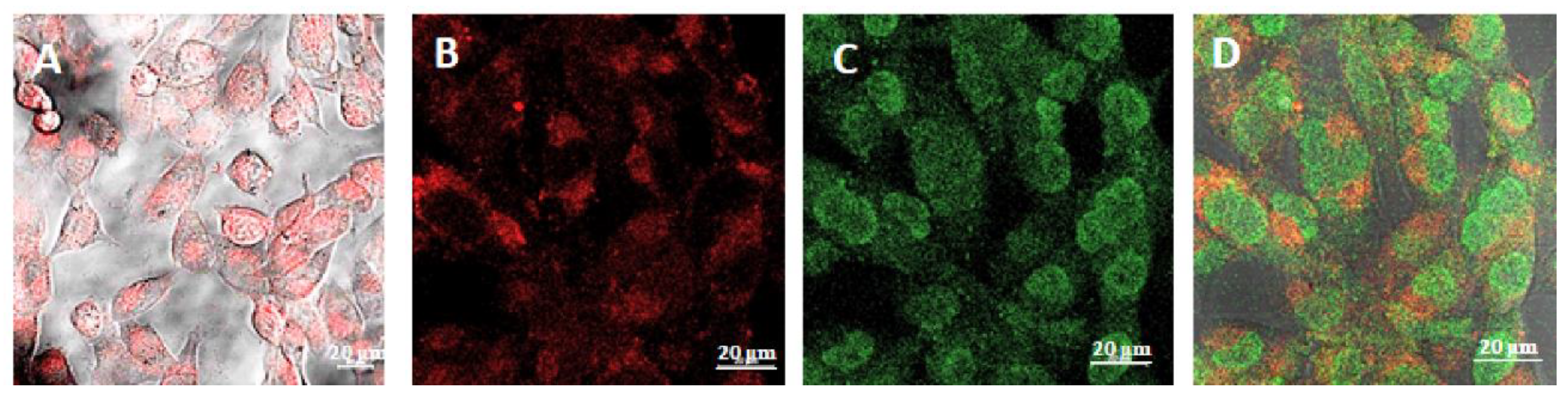
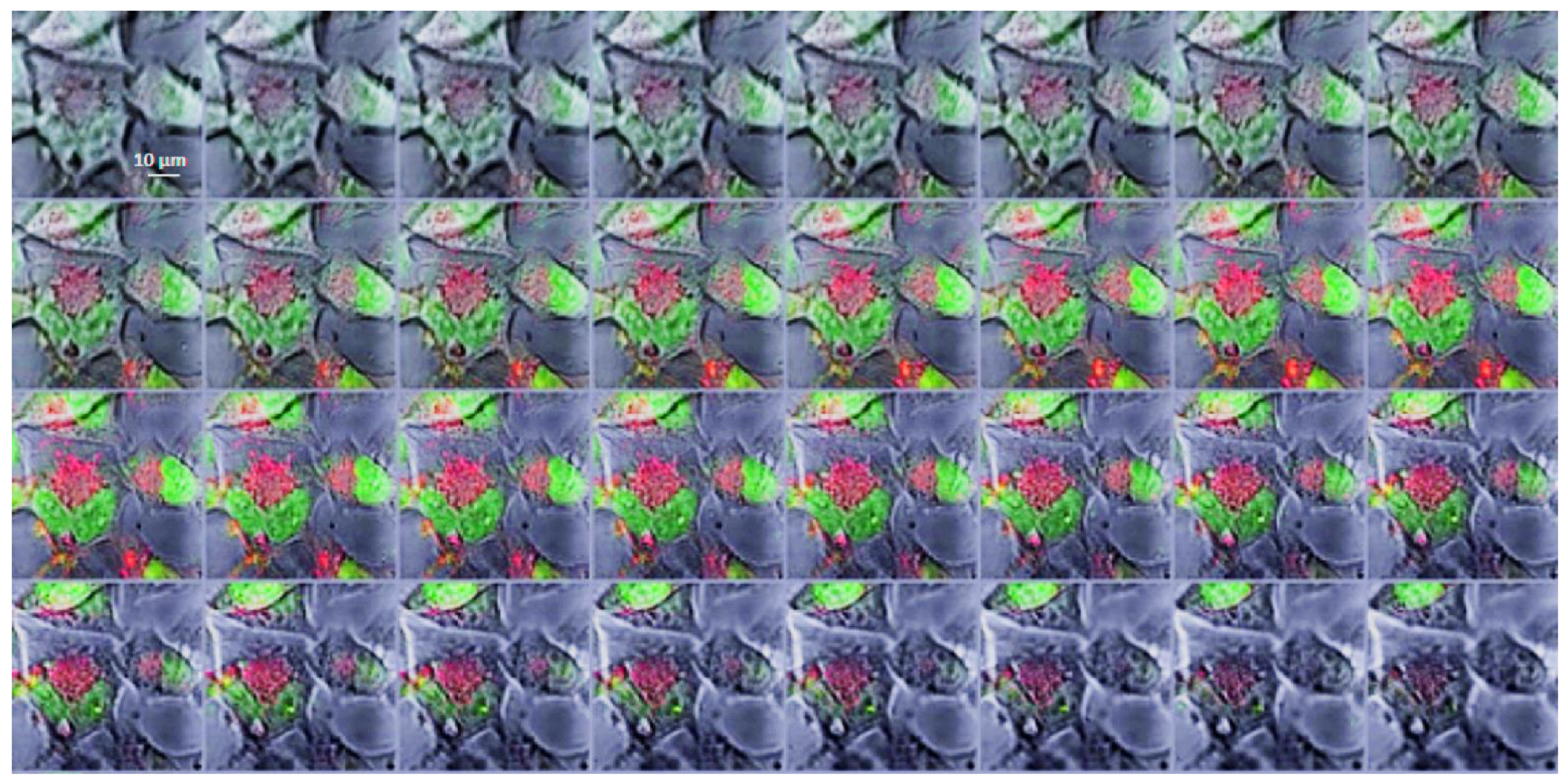
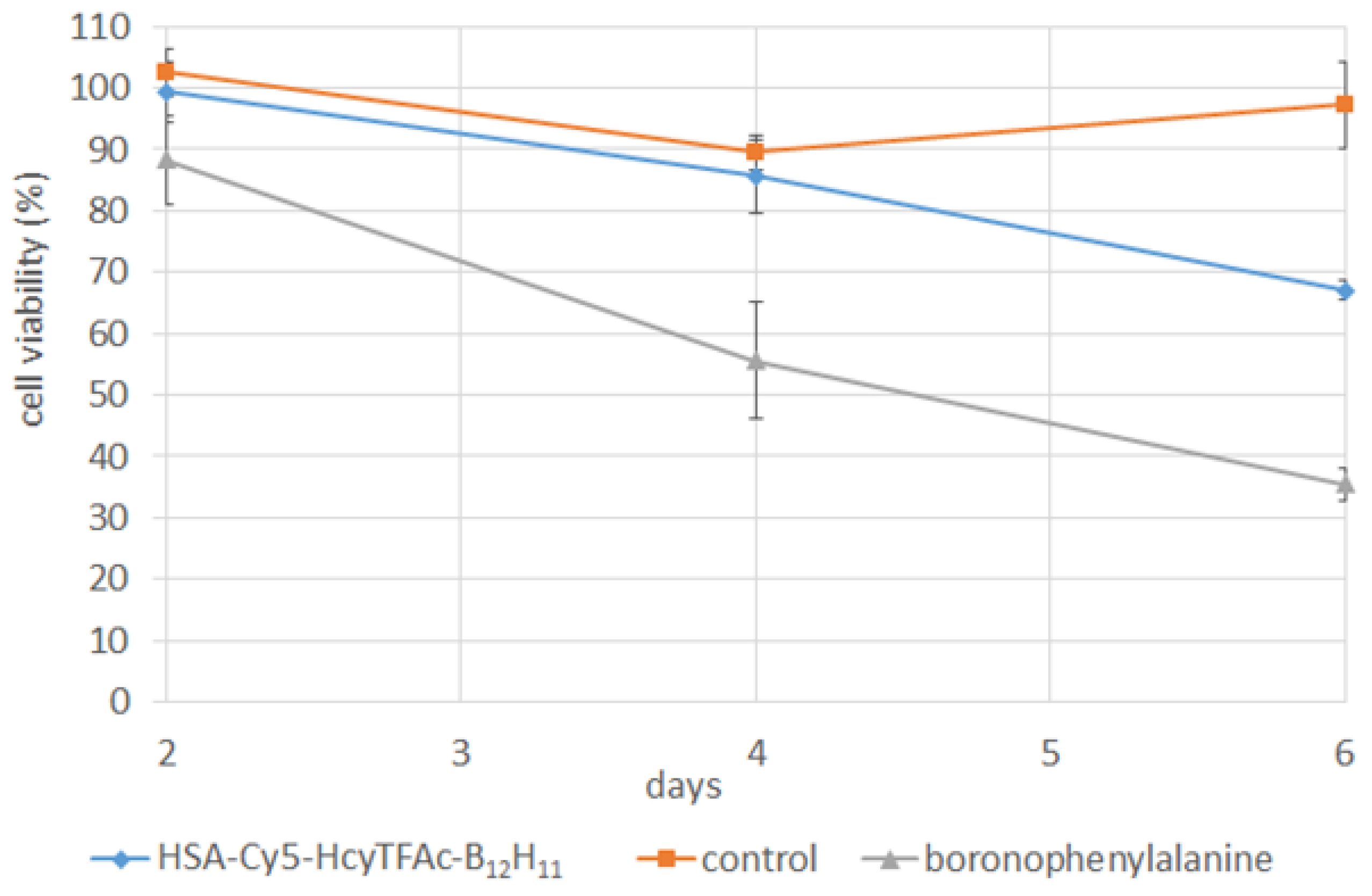
2.2. In Vitro Studies
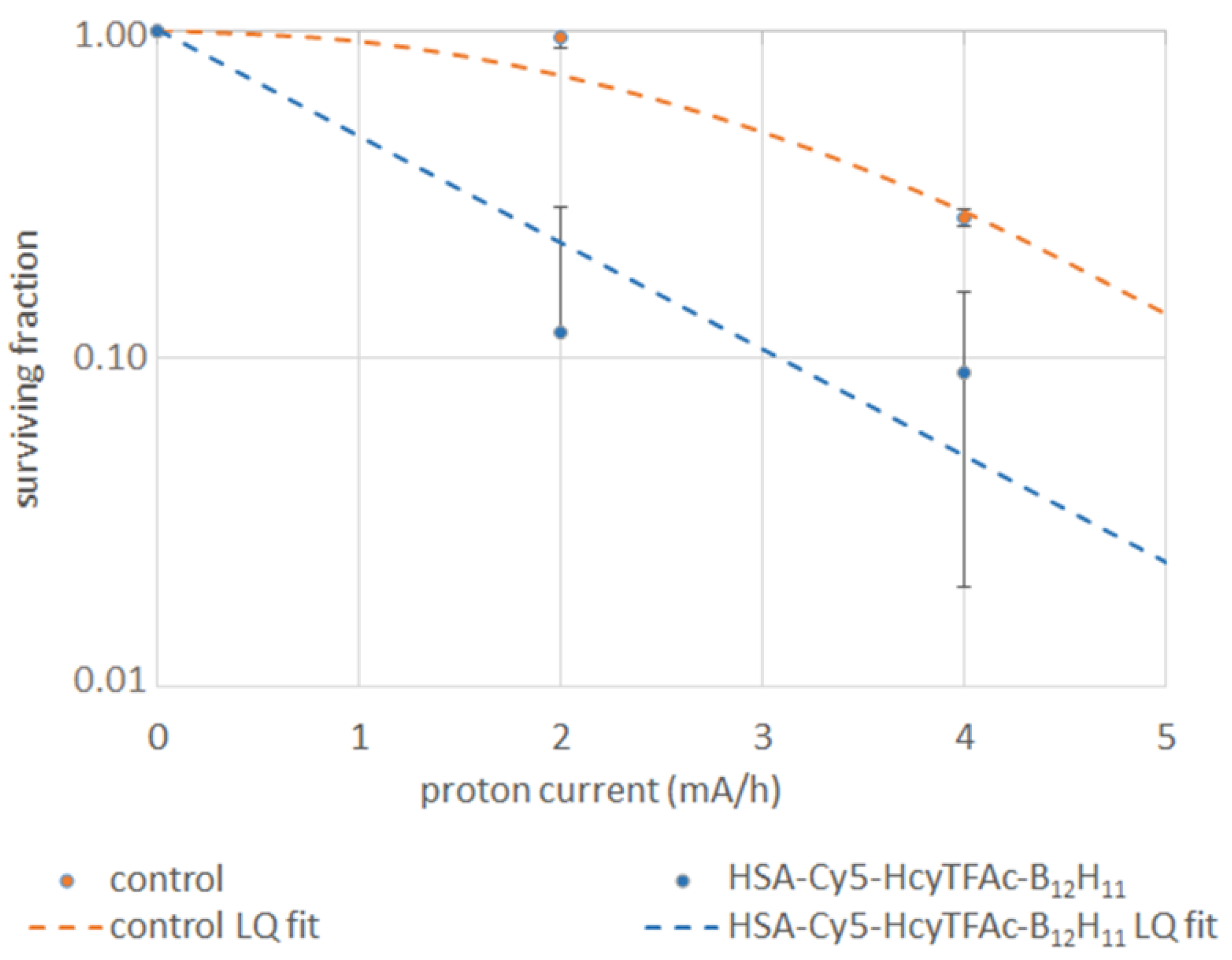
2.3. Neutron Irradiation Experiments
3. Materials and Methods
3.1. Chemicals, Reagents, Cancer Cells, and Facilities
3.2. Synthesis and Characterization of Multifunctional Human Serum Albumin-Therapeutic Conjugates
3.2.1. Synthesis and Characterization of Theranostic Conjugate HSA-Cy5-HcyTFAc-B12H11
3.2.2. Synthesis and Characterization of Multifunctional Human Serum Albumin-Therapeutic Conjugate HAS-Cy5-HcyAc-B12H11-TTFA
3.3. Cell Viability Assay (MTT Test)
3.4. Intracellular Distribution of Boronated Albumin Theranostic In Vitro
3.5. Flow Cytometry
3.6. Neutron Irradiation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Zhang, Z.; Liu, T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun. 2018, 38, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Pooh, K.; Kobayashi, T.; Kageji, T.; Uyama, S.; Matsumura, A.; Kumada, H. Clinical review of the Japanese experience with boron neutron capture therapy and a proposed strategy using epithermal neutron beams. J. Neurooncol. 2003, 62, 87–99. [Google Scholar] [CrossRef]
- Miyatake, S.; Kawabata, S.; Kajimoto, Y.; Aoki, A.; Yokoyama, K.; Yamada, M.; Kuroiwa, T.; Tsuji, M.; Imahori, Y.; Kirihata, M.; et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: An efficacy study based on findings on neuroimages. J. Neurosurg. 2005, 103, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.; Kawabata, S.; Yokoyama, K.; Kuroiwa, T.; Michiue, H.; Sakurai, Y.; Kumada, H.; Suzuki, M.; Maruhashi, A.; Kirihata, M.; et al. Survival benefit of boron neutron capture therapy for recurrent malignant gliomas. J. Neurooncol. 2009, 91, 199–206. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Saarilahti, K.; Makitie, A.; Valimaki, P.; Tenhunen, M.; Joensuu, H. Boron neutron capture therapy (BNCT) followed by intensity modulated chemoradiotherapy as primary treatment of large head and neck cancer with intracranial involvement. Radiother. Oncol. 2011, 99, 98–99. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Välimäki, P.; et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: Final analysis of a phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e67–e75. [Google Scholar] [CrossRef]
- Ariyoshi, Y.; Miyatake, S.; Kimura, Y.; Shimahara, T.; Kawabata, S.; Nagata, K.; Suzuki, M.; Maruhashi, A.; Ono, K.; Shimahara, M. Boron neuron capture therapy using epithermal neutrons for recurrent cancer in the oral cavity and cervical lymph node metastasis. Oncol. Rep. 2007, 18, 861–866. [Google Scholar] [CrossRef]
- Kimura, Y.; Ariyoshi, Y.; Miyatake, S.; Shimahara, M.; Kawabata, S.; Ono, K. Boron neutron capture therapy for papillary cystadenocarcinoma in the upper lip: A case report. Int. J. Oral. Maxillofac. Surg. 2009, 38, 293–295. [Google Scholar] [CrossRef]
- Kimura, Y.; Ariyoshi, Y.; Shimahara, M.; Miyatake, S.; Kawabata, S.; Ono, K.; Suzuki, M.; Maruhashi, A. Boron neutron capture therapy for recurrent oral cancer and metastasis of cervical lymph node. Appl. Radiat. Isot. 2009, 67, S47–S49. [Google Scholar] [CrossRef]
- Aihara, T.; Hiratsuka, J.; Morita, N.; Uno, M.; Sakurai, Y.; Maruhashi, A.; Ono, K.; Harada, T. First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck 2006, 28, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Fujita, Y.; Maruhashi, A.; Kumada, H.; Ohmae, M.; Kirihata, M.; Imahori, Y.; Suzuki, M.; Sakrai, Y.; Sumi, T.; et al. Effectiveness of boron neutron capture therapy for recurrent head and neck malignancies. Appl. Radiat. Isot. 2009, 67, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Honda, C.; Ichihashi, M.; Obara, H.; Hiratsuka, J.; Fukuda, H.; Karashima, H.; Kobayashi, T.; Kanda, K.; Yoshino, K. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound. Lancet 1989, 2, 388–389. [Google Scholar] [CrossRef]
- Mishima, Y. (Ed.) Selective thermal neutron capture therapy of cancer cells using their specific metabolic activities–melanoma as prototype. In Cancer Neutron Capture Therapy; Plenum Press: New York, NY, USA, 1996; pp. 1–26. [Google Scholar]
- Yong, Z.; Song, Z.; Zhou, Y.; Liu, T.; Zhang, Z.; Zhao, Y.; Chen, Y.; Jin, C.; Chen, X.; Lu, J.; et al. Boron neutron capture therapy for malignant melanoma: First clinical case report in China. Chin. J. Cancer Res. 2016, 28, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, J.F. Malignant melanoma. In Neutron Capture Therapy; Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 433–448. [Google Scholar]
- Hiratsuka, J.; Kamitani, N.; Tanaka, R.; Yoden, E.; Tokiya, R.; Suzuki, M.; Barth, R.F.; Ono, K. Boron neutron capture therapy for vulvar melanoma and extramammary Paget’s disease of the genital regions with curative clinical responses. Chin. J. Cancer 2018, 38, 1–10. [Google Scholar]
- Luderer, M.J.; Puente, P.; Azab, A.K. Advancements in tumor targeting strategies for boron neutron capture therapy. Pharm. Res. 2015, 32, 2824–2836. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.L.A.; Sumerlin, B.S. Synthesis and applications of boronic acid-containing polymers: From materials to medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef]
- Kawasaki, R.; Sasaki, Y.; Akiyoshi, K. Intracellular delivery and passive tumor targeting of a self-assembled nanogel containing carborane clusters for boron neutron capture therapy. Biochem. Biophys. Res. Commun. 2017, 483, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, T.; Inoue, Y.; Yao, Y.; Suzuki, M.; Kanamori, K.; Takemoto, H.; Matsui, M.; Tomoda, K.; Nishiyama, N. Poly(vinyl alcohol) boosting therapeutic potential of p-boronophenylalanine in neutron capture therapy by modulating metabolism. Sci. Adv. 2020, 6, eaaz1722. [Google Scholar] [CrossRef]
- Yoneoka, S.; Nakagawa, Y.; Uto, K.; Sakura, K.; Tsukahara, T.; Ebara, M. Boron-incorporating hemagglutinating virus of Japan envelope (HVJ-E) nanomaterial in boron neutron capture therapy. Sci. Technol. Adv. Mater. 2019, 20, 291–304. [Google Scholar] [CrossRef]
- Nakamura, H.; Ueda, N.; Ban, H.S.; Ueno, M.; Tachikawa, S. Design and synthesis of fluorescence-labeled closo-dodecaborate lipid: Its liposome formation and in vivo imaging targeting of tumors for boron neutron capture therapy. Org. Biomol. Chem. 2012, 10, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Khanadeev, V.; Khlebtsov, B.; Khlebtsov, N.; Gopinath, P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv. Colloid Interface Sci. 2017, 246, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Kulluru, L.P.; Rizvi, S.A.; D’Souza, M.; D’Souza, M. Formulation development of albumin based theranostic nanoparticles as a potential delivery system for tumor targeting. J. Drug Target. 2013, 21, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Lee, J.; Chang, H.; Jeewon, L.; Kwon, I.C.; Kim, K. Molecular imaging and targeted drug delivery using albumin-based nanoparticles. Curr. Pharm. Des. 2015, 21, 1889–1898. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, S.N. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387–6406. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Schäffler, M.; Sousa, F.; Wenk, A.; Sitia, L.; Hirn, S.; Schleh, C.; Haberl, N.; Violatto, M.; Canovi, M.; Andreozzi, P.; et al. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials 2014, 35, 3435–3466. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H.; Brekken, R.A. Mini review SPARC, a matricellular protein: At the crossroads of cell matrix SPARC, a matricellular protein: At the crossroads of cell matrix communication. Matrix Biol. 2001, 19, 815–827. [Google Scholar] [CrossRef]
- Kouros, M. SPARC (osteonectin/BM-40). Int. J. Biochem. Cell Biol. 1999, 31, 1363–1366. [Google Scholar]
- Podhajcer, O.L.; Benedetti, L.G.; Girotti, M.R.; Prada, F.; Salvatierra, E.; Llera, A.S. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008, 27, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Bao, S.; Song, L.; Wu, Q.; Bigner, D.D.; Hjelmeland, A.B.; Rich, J.N. Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene 2007, 26, 4084–4094. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Kanoh, D.; Sato, S.; Sakurai, Y.; Suzuki, M.; Nakamura, H. Maleimide-functionalized closo-dodecaborate albumin conjugates (MID-AC): Unique ligation at cysteine and lysine residues enables efficient boron delivery to tumor for neutron capture therapy. J. Control. Release 2016, 237, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Sato, S.; Asami, H.; Hasegawa, T.; Kohno, J.; Nakamura, H. Design of S-S bond containing maleimide-conjugated closo-dodecaborate (SSMID): Identification of unique modification sites on albumin and investigation of intracellular uptake. Org. Biomol. Chem. 2019, 17, 5496–5499. [Google Scholar] [CrossRef]
- Nakamura, H.; Kikuchi, S.; Kaway, K.; Ishii, S.; Sato, S. closo-Dodecaborate-conjugated human serum albumins: Preparation and in vivo selective boron delivery to tumor. Pure Appl. Chem. 2018, 90, 745–753. [Google Scholar] [CrossRef]
- Sato, S.; Ishii, H.; Nakamura, H. Development of albumin-closo-dodecaborate conjugates as boron carriers for neutron-capture therapy by Ru(bpy)3-photocatalyzed modification of tyrosine. Eur. J. Inorg. Chem. 2017, 2017, 4406–4410. [Google Scholar] [CrossRef]
- Pulagam, K.R.; Gona, K.B.; Gómez-Vallejo, V.; Meijer, J.; Zilberfain, C.; Estrela-Lopis, I.; Baz, Z.; Cossio, U.; Llop, J. Gold nanoparticles as boron carriers for boron neutron capture therapy: Synthesis, Radiolabelling and in vivo evaluation. Molecules 2019, 24, 3609. [Google Scholar] [CrossRef] [PubMed]
- Frigell, J.; García, I.; Gómez-Vallejo, V.; Llop, J.; Penadés, S. 68Ga-labeled gold glyconanoparticles for exploring blood-brain barrier permeability: Preparation, biodistribution studies, and improved brain uptake via neuropeptide conjugation. J. Am. Chem. Soc. 2014, 136, 449–457. [Google Scholar] [CrossRef]
- Ishiwata, K. 4-Borono-2-18F-fluoro-L-phenylalanine PET for boron neutron capture therapy-oriented diagnosis: Overview of a quarter century of research. Ann. Nucl. Med. 2019, 33, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, S.; Kortesniemi, M.; Timonen, M.; Reijonen, V.; Kuusela, L.; Uusi-Simola, J.; Salli, E.; Koivunoro, H.; Seppälä, T.; Lönnroth, N.; et al. Boron neutron capture therapy (BNCT) in Finland: Technological and physical prospects after 20 years of experiences. Phys. Med. 2013, 29, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, J.; Ma, S.; Liu, Q.; Huang, L.; Chen, X.; Lou, K.; Wang, W. Recent developments in multimodality fluorescence imaging probes. Acta Pharm. Sin. B 2018, 8, 320–338. [Google Scholar] [CrossRef]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef]
- Chubarov, A.S.; Zakharova, O.D.; Koval, O.A.; Romaschenko, A.V.; Akulov, A.E.; Zavjalov, E.L.; Razumov, I.A.; Koptyug, I.V.; Knorre, D.G.; Godovikova, T.S. Design of protein homocystamides with enhanced tumor uptake properties for 19F magnetic resonance imaging. Bioorg. Med. Chem. 2015, 23, 6943–6954. [Google Scholar] [CrossRef] [PubMed]
- Lisitskiy, V.A.; Khan, H.; Popova, T.V.; Chubarov, A.S.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Koptyug, I.V.; Ahmad, S.; et al. Multifunctional human serum albumin-therapeutic nucleotide conjugate with redox and pH-sensitive drug release mechanism for cancer theranostics. Bioorg. Med. Chem. Lett. 2017, 27, 3925–3930. [Google Scholar] [CrossRef]
- Fasano, F.; Campanella, R.; Migneco, L.M.; Pastore, F.S.; Maraviglia, B. In vivo F-19 MRI and F-19 MRS of F-19-labelled borophenylalanine-fructose complex on a C6 rat glioma model to optimize boron neutron capture therapy (BNCT). Phys. Med. Biol. 2008, 53, 6979–6989. [Google Scholar]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Caspersen, M.B.; Kuhlmann, M.; Nicholls, K.; Saxton, M.J.; Andersen, B.; Bunting, K.; Cameron, J.; Howard, K.A. Albumin-based drug delivery using cysteine 34 chemical conjugates–important considerations and requirements. Ther. Deliv. 2017, 8, 511–519. [Google Scholar] [CrossRef]
- Peters, T., Jr. The Albumin molecule: Its structure and chemical properties. In All about Albumin: Biochemistry, Genetics, and Molecular Applications; Academic Press: San Diego, CA, USA, 1996; p. 432. [Google Scholar]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef] [PubMed]
- An, F.-F.; Zhang, X.-H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.V.; Pyshnaya, I.A.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Poletaeva, J.; Zavjalov, E.L.; Silnikov, V.N.; Ryabchikova, E.I.; Godovikova, T.S. Rational design of albumin theranostic conjugates for gold nanoparticles anticancer drugs: Where the seed meets the soil? Biomedicines 2021, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Wanigasekara, M.S.K.; Huang, X.; Chakrabarty, J.K.; Bugarin, A.; Chowdhury, S.M. Arginine-Selective chemical labeling approach for identification and enrichment of reactive arginine residues in proteins. ACS Omega 2018, 3, 14229–14235. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Araya-Maturana, R.; Urra, F.A. Regulation of mitochondrial function as a promising target in platelet activation-related diseases. Free Radic. Biol. Med. 2019, 136, 172–182. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Sang, H.; Zhou, Y.; Shang, C.; Wang, Y.; Zhu, H. Effects of hyperglycemia on the progression of tumor diseases. J. Exp. Clin. Cancer Res. 2019, 38, 327. [Google Scholar] [CrossRef] [PubMed]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 7467, 338–345. [Google Scholar] [CrossRef]
- Turner, N.C.; Reis-Filho, J.S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012, 13, 178–185. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharm. 2012, 83, 1104–1111. [Google Scholar] [CrossRef]
- Bregadze, V.; Sivaev, I.; Glazun, S. Polyhedral boron compounds as potential diagnostic and therapeutic antitumor agents. Anti-Cancer Agents Med. Chem. 2006, 6, 75–109. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with maleimides: A useful tool for chemical biology. Chem. Eur. J. 2018, 24, 1–18. [Google Scholar] [CrossRef]
- Janatova, J.; Fuller, J.K.; Hunter, M.J. The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content. J. Biol. Chem. 1968, 243, 3612–3622. [Google Scholar] [CrossRef]
- Jakubowski, H. Homocysteine in Protein Structure/Function and Human Disease: Chemical Biology of Homocysteine-containing Proteins; Springer: Wien, Austria, 2013; pp. 1–166. [Google Scholar]
- Eriksson, U.J.; Borg, L.A. Protection by free oxygen radical scavenging enzymes against glucose-induced embryonic malformations in vitro. Diabetologia 1991, 34, 325–331. [Google Scholar] [CrossRef]
- Pisanti, F.A.; Frascatore, S.; Vuttariello, E.; Grillo, A. Influence of acetyl homocysteine thiolactone on erythrocyte superoxide dismutase activity. Biochem. Med. Metab. Biol. 1987, 37, 265–267. [Google Scholar] [CrossRef]
- Papaccio, G.; Pisanti, F.A.; Frascatore, S. Acetyl-homocysteine-thiolactone-induced increase of superoxide dismutase counteracts the effect of subdiabetogenic doses of streptozocin. Diabetes 1986, 35, 470–474. [Google Scholar] [CrossRef]
- Mbiya, W.; Choi, B.; Martincigh, B.S.; Morakinyo, M.K.; Simoyi, R.H. Oxyhalogen-sulfur chemistry: Kinetics and mechanism of oxidation of N-acetyl homocysteine thiolactone by acidified bromate and aqueous bromine. J. Phys. Chem. A 2013, 117, 13059–13069. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival–application to proliferation and cyto-toxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: Effect of size and surface charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef] [PubMed]
- Martucci, N.M.; Migliaccio, N.; Ruggiero, I.; Albano, F.; Calì, G.; Romano, S.; Terracciano, M.; Rea, I.; Arcari, P.; Lamberti, A. Nanoparticle-based strategy for personalized B-cell lymphoma therapy. Int. J. Nanomed. 2016, 11, 6089–6101. [Google Scholar] [CrossRef]
- Kreiner, A.J.; Bergueiro, J.; Cartelli, D.; Baldo, M.; Castell, W.; Asoia, J.G.; Padulo, J.; Sandín, J.C.S.; Igarzabal, M.; Erhardt, J.; et al. Present status of accelerator-based BNCT. Rep. Pract. Oncol. Radiother. 2016, 21, 95–101. [Google Scholar] [CrossRef]
- Taskaev, S. Development of an accelerator-based epithermal neutron source for boron neutron capture therapy. Phys. Part. Nucl. 2019, 50, 569–575. [Google Scholar] [CrossRef]
- Sato, E.; Zaboronok, A.; Yamamoto, T.; Nakai, K.; Taskaev, S.; Volkova, O.; Mechetina, L.; Taranin, A.; Kanygin, V.; Isobe, T.; et al. Radiobiological response of U251MG, CHO-K1 and V79 cell lines to accelerator-based boron neutron capture therapy. J. Radiat. Res. 2018, 59, 101–107. [Google Scholar] [CrossRef]
- Zaboronok, A.; Byvaltsev, V.A.; Kanygin, V.V.; Iarullina, A.I.; Kichigin, A.I.; Taranin, A.V.; Volkova, O.Y.; Mechetina, L.V.; Taskaev, S.Y.; Muhamadiyarov, R.A.; et al. Boron-neutron capture therapy in Russia: Preclinical evaluation of efficacy and perspectives of its application in neurooncology. New Armen. Med. J. 2017, 11, 6–15. [Google Scholar]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Sivaev, I.B.; Semioshkin, A.A.; Brellochs, B.; Sjoberg, S.; Bregadze, V.I. Synthesis of oxonium derivatives of the dodecahydro-closo-dodecaborate anion [B12H12]2−. Tetramethylene oxonium derivative of [B12H12]2− as a convenient precursor for the synthesis of functional compounds for boron neutron capture therapy. Polyhedron 2000, 19, 627–632. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, T.; Dymova, M.A.; Koroleva, L.S.; Zakharova, O.D.; Lisitskiy, V.A.; Raskolupova, V.I.; Sycheva, T.; Taskaev, S.; Silnikov, V.N.; Godovikova, T.S. Homocystamide Conjugates of Human Serum Albumin as a Platform to Prepare Bimodal Multidrug Delivery Systems for Boron Neutron Capture Therapy. Molecules 2021, 26, 6537. https://doi.org/10.3390/molecules26216537
Popova T, Dymova MA, Koroleva LS, Zakharova OD, Lisitskiy VA, Raskolupova VI, Sycheva T, Taskaev S, Silnikov VN, Godovikova TS. Homocystamide Conjugates of Human Serum Albumin as a Platform to Prepare Bimodal Multidrug Delivery Systems for Boron Neutron Capture Therapy. Molecules. 2021; 26(21):6537. https://doi.org/10.3390/molecules26216537
Chicago/Turabian StylePopova, Tatyana, Maya A. Dymova, Ludmila S. Koroleva, Olga D. Zakharova, Vladimir A. Lisitskiy, Valeria I. Raskolupova, Tatiana Sycheva, Sergei Taskaev, Vladimir N. Silnikov, and Tatyana S. Godovikova. 2021. "Homocystamide Conjugates of Human Serum Albumin as a Platform to Prepare Bimodal Multidrug Delivery Systems for Boron Neutron Capture Therapy" Molecules 26, no. 21: 6537. https://doi.org/10.3390/molecules26216537
APA StylePopova, T., Dymova, M. A., Koroleva, L. S., Zakharova, O. D., Lisitskiy, V. A., Raskolupova, V. I., Sycheva, T., Taskaev, S., Silnikov, V. N., & Godovikova, T. S. (2021). Homocystamide Conjugates of Human Serum Albumin as a Platform to Prepare Bimodal Multidrug Delivery Systems for Boron Neutron Capture Therapy. Molecules, 26(21), 6537. https://doi.org/10.3390/molecules26216537






