Preparation and Self-Cleaning Performance of Carbon-Based Superhydrophobic Coatings Based on Non-Fluorine and Non-Toxic Corn Straw
Abstract
:1. Introduction
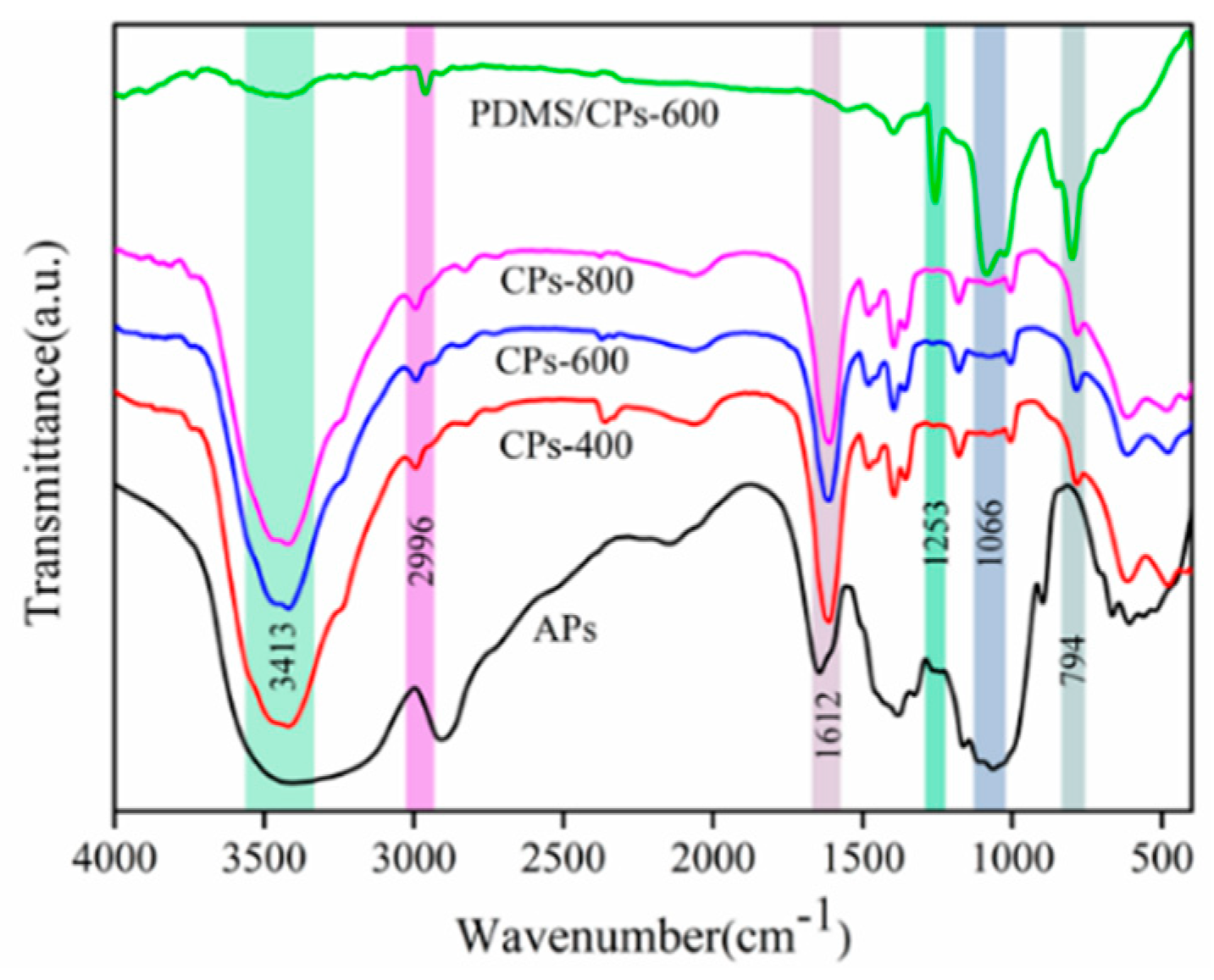
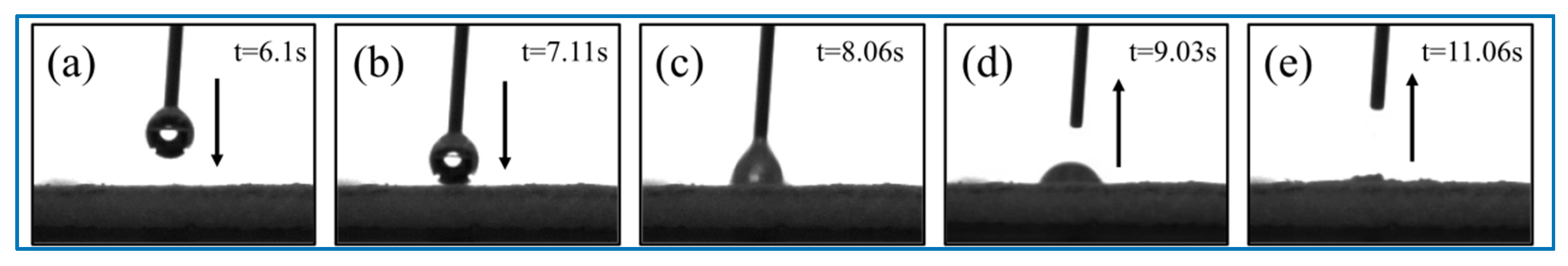
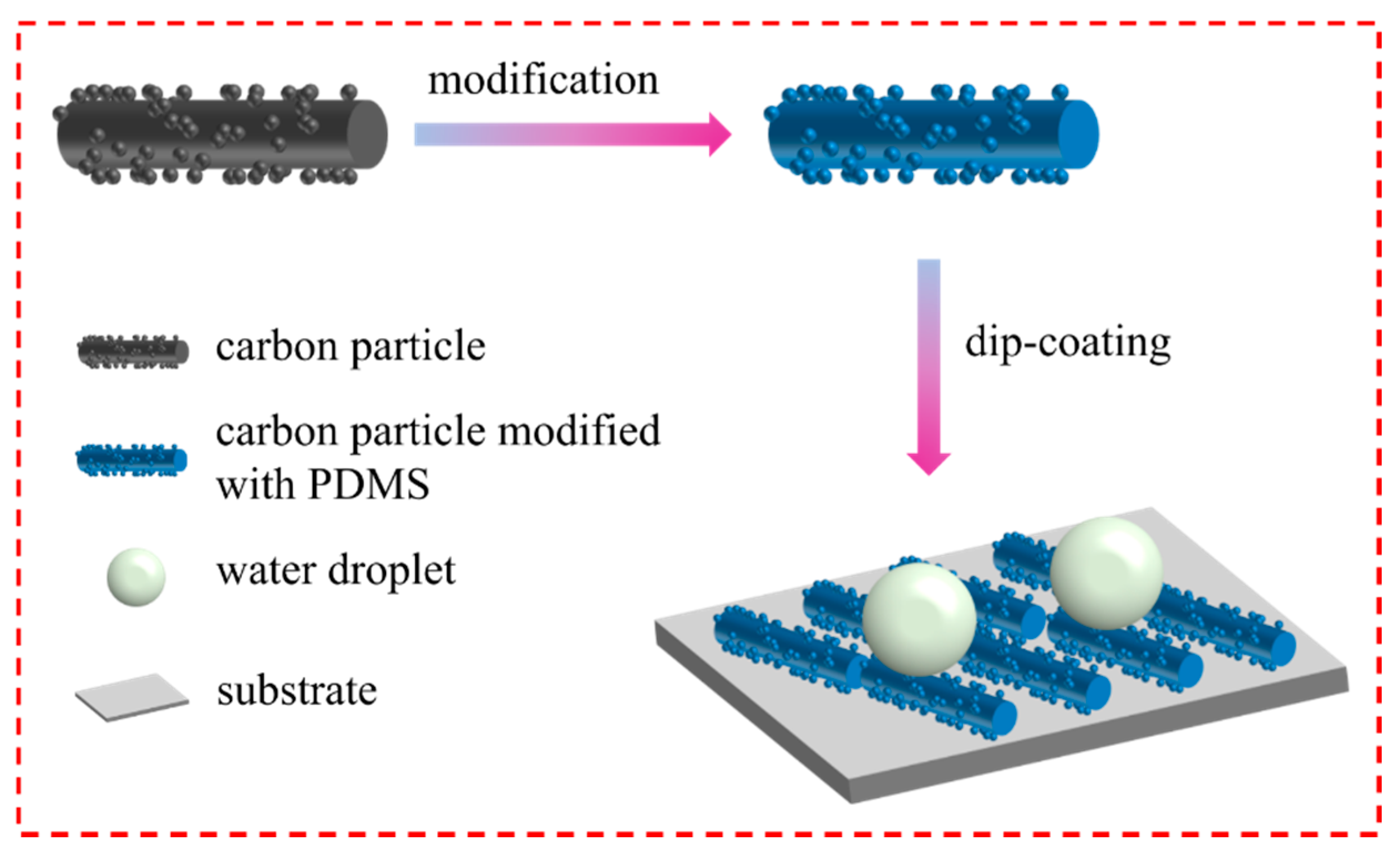
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Chemicals
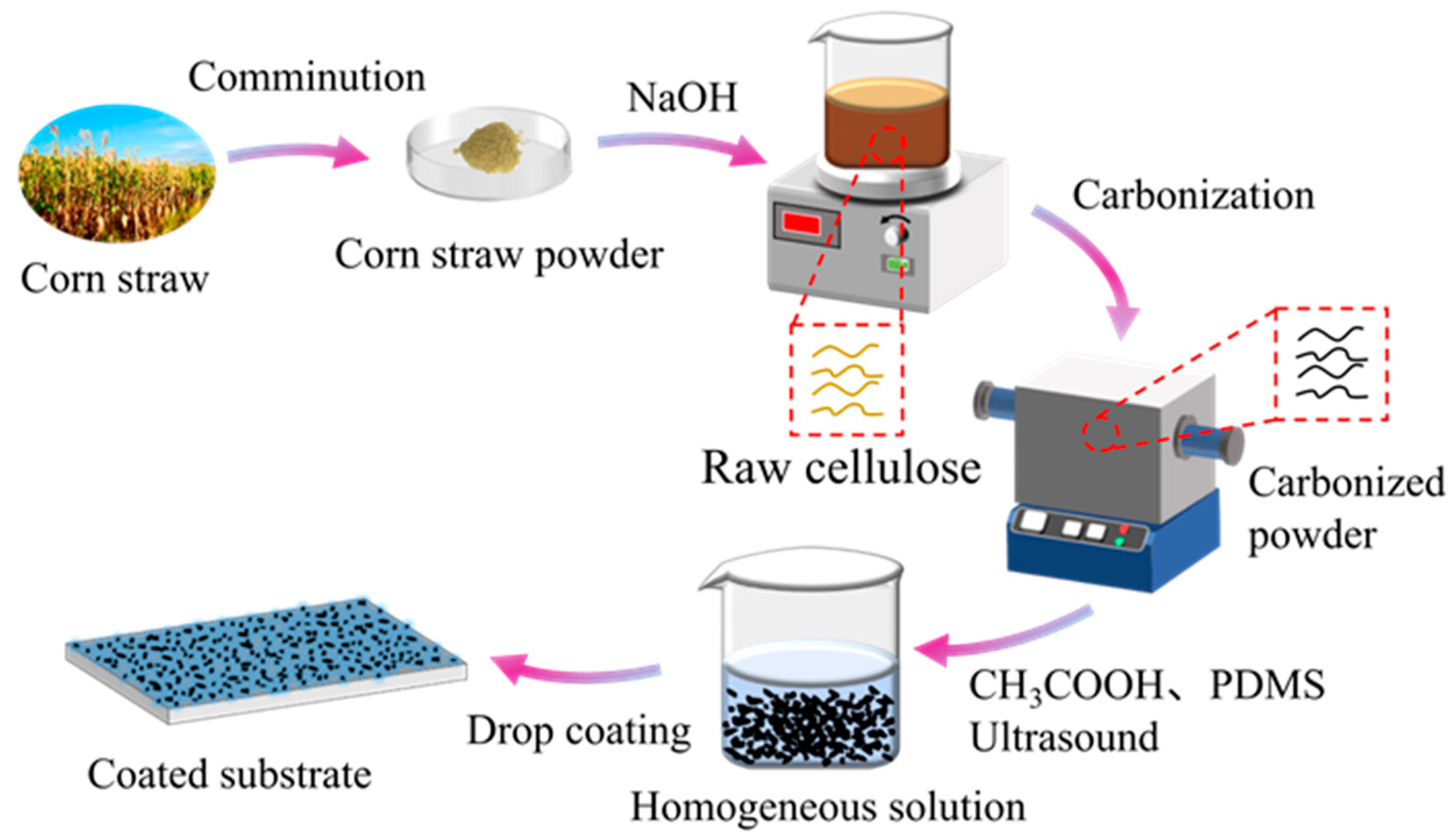
3.2. Pre-Treatment of the Corn Straw
3.3. Preparation of Corn Straw Based Carbon Particles (CPs)
3.4. Fabrication of Coating Suspension
3.5. Fabrication of Superhydrophobic Coatings
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Garlisi, C.; Trepci, E.; Li, X.; Al Sakkaf, R.; Al-Ali, K.; Nogueira, R.P.; Zheng, L.; Azar, E.; Palmisano, G. Multilayer thin film structures for multifunctional glass: Self-cleaning, antireflective and energy-saving properties. Appl. Energy 2020, 264, 114697. [Google Scholar] [CrossRef]
- Murugan, K.; Subasri, R.; Rao, T.N.; Gandhi, A.S.; Murty, B.S. Synthesis, characterization and demonstration of self-cleaning TiO2 coatings on glass and glazed ceramic tiles. Prog. Org. Coat. 2013, 76, 1756–1760. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; El-Sockary, M.A.; Hashem, A.I.; Elenien, O.M.A.; El-Saeed, A.M.; Fatthallah, N.A. Smart photo-induced silicone/TiO2 nanocomposites with dominant [110] exposed surfaces for self-cleaning foul-release coatings of ship hulls. Mater. Des. 2016, 101, 218–225. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Shenashen, M.A.; Higazy, S.A.; Elmarakbi, A. Progress in biomimetic leverages for marine antifouling using nanocomposite coatings. J. Mater. Chem. B 2020, 8, 3701–3732. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired Surfaces with Special Wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef]
- Feng, X.J.; Jiang, L. Design and Creation of Superwetting/Antiwetting Surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Lin, F.; Li, S.-H.; Li, Y.; Li, H.-J.; Zhu, D.-B. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2003, 14, 1857–1860. [Google Scholar]
- Gao, X.; Jiang, L. Biophysics: Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-Inspired Self-Cleaning Surfaces. Annu. Rev. Mater. Res. 2012, 42, 231–263. [Google Scholar] [CrossRef]
- Hansen, W.R.; Autumn, K. Evidence for self-cleaning in gecko setae. Proc. Natl. Acad. Sci. USA 2005, 102, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Zheng, L.; Zhao, C.; Liu, H. Micro-/Nanostructured Interface for Liquid Manipulation and Its Applications. Small 2020, 16, 1903849. [Google Scholar] [CrossRef]
- Liu, R.; Chi, Z.; Cao, L.; Weng, Z.; Wang, L.; Li, L.; Saeed, S.; Lian, Z.; Wang, Z. Fabrication of biomimetic superhydrophobic and anti-icing Ti6Al4V alloy surfaces by direct laser interference lithography and hydrothermal treatment. Appl. Surf. Sci. 2020, 534, 147576. [Google Scholar] [CrossRef]
- Zhang, J.; Rosenkranz, A.; Zhang, J.; Guo, J.; Li, X.; Chen, X.; Xiao, J.; Xu, J. Modified Wettability of Micro-structured Steel Surfaces Fabricated by Elliptical Vibration Diamond Cutting. Int. J. Precis. Eng. Manuf.-Green Technol. 2021. [Google Scholar] [CrossRef]
- Wei, D.; Wang, J.; Liu, Y.; Wang, D.; Li, S.; Wang, H. Controllable superhydrophobic surfaces with tunable adhesion on Mg alloys by a simple etching method and its corrosion inhibition performance. Chem. Eng. J. 2021, 404, 126444. [Google Scholar] [CrossRef]
- Mayoussi, F.; Doeven, E.H.; Kick, A.; Goralczyk, A.; Thomann, Y.; Risch, P.; Guijt, R.M.; Kotz, F.; Helmer, D.; Rapp, B.E. Facile fabrication of micro-/nanostructured, superhydrophobic membranes with adjustable porosity by 3D printing. J. Mater. Chem. A 2021, 9, 21379–21386. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Pan, Y.; Wang, C.; Liu, C.; Shen, C.; Pan, C.; Liu, X. Asymmetric Superhydrophobic Textiles for Electromagnetic Interference Shielding, Photothermal Conversion, and Solar Water Evaporation. ACS Appl. Mater. Interfaces 2021, 13, 28996–29007. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Torun, I.; Ruzi, M.; Esidir, A.; Onses, M.S. Fabrication of robust superhydrophobic surfaces by one-step spray coating: Evaporation driven self-assembly of wax and nanoparticles into hierarchical structures. Chem. Eng. J. 2020, 396, 125230. [Google Scholar] [CrossRef]
- Duan, Z.; Qu, L.; Hu, Z.; Liu, D.; Liu, R.; Zhang, Y.; Zheng, X.; Zhang, J.; Wang, X.; Zhao, G. Fabrication of micro-patterned ZrO2/TiO2 composite surfaces with tunable super-wettability via a photosensitive sol-gel technique. Appl. Surf. Sci. 2020, 529, 147136. [Google Scholar] [CrossRef]
- Zhong, Y.; Gu, L.; Wang, S.; Jin, Y.; Xiao, H. Green and Superhydrophobic Coatings Based on Tailor-Modified Lignocellulose Nanofibrils for Self-Cleaning Surfaces. Ind. Eng. Chem. Res. 2019, 58, 20323–20330. [Google Scholar] [CrossRef]
- Chen, B.; Qiu, J.; Sakai, E.; Kanazawa, N.; Liang, R.; Feng, H. Robust and Superhydrophobic Surface Modification by a “Paint + Adhesive” Method: Applications in Self-Cleaning after Oil Contamination and Oil-Water Separation. ACS Appl. Mater. Interfaces 2016, 8, 17659–17667. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Johnson, L.; Tran, D.N.; Losic, D. Robust Superhydrophobic Graphene-Based Composite Coatings with Self-Cleaning and Corrosion Barrier Properties. ACS Appl. Mater. Interfaces 2015, 7, 28482–28493. [Google Scholar] [CrossRef]
- Lv, C.; Wang, H.; Liu, Z.; Wang, C.; Zhang, W.; Li, M.; Zhu, Y. Fabrication of durable fluorine-free polyphenylene sulfide/silicone resin composite superhydrophobic coating enhanced by carbon nanotubes/graphene fillers. Prog. Org. Coat. 2019, 134, 1–10. [Google Scholar] [CrossRef]
- Mates, J.E.; Ibrahim, R.; Vera, A.; Guggenheim, S.; Qin, J.; Calewarts, D.; Waldroup, D.E.; Megaridis, C.M. Environmentally-safe and transparent superhydrophobic coatings. Green Chem. 2016, 18, 2185–2192. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbuehler, K. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environ. Int. 2015, 75, 172–179. [Google Scholar] [CrossRef]
- Kota, A.K.; Mabry, J.M.; Tuteja, A. Superoleophobic surfaces: Design criteria and recent studies. Surf. Innov. 2013, 1, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Wu, X.; Lou, Z.; Wu, D. Superhydrophobicity from microstructured surface. Chin. Sci. Bull. 2004, 49, 1779–1787. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Z. Comparison of hydrochar- and pyrochar-based solid acid catalysts from cornstalk: Physiochemical properties, catalytic activity and deactivation behavior. Bioresour. Technol. 2020, 297, 122477. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Mu, J.; Chu, D.; Zang, X. Converting industrial waste cork to biochar as Cu (II) adsorbent via slow pyrolysis. Waste Manag. 2020, 105, 102–109. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, T.; Huang, J.; Chen, J.; Li, J. Fabrication and characterization of superhydrophobic PDMS composite membranes for efficient ethanol recovery via pervaporation. Sep. Purif. Technol. 2020, 241, 116675. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, P.; Wang, M.; Wu, G.; Dong, C.; Wu, A. Biomass-Derived Porous Carbonaceous Aerogel as Sorbent for Oil-Spill Remediation. ACS Appl. Mater. Interfaces 2016, 8, 32862–32868. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.W.; Ahn, K.H.; Lee, Y.; Kim, K.P.; Rhee, J.S.; Park, J.T.; Paeng, K.J. Adsorption characteristics of phenol and chlorophenols on granular activated carbons (GAC). Microchem. J. 2001, 70, 123–131. [Google Scholar] [CrossRef]
- Beshkar, F.; Khojasteh, H.; Salavati-Niasari, M. Recyclable magnetic superhydrophobic straw soot sponge for highly efficient oil/water separation. J. Colloid Interface Sci. 2017, 497, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, N.; Yamada, Y.; Shiraishi, M.; Kaneko, K.; Setoyama, N. Evaluation of accessible and inaccessible microporosities of microporous carbons. J. Chem. Soc. Faraday Trans. 1996, 92, 2297–2302. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.; Li, C. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Chabalala, V.P.; Wagner, N.; Potgieter-Vermaak, S. Investigation into the evolution of char structure using Raman spectroscopy in conjunction with coal petrography; Part 1. Fuel Process. Technol. 2011, 92, 750–756. [Google Scholar] [CrossRef]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Dixon, D.E. Morphological Characterization of Soot from the Atmospheric Combustion of Kerosene. J. Chem. 2011, 8, 1068–1073. [Google Scholar]
- Tong, W.; Xiong, D.; Tian, T.; Liu, Y. Superhydrophobic surface on aeronautical materials via the deposition of nanoparticles and a PDMS seal. Appl. Phys. A 2019, 125, 177. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Farshchian, B.; Gatabi, J.R.; Bernick, S.M.; Park, S.; Lee, G.-H.; Droopad, R.; Kim, N. Laser-induced superhydrophobic grid patterns on PDMS for droplet arrays formation. Appl. Surf. Sci. 2017, 396, 359–365. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Yu, L.; Duan, Q.; Chen, L. Superhydrophobic Modification on Starch Film Using PDMS and Ball-Milled MMT Coating. ACS Sustain. Chem. Eng. 2020, 8, 10423–10430. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Hussain, S.Z.; Subhani, T.; Hussain, I.; Hussain, I. Mechanically robust superhydrophobic coating from sawdust particles and carbon soot for oil/water separation. Colloids Surf. A 2018, 539, 391–398. [Google Scholar] [CrossRef]





| Sample | Ra (μm) | Mean Value (μm) | Standard Deviation (μm) | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| PDMS/CPs-400 | 2.74 | 2.83 | 3.05 | 2.87 | 0.13 |
| PDMS/CPs-600 | 3.35 | 3.14 | 3.52 | 3.34 | 0.16 |
| PDMS/CPs-800 | 1.83 | 2.68 | 2.36 | 2.29 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Kang, L.; Li, Z.; Su, Q.; Pang, S.; Liang, L.; Wang, D.; Cao, S. Preparation and Self-Cleaning Performance of Carbon-Based Superhydrophobic Coatings Based on Non-Fluorine and Non-Toxic Corn Straw. Molecules 2021, 26, 6401. https://doi.org/10.3390/molecules26216401
Wang Y, Kang L, Li Z, Su Q, Pang S, Liang L, Wang D, Cao S. Preparation and Self-Cleaning Performance of Carbon-Based Superhydrophobic Coatings Based on Non-Fluorine and Non-Toxic Corn Straw. Molecules. 2021; 26(21):6401. https://doi.org/10.3390/molecules26216401
Chicago/Turabian StyleWang, Yanbin, Lihui Kang, Zhaoxia Li, Qiong Su, Shaofeng Pang, Lichun Liang, Dian Wang, and Shijun Cao. 2021. "Preparation and Self-Cleaning Performance of Carbon-Based Superhydrophobic Coatings Based on Non-Fluorine and Non-Toxic Corn Straw" Molecules 26, no. 21: 6401. https://doi.org/10.3390/molecules26216401
APA StyleWang, Y., Kang, L., Li, Z., Su, Q., Pang, S., Liang, L., Wang, D., & Cao, S. (2021). Preparation and Self-Cleaning Performance of Carbon-Based Superhydrophobic Coatings Based on Non-Fluorine and Non-Toxic Corn Straw. Molecules, 26(21), 6401. https://doi.org/10.3390/molecules26216401






