Nano-Sized NiO Immobilized on Au/CNT for Benzyl Alcohol Oxidation: Influences of Hybrid Structure and Interface
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selective Oxidation of Benzyl Alcohol
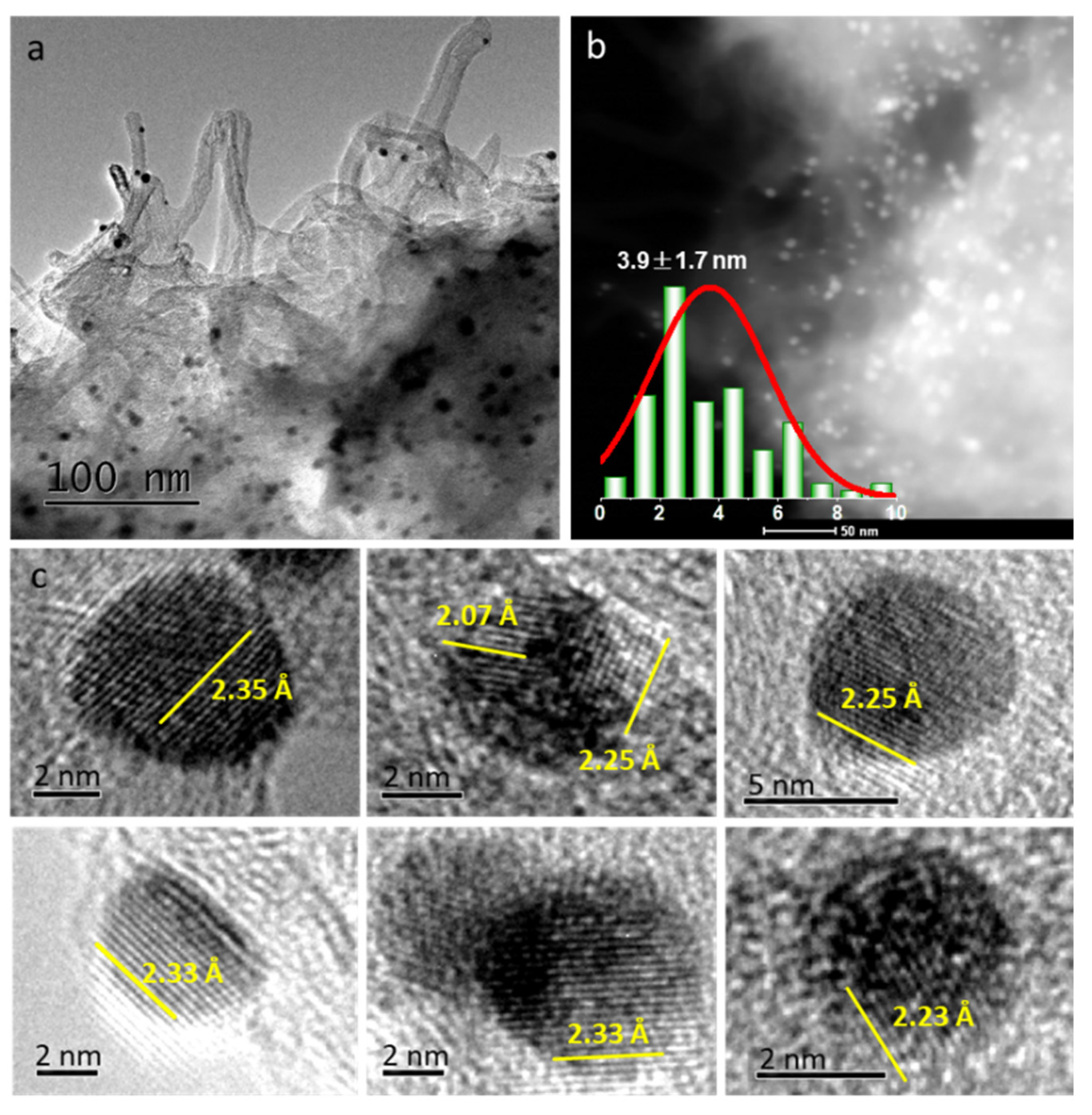
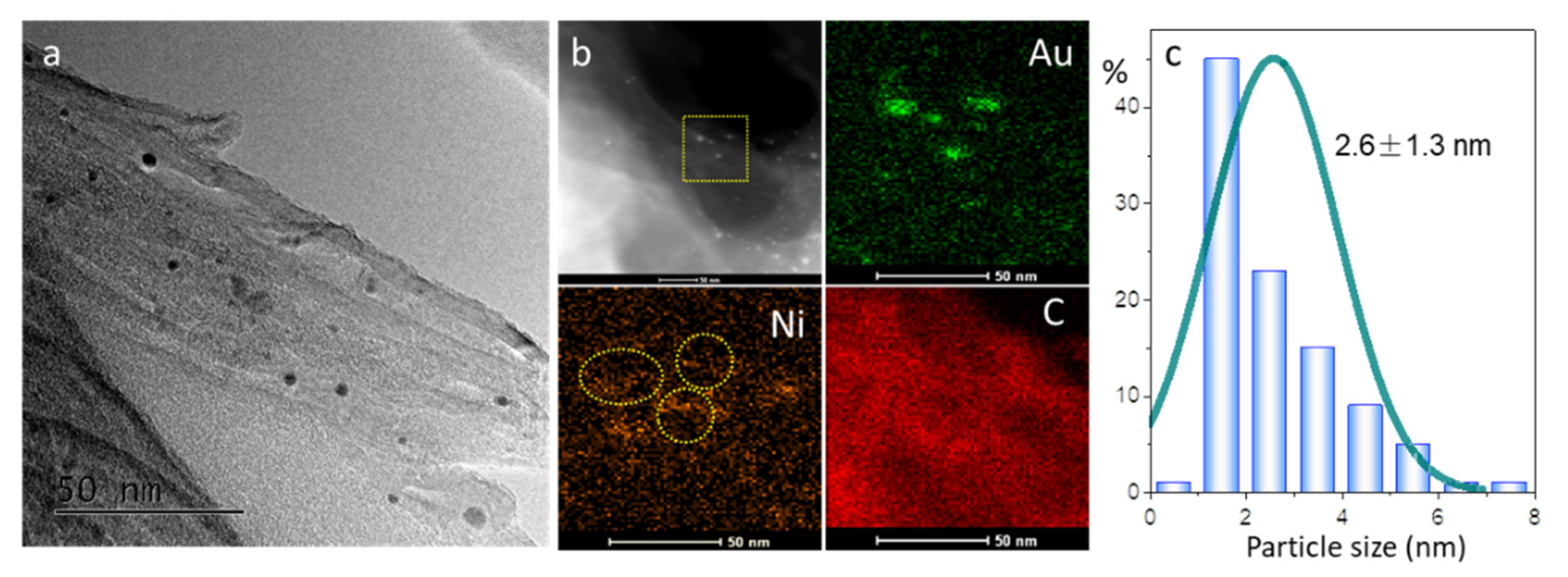
2.2. Structure and Morphology of Catalysts
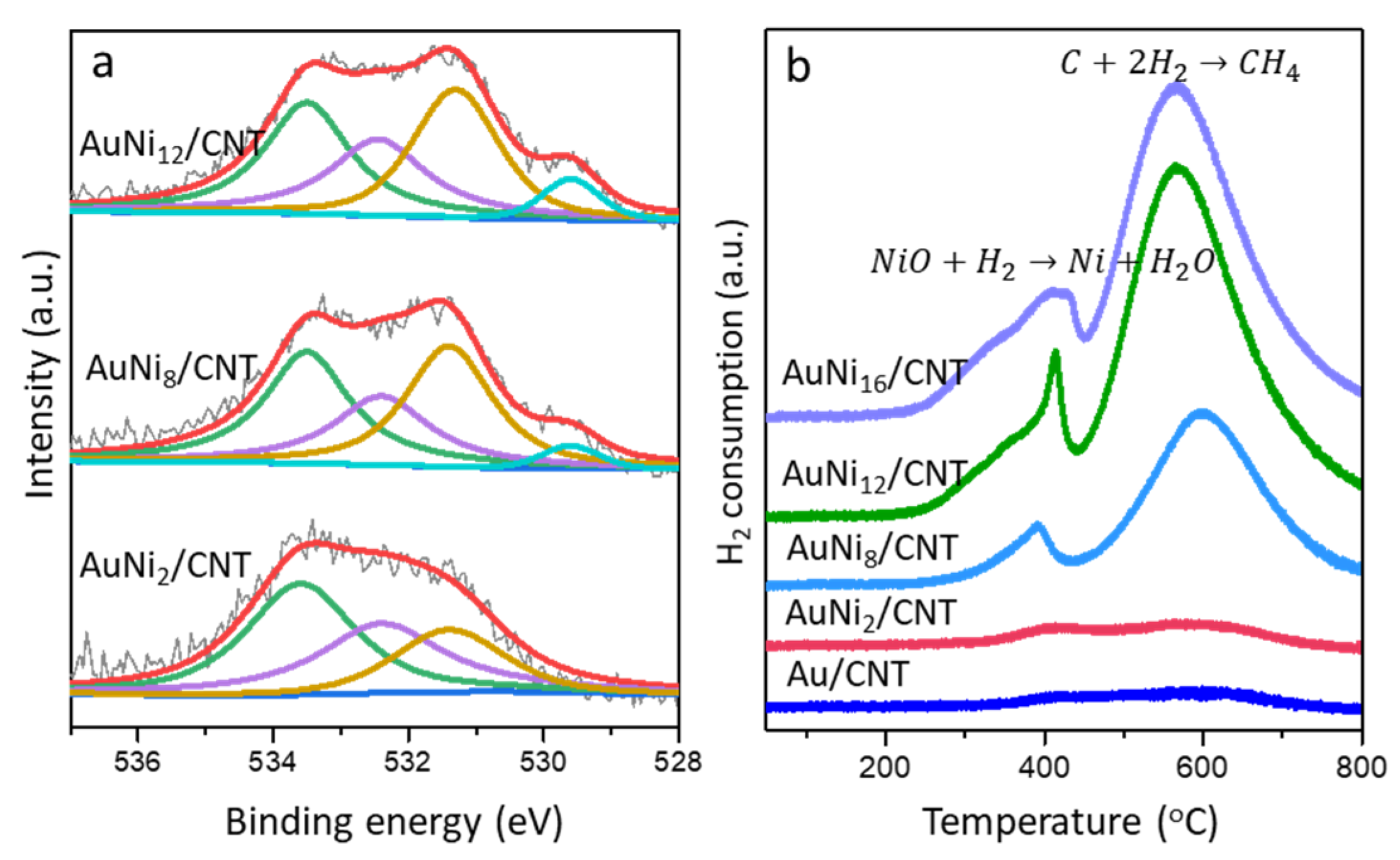
2.3. Surface Chemistry and Properties
3. Materials and Methods
3.1. Materials
3.2. Characterizations
3.3. Evaluation of Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold Catalysts Prepared by Coprecipitation for Low-Temperature Oxidation of Hydrogen and of Carbon-Monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Song, Z.; Li, W.; Niu, F.; Xu, Y.; Niu, L.; Yang, W.; Wang, Y.; Liu, J. A Novel Method to Decorate Au Clusters onto Graphene via a Mild Co-reduction Process for Ultrahigh Catalytic Activity. J. Mater. Chem. A. 2017, 5, 230–239. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Gold Nanoparticles Deposited on Surface Modified Carbon Materials as Reusable Catalysts for Hydrocarboxylation of Cyclohexane. Appl. Catal. A Gen. 2017, 547, 124–131. [Google Scholar] [CrossRef]
- Mao, J.B.; Zhao, B.; Zhou, J.X.; Zhang, L.; Yang, F.; Guo, X.W.; Zhang, Z.C. Identification and Characteristics of Catalytic Quad-Functions on Au/Anatase TiO2. ACS Catal. 2019, 9, 7900–7911. [Google Scholar] [CrossRef]
- Ding, S.T.; Jiao, N. N,N-Dimethylformamide: A Multipurpose Building Block. Angew. Chem. Int. Ed. 2012, 51, 9226–9237. [Google Scholar] [CrossRef]
- Lorencon, E.; Ferreira, D.C.; Resende, R.R.; Krambrock, K. Amphiphilic Gold Nanoparticles Supported on Carbon Nanotubes: Catalysts for the Oxidation of Lipophilic Compounds by Wet Peroxide in Biphasic Systems. Appl. Catal. A Gen. 2015, 505, 566–574. [Google Scholar] [CrossRef]
- Chen, B.B.; Shi, C.A.; Crocker, M.; Wang, Y.; Zhu, A.M. Catalytic Removal of Formaldehyde at Room Temperature over Supported Gold Catalysts. Appl. Catal. B-Environ. 2013, 132, 245–255. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Wang, L.; Ali, S.; Wang, C.T.; Wang, L.X.; Meng, X.J.; Li, B.; Su, D.S.; Xiao, F.S. Wet-Chemistry Strong Metal-Support Interactions in Titania-Supported Au Catalysts. J. Am. Chem. Soc. 2019, 141, 2975–2983. [Google Scholar] [CrossRef]
- Kiraly, B.; Liu, X.L.; Wang, L.Q.; Zhang, Z.H.; Mannix, A.J.; Fisher, B.L.; Yakobson, B.I.; Hersam, M.C.; Guisinger, N.P. Borophene Synthesis on Au(111). ACS Nano 2019, 13, 3816–3822. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, H.C. Transition-Metal-Ions-Induced Coalescence: Stitching Au Nanoclusters into Tubular Au-Based Nanocomposites. Small 2016, 12, 2652–2664. [Google Scholar] [CrossRef]
- Linden, M.; Bunningen, A.J.; Amidani, L.; Bransen, M.; Elnaggar, H.; Glatzel, P.; Meijerink, A.; Groot, F.M.F. Single Au Atom Doping of Silver Nanoclusters. ACS Nano 2018, 12, 12751–12760. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Tumkur, T.; Yang, J.; Lou, M.H.; Dong, L.L.; Zhou, L.N.; Nordlander, P.; Halas, N.J. Optical-Force-Dominated Directional Reshaping of Au Nanodisks in Al-Au Heterodimers. Nano Lett. 2018, 18, 6509–6514. [Google Scholar] [CrossRef]
- Xia, S.; Fang, L.; Meng, Y.; Zhang, X.; Zhang, L.; Yang, C.; Ni, Z. Water-gas Shift Reaction Catalyzed by Layered Double Hydroxides Supported Au-Ni/Cu/Pt Bimetallic Alloys. Appl. Catal. B-Environ. 2020, 272, 118949. [Google Scholar] [CrossRef]
- Wrasman, C.J.; Riscoe, A.R.; Lee, H.; Cargnello, M. Dilute Pd/Au Alloys Replace Au/TiO2 Interface for Selective Oxidation Reactions. ACS Catal. 2020, 10, 1716–1720. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kawamoto, D.; Yamamoto, Y.; Ishida, T.; Ohashi, H.; Akita, T.; Honma, T.; Oji, H.; Kobayashi, Y.; Hamasaki, A.; et al. Promotional Effect of Au on Reduction of Ni(II) to Form Au–Ni Alloy Catalysts for Hydrogenolysis of Benzylic Alcohols. J. Catal. 2013, 307, 254–264. [Google Scholar] [CrossRef]
- Beniya, A.; Ikuta, Y.; Isomura, N.; Hirata, H.; Watanabe, Y. Synergistic Promotion of NO-CO Reaction Cycle by Gold and Nickel Elucidated using a Well-Defined Model Bimetallic Catalyst Surface. ACS Catal. 2017, 7, 1369–1377. [Google Scholar] [CrossRef]
- Kyriakou, G.; Marquez, A.M.; Holgado, J.P.; Taylor, M.J.; Wheatley, A.E.H.; Mehta, J.P.; Fernandez Sanz, J.; Beaumont, S.K.; Lambert, R.M. Comprehensive Experimental and Theoretical Study of the CO + NO Reaction Catalyzed by Au/Ni Nanoparticles. ACS Catal. 2019, 9, 4919–4929. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Fu, Q.; Guo, X.G.; Bao, X.H. A Highly Active “NiO-on-Au” Surface Architecture for CO Oxidation. ACS Catal. 2013, 3, 1810–1818. [Google Scholar] [CrossRef]
- Lolli, A.; Albonetti, S.; Utili, L.; Amadori, R.; Ospitali, F.; Lucarelli, C.; Cavani, F. Insights into the Reaction Mechanism for 5-Hydroxymethylfurfural Oxidation to FDCA on Bimetallic Pd-Au Nanoparticles. Appl. Catal. A Gen. 2015, 504, 408–419. [Google Scholar] [CrossRef]
- Yan, H.; Su, C.; He, J.; Chen, W. Single-Atom Catalysts and their Applications in Organic Chemistry. J. Mater. Chem. A. 2018, 6, 8793–8814. [Google Scholar] [CrossRef]
- Majhi, S.M.; Naik, G.K.; Lee, H.-J.; Song, H.-G.; Lee, C.-R.; Lee, I.-H.; Yu, Y.-T. Au@NiO Core-Shell Nanoparticles as a p-Type Gas Sensor: Novel Synthesis, Characterization, and their Gas Sensing Properties with Sensing Mechanism. Sens. Actuators B Chem. 2018, 268, 223–231. [Google Scholar] [CrossRef]
- Faqeeh, A.J.; Ali, T.T.; Basahel, S.N.; Narasimharao, K. Nanosized Samarium Modified Au-Ce0.5Zr0.5O2 Catalysts for Oxidation of Benzyl Alcohol. Mol. Catal. 2018, 456, 10–21. [Google Scholar] [CrossRef]
- Bruno, J.E.; Dwarica, N.S.; Whittaker, T.N.; Hand, E.R.; Guzman, C.S.; Dasgupta, A.; Chen, Z.; Rioux, R.M.; Chandler, B.D. Supported Ni–Au Colloid Precursors for Active, Selective, and Stable Alkyne Partial Hydrogenation Catalysts. ACS Catal. 2020, 10, 2565–2580. [Google Scholar] [CrossRef]
- Zhang, M.M.; Yan, Z.X.; Xie, J.M. Core/Shell Ni@Pd Nanoparticles Supported on MWCNTs at Improved Electrocatalytic Performance for Alcohol Oxidation in Alkaline Media. Electrochim. Acta 2012, 77, 237–243. [Google Scholar] [CrossRef]
- Yang, J.L.; Mou, C.Y. Ordered Mesoporous Au/TiO2 Nanospheres for Solvent-free Visible-Light-Driven Plasmonic Oxidative Coupling Reactions of Amines. Appl. Catal. B-Environ. 2018, 231, 283–291. [Google Scholar] [CrossRef]
- Fang, Q.H.; Qin, Z.X.; Shi, Y.Y.; Liu, F.; Barkaoui, S.; Abroshan, H.; Li, G. Au/NiO Composite: A Catalyst for One-Pot Cascade Conversion of Furfural. ACS Appl. Energy Mater. 2019, 2, 2654–2661. [Google Scholar] [CrossRef]
- Li, R.T.; Xu, X.Y.; Zhu, B.E.; Li, X.Y.; Ning, Y.X.; Mu, R.T.; Du, P.F.; Li, M.W.; Wang, H.K.; Liang, J.J.; et al. In Situ Identification of the Metallic State of Ag Nanoclusters in Oxidative Dispersion. Nat. Commun. 2021, 12, 1460. [Google Scholar]
- Aitbekova, A.; Wu, L.H.; Wrasman, C.J.; Boubnov, A.; Hoffman, A.S.; Goodman, E.D.; Bare, S.R.; Cargnello, M. Low-Temperature Restructuring of CeO2-Supported Ru Nanoparticles Determines Selectivity in CO2 Catalytic Reduction. J. Am. Chem. Soc. 2018, 140, 13736–13745. [Google Scholar] [CrossRef]
- Luo, J.; Dong, Y.; Petit, C.; Liang, C. Development of Gold Catalysts Supported by Unreducible Materials: Design and Promotions. Chin. J. Catal. 2021, 42, 670–693. [Google Scholar] [CrossRef]
- Luo, J.; Liu, Y.; Zhang, L.; Ren, Y.; Miao, S.; Zhang, B.; Su, D.S.; Liang, C. Atomic-Scale Observation of Bimetallic Au-CuOx Nanoparticles and their Interfaces for Activation of CO Molecules. ACS Appl. Mater. Interfaces 2019, 11, 35468–35478. [Google Scholar] [CrossRef]
- Liu, S.; Xu, W.; Niu, Y.; Zhang, B.; Zheng, L.; Liu, W.; Li, L.; Wang, J. Ultrastable Au Nanoparticles on Titania through an Encapsulation Strategy under Oxidative Atmosphere. Nat. Commun. 2019, 10, 5790. [Google Scholar] [CrossRef]
- Wang, J.; Tan, H.; Yu, S.; Zhou, K. Morphological Effects of Gold Clusters on the Reactivity of Ceria Surface Oxygen. ACS Catal. 2015, 5, 2873–2881. [Google Scholar] [CrossRef]
- Yang, M.; Allard, L.F.; Flytzani-Stephanopoulos, M. Atomically Dispersed Au–(OH)x Species Bound on Titania Catalyze the Low-Temperature Water-Gas Shift Reaction. J. Am. Chem. Soc. 2013, 135, 3768–3771. [Google Scholar] [CrossRef]
- Ioannidou, E.; Neofytidis, C.; Sygellou, L.; Niakolas, D.K. Au-doped Ni/GDC as an Improved Cathode Electrocatalyst for H2O Electrolysis in SOECs. Appl. Catal. B-Environ. 2018, 236, 253–264. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.J.; Park, J.; Han, S.W.; Kim, S.M. Purification Effect of Carbon Nanotube Fibers on their Surface Modification to Develop a High-Performance and Multifunctional Nanocomposite Fiber. Carbon 2021, 173, 376–383. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, J.; Jiang, Z.; Zhang, S.; Chen, L.; Lu, Y. Microstructured Au/Ni-Fiber Catalyst for Low-Temperature Gas-Phase Alcohol Oxidation: Evidence of Ni2O3–Au+ Hybrid Active Sites. Appl. Catal. B-Environ. 2013, 140–141, 249–257. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Chen, S.; Quan, X. The Role of Lattice Oxygen on the Activity and Selectivity of the OMS-2 Catalyst for the Total Oxidation of Toluene. Chem. Eng. J. 2015, 270, 58–65. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, Z.; Zeng, G.; Lai, C.; Duan, A.; Xiao, R.; Huang, D.; Fu, Y.; Yi, H.; Li, B.; et al. Cooperative Catalytic Performance of Bimetallic Ni-Au Nanocatalyst for Highly Efficient Hydrogenation of Nitroaromatics and Corresponding Mechanism Insight. Appl. Catal. B-Environ. 2019, 259, 118035. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Szynkowska, M.I.; Maniecki, T.P. Bimetallic Au-Cu, Au-Ni Catalysts Supported on MWCNTs for Oxy-steam Reforming of Methanol. Appl. Catal. B-Environ. 2016, 185, 281–294. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Costa, D.S.; Teixeira, I.F.; Parreira, L.A.; Menini, L.; Gusevskaya, E.V.; Moura, F.C.C. Red Mud Based Gold Catalysts in the Oxidation of Benzyl Alcohol with Molecular Oxygen. Catal. Today 2017, 289, 89–95. [Google Scholar] [CrossRef]
- Olmos, C.M.; Chinchilla, L.E.; Villa, A.; Delgado, J.J.; Hungria, A.B.; Blanco, G.; Prati, L.; Calvino, J.J.; Chen, X.W. Size, Nanostructure, and Composition Dependence of Bimetallic Au-Pd Supported on Ceria-zirconia Mixed Oxide Catalysts for Selective Oxidation of Benzyl Alcohol. J. Catal. 2019, 375, 44–55. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, J.J.; Li, X.L.; Oh, R.N.; Shi, D.D.; Akdim, O.; Xia, M.; Zhao, L.; Huang, X.Y.; Zhang, G.J. Au-Pd Nanoparticles Immobilized on TiO2 Nanosheet as an Active and Durable Catalyst for Solvent-free Selective Oxidation of Benzyl Alcohol. J. Colloid Interfaces Sci. 2021, 588, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Shadravan, V.; Bukas, V.J.; Gunasooriya, G.T.K.K.; Waleson, J.; Drewery, M.; Karibika, J.; Jones, J.; Kennedy, E.; Adesina, A.; Nørskov, J.K.; et al. Effect of Manganese on the Selective Catalytic Hydrogenation of COx in the Presence of Light Hydrocarbons Over Ni/Al2O3: An Experimental and Computational Study. ACS Catal. 2020, 10, 1535–1547. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.J.; Wei, H.; Liu, Y.F.; Zhang, D.; Zhang, B.S.; Chu, W.; Pham-Huu, C.; Su, D.S. Oxygenated Groups and Structural Defects Enriched Carbon Nanotubes for Immobilizing Gold Nanoparticles. Chem. Commun. 2017, 53, 12750–12753. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Shan, F.; Yang, S.; Luo, J.; Liang, C. Nano-Sized NiO Immobilized on Au/CNT for Benzyl Alcohol Oxidation: Influences of Hybrid Structure and Interface. Molecules 2021, 26, 6276. https://doi.org/10.3390/molecules26206276
Zhou Y, Shan F, Yang S, Luo J, Liang C. Nano-Sized NiO Immobilized on Au/CNT for Benzyl Alcohol Oxidation: Influences of Hybrid Structure and Interface. Molecules. 2021; 26(20):6276. https://doi.org/10.3390/molecules26206276
Chicago/Turabian StyleZhou, Yixue, Fengxiang Shan, Sihan Yang, Jingjie Luo, and Changhai Liang. 2021. "Nano-Sized NiO Immobilized on Au/CNT for Benzyl Alcohol Oxidation: Influences of Hybrid Structure and Interface" Molecules 26, no. 20: 6276. https://doi.org/10.3390/molecules26206276
APA StyleZhou, Y., Shan, F., Yang, S., Luo, J., & Liang, C. (2021). Nano-Sized NiO Immobilized on Au/CNT for Benzyl Alcohol Oxidation: Influences of Hybrid Structure and Interface. Molecules, 26(20), 6276. https://doi.org/10.3390/molecules26206276





