Abstract
The objective of this study was to assess the biological activity of essential oils (EOs) of four Juniperus species obtained via two different distillation methods and their potential as biopesticides. The studied factors were juniper species (Juniperus communis L., J. oxycedrus L., J. pygmaea C. Koch., and J. sibirica Burgsd), plant sex (male (M) and female (F)), and distillation method (hydrodistillation via a standard Clevenger apparatus (ClevA) and semi-commercial (SCom) steam distillation). The hypothesis was that the EO will have differential antioxidant, antimicrobial, and insecticidal activities as a function of plant species, plant sex, and distillation method. The two distillation methods resulted in similar EO composition within a given species. However, there were differences in the EO content (yield) due to the sex of the plant, and also differences in the proportions of some EO components. The concentration of α-pinene, β-caryophyllene, δ-cadinene and δ-cadinol was dissimilar between the EO of M and F plants within all four species. Additionally, M and F plants of J. pygmaea, and J. sibirica had significantly different concentrations of sabinene within the respective species. The EOs obtained via ClevA extraction showed higher antioxidant capacity within a species compared with those from SCom extraction. All of the tested EOs had significant repellent and insecticidal activity against the two aphid species Rhopalosiphum padi (bird cherry-oat aphid) and Sitobion avenae (English grain aphid) at concentrations of the EO in the solution of 1%, 2.5%, and 5%. The tested EOs demonstrated moderate activity against selected pathogens Fusarium spp., Botrytis cinerea, Colletotrichum spp., Rhizoctonia solani and Cylindrocarpon pauciseptatum. The results demonstrate that the standard ClevA would provide comparable EO content and composition in comparison with SCom steam distillation; however, even slight differences in the EO composition may translate into differential bioactivity.
1. Introduction
Essential oils (EOs) are important natural products (NP) utilized in the development of new products, including environmentally safe pesticides [1]. They have shown significant biological activities such as antibacterial, antimicrobial, antifungal, antiviral and insecticidal, and play a notable role in allelopathic communication between plants [1,2,3,4,5,6]. Juniper species are important as a source of complex mixtures of secondary metabolites, especially EO. The main components of junipers oils are monoterpene hydrocarbons (α-pinene, β-pinene, δ-3-carene, and limonene) [7,8,9,10]. According to Flora of Bulgaria [11], in the Bulgarian flora, there are six species of genus Jiniperus (J. excelsa; J. communis; J. oxycedrus; J. sibirica; J. sabina; J. pygmaea). Two of them are of limited distribution (J. excelsa; J. sabina), but the others (J. communis; J. oxycedrus; J. sibirica; J. pygmaea) are widely distributed, and they have been known to encroach on pasture lands, especially in the mountains. For example, J. communis and J. sibirica have a dominant distribution, spreading intensively (and invasively) in most of the mountains of Bulgaria (Stara Planina, Rila, Pirin, Slavyanka) [12]. This intensive distribution of juniper species adversely affects the ecological balance of the ecosystems because: (1) the distribution of other plant species is restricted, and (2) the spread of junipers reduces the usable pastures. In some countries and states, they were declared unwanted species in such habitats. Many farmers are trying to limit the spread of J. communis and J. sibirica by means of various chemical and physical methods such as cutting or burning juniper bushes. On the other hand, junipers are potential resources for high-value EO. Currently, juniper EO has an expanding market; it is highly valued as an aroma agent and there is a significant production of juniper EO in Europe, North America, and Asia. Therefore, juniper removal can be coupled with juniper EO production. A literature review showed several biological activities of juniper EO, such as antibacterial, antifungal, antiviral, and antioxidant activities [7,8,9,10,13,14,15]. The biological activity of EOs is influenced by a number of factors, such as the composition of the EO, the distillation timeframe, the extraction method, and plant species [8,14,16,17,18,19]. Juniperus communis is one of the most widely distributed species on the planet [20], and therefore, among all juniper species, its EO composition has been reported most often. The antimicrobial activity of J. communis galbuli EO obtained with different distillation timeframes was demonstrated against S. aureus subsp. aureus, C. glabrata and K. pneumonia [14]. The antimicrobial activity of the EOs of fruits (galbuli/berries) and leaves (needles) of J. communis subsp. hemisphaerica and J. oblonga were investigated against B. subtilis, S. aureus, E. coli, P. aeroginosa and C. albicans [21]. Angioni et al. [13] tested the EOs and their major compounds of ripe and unripe berries and leaves of J. oxycedrus L. ssp. oxycedrus, J. phoenicea ssp. turbinata and J. communis ssp. communis against C. albicans, S. aureus, E. coli, and P. aeruginosa in Italy, and concluded that the EOs of J. phoenicea ssp. turbinata and the EO from leaves of J. oxycedrus ssp. oxycedrus exhibit either good or weak activity against C. albicans and S. aureus. The leaf EOs of M J. communis, J. sibirica, and J. pygmaea showed higher antimicrobial activity against E. coli, H. influenzae, S. sonei, Y. enterocolitica, S. aureus subs. aureus, S. pneumonia, compared to the EOs of female junipers [7].
Some EOs have the potential to scavenge free radicals of cells and may play an important role in the prevention of some diseases [22]. The antioxidant activity of the EOs from the juniper leaves and galbuli have been previously reported [14,23,24]. There are some previous reports on the composition of the EO from the berries and leaves of the Juniperus species; however, very few studies reported the antifungal and insecticidal activity of juniper EOs. The terpenoid compounds of EO of J. saltuaria, J. squamata var. fargesii and J. squamata var. morrisonicola, and J. communis were found to be highly toxic to insects [15,25,26]. Juniper EOs have the potential to be used as an ingredient in the development of new environmentally friendly insecticides, and therefore reduce the use of synthetic pesticides [1,10,15].
The objective of this study was to assess the antioxidant, antimicrobial, and insecticidal activities of M and F J. communis, J. oxycedrus, J. pygmaea, and J. sibirica EOs obtained by means of two distillation methods (ClevA and SCom). The hypothesis was that EOs of these four juniper species will have different antioxidant, antimicrobial, insecticidal and antifungal activities, and the standard method of ClevA hydrodistillation would provide dissimilar EO profiles when compared to a commercial steam distillation unit.
2. Results and Discussion
2.1. Total Essential Oil (EO) Content (Yield)
Overall, there was a substantial variation in the EO yield between M and F plants, plant species, and extraction methods (ClevA and SCom) (Table 1). The EO yield varied from 0.05% in J. oxycedrus (F) to 1.63% in J. communis (F). The highest yield of EO was obtained from J. communis (F) and J. pygmaea (F) with ClevA and SCom extraction (Table 1). Previous reports [27,28] have shown that the EO yield of Juniperus may depend on the gender of the plants, genetic characteristics, soil, and climatic factors. In this study, higher EO yields were obtained using ClevA in comparison with SCom distillation.

Table 1.
Essential oil (EO) content of Juniperus oxycedrus, J. communis, J. pygmaea, and J. sibirica as a function of the sex of the tree and the extraction method.
2.2. Composition of the Juniper Essential Oil (EO)
Gas chromatography (GC) and mass spectroscopy (MS) analyses of each of the four junipers EOs identified at least 35 constituents (Supplemental Tables S1–S4). Among the identified classes, the main ones were monoterpenes hydrocarbons (MH), oxygenated monoterpenes (OM), phenolic monoterpenes (PhM), bicyclic oxygenated monoterpenes (BOM), sesquiterpenes hydrocarbons (SH), oxygenated sesquiterpenes (OS), tricyclic oxygenated sesquiterpenes (TOS), bicyclic sesquiterpene hydrocarbons (BSH), oxygenated bicyclic sesquiterpenes (OBS) and diterpenes (D) (Table S5). The monoterpenes were the main class of EOs in all four junipers (Table S5).
As shown in Table 2, α-pinene was the predominant constituent of the monoterpenes in all four species (J. communis, J. pygmaea, J. sibirica, J. oxycedrus), with the highest concentration being in J. communis (F) EO. α-pinene varied from 14.9% (in J. sibirica F) to 34.9% (in J. communis F). The concentration of sabinene was generally above 9% of the EO, except in J. oxycedrus (M, F) (Table 2). Overall, the concentration of limonene was higher in J. communis (M) and J. oxycedrus (M) and lower in J. sibirica (M, F) (Table 3). α-terpinene, p-cymene, bornyl acetate, β-elemene were minor (below 2% of the EO) constituents in the EOs of all four species (Table 2 and Table 4). β-myrcene and terpinen-4-ol were found at higher concentrations in the EO of J. communis (Table 2 and Table 3). Overall, the monoterpenes in the EOs obtained via ClevA were slightly higher compared to those in the EO obtained through SCom. One sample of J. communis-M obtained through ClevA was an exception (Table S5).

Table 2.
Mean concentration (%) of α-pinene, camphene, sabinene, β-pinene, β-myrcene, α-terpinene, and p-cymene (extracted using the commercial steam extraction method) obtained from the eight combinations of species and sex.

Table 3.
Mean concentration (%) of limonene, γ-terpinene, α-terpinolene, terpinen-4-ol, bornyl acetate, β-elemene, and β-caryophyllene (extracted using the commercial steam extraction method) obtained from the eight combinations of species and sex.

Table 4.
Mean concentration (%) of α-humulene, germacrene D, γ-cadinene, δ-cadinene, δ-cadinol, tau-cadinol, and α-cadinol (extracted using the commercial steam extraction method) obtained from the eight combinations of species and sex.
In general, the values of sesquiterpenes in the four juniper species were higher in the EO obtained via SCom (Table S5). The monocyclic sesquiterpene hydrocarbons (MSH) and bicyclic sesquiterpene hydrocarbons (BSH) were the most represented of this class (Table S5). Germacrene D and β-elemene were the predominant compounds of the monocyclic sesquiterpene hydrocarbons (MSH). The highest amount of germacrene D was found in J. oxycedrus (17.8% F), followed by J. sibirica (13.1% M), J. pygmaea (11.4% F), and J. communis (6.9% M) (Table 4). β-elemene was higher in J. sibirica (F) EO (6.8%), followed by J. pygmaea (F) (4.8%) (Table 3). β-caryophyllene, γ-cadinene, δ-cadinene, and α-humulene were predominant among the bicyclic sesquiterpene hydrocarbons (BSH) (Table 3 and Table 4). The highest γ-cadinene values were obtained from J. oxycedrus (M, F), followed by J. pygmaea F (3.2%) (Table 4), while α-humulene was highest (2.98%) in J. pygmaea (F) EO. The compositions of J. communis EO extracted by SCom steam distillation were specific and contained δ-cadinol, tau-cadinol, tau-muurolol, α-cadinol. Acyclic sesquiterpene hydrocarbons were found only in J. sibirica (farnesol and farnesal) (Table S5).
The diterpenes class was the highest in J. oxycedrus (M) EO (~20%) (Table S5). Oxygenated diterpenes were found only in J. oxycedrus and J. communis EO, while monocyclic diterpenes were found in J. sibirica EO.
Generally, the EO composition of the respective samples obtained by the two extraction methods (ClevA and SCom) was very similar. We found differences in the proportions of EO constituents, with the exception of the J. communis EO. As mentioned in the Introduction section, J. communis is one of the most widely distributed species on the planet [20], and therefore its EO composition has been reported most often. Previous studies on J. communis utilized different EO extractions methods [29,30,31,32,33]. It has been demonstrated that supercritical fluid extraction and hydrodistillation methods result in dissimilar leaf EO composition [32].
Our results disprove the working hypothesis, because the EO profile of the samples extracted via the two different methods was very similar within a juniper species. In this study, the main EO constituents of the different extraction methods were α-pinene, sabinene, limonene, β-myrcene. Previous research identified the same composition of EOs obtained by micro-distillation and extraction, and supercritical carbon dioxide extraction from juniper needles [31]. Differences in the EO composition of J. communis have been reported following supercritical fluid extraction (SFE) and hydrodistilled EOs [32]. The cited authors found a total of 22 compounds in the EOs from SFE, and only 11 in the hydrodistilled EO [32]. The EO of J. communis berries (galbuli) had different qualitative compositions when extracted via hydrodistillation and hexane extraction methods [30]. The concentrations of α-pinene, sabinene, myrcene were higher in the hydrodistilled EO, while some less volatile compounds were present in the extracts, especially in the hexane extract [30].
2.3. Insecticidal Action and Repellent Activities of the EOs from the Semi-Commercial Steam Extraction against Rhopalosiphum padi (Bird Cherry—Oat Aphid) and Sitobion avenae (English Grain Aphid)
Aphids are some of the main pests on cereal crops and can significantly reduce yields [34,35]. The interaction effect of species-sex and EO concentration was highly significant on % number (nb) for the repellent action of EO on leaf aphids (Table 5), which suggests that the ideal EO concentration that needs to be used in repellent varies with the combination of the species and the sex of the plant. There are no previous reports concerning repellent and insecticidal action of the EOs of Bulgarian conifers.

Table 5.
ANOVA p-values that show the significance of the main effects and the interaction effect of species-sex and concentration (Conc) on nb./leaf of Rhopalosiphum padi and nb./leaf of Sitobion avenae for repellent action.
2.3.1. Repellent Activity of EOs of J. oxycedrus, J. communis, J. pygmaea, and J. sibirica
The insect repellent activity of EOs is commonly used to deter insects. The results of the repellent activity for the EO and constituents against Rhopalosiphum padi and Sitobion avenae are presented in Table 6. The data of the tested EO of Rh. padi showed that at a 5% concentration, the EOs of J. oxycedrus (M), J. sibirica (M) and J. pygmaea (F, M) had a strong repellent activity (Table 6), where the main compound EO in all three species was α-pinene (Table 2). However, at concentrations of 1–2.5%, the EOs J. communis (M), J. sibirica (F) (1%), J. oxycedrus (M), and J. sibirica (F) (2.5%) demonstrated a lower repellent action (Table 6). Similar results were obtained by Carroll et al. [36]. The cited authors tested junipers EOs (J. chinensis, J. communis) for repellency for Aedes aegypti, Amblyomma americanum and Ixodes scapularis and concluded that the oils were repellent to both species of ticks, but EO of J. communis had a minimum effective dosage for repellency [36]. However, in this study, S. avenae was more sensitive to the tested EOs at lower concentrations. The three tested concentrations (1%, 2.5% and 5%) of the EOs of J. communis (F), J. sibirica (F), and J. oxycedrus (M) had a strong repellent activity, and α-pinene and sabinene were main EO constituents in the three species. The oils of J. communis (M) (2.5% and 5%), J. pygmaea (F) (1% and 5%), J. pygmaea (M) (2.5% and 5%), and J. sibirica (M) (2.5% and 5%) had a lower repellent activity (Table 6).

Table 6.
Mean Rhopalosiphum padi and Sitobion avenae for the repellent action obtained from the 28 combinations of species-sex and EO concentration (Conc).
Overall, in this study, the EOs from J. communis (F, M), J. pygmaea (F, M) and J. sibirica (F, M) were found to have the strongest repellent activity on both aphid species (Rh. padi and S avenae). The main compounds in the EOs of J. communis (F, M), J. pygmaea (F, M) and J. sibirica (F, M) were α-pinene and sabinene (Table 2). Over 50% of the composition of the J. communis, J. pygmaea and J. sibirica oils consists of α-pinene, sabinene, germacrene D, and δ-cadinene. α-pinene was reported as a repellent in an insect activity study [37]. Obviously, the synergism between the individual compounds in the EOs of J. communis, J. pygmaea and J. sibirica is the reason for their activity. Current thinking is that although some specific compounds may have a repellent effect, in some instances, the interaction between them causes greater bioactivity [38]. The EOs of junipers can be used in lower doses to achieve a repellent effect in agricultural management practices.
2.3.2. Insecticidal Activity of EOs of J. oxycedrus, J. communis, J. pygmaea, and J. sibirica
The insecticidal activity of the EOs obtained from the four juniper species (M, F) was established by testing oil efficacy at different concentrations. The effect was calculated using Abbot’s formula [39]. The tested EOs demonstrated a very good insecticidal effect 24 h after the treatment of the aphids (Table 7). The EOs most often act as neurotoxins on the insects, and they affect their physiological processes [40,41]. The EOs’ efficacy was 100% on both aphids (S. avenae and Rh. padi). An exception was the EO of J. sibirica (M) at 2.5% and J. communis (F) at 1%, with efficiencies around 90%. After 72 h, the efficiency in all treatments and concentrations was 100% (Table 7). The juniper EOs have mostly been tested for the control of mosquitoes and ticks, and less for the control of agricultural pests. Athanassiou et al. [42] suggested that the simultaneous use of silica gel and EO of J. oxycedrus ssp. oxycedrus significantly enhanced the activity against Sitophilus oryzae. Therefore, the results suggest that the tested EOs could be utilized at their lowest dose (1%) to achieve a very good insecticidal effect [42]. We observed the excellent insecticidal effect of juniper EOs on both species of aphids. The efficacy was 100% after 24 h of the EO applications. These results are not accidental, because α-pinene, sabinene, limonene, β-myrcene are the main constituents of monoterpenes in juniper species and they have been reported to have insecticidal activity [43,44,45]. Monoterpenes and sesquiterpenes (α-pinene, terpineol, 1,8-cineole, p-cymene, limonene, α-terpinene, thymol, carvacrol) were found to have a high fumigant activity against Musca domestica, Tribolium confusum and Sitophilus oryzae [43,45].

Table 7.
The insecticidal effect of semi-commercial extraction EOs of J. pygmaea (M, F), J. oxycedrus (M, F), J. sibirica (M, F) and J. communis (M, F) on two aphids (Rhopalosiphum padi, Sitobion avenae).
2.4. Antifungal Activity of Juniper EOs on Plant Pathogenic Fungi
Screening of the antifungal activity of the four juniper species EOs (1 µL mL−1) on the studied plant pathogens showed a varying positive effect (Table 8). The most substantial inhibitory effect on the radial mycelial growth was found against C. pauciseptatum and Fusarium sp. Significantly different inhibitory effects (p < 0.05) on the mycelial growth of Fusarium sp. (36.6% and 34.5%) were observed after the application of the EOs from J. sibirica (F) and J. pygmaea (M). Regarding the other tested EOs, the closest values to that already mentioned were obtained from the EOs of J. oxycedrus (M) and J. communis (M), and the differences in their antifungal activity were small. The largest difference in the antifungal activity of EOs obtained from M and F juniper species was between EOs from J. pygmaea (M) and J. pygmaea (F) (16.9%). The weakest inhibitory effect was found for the J. communis (F) EO against Fusarium spp. (Table 8).

Table 8.
Inhibitory effect (%) of juniper essential oils on plant pathogenic fungi at concentration of 1 µL/mL.
The highest antifungal efficacy against C. pauciseptatum was observed in J. oxycedrus (M), J. pygmaea (M), J. sibirica (F), J. sibirica (M) and J. pygmaea (F). The inhibition coefficient varied from 29.8% to 24.7%. A statistically similar inhibitory effect was found in J. oxycedrus (M) and J. pygmaea (M), as well as in J. pygmaea (F) and J. sibirica (M). A significantly different inhibitory effect was found among J. oxycedrus (M), J. pygmaea (F), J. communis (F) and J. communis (M). Statistically significant difference (10.96%) was established in the percent inhibition of the EOs from sexually different species (J. communis F and J. communis M) on the mycelial growth of C. pauciseptatum. The inhibition coefficient of J. pygmaea (M) on the mycelial growth of R. solani and C. pauciseptatum was the highest and significant compared to the other studied EOs.
Juniperus pygmaea (M) EO showed a strong inhibitory efficacy against four of the studied pathogens (Fusarium sp., R. solani, C. pauciseptatum and Colletotrichum sp.), while J. sibirica (F) showed a similar effect against Fusarium sp. and C. pauciseptatum (Table 8). As shown in Table 2, the concentrations of sabinene (25.06), terpinen-4-ol (6.01) and α-pinene (19.64) were higher in J. pygmaea (M), and these compounds probably interact with each other. Botrytis cinerea was the most resistant species against the used juniper EOs. None of the tested EOs showed a significant inhibitory effect on mycelial growth of B. cinerea.
The available information on the biological activity of the tested juniper EOs against phytopathogenic fungi is scarce. Zabka et al. [46] reported relatively low inhibitory activity of EO from J. communis L. on Alternaria alternata, Cladosporium cladosporioides and Aspergilus niger, 11.8%, 31.0% and 1.8%, respectively, at 1 μL/mL. According to these authors, the high antifungal effect of Origanum vulgare, Thymus vulgaris and Pimenta racemosa EO of the 20 studied types of oils was due to the high content of the phenolic monoterpenes thymol, carvacrol or eugenol. Gleń-Karolczyk and Boligłowa [47] reported a strong inhibitory effect of J. communis EO, ranging from 87.1% at 0.1 mm3 cm−3 to 93.1% at 1 mm3 cm−3 on R. solani and from 42% at 0.1 mm3 cm−3 to 85.4% at 1 mm3 cm−3 on C. destructans isolated from horseradish seedlings. Analyzing EOs from green and ripe berries of J. communis, an antifungal effect was found, manifested against Sclerotium rolfsii [48]. Based on their own results and on those obtained by Jing et al. [49], the latter authors suggested that sesquiterpenes were the phytochemical components that show a toxic effect on fungal pathogens. It can be seen that EOs mainly from J. communis were tested to establish the antifungal effect against phytopathogens.
In the present study, the most significant inhibition of the radial mycelial growth of the studied phytopathogens was demonstrated by the EOs of J. sibirica (F) (36.6%) and J. pygmaea (M) (34.5%) on Fusarium spp., by the EO of J. oxycedrus (M, 29.8%) and J. pygmaea (M, 29.0%) on C. pauciseptatum, as well as by the EO of J. pygmaea (M, 31.97%) on R. solani.
In most cases, there were statistically significant differences between the means of the inhibition coefficient of the juniper EO from M and F plants against the tested pathogens (Table 8). This was probably due to the different chemical composition of the EOs obtained from M and F plants. An exception was found in EO from J. communis against C. pauciseptatum and J. sibirica against R. solani and C. pauciseptatum, in which, under the conditions of the present experiment, the differences between the inhibitory effect of EO obtained from M and F juniper plants were not significant.
Overall, we cannot find a clear pattern between the chemical composition of EOs of Juniperus species and their inhibitory effect. J. pygmaea and J. sibirica are characterized with a high content of sabinene (Table 2). In the studied species, the predominant component of EOs was the class monoterpenes, especially α-pinene. In order to determine exactly which compounds exhibit antifungal efficacy, these compounds should be tested individually. As indicated by Tripathietii et al. [50] and Sharma and Tripathi [51], the fungicidal activity of EO is often the result of the synergistic activity of their compounds.
The results from this study on the inhibitory effect of EOs from four species of juniper, M and F plants (1 µL/mL) on phytopathogens and polyphages, causing diseases both during vegetation and the storage of plant products, are encouraging and call for further research.
2.5. Antimicrobial Activity
The antimicrobial activity of the F and M Juniperus EOs (J. communis, J. oxycedrus, J. pygmaea, J. sibirica) obtained through the commercial steam extraction method was evaluated in this study. Among the combinations of the levels of the two factors (sex, species), the highest antimicrobial activity was observed in J. communis (M) and J. sibirica (M) on S. pneumoniae; J. sibirica (F), J. communis (M), J. pygmaea (M) on C. perfringens; J. pygmaea (M, F) on L. monocytogenes; J. sibirica (F) on E. faecalis; J. sibirica (F) on S. enterica; J. pygmaea (F), J. sibirica (M, F) on H. influenzae; J. sibirica (F) on P. aeruginosa; and J. sibirica (F) on Y. enterocolitica (Table 9 and Table 10). High antimicrobial activity was observed for the EO of J. sibirica (M, F) on the seven tested bacterial species (S. pneumoniae, C. perfringens, E. faecalis, S. enterica, H. influenzae, P. aeruginosa, and Y. enterocolitica), followed by the EOs of J. communis (M) and J. pygmaea (M, F). Similar antimicrobial activity was observed for the EOs of J. communis on Gram-positive and Gram-negative bacterial species and yeasts [14,52], while Glišič et al. [53] found higher antimicrobial activity for Juniperus EOs compared with that of commercial antibiotics. Overall, the results of this study confirm our working hypothesis.

Table 9.
Mean antimicrobial activity (inhibition zones in mm) of S. pneumoniae, C. perfringens, L. monocytogenes, and E. faecalis obtained from the eight combinations of species and sex.

Table 10.
Mean antimicrobial activity (inhibition zones in mm) of S. enterica, H. influenzae, P. aeruginosa, Y. enterocolitica, and S. pneumoniae obtained from the eight combinations of species and sex.
2.6. Antioxidant Activity
Essential oils, including those from some of the juniper species, have been utilized in the pharmaceutical, cosmetic and food industries [5]. The antioxidant capacity (ORAC) of J. communis, J. oxycedrus, J. pygmaea, and J. sibirica EOs obtained by different extraction methods were evaluated in this study. The ORAC values of Clevenger method EOs were higher than that of semi-commercial method EOs (Figure 1). The results on the antioxidant capacity confirm our working hypothesis that the EOs extracted via different methods would have dissimilar bioactivity. Overall, the values of monoterpenes in the tested EOs obtained by the Clevenger method were higher than the concentration of monoterpenes in the EOs obtained using the semi-commercial method, and α-pinene was the predominant constituent. Reports on the antioxidant capacity of junipers EOs can be found in the literature [7,8,9,10,14,23,24]. It is well known that the qualitative and quantitative profiles of junipers can be influenced by a number of factors, including genetic, environmental, and post-harvest processing such as drying and extraction. The higher antioxidant capacity of the galbuli EO from J. sibirica and J. communis were reported by Zheljazkov et al. [14], where α-pinene was the dominant compound in the EOs. Due to the high level of γ-terpinene, Emami et al. [54] reported the strong antioxidant capacity of the leaves J. communis subsp. hemisphaerica [54]. In this study, the highest ORAC value was obtained from EOs of J. oxycedrus (M) (Figure 1), where α-pinene and limonene were the dominant compounds (Table 2). Current thinking is that the antioxidant capacity in EOs may depend strongly on the interaction between several compounds. The demonstrated high antioxidant capacity of some of the juniper oils opens the door for their wider utilization in various products.
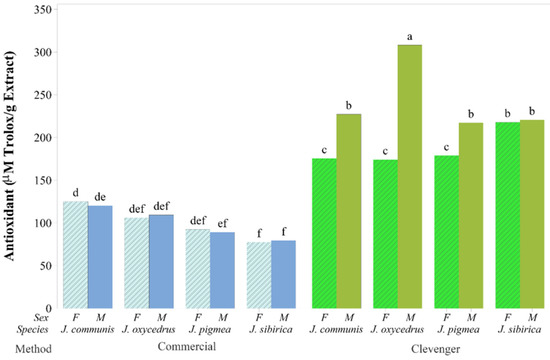
Figure 1.
Bar graph of mean antioxidant capacity in μmol Trolox g−1 obtained from the 16 combinations of the extraction method, species and sex. Means sharing the same letter (at the top of the bars) are not significantly different.
3. Materials and Methods
3.1. Plant Material
In this study, plant materials of Juniperus communis L., J. oxycedrus L., J. pygmaea C. Koch., and J. sibirica Burgsd. were used. Samples of the four species were collected from natural populations as follows: (а) J. communis (F, M) samples were collected above the village of Markovo in the Rhodope Mountains, 42°02′35.2″ N; 024°42′05.6″ E, at 587 masl; (b) J. oxycedrus (F, M) samples were also collected above the village of Markovo in the Rhodope Mountains, 42°02′32.6″ N; 024°42′05.7″ E, at 613 masl; (c) J. pygmaea (F, M) samples were collected above the village of Dobrostan in the Rhodope Mountains, 41°54′12.4″ N; 024°55′02.05″ E, at 1314 masl; and (d) J. sibirica (F, M) samples were collected in the Trojan mountain pass (Beklemeto) of Stara Planina (The Balkans) Mountains, 42°46′16.4″ N; 024°36′43.6″ E, at 1485 masl. Voucher specimens of J. communis, J. oxycedrus, J. pygmaea, and J. sibirica (small branches with needles) were deposited at the Herbarium of the Agricultural University, Plovdiv, Bulgaria (SOA) [55].
3.2. Preparation of Juniperus Samples for the Extraction of the Essential Oil (EO)
The samples of the four Juniper species were collected in June and were placed in a shady area at a temperature of below 35 °C for air-drying.
3.3. Hydrodistillation Extraction of EO
The Clevenger-type (ClevA) hydrodistillation extraction of EO was performed according to the British Pharmacopoeia [56] by using 800 mL of water in 2 L Clevenger-type hydrodistillation units. The plant tissue samples consisted of 100 g of air-dried leaves in 2 L flasks for the ClevA distillation. All samples were distilled for 2 h in two replicates.
3.4. Steam Distillation Semi-Commercial Extraction (SCom) of EO
The SCom steam distillation was conducted in a semi-commercial 100 L steam distillation units using 20 kg of leaves and small twigs, steam distilled for 3 h, the usual steam distillation time used by industry for junipers. The resulting EO from the above extractions was collected, separated from the remaining water, and kept in a freezer until the gas chromatography (GC)-mass spectroscopy (MS) analyses were performed. The EO was measured both by volume and by weight.
3.5. Gas Chromatography (GC)—Mass Spectroscopy (MS) Analyses of the Essential Oils (EO)
The chemical profile of the four Juniperus EOs in three replications was determined by GC-FID and GC/MS techniques, according to the methods described previously [14]. Identification of the components present in the EO samples was performed by comparing the mass spectra of components in the EOs with those from the National Institute of Standards and Technology (NIST 08) and Adams mass spectra libraries [57], by AMDIS (Automated Mass spectral Deconvolution and Identification System) and by comparing the literature and estimated Kovat′s (retention) indices that were determined using mixtures of homologous series of normal alkanes from C8 to C40 in hexane, under the same conditions mentioned above. The percentage ratio of EO components was computed using the normalization method of the GC/FID peak areas (Supplementary Materials).
3.6. Testing the Repellent and Insecticidal Action of EOs Obtained via Semi-Commercial Steam Extraction against Rhopalosiphum padi (Bird Cherry—Oat Aphid) and Sitobion avenae (English Grain Aphid)
3.6.1. Colonization of Rhopalosiphum padi and Sitobion avenae
The experiment was conducted at the entomology laboratory of the Institute of Agriculture in Karnobat, Bulgaria. Wingless female aphids of the above species were used in the experiment. The Rhopalosiphum padi and Sitobion avenae aphids were collected from the barley fields in the area of Karnobat, Bulgaria (42°38′54.51″ N, 27°21′60.56″ E). The aphids were kept on Hordeum vulgare Jess. subsp. distichum L., var. erectum, cv. Obzor plants which were grown in containers under controlled environment conditions. After the young barley plants reached the 3rd leaf stage, the aphids were introduced and infested the experimental plants. The experimental conditions included a temperature of 23–24 °C, 65% RH, and a light:dark (L:D) cycle of 8:16 h.
3.6.2. Repellency Tests
Each of the EOs extracted by SCom steam distillation of J. communis (F, M), J. oxycedrus (M), J. pygmaea (F, M), and J. sibirica (F, M) were tested for their repellent activity against Rh. padi and S. avenae. The repellence of the EO was assessed by using the Petri dish assay [58]. The seven EOs were diluted with an aqueous solution with an emulsifier, 0.1% polysorbate 80. Two microliters of each EO of the target species with 0%, 1%, 2.5%, and 5% concentrations in three replicates were used, according to the methods described previously [10]. One treated leaf and one non-treated leaf plus 10 leafless aphids were introduced into each Petri dish. After that, the Petri dishes were covered with cheesecloth (44 g/m2). The repellent effect was observed and recorded after 24 h.
3.6.3. Testing the Insecticidal Action of the Essential Oils (EO) from the SCom Steam Distillation against Rhopalosiphum padi and Sitobion avenae
The insecticidal activity of EOs was determined following a method described by Konstantopoulou et al. [59]. Each of the seven EOs was tested on the adult wingless forms of two aphid species at a concentration of 0%, 1%, 2.5%, and 5% in three replicates. The insecticidal actions of the EO on pests were evaluated on leaves of H. vulgare subsp. distichum var. erectum, cv. Obzor. The EOs were diluted in aqueous solution with an emulsifier of 0.1% polysorbate 80. The control (0%) was treated with a 0.1% aqueous solution of polysorbate 80. Two microliters of the solution (0%, 1%, 2.5%, and 5%) were applied directly on barley leaves with the aphid colonies. After the treatments, the leaves were dried on filter paper and transferred to Petri dishes [59]. The Petri dishes were covered with cheesecloth (44 g/m2). The effect of the application (knock-down or mortality) was observed after 24 and 72 h. The results (knock-down or mortality) were compared with the controls. The effect of the application of EOs at different concentrations was calculated using Abbott’s formula [38]:
where E is aphid mortality; x is the percentage of live aphids in the control; and y is % of live aphids in the treatment with the EOs.
E = (x − y)/x × 100
3.7. Antifungal Activity of the Juniper EO on Plant Pathogenic Fungi
3.7.1. Fungal Plant Pathogenic Strains
Five plant pathogenic fungal strains were obtained from a culture collection maintained in the Department of Phytopathology, Agricultural University, Plovdiv, Bulgaria. Fusarium sp. (fusarium dry rot) and Rhizoctonia solani (stem canker and black scurf) were isolated from stored potato tubers (Solanum tuberosum L.), Botrytis cinerea (grey mould) strain was isolated from infected stored tomato fruits (Lycopersicon esculentum Mill). The strain of a Colletotrichum sp. was isolated from anthracnose of banana fruit (Musa sp.), whereas Cylindrocarpon pauciseptatum strain was obtained from black foot disease of grapevine (Vitis vinifera L.). All strains were identified using cultural and morphological characteristics, as well as pathogenicity test. The Cylindrocarpon pauciseptatum was confirmed by DNA sequences [60]. Strains were preserved on potato dextrose agar (PDA) at 4 °C.
3.7.2. Agar Dilution Method (Antifungal Activity of Juniper EOs)
Preliminary study of inhibitory effect of seven juniper essential oils (J. oxycedrus L. M, J. communis L. M and F, J. pygmaea C. Koch., M and F, and J. sibirica Burgsd. M and F) on the mycelial radial growth of plant pathogenic fungi were tested by the agar dilution method [46]. The essential oils were diluted in PDA at 1 µL mL−1 concentration. Discs (5 mm/d) were cut out from the periphery of a 10 day-old culture of tested fungi and aseptically placed in the prepared Petri dishes (9 cm/d) with PDA and EO. Pure PDA medium (without essential oil) was used as the control. The inoculated Petri dishes were placed for incubation at 22 °C for 10 days. The percent inhibition of the radial mycelial growth of the tested fungal pathogens was calculated using the formula:
where DC is the diameter of the colony of the control, and DT is the diameter of the colony of the treatment. The experiment was conducted in four replications.
(DC − DT)/DC × 100%
3.8. Antioxidant Activity
The antioxidant activity of EOs of J. communis, J. oxycedrus, J. pygmaea and J. sibirica (Clevenger; Commercial) were analyzed at the University of Nebraska-Lincoln, Small Molecule Analysis Laboratory. The oxygen radical absorbance capacity (ORAC oil) was detected according to Huang et al. [61,62]. Briefly, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a polar derivative of vitamin E, was used as a standard, and the results were reported as µmole Trolox g−1. All samples of EOs were prepared by mixing 10 ± 1 mg oil with 1 mL of water and acetone (1:1) with 7% methyl-β-cyclodextrins (w:v). The activity was started in a 96-well plate by first transferring 25 μL of 74 mM phosphate buffer, with pH 7.4, to each well. Thereafter the EO samples (25 µL) or Trolox (25 μL) were added at concentrations of 0.2, 0.4, 3.3, 6.5, 10, 13, 25, and 50 μg/mL, followed by 150 μL of fluorescein (8.16 × 10−5 mM). Each sample was incubated at 37 °C for 10 min, with 3 min of alternating shaking. The 153 mM 2,2′-azobis (2-amidinopropane) hydrochloride (25 μL) was used for reaction activation. The standards and tested EO were monitored with a BMG Labtech FLUOstar Optima microplate reader (Durham, NC, USA). We measured fluorescence every 1.5 min at an excitation and emission wavelength of 485 and 520 nm, respectively, until the decreasing fluorescence values plateaued. The area under the curve was calculated. The EOs of the four junipers were analyzed in triplicate. The averages of the tested EOs in triplicate were used for the statistical analysis.
3.9. Antimicrobial Activity
3.9.1. Microorganisms
The four Gram-negative bacteria (Salmonella enterica susp. enterica CCM 3807, Haemophilus influenzae CCM 4456, Pseudomonas aeruginosa CCM 1959, and Yersinia enterocolitica CCM CCM 5671), four Gram-positive bacteria (Clostridium perfringens CCM 4435, Enterococcus faecalis CCM 4224, Listeria monocytogenes CCM 4699 and Streptococcus pneumoniae CCM 4501), were used for the antimicrobial activity testing. The microorganisms were used from the Czech Collection of Microorganisms, Brno, Czech Republic. The cultures were incubated in Mueller–Hinton broth (MHB, Oxoid, Basingstoke, UK) at 37 °C overnight.
3.9.2. Disc Diffusion Method
One hundred microliters of bacterial suspension after incubation were spread on the Mueller–Hinton agar (MHA, Oxoid) for the agar disc diffusion method. The filter paper discs (6 mm diameter) were infused with 15 µL of the EO, tested, and placed on the inoculated MHA. The MHA was kept at 4 °C for 2 h and then at 37 °C for 24 h aerobically. For yeasts, 100 µL of the microbial suspension was spread onto Sabouraud agar (Oxoid) and cultivated at 37 °C for 24 h. After the incubation period, the diameter of the inhibition zones was measured (mm). The growth inhibition was compared with standard drugs. Tests were performed in three replications.
3.10. Statistical Analyses
3.10.1. Statistical Methods for Constituents, Antimicrobial Activities, and Antioxidant Capacity
The effect of juniper species (4 levels: J. communis, J. oxycedrus, J. pygmaea, and J. sibirica) and sex (2 levels: F and M) on (1) the concentrations of 21 constituents (α-pinene, camphene, sabinene, β-pinene, β-myrcene, α-terpinene, p-cymene, limonene, γ-terpinene, α-terpinolene, terpinen-4-ol, bornyl acetate, β-elemene, β-caryophyllene, α-humulene, germacrene D, γ-cadinene, δ-cadinene, δ-cadinol, tau.-cadinol, and α-cadinol), and (2) eight antimicrobial activities (S. pneumoniae, C. perfringens, L. monocytogenes, E. faecalis, S. enterica, H. influenzae, P. aeruginosa, and Y. enterocolitica) where the extraction method was commercial was determined using a 4 × 2 factorial design. For antioxidant capacity response, in addition to the above two factors, a third factor, namely extraction method (2 levels: ClevA and SCom), was added, which made the design a 4 × 2 × 2 factorial.
3.10.2. Statistical Methods for Aphids
The effect of the combinations of juniper species and sex (7 levels: J. communis-F, J. communis-M, J. oxycedrus-M, J. pygmaea-F, J. pygmaea-M, J. sibirica-F, and J. sibirica-M) and the concentration of their EOs (4 levels: 0%, 1%, 2.5%, and 5%) on nb/leaf of Sitobion avenae and Rhopalosiphum padi for repellent action was determined using a 7 × 4 factorial design with 3 replications. However, for the insecticidal action, only three concentrations (1%, 2.5%, and 5%) were used for each of the seven species by sex combinations. Additionally, the antifungal activity effect of juniper species and sex (7 levels) on plant pathogens was determined using a completely randomized design (a single factor with 7 levels).
The analysis was completed using the GLM procedure of SAS [63], and the validity of model assumptions (normal distribution and constant variance of the error terms) was verified by examining the residuals as described in Montgomery [64]. Since the two-way interaction effect for all constituents, antimicrobial activities, and aphids and the three-way interaction effect for antioxidant capacity were highly significant, a multiple means comparison of the corresponding treatment combinations was completed and letter groupings generated using the least squares means at the 1% level of significance to protect the Type I experiment-wise error rate from overinflation.
4. Conclusions
Generally, the EOs of J. communis, J. pygmaea, J. sibirica, and J. oxycedrus had similar chemical profiles between the two extraction methods (ClevA, SCom) within a species, but they had a different antioxidant capacity. The EOs obtained via ClevA extraction showed greater antioxidant capacity within a species compared with those from SCom extraction. Overall, the EOs among the two factors (sex, species) had different antimicrobial activity. All of the tested EOs had significant repellent and insecticidal activity against the two aphid species Rhopalosiphum padi (bird cherry-oat aphid) and Sitobion avenae (English grain aphid) at concentrations of the EO in the solution at 1%, 2.5%, and 5%. Most of juniper EOs were effective against the tested pathogens, Fusarium spp., Cylindrocarpon pauciseptatum Colletotrichum spp. and Rhizoctonia solani, especially J. pygmaea (M) and J. sibirica (F). These EOs show promise as alternatives to conventional synthetic pesticides and could be used in the development of biopesticide products.
Supplementary Materials
The following are available online, Table S1: Constituents and concentrations of J. communis extracted using the Clevenger and Commercial extraction methods; Table S2: Constituents and concentrations of J. sibirica extracted using the Clevenger and commercial extraction methods; Table S3: Constituents and concentrations of J. pygmaea extracted using the Clevenger and commercial extraction methods; Table S4: Constituents and concentrations of J. oxycedrus extracted using the Clevenger and Commercial extraction methods; Table S5: Main class of compounds in the essential oil (EO) of J. oxycedrus, J. communis, J. pygmaea, J. sibirica.
Author Contributions
Conceptualization, V.D.Z., I.S.; methodology, I.D., V.M., T.A., N.P. and M.K.; resources, T.A., I.S., T.R. and V.D.Z.; writing—original draft preparation, I.S., V.D.Z.; writing—review and editing, V.D.Z., I.S., T.R., V.M., M.K., T.A., I.D.; supervision, V.D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agricultural University Plovdiv, Bulgaria [Project № 11-17], This work was supported by the Bulgarian Ministry of Education and Science under the National Research Program “Healthy Foods for a Strong Bio-Economy and Quality of Life” approved by DCM # 577/17.08.2018” and in part by the Oregon State University, USA.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable for studies not involving humans.
Data Availability Statement
Data of the compounds are available from the authors.
Acknowledgments
This study was supported in part by the Agricultural University Plovdiv, Bulgaria [Project № 11-17]. This work was supported by the Bulgarian Ministry of Education and Science under the National Research Program “Healthy Foods for a Strong Bio-Economy and Quality of Life” approved by DCM # 577/17.08.2018” and in part by the Oregon State University funds awarded to Valtcho Jeliazkov (Zheljazkov). The authors thank Yulia Yonkova for helping with the essential oil extraction.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Data of the compounds are available from the authors.
Abbreviations
MH—monoterpenes hydrocarbons; OM—oxygenated monoterpenes; PhM—phenolic monoterpenes; BOM—bicyclic oxygenated monoterpenes; SH—sesquiterpenes hydrocarbons; OS—oxygenated sesquiterpenes; D—diterpenes; OD—oxygenated diterpenes; TOS—tricyclic oxygenated sesquiterpenes; BSH—bicyclic sesquiterpenes hydrocarbons; OBS—oxygenated bicyclic sesquiterpenes; ClevA—Clevenger apparatus; SCom—semi-commercial steam distillation; F—female; M—male.
References
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2019, 19, 235–241. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Camilo, C.J.; Alves Nonato, C.D.F.; Galvão-Rodrigues, F.F.; Costa, W.D.; Clemente, G.G.; Sobreira Macedo, M.A.C.; Galvão Rodrigues, F.F.; da Costa, J.G.M. Acaricidal activity of essential oils: A review. Trends Phytochem. Res. 2017, 1, 183–198. [Google Scholar]
- Ibrahim, M.; Kainulainen, P.; Aflatuni, A. Insecticidal, repellent, antimicrobial activity and phytotoxicity of essential oils: With special reference to limonene and its suitability for control of insect pests. Agric. Food Sci. 2001, 10, 243–259. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties—An Overview. Complement. Med. Res. 2009, 16, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Isman, M.B.; Tak, J.-H. Insecticidal Activity of 28 Essential Oils and a Commercial Product Containing Cinnamomum cassia Bark Essential Oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Radoukova, T.; Zheljazkov, V.; Semerdjieva, I.; Dincheva, I.; Stoyanova, A.; Kačániová, M.; Marković, T.; Radanović, D.; Astatkie, T.; Salamon, I. Differences in essential oil yield, composition, and bioactivity of three juniper species from Eastern Europe. Ind. Crops Prod. 2018, 124, 643–652. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Zheljazkov, V.; Radoukova, T.; Radanović, D.; Marković, T.; Dincheva, I.; Stoyanova, A.; Astatkie, T.; Kačániová, M. Essential oil yield, composition, bioactivity and leaf morphology of Juniperus oxycedrus L. from Bulgaria and Serbia. Biochem. Syst. Ecol. 2019, 84, 55–63. [Google Scholar] [CrossRef]
- Semerdjieva, I.B.; Zheljazkov, V.D.; Dincheva, I.; Astatkie, T.; Kačániová, M. Chemotypes of Juniperus oxycedrus in Bulgaria and the antimicrobial activity of galbuli essential oils. Ind. Crops Prod. 2020, 158, 113005. [Google Scholar] [CrossRef]
- Zheljazkov, V.; Cantrell, C.; Semerdjieva, I.; Radoukova, T.; Stoyanova, A.; Maneva, V.; Kačániová, M.; Astatkie, T.; Borisova, D.; Dincheva, I.; et al. Essential Oil Composition and Bioactivity of Two Juniper Species from Bulgaria and Slovakia. Molecules 2021, 26, 3659. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, D.; Stoayanov, N.; Ahtarov, B.; Kitanov, B.; Valev, S.; Ganchev, I.; Penev, I.; Georgiev, T.; Hinkova, C.; Velchev, V. Genus Juniperus. In Flora Na NR Bulgaria; Yordanov, D., Ed.; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1963; Volume 1. (In Bulgarian) [Google Scholar]
- Meshinev, T.; Apostolova, I.; Koleva, E. Influence of warming on timberline rising: A case study on Pinus peuce Griseb. in Bulgaria. Phytocoenologia 2000, 30, 431–438. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessí, S.; Cabras, P. Chemical Composition of the Essential Oils of Juniperus from Ripe and Unripe Berries and Leaves and Their Antimicrobial Activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Semerdjieva, I.B.; Dincheva, I.; Kacaniova, M.; Astatkie, T.; Radoukova, T.; Schlegel, V. Antimicrobial and antioxidant activity of Juniper galbuli essential oil constituents eluted at different times. Ind. Crops Prod. 2017, 109, 529–537. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Mazzara, E.; Torresi, J.; Cianfaglione, K.; Benelli, G.; Canale, A. Prolonged sublethal effects of essential oils from non-wood parts of nine conifers on key insect pests and vectors. Ind. Crops Prod. 2021, 168, 113590. [Google Scholar] [CrossRef]
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential Oils from Wild Populations of Algerian Lavandula stoechas L.: Composition, Chemical Variability, and in vitro Biological Properties. Chem. Biodivers. 2011, 8, 937–953. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Said, L.A.; Zahlane, K.; Ghalbane, I.; El Messoussi, S.; Romane, A.; Cavaleiro, C.; Salgueiro, L. Chemical composition and antibacterial activity of Lavandula coronopifoli aessential oil against antibiotic-resistant bacteria. Nat. Prod. Res. 2014, 29, 582–585. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Adams, R.P. Junipers of the World: The Genus Juniperus, 4th ed.; Trafford Publishing: Vancouver, BC, Canada, 2014; p. 422. ISBN 9781490723259. [Google Scholar]
- Asili, J.; Emami, S.; Rahimizadeh, M.; Fazly-Bazzaz, B.; Hassanzadeh, M. Chemical and Antimicrobial Studies of Juniperus communis subsp. hemisphaerica and Juniperus oblonga Essential Oils. J. Essent. Oil Bear. Plants 2008, 11, 96–105. [Google Scholar] [CrossRef]
- Miguel, M.G.; Cruz, C.; Faleiro, L.; Simões, M.T.F.; Figueiredo, A.C.; Barroso, J.; Pedro, L. Foeniculum vulgare Essential Oils: Chemical Composition, Antioxidant and Antimicrobial Activities. Nat. Prod. Commun. 2010, 5, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheljazkov, V.D.; Astatkie, T.; Jeliazkova, E.A.; Tatman, A.O.; Schlegel, V. Distillation time alters essential oil yield, composition and antioxidant activity of female Juniperus scopulorum trees. J. Essent. Oil Res. 2013, 25, 62–69. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Kacaniova, M.; Dincheva, I.; Radoukova, T.; Semerdjieva, I.; Astatkie, T.; Schlegel, V. Essential oil composition, antioxidant and antimicrobial activity of the galbuli of six juniper species. Ind. Crops Prod. 2018, 124, 449–458. [Google Scholar] [CrossRef]
- Teng, C.-M.; Lin, C.-H.; Kuo, Y.-H.; Lin, Y.-L.; Huang, T.-F. Antiplatelet and Vasorelaxing Actions of the Acetoxy Derivative of Cedranediol Isolated from Juniperus squamata. Planta Med. 1994, 60, 209–213. [Google Scholar] [CrossRef]
- Wedge, D.E.; Tabanca, N.; Sampson, B.J.; Werle, C.; Demirci, B.; Baser, K.H.C.; Nan, P.; Duan, J.; Liu, Z. Antifungal and Insecticidal Activity of two Juniperus Essential Oils. Nat. Prod. Commun. 2009, 4, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantrell, C.L.; Zheljazkov, V.D.; Osbrink, W.L.; Castro, A.; Maddox, V.; Craker, L.E.; Astatkie, T. Podophyllotoxin and essential oil profile of Juniperus and related species. Ind. Crops Prod. 2013, 43, 668–676. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Jeliazkova, E. Year-round Variations in Essential Oil Content and Composition of Male and Female Juniper. HortScience 2013, 48, 883–886. [Google Scholar] [CrossRef] [Green Version]
- Chatzopoulou, P.; De Haan, A.; Katsiotis, S.T. Investigation on the Supercritical CO2 Extraction of the Volatile Constituents from Juniperus communis Obtained under Different Treatments of the “Berries” (Cones). Planta Med. 2002, 68, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, B.M.; Skala, D.; Petrovic-Djakov, D.; Baras, J. A Comparison Between the Oil, Hexane Extract and Supercritical Carbon Dioxide Extract of Juniperus communis L. J. Essent. Oil Res. 2003, 15, 90–92. [Google Scholar] [CrossRef]
- Orav, A.; Koel, M.; Kailas, T.; Müürisepp, M. Comparative analysis of the composition of essential oils and supercritical carbon dioxide extracts from the berries and needles of Estonian juniper (Juniperus communis L.). Procedia Chem. 2010, 2, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Pourmortazavi, S.M.; Baghaee, P.; Mirhosseini, M.A. Extraction of volatile compounds from Juniperus communis L. leaves with super critical fluid carbon dioxide: Comparison with hydrodistillation. Flavour Fragr. J. 2004, 19, 417–420. [Google Scholar] [CrossRef]
- Joshi, S.; Sati, S.C. Antibacterial potential of leaf extracts of Juniperus communis L. from Kumaun Himalaya. Afr. J. Microbiol. Res. 2010, 4, 1291–1294. [Google Scholar]
- Dixon, A.F.G. Cereal aphids as an applied problem. Agric. Zool. Rev. 1987, 2, 1–57. [Google Scholar]
- Jackman, J.A.; Drees, B.M. A Field Guide to Common Texas Insects; Taylor Trade Publishing: Lanham-Seabrook, MD, USA, 1998. [Google Scholar]
- Carroll, J.F.; Tabanca, N.; Kramer, M.; Elejalde, N.M.; Wedge, D.E.; Bernier, U.R.; Coy, M.; Becnel, J.J.; Demirci, B.; Başer, K.H.C.; et al. Essential oils of Cupressus funebris, Juniperus communis, and J. chinensis (Cupressaceae) as repellents against ticks (Acari: Ixodidae) and mosquitoes (Diptera: Culicidae) and as toxicants against mosquitoes. J. Vector Ecol. 2011, 36, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Hummelbrunner, L.A.; Isman, M.B. Acute, Sublethal, Antifeedant, and Synergistic Effects of Monoterpenoid Essential Oil Compounds on the Tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Èntomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Èntomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Chiriloaie, A.; Vassilakos, T.N.; Fãtu, V.; Drosu, S.; Ciobanu, M.; Dudoiu, R. In-secticidal efficacy of natural diatomaceous earth deposits from Greece and Romania against four stored grain beetles: The effect of temperature and relative humidity. Bull. Insectol. 2016, 69, 25–34. [Google Scholar]
- Ojimelukwe, P.C.; Adler, C. Potential of Zimtaldehyde, 4-allyl-anisol, linalool, terpineol and other phytochemicals for the control of confused flour beetle (Tribolium confusum J.D.V) (Col; Tenebrionidae). J. Pestic. Sci. 1999, 72, 81–86. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Karamanoli, K.I.; Stamopoulos, D.C.; Menkissoglu-Spiroudi, U. The relationship between the chemical composition of three essential oils and their insecticidal activity against Acanthoscelides obtectus (Say). Pest Manag. Sci. 2004, 60, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.J.; Coats, J.R. Insecticidal Properties of Several Monoterpenoids to the House Fly (Diptera: Muscidae), Red Flour Beetle (Coleoptera: Tenebrionidae), and Southern Corn Rootworm (Coleoptera: Chrysomelidae). J. Econ. Èntomol. 1994, 87, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Zabka, M.; Pavela, R.; Prokinova, E. Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere 2014, 112, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Gleń-Karolczyk, K.; Boligłowa, E. Fungicidal activity of juniper essential oil (Juniperus communis seedlings L.) against the fungi infecting horseradish seedlings. J. Res. Appl. Agric. Eng. 2016, 61, 119–125. [Google Scholar]
- Falasca, A.; Caprari, C.; De Felice, V.; Fortini, P.; Saviano, G.; Zollo, F.; Iorizzi, M. GC-MS analysis of the essential oils of Juniperus communis L. berries growing wild in the Molise region: Seasonal variability and in vitro antifungal activity. Biochem. Syst. Ecol. 2016, 69, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.; Lei, Z.; Li, L.; Xie, R.; Xi, W.; Guan, Y.; Sumner, L.; Zhou, Z. Antifungal Activity of Citrus Essential Oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef]
- Tripathietii, P.; Dubey, N.K.; Banerji, R.; Chansouria, J.P.N. Evaluation of some essential oils as botanical fungitoxicants in management of post-harvest rotting of citrus fruits. World J. Microbiol. Biotechnol. 2004, 20, 317–321. [Google Scholar] [CrossRef]
- Sharma, N.; Tripathi, A. Fungitoxicity of the essential oil of Citrus sinensis on post-harvest pathogens. World J. Microbiol. Biotechnol. 2006, 22, 587–593. [Google Scholar] [CrossRef]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blazevich, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar]
- Glisic, S.; Milojevic, S.; Dimitrijevic-Brankovic, S.; Orlovic, A.; Skala, D. Antimicrobial activity of the essential oil and different fractions of Juniperus communis L. and a comparison with some commercial antibiotics. J. Serb. Chem. Soc. 2007, 72, 311–320. [Google Scholar] [CrossRef]
- Emami, S.A.; Javadi, B.; Hassanzadeh, M. Antioxidant Activity of the Essential Oils of Different Parts of Juniperus communis. subsp. hemisphaerica. and Juniperus oblonga. Pharm. Biol. 2007, 45, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. 2017. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 20 April 2021).
- British Pharmacopoeia. Medicines Commission; Her Majesty’s Stationery Office: London, UK, 1988; pp. 609–1139. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Jiang, H.; Wang, J.; Song, L.; Cao, X.; Yao, X.; Tang, F.; Yue, Y. GC×GC-TOFMS Analysis of Essential Oils Composition from Leaves, Twigs and Seeds of Cinnamomum camphora L. Presl and Their Insecticidal and Repellent Activities. Molecules 2016, 21, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantopoulou, I.; Vassilopoulou, L.; Mavragani-Tsipidou, P.; Scouras, Z.G. Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Experientia 1992, 48, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Piperkova, N.; Cabral, A.; Mohamedova, M.; Milusheva, S.; Oliveira, H. First Report of Dactylonectria pauciseptata Associated with Black Foot of Grapevine and Root Rot of Plum in Bulgaria. Plant Dis. 2017, 101, 2146. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, A.J.A.; Deemer, E.K. Development and Validation of Oxygen Radical Absorbance Capacity Assay for Lipophilic Antioxidants Using Randomly Methylated β-Cyclodextrin as the Solubility Enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 10th ed.; Wiley & Sons: New York, NY, USA, 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).