Pentacyclic Cytochalasins and Their Derivatives from the Endophytic Fungus Phomopsis sp. xz-18
Abstract
:1. Introduction
2. Results and Experiments
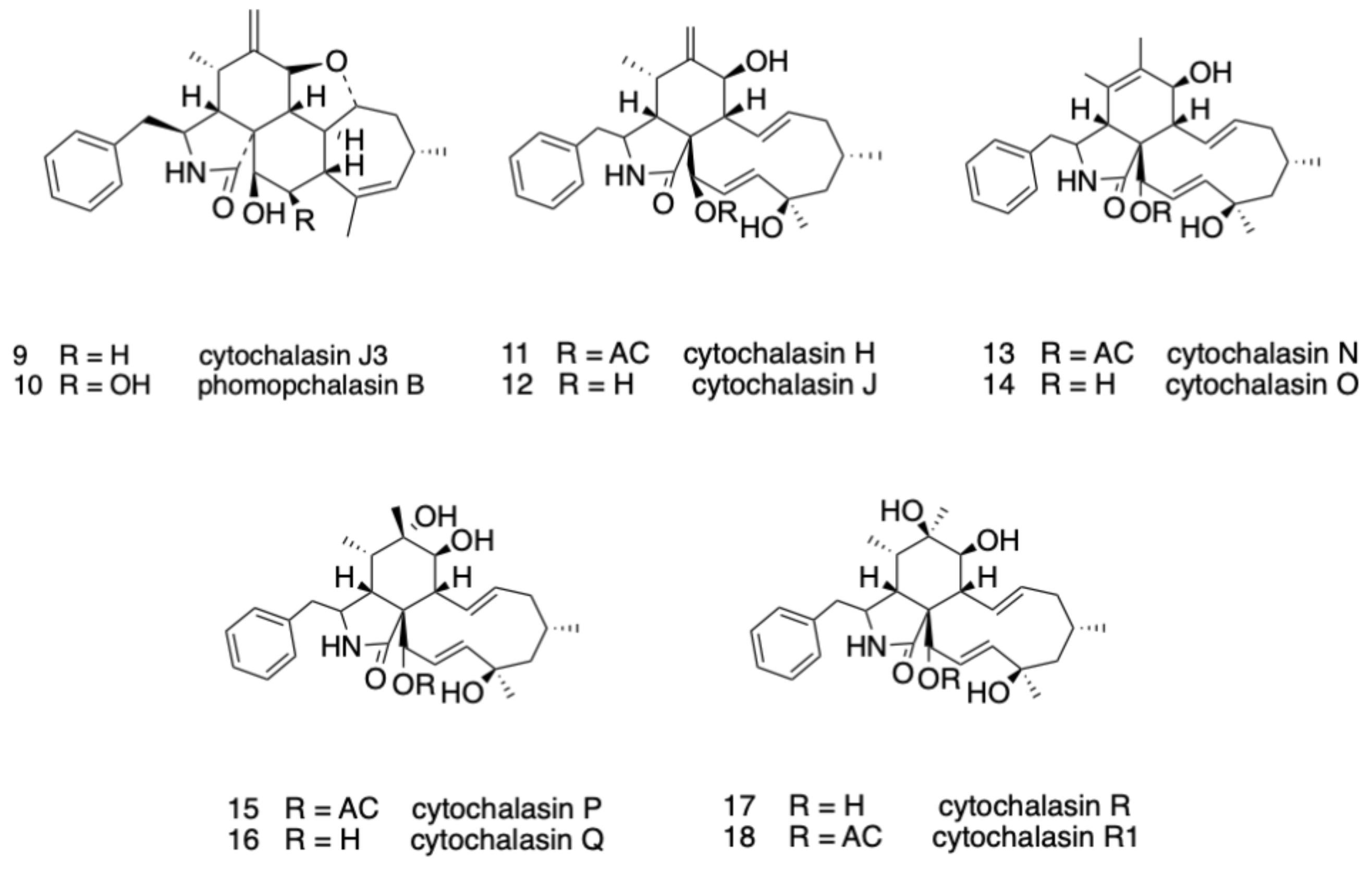
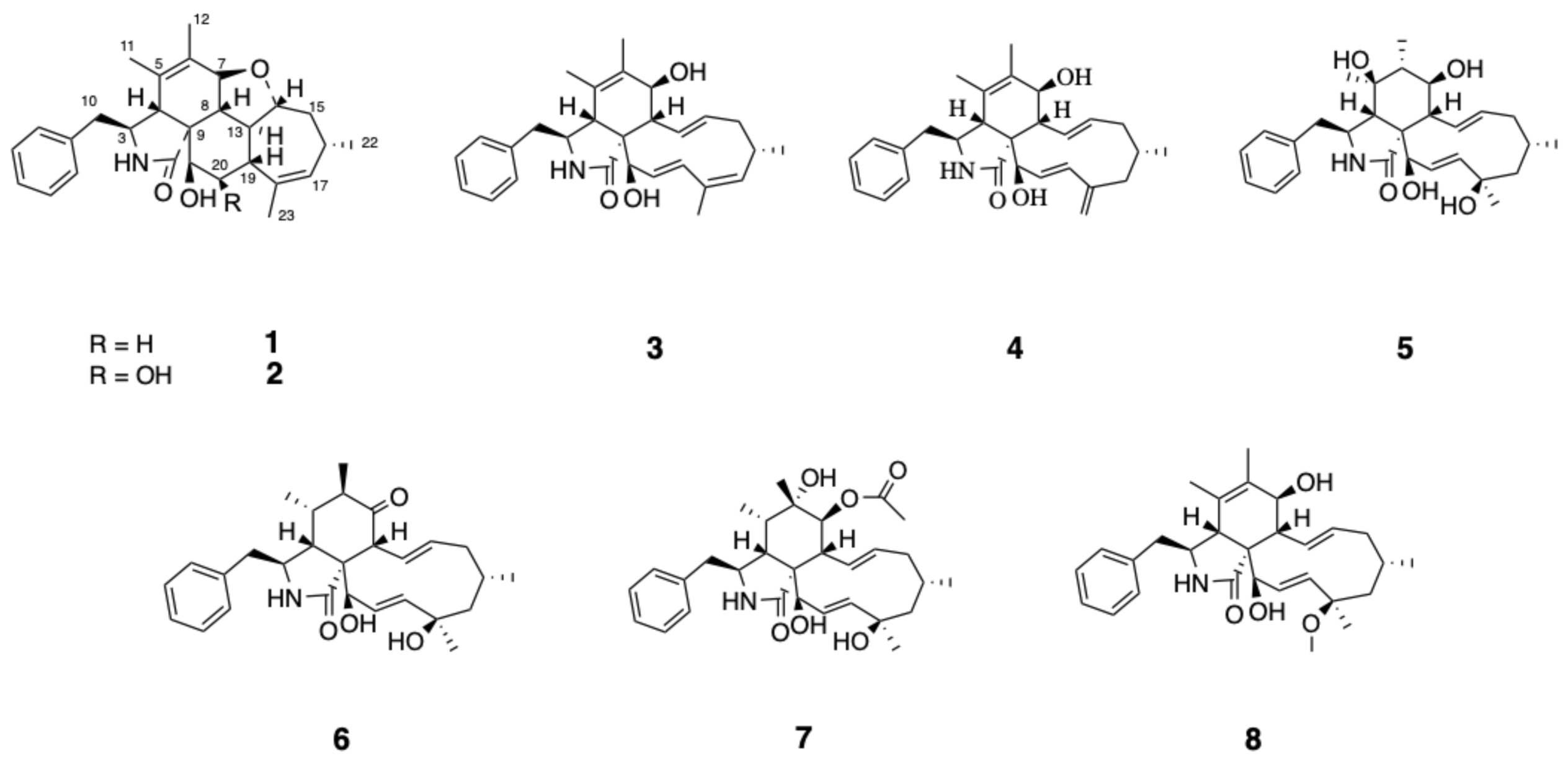
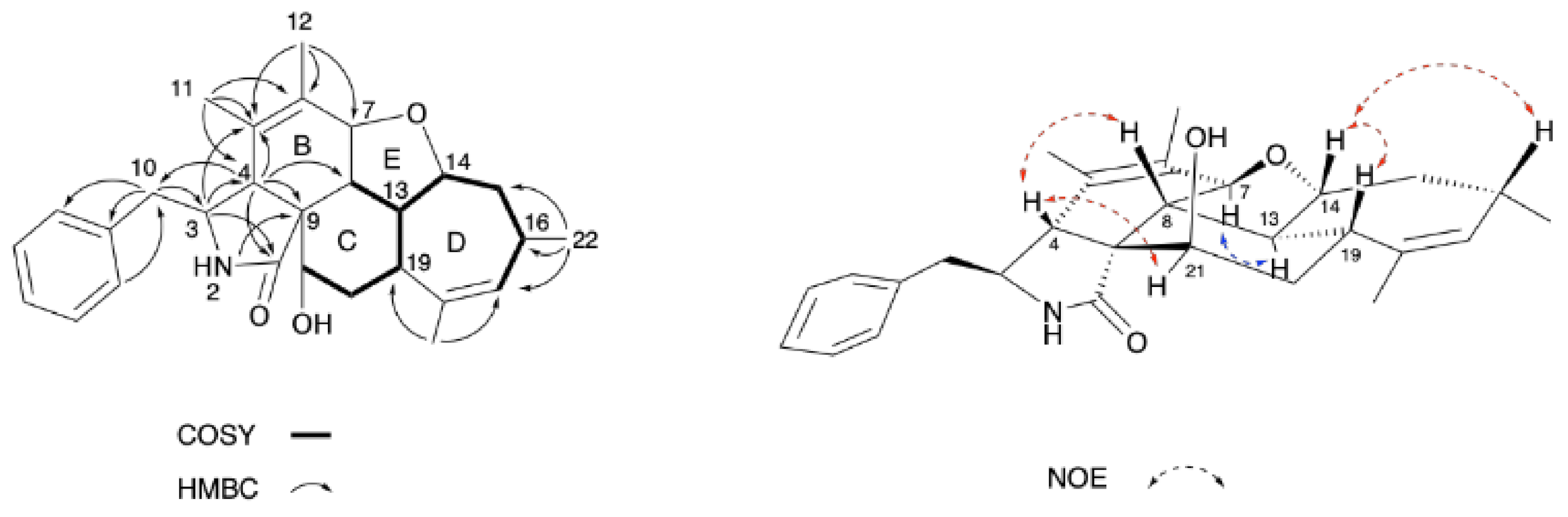
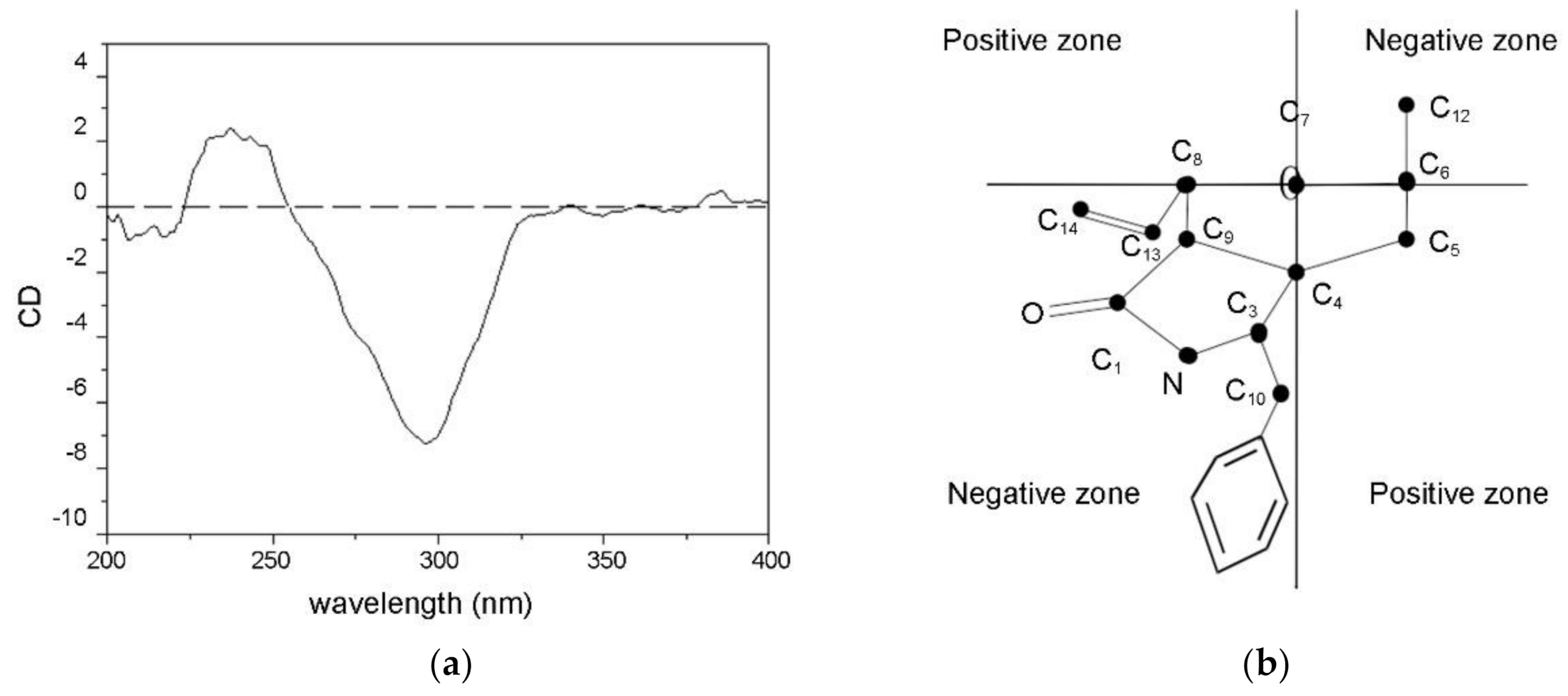
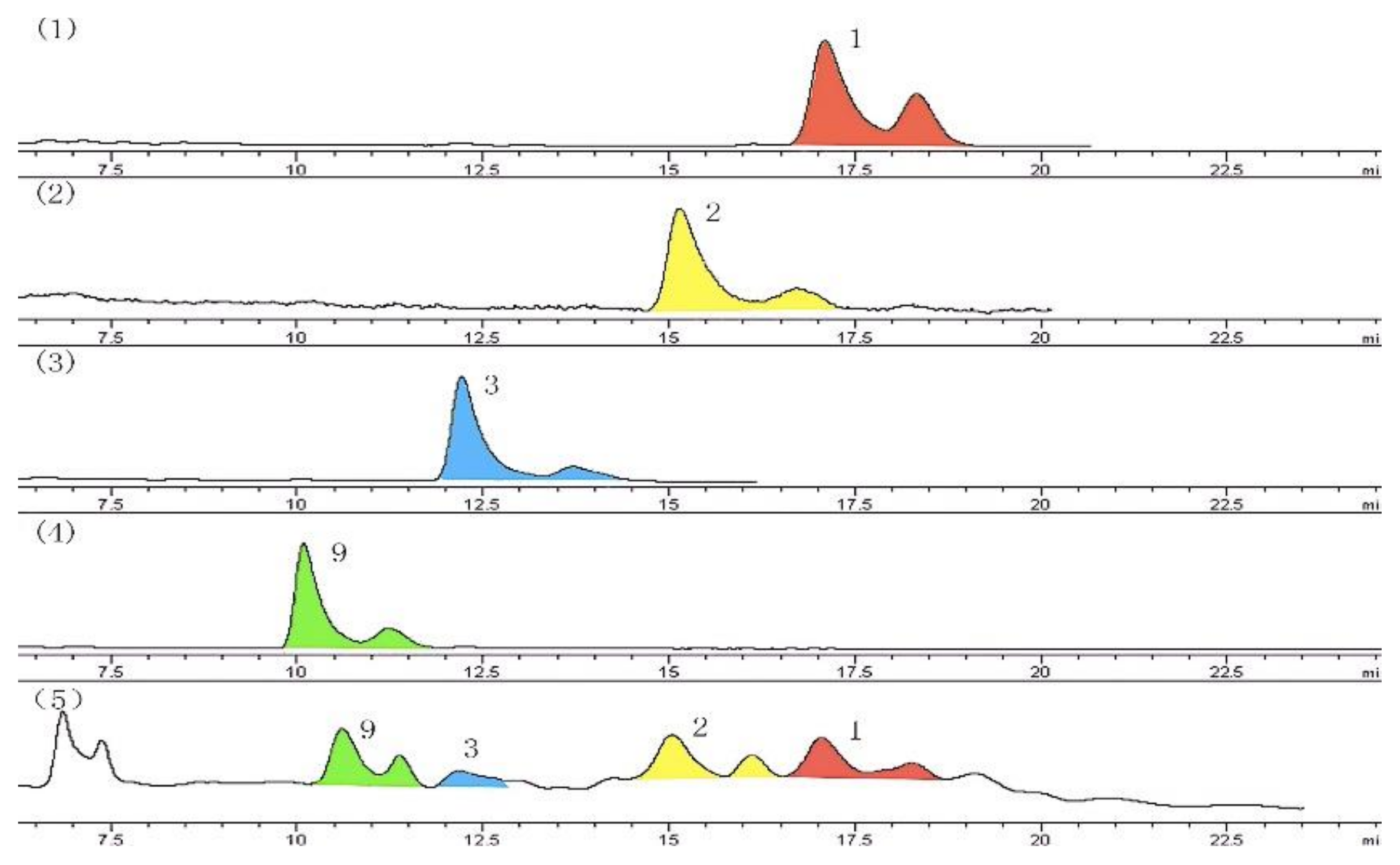
2.1. Structure Elucidation
2.2. Bioactivity
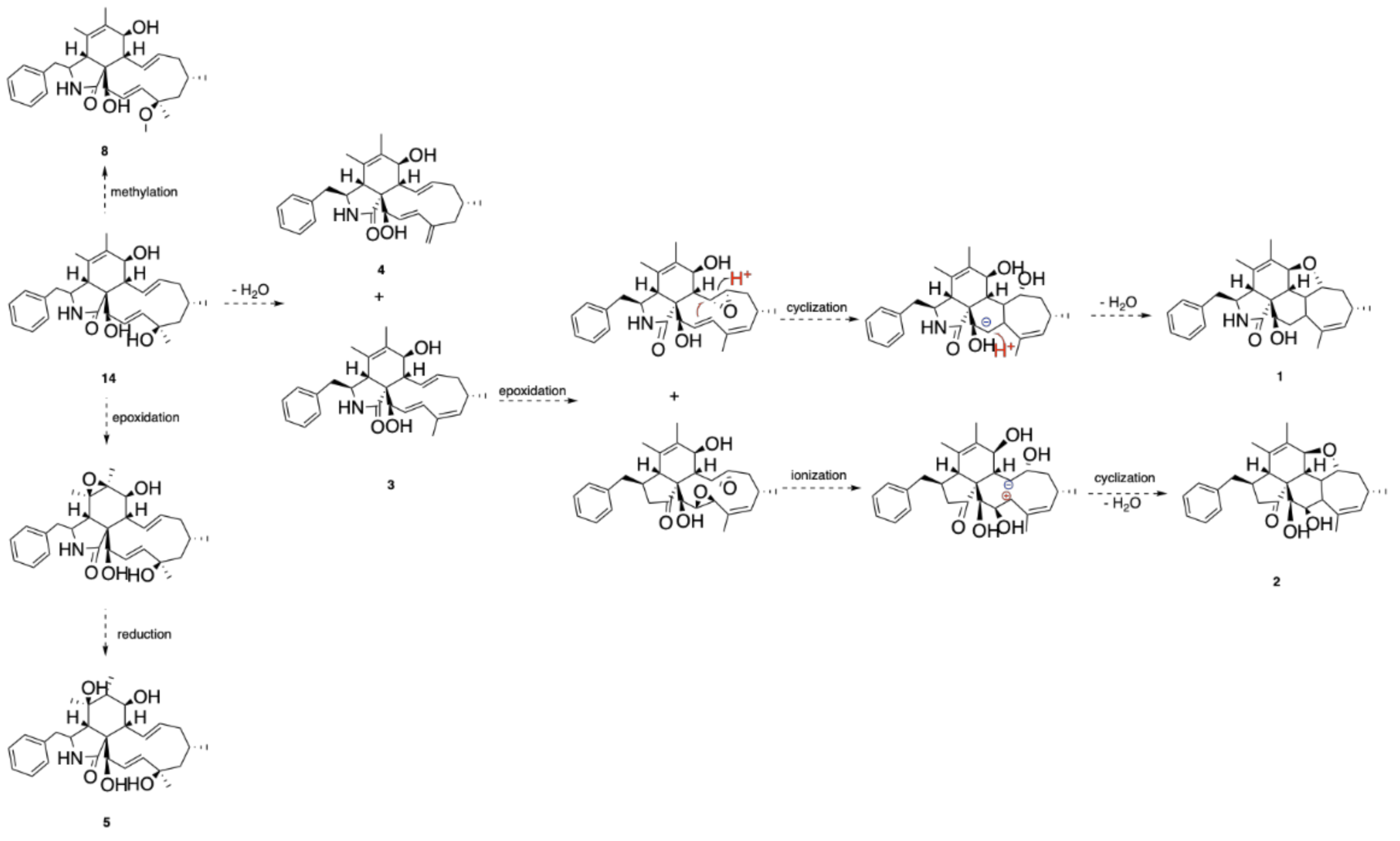
2.3. Proposed Biosynthetic Pathway of 1–5 and 8
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Experimental Biological Material
4.3. Fungal Production and Cultivation
4.4. Extraction and Purification
4.5. MTT Cytotoxicity Assay
4.6. Disk Difusion Methodology
4.7. Identification of Metabolites
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hirose, T.; Izawa, Y.; Koyama, K.; Natori, S.; Iida, K.; Yahara, I.; Shimaoka, S.; Maruyama, K. The effect of new cytochalasin from Phomopsis sp. and the derivatives on cellular structure and actin polymerization. Chem. Pharm. Bull. 1990, 38, 971–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Izawa, Y.; Hirose, T.; Shimizu, T.; Koyama, K.; Natori, S. Six new 10-phenyl-[11]cytochalasans, ctysochalasins N-S from phono-sis sp. Tetrahedron 1989, 8, 2323–2335. [Google Scholar] [CrossRef]
- Robert, J.L.; Tamm, C. Biosynthesis of cytochalasans. Part 5. The incorporation of deoxaphomin into cytochalasin B (Phomin). Helv. Chim. Acta 1975, 28, 2501–2504. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Shao, C.L.; Wu, L.Y.; Chen, M.C.; Wang, K.L.; Zhao, D.L.; Sun, X.P.; Chen, G.Y.; Wang, C.Y. Bioactive phenylalanine derivatives and cytochalasins from the soft coral-derived fungus, Aspergillus elegans. Mar. Drugs 2013, 11, 2054–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.J.; Zhang, G.J.; Zhu, T.J.; Liu, R.; Wei, H.J.; Gu, Q.Q. Bioactive cytochalasins from Aspergillus flavipes, an endophytic fungus associated with the mangrove plant Acanthus ilicifolius. Helv. Chim. Acta 2009, 92, 1538–1544. [Google Scholar] [CrossRef]
- Yan, B.C.; Wang, W.G.; Hu, D.B.; Sun, X.; Kong, L.M.; Li, X.L.; Du, X.; Luo, S.H.; Li, Y.; Sun, H.D.; et al. Phomopchalasins A and B, two cytochalasans with polycyclic-fused skeletons from the endophytic fungus Phomopsis sp. shj2. J. Org. Lett. 2016, 18, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Raju, R.; Salim, A.A.; Khalil, Z.G.; Capon, R.J. Cytochalasins from an australian marine sediment-derived Phomopsis sp. (CMB-M0042F): Acid-mediated intramolecular cycloadditions enhance chemical diversity. J. Org. Chem. 2017, 82, 9704–9709. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A.; Widdas, W.F. Competitive inhibition of hexose transfer in human erythrocytes by cytochalasin B. J. Physiol. 1977, 265, 39–40. [Google Scholar]
- Luo, Y.F.; Zhang, M.; Dai, J.G.; Pedpradab, P.; Wang, W.J.; Wu, J. Cytochalasins from mangrove endophytic fungi Phomopsis spp. xy21 and xy22. Phytochem. Lett. 2016, 17, 162–166. [Google Scholar] [CrossRef]
- Edwards, R.L.; Maitland D., J. Metabolites of the higher fungi. Part 24. Cytochalasin N, O, P, Q, and R. New cytochalasins from the fungus Hypoxylon terricola Mill. J. Chem. Soc. Perkin Trans. 1 1989, 57–65. [Google Scholar] [CrossRef]
- Su, J.Y.; Zeng, L.M. Organo-Stereochemistry; Sun Yat-Sen University Publishing House: Guangzhou, China, 1999; p. 345. [Google Scholar]
- Zhan, F.; Li, X.Y.; Wu, L.W.; Yang, T.; Han, Y.P.; Li, G.Y. Cytochalasins from the endophytic fungus Phomopsis sp. cib-109. Chem. Nat. Compd. 2013, 49, 696–698. [Google Scholar] [CrossRef]
- Skellam, E. The biosynthesis of cytochalasans. Nat. Prod. Rep. 2017, 34, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.Z.; Tan, D.D.; Chen, C.M.; Tong, Q.Y.; Li, X.L.; Huang, J.F.; Liu, J.J.; Xue, Y.B.; Wang, J.P.; Luo, Z.W.; et al. Flavichalasines A–M, cytochalasan alkaloids from Aspergillus flavipes. Sci. Rep. 2017, 7, 42434. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, D.C.; Armstrong, J.J.; Speake, R.N.; Turner, W.B. The cytochalasins, a new class of biologically active mould metabolites. Chem. Commun. 1967, 1, 26–27. [Google Scholar] [CrossRef]
| Position | Phomopchalasin C1 (1) | Phomopchalasin C2 (2) | |||
|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | HMBC (H→C) | δC, Type | δH (J in Hz) | |
| 1 | 176.0, C | 176.5, C | |||
| 2 | 5.70, s | 4, 9 | 6.12, s | ||
| 3 | 58.5, CH | 3.46, dd (6.7, 7.7) | 1, 4, 7 | 59.3, CH | 3.44, dd (6.5,7.3) |
| 4 | 49.1, CH | 3.03, s | 1, 5, 9, 10 | 48.7, CH | 3.09, s |
| 5 | 126.9, C | 126.8, C | |||
| 6 | 135.0, C | 134.4, C | |||
| 7 | 77.2, CH | 4.07, d (10.7) | 76.3, CH | 3.96, dd (1.1,11.0) | |
| 8 | 47.1, CH | 2.03, m | 45.5, CH | 2.17, dd (9.8,14.4) | |
| 9 | 47.3, C | 47.6, C | |||
| 10 | 45.4, CH2 | 2.81, dd (8.1, 13.3) | 3, 2′ | 45.4, CH2 | 2.81, dd (7.7, 13.3) |
| 2.92, dd (6.1, 13.3) | 1′ | 2.88, dd (6.5, 13.3) | |||
| 11 | 16.9, CH3 | 1.50, s | 4, 5, 6 | 16.9, CH3 | 1.44, s |
| 12 | 13.5, CH3 | 1.78, s | 5, 6, 7 | 13.5, CH3 | 1.75, s |
| 13 | 45.3, CH | 2.14, t (9.7) | 42.9, CH | 2.01, m | |
| 14 | 87.9, CH | 3.66, ddd (2.9, 9.2, 12.0) | 89.3, CH | 3.63, ddd (3.3, 9.6, 11.8) | |
| 15 | 40.4, CH2 | 1.46, d (11.6) | 39.1, CH2 | 1.28, dd (3.0, 11.4) | |
| 2.01, m | 1.87, d (11.5) | ||||
| 16 | 30.5, CH | 2.15, dd (7.1, 9.7) | 29.5, CH | 2.07, m | |
| 17 | 133.7, CH | 5.29, s | 135.1, CH | 5.30, d (1.8) | |
| 18 | 137.5, C | 138.4, C | |||
| 19 | 34.6, CH | 2.32, dd (3.3, 11.7) | 40.8, CH | 2.30, t (10.8) | |
| 20 | 34.4, CH2 | 2.07, dt (3.3, 13.7) | 13 | 71.8, CH | 4.57, dd (3.2,10.2) |
| 2.38, td (2.3, 13.5) | |||||
| 21 | 70.3, CH | 3.96, d (2.7) | 73.8, CH | 3.81, d (3.3) | |
| 22 | 24.6, CH3 | 1.14, d (7.1) | 15, 16, 17 | 23.9, CH3 | 1.09, d (7.1) |
| 23 | 23.8, CH3 | 1.77, s | 17, 18, 19 | 22.8, CH3 | 2.00, s |
| 1′ | 137.0, C | 137.0, C | |||
| 2′, 6′ | 129.4, CH | 7.21, d (7.0) | 10, 3′, 4′ | 129.4, CH | 7.22, d (7.1) |
| 3′, 5′ | 128.8, CH | 7.36, t (7.2) | 1′ | 128.8, CH | 7.34, t (7.5) |
| 4′ | 127.1, CH | 7.29, d (7.2) | 127.1, CH | 7.28, t (7.5) | |
| OH (20) | 3.35, s | ||||
| OH (21) | 1.64, brs | 2.58, s | |||
| Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|
| Compound | B. subtilis | B. pumilus | C. albicans | A. niger |
| 1 | / | / | / | 8 |
| 3 | / | 9 | 10 | / |
| 4 | 7 | 8 | / | / |
| Gentamicin | 16 | 16 | / | / |
| Amphotericin B | / | / | 15 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Lin, W.; Li, H.; Tang, Q.; Hu, Z.; Huang, H.; Deng, X.; Xu, Q. Pentacyclic Cytochalasins and Their Derivatives from the Endophytic Fungus Phomopsis sp. xz-18. Molecules 2021, 26, 6505. https://doi.org/10.3390/molecules26216505
Huang G, Lin W, Li H, Tang Q, Hu Z, Huang H, Deng X, Xu Q. Pentacyclic Cytochalasins and Their Derivatives from the Endophytic Fungus Phomopsis sp. xz-18. Molecules. 2021; 26(21):6505. https://doi.org/10.3390/molecules26216505
Chicago/Turabian StyleHuang, Guichon, Weiwen Lin, Hanpeng Li, Qian Tang, Zhiyu Hu, Huiying Huang, Xianming Deng, and Qingyan Xu. 2021. "Pentacyclic Cytochalasins and Their Derivatives from the Endophytic Fungus Phomopsis sp. xz-18" Molecules 26, no. 21: 6505. https://doi.org/10.3390/molecules26216505
APA StyleHuang, G., Lin, W., Li, H., Tang, Q., Hu, Z., Huang, H., Deng, X., & Xu, Q. (2021). Pentacyclic Cytochalasins and Their Derivatives from the Endophytic Fungus Phomopsis sp. xz-18. Molecules, 26(21), 6505. https://doi.org/10.3390/molecules26216505





