In Silico Exploration of Potential Natural Inhibitors against SARS-Cov-2 nsp10
Abstract
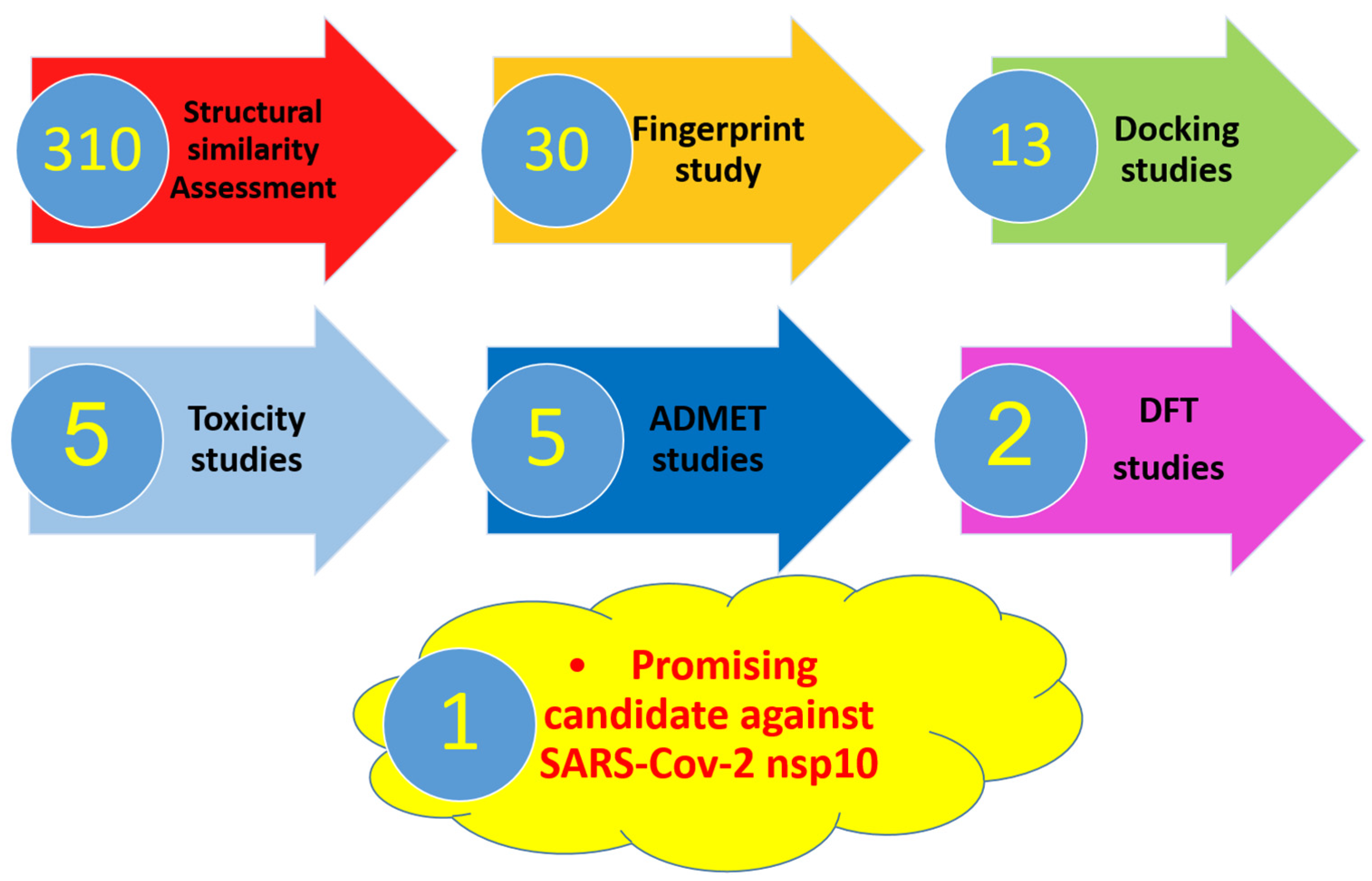
:1. Introduction
2. Results and Discussion
2.1. Molecular Similarity against SAM
2.2. Filter Using Fingerprints
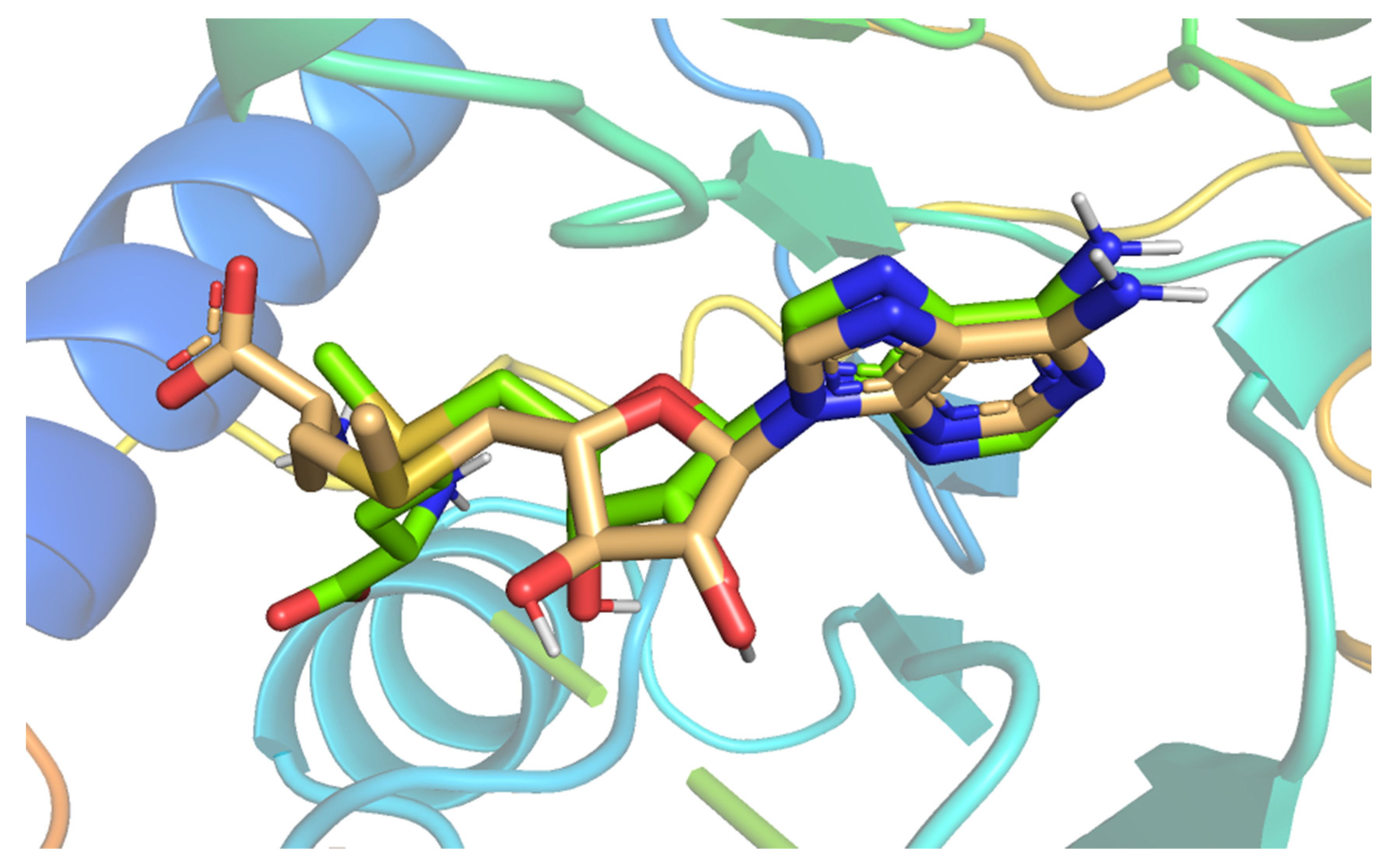
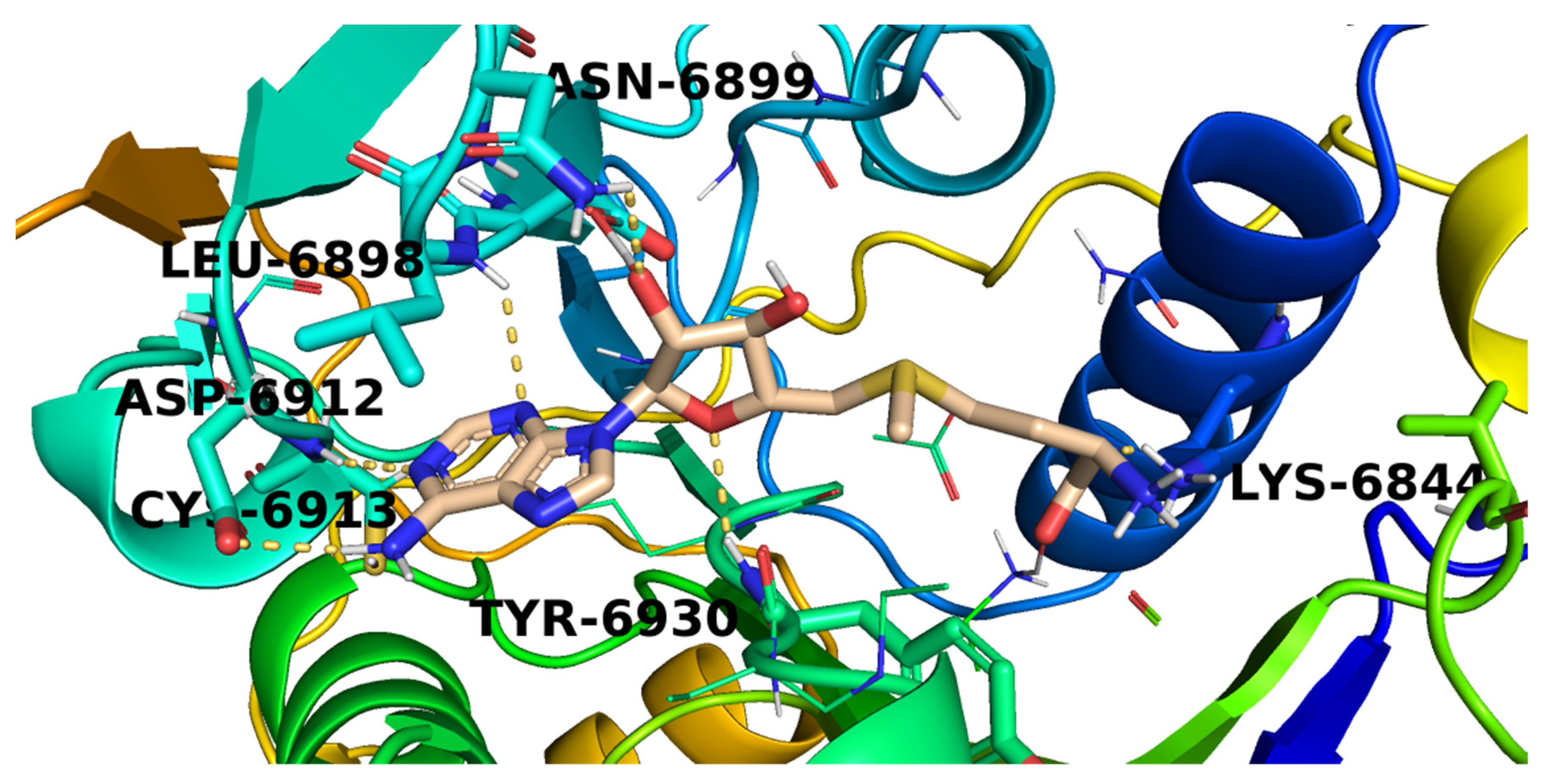
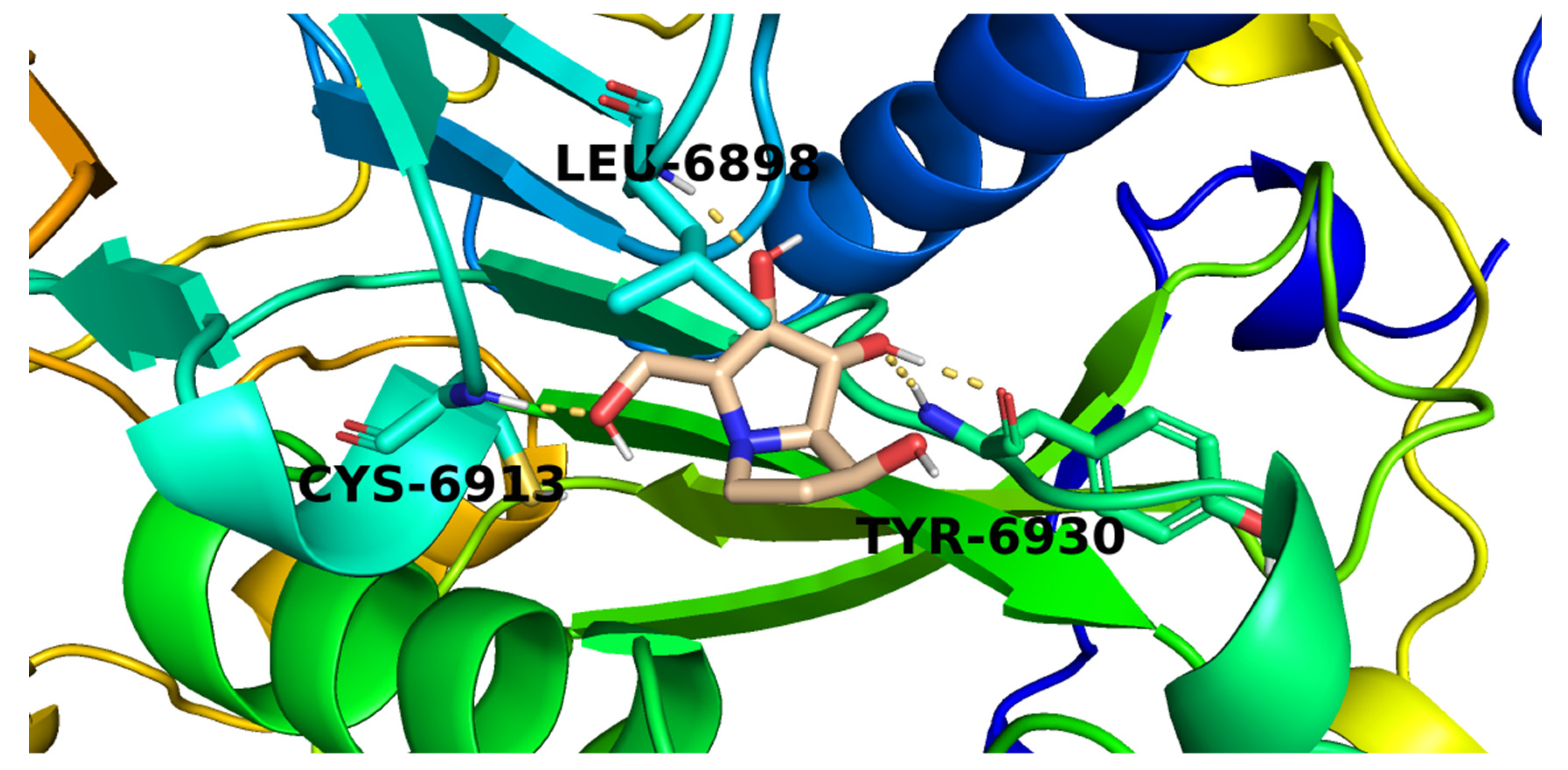
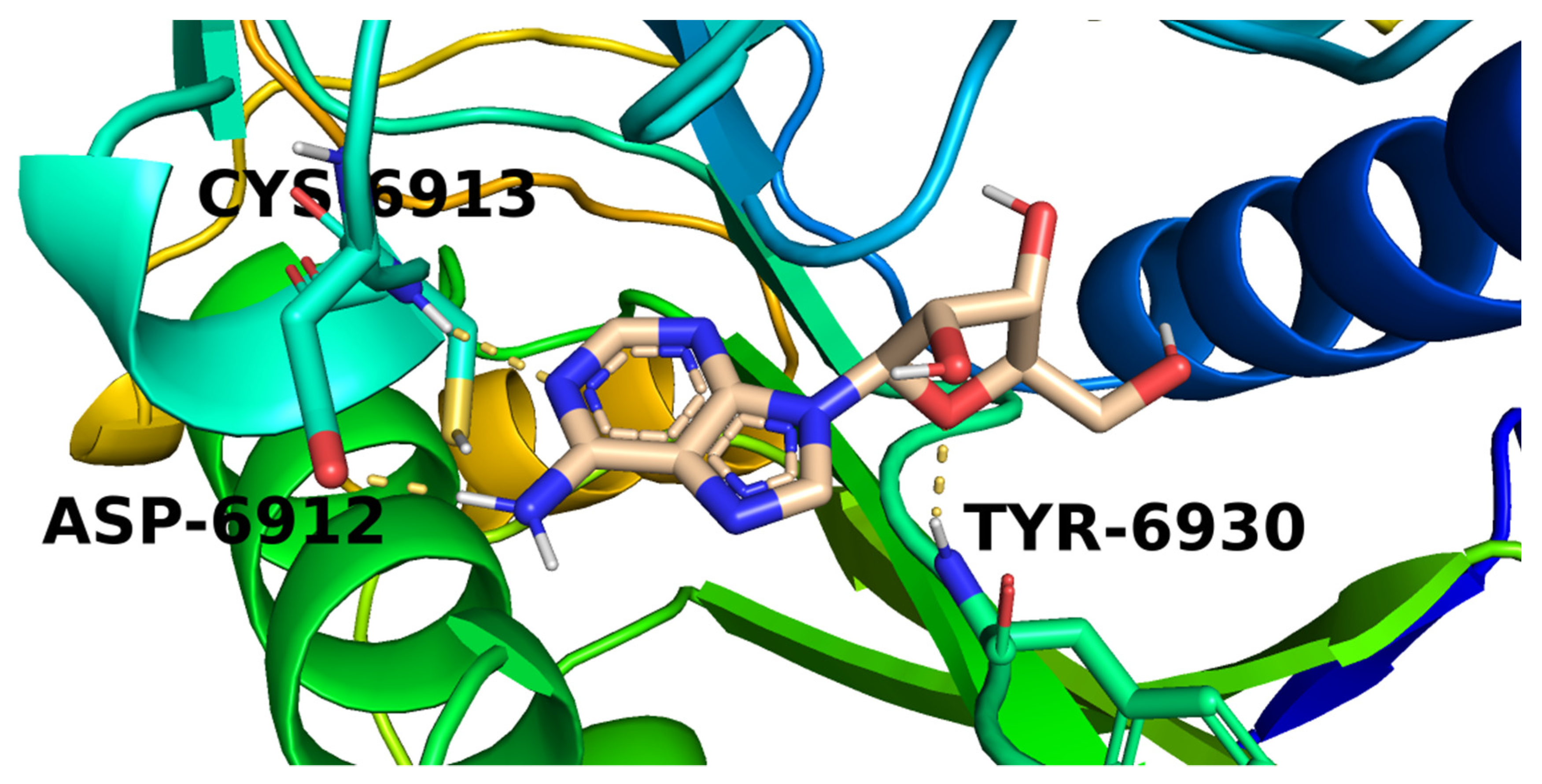
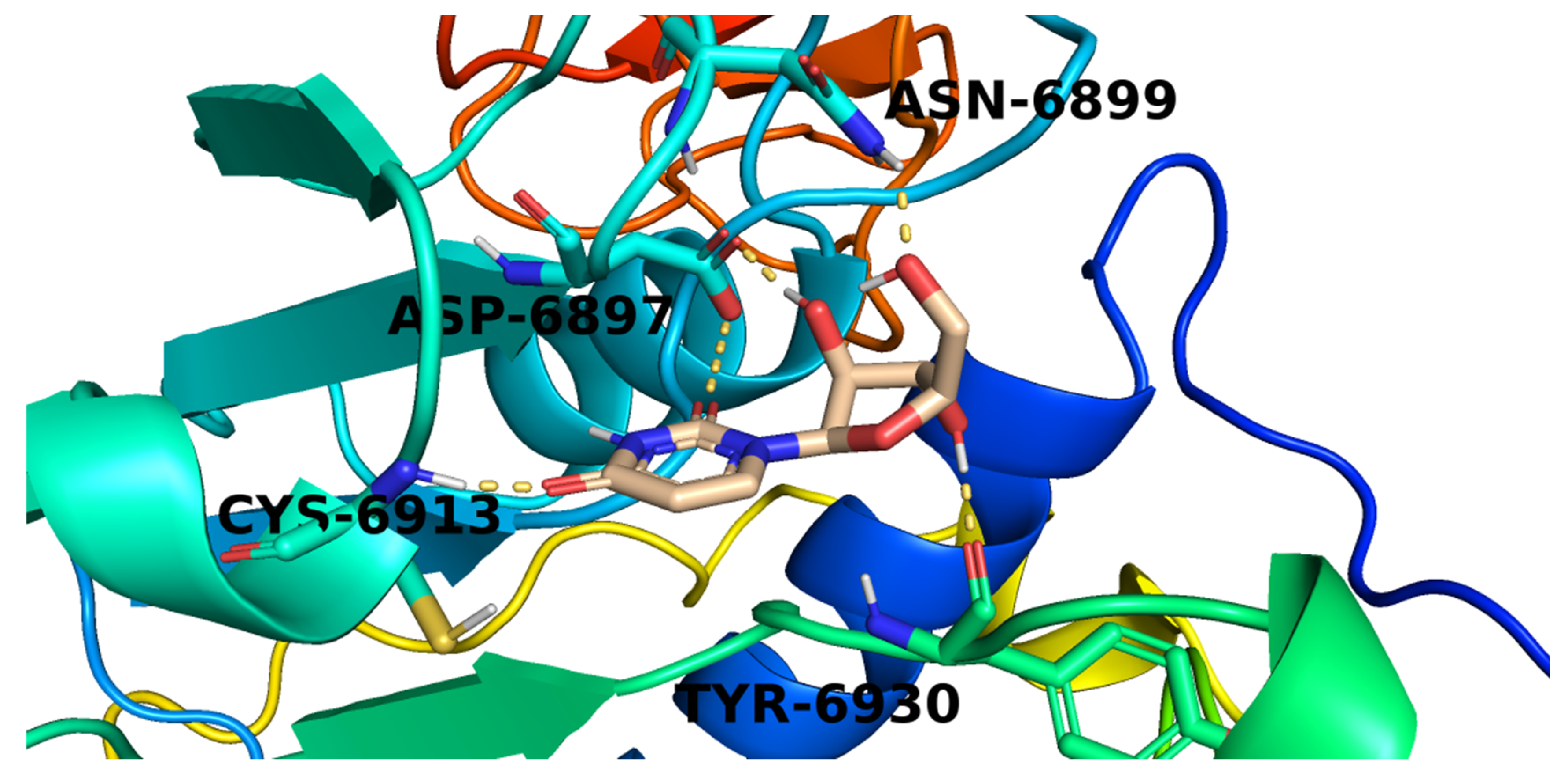
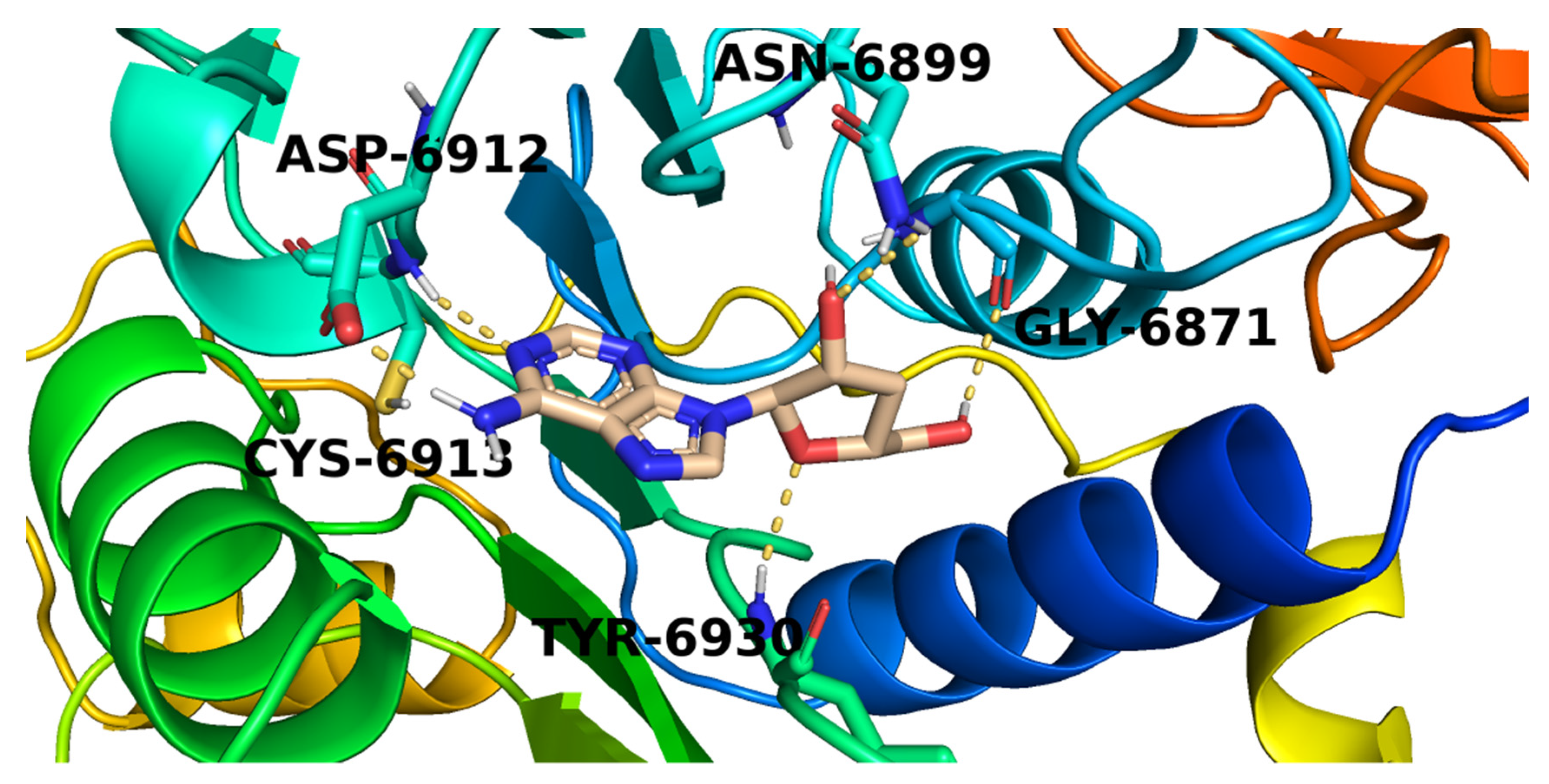
2.3. Docking Studies
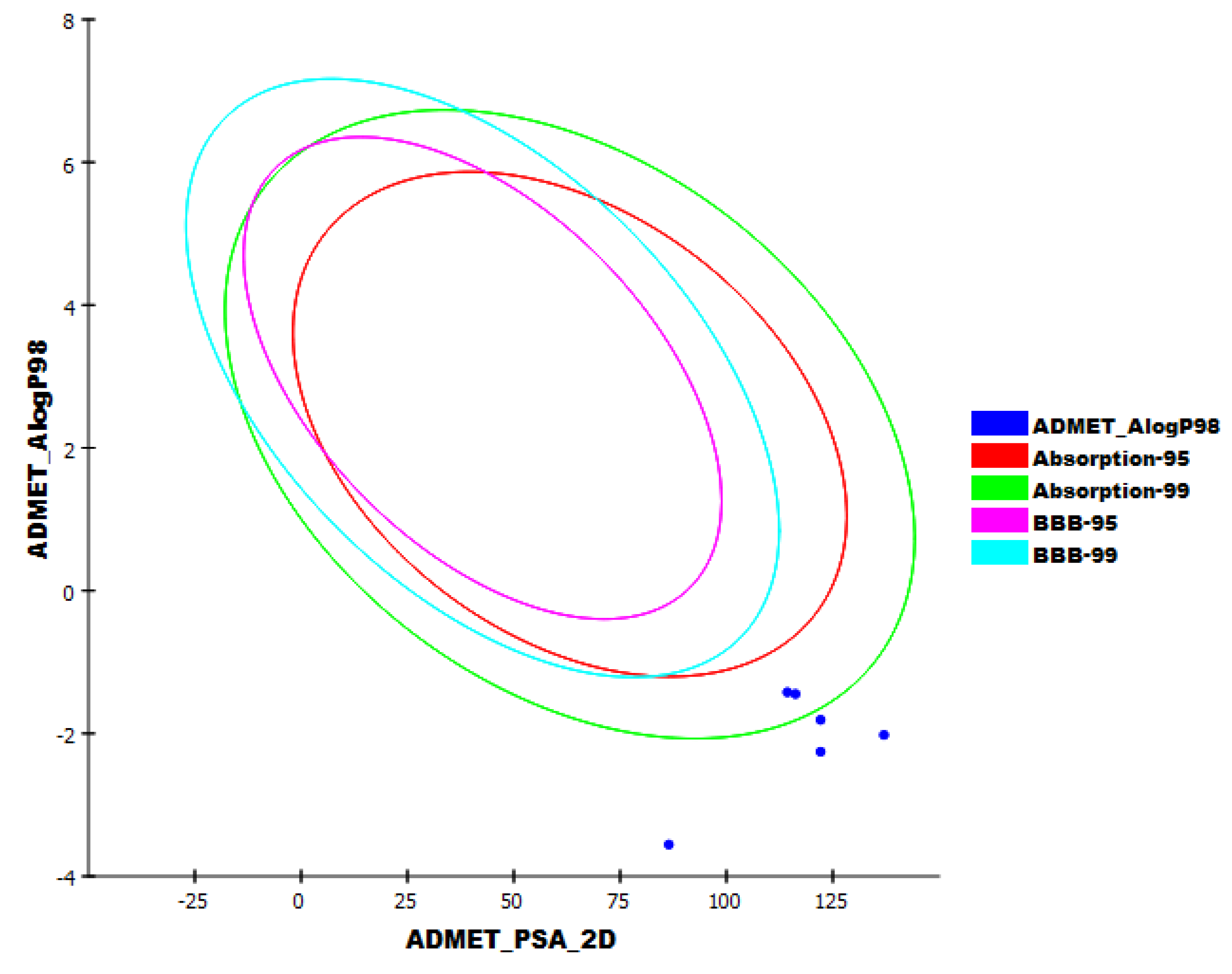
2.4. In Silico ADMET Analysis
2.5. In Silico Toxicity Studies
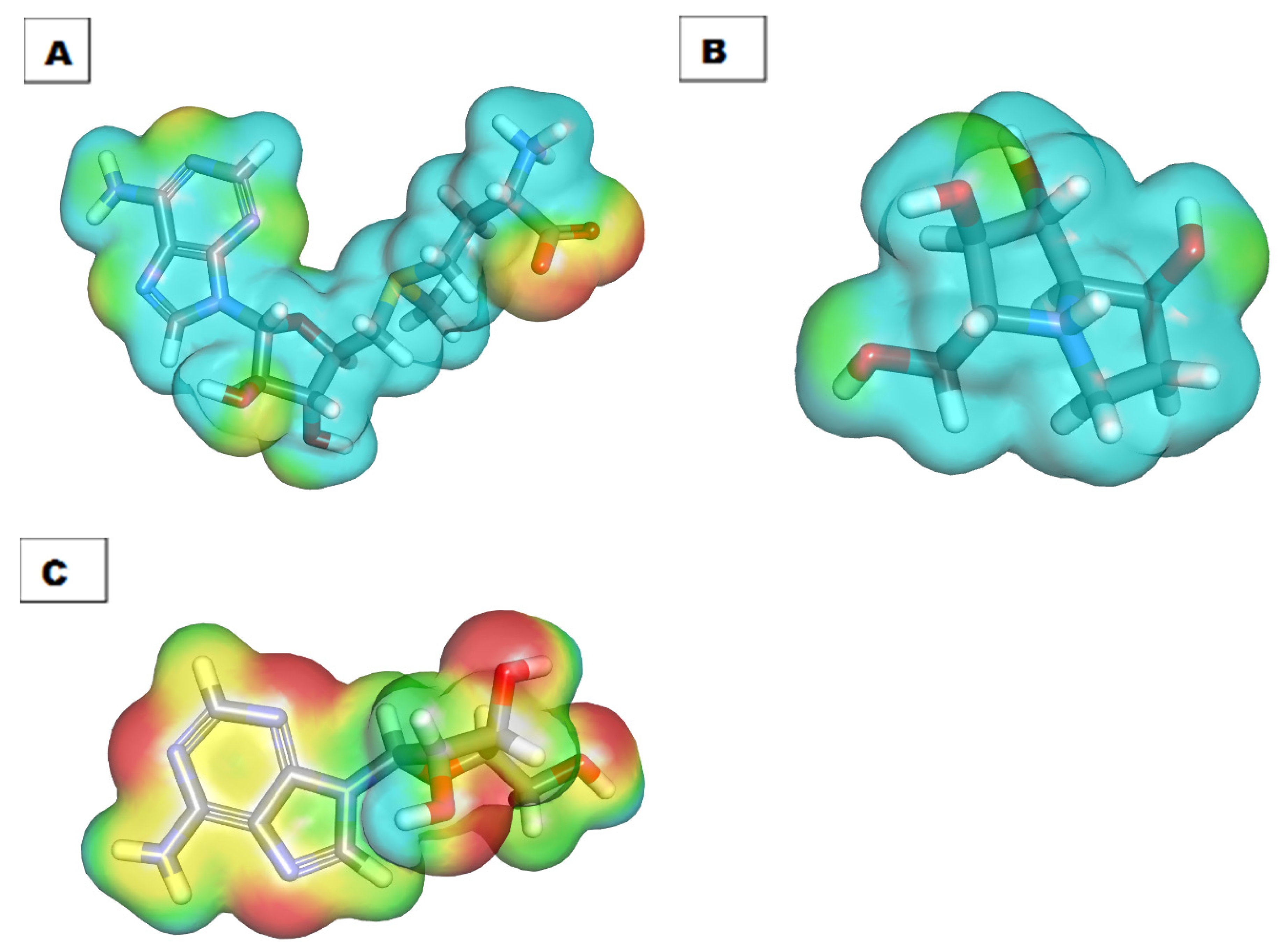
2.6. DFT Studies
2.6.1. Molecular Orbital Analysis
2.6.2. Molecular Electrostatic Potential Maps (MEP)
3. Method
3.1. Molecular Similarity Detection
3.2. Pharmacophoric Study
3.3. Docking Studies
3.4. ADMET Analysis
3.5. Toxicity Studies
3.6. DFT Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 2 September 2021).
- Prieto-Martínez, F.D.; López-López, E.; Juárez-Mercado, K.E.; Medina-Franco, J.L. Computational drug design methods—Current and future perspectives. In In Silico Drug Design; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–44. [Google Scholar]
- Nasser, A.A.; Eissa, I.H.; Oun, M.R.; El-Zahabi, M.A.; Taghour, M.S.; Belal, A.; Saleh, A.M.; Mehany, A.B.; Luesch, H.; Mostafa, A.E.; et al. Discovery of new pyrimidine-5-carbonitrile derivatives as anticancer agents targeting EGFR WT and EGFR T790M. Org. Biomol. Chem. 2020, 18, 7608–7634. [Google Scholar] [CrossRef] [PubMed]
- Abbass, E.M.; Khalil, A.K.; Mohamed, M.M.; Eissa, I.H.; El-Naggar, A.M. Design, efficient synthesis, docking studies, and anticancer evaluation of new quinoxalines as potential intercalative Topo II inhibitors and apoptosis inducers. Bioorg. Chem. 2020, 104, 104255. [Google Scholar] [CrossRef]
- Alanazi, M.M.; Mahdy, H.A.; Alsaif, N.A.; Obaidullah, A.J.; Alkahtani, H.M.; Al-Mehizia, A.A.; Alsubaie, S.M.; Dahab, M.A.; Eissa, I.H. New bis ([1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4] triazolo)[4,3-a:3′,4′-c] quinoxaline derivatives as VEGFR-2 inhibitors and apoptosis inducers: Design, synthesis, in silico studies, and anticancer evaluation. Bioorg. Chem. 2021, 112, 104949. [Google Scholar] [PubMed]
- El-Helby, A.-G.A.; Sakr, H.; Ayyad, R.R.; Mahdy, H.A.; Khalifa, M.M.; Belal, A.; Rashed, M.; El-Sharkawy, A.; Metwaly, A.M.; Elhendawy, M.A.; et al. Design, synthesis, molecular modeling, in vivo studies and anticancer activity evaluation of new phthalazine derivatives as potential DNA intercalators and topoisomerase II inhibitors. Bioorg. Chem. 2020, 103, 104233. [Google Scholar] [CrossRef]
- Eissa, I.H.; Ibrahim, M.K.; Metwaly, A.M.; Belal, A.; Mehany, A.B.; Abdelhady, A.A.; Elhendawy, M.A.; Radwan, M.M.; ElSohly, M.A.; Mahdy, H.A. Design, molecular docking, in vitro, and in vivo studies of new quinazolin-4 (3H)-ones as VEGFR-2 inhibitors with potential activity against hepatocellular carcinoma. Bioorg. Chem. 2021, 107, 104532. [Google Scholar] [CrossRef]
- Abo-Ashour, M.F.; Eldehna, W.M.; Nocentini, A.; Bonardi, A.; Bua, S.; Ibrahim, H.S.; Elaasser, M.M.; Kryštof, V.; Jorda, R.; Gratteri, P.; et al. 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity: Synthesis, in vitro biological evaluation and in silico insights. Eur. J. Med. Chem. 2019, 184, 111768. [Google Scholar] [CrossRef]
- Marrone, T.J.; Briggs, A.; James, M.; McCammon, J.A. Structure-based drug design: Computational advances. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 71–90. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wang, Y.; Li, W.; Li, H.; Yang, L.; Wang, J.; Mahdy, H.A.; Mehany, A.; Jaiash, D.A.; Santali, E.Y.; et al. Screening of Some Sulfonamide and Sulfonylurea Derivatives as Anti-Alzheimer’s Agents Targeting BACE1 and PPARγ. J. Chem. 2020, 2020, 1631243. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Eldehna, W.M.; Fares, M.; Al-Rashood, S.T.; Al-Rashood, K.A.; Abdel-Aziz, M.M.; Soliman, D.H. Synthesis, biological evaluation and 2D-QSAR study of halophenyl bis-hydrazones as antimicrobial and antitubercular agents. Int. J. Mol. Sci. 2015, 16, 8719–8743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kairys, V.; Baranauskiene, L.; Kazlauskiene, M.; Matulis, D.; Kazlauskas, E. Binding affinity in drug design: Experimental and computational techniques. Expert Opin. Drug Discov. 2019, 14, 755–768. [Google Scholar] [CrossRef]
- Al-Warhi, T.; El Kerdawy, A.M.; Aljaeed, N.; Ismael, O.E.; Ayyad, R.R.; Eldehna, W.M.; Abdel-Aziz, H.A.; Al-Ansary, G.H. Synthesis, biological evaluation and in silico studies of certain oxindole–indole conjugates as anticancer CDK inhibitors. Molecules 2020, 25, 2031. [Google Scholar] [CrossRef]
- El-Metwally, S.A.; Abou-El-Regal, M.M.; Eissa, I.H.; Mehany, A.B.; Mahdy, H.A.; Elkady, H.; Elwan, A.; Elkaeed, E.B. Discovery of thieno [2,3-d] pyrimidine-based derivatives as potent VEGFR-2 kinase inhibitors and anti-cancer agents. Bioorg. Chem. 2021, 112, 104947. [Google Scholar] [CrossRef]
- Alanazi, M.M.; Eissa, I.H.; Alsaif, N.A.; Obaidullah, A.J.; Alanazi, W.A.; Alasmari, A.F.; Albassam, H.; Elkady, H.; Elwan, A. Design, synthesis, docking, ADMET studies, and anticancer evaluation of new 3-methylquinoxaline derivatives as VEGFR-2 inhibitors and apoptosis inducers. J. Enzym. Inhib. Med. Chem. 2021, 36, 1760–1782. [Google Scholar] [CrossRef]
- Alanazi, M.M.; Alaa, E.; Alsaif, N.A.; Obaidullah, A.J.; Alkahtani, H.M.; Al-Mehizia, A.A.; Alsubaie, S.M.; Taghour, M.S.; Eissa, I.H. Discovery of new 3-methylquinoxalines as potential anti-cancer agents and apoptosis inducers targeting VEGFR-2: Design, synthesis, and in silico studies. J. Enzym. Inhib. Med. Chem. 2021, 36, 1732–1750. [Google Scholar] [CrossRef]
- Alsaif, N.A.; Taghour, M.S.; Alanazi, M.M.; Obaidullah, A.J.; Al-Mehizia, A.A.; Alanazi, M.M.; Aldawas, S.; Elwan, A.; Elkady, H. Discovery of new VEGFR-2 inhibitors based on bis ([1,2,4]triazolo)[4,3-a: 3′,4′-c] quinoxaline derivatives as anticancer agents and apoptosis inducers. J. Enzym. Inhib. Med. Chem. 2021, 36, 1093–1114. [Google Scholar] [CrossRef]
- Alsaif, N.A.; Dahab, M.A.; Alanazi, M.M.; Obaidullah, A.J.; Al-Mehizia, A.A.; Alanazi, M.M.; Aldawas, S.; Mahdy, H.A.; Elkady, H. New quinoxaline derivatives as VEGFR-2 inhibitors with anticancer and apoptotic activity: Design, molecular modeling, and synthesis. Bioorg. Chem. 2021, 110, 104807. [Google Scholar] [CrossRef]
- El-Adl, K.; Ibrahim, M.-K.; Alesawy, M.S.; Eissa, I.H. [1,2,4]Triazolo [4,3-c]quinazoline and bis([1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4][1,2,4]triazolo)[4,3-a:4′,3′-c] quinazoline derived DNA intercalators: Design, synthesis, in silico ADMET profile, molecular docking and anti-proliferative evaluation studies. Bioorg. Med. Chem. 2021, 30, 115958. [Google Scholar]
- March-Vila, E.; Pinzi, L.; Sturm, N.; Tinivella, A.; Engkvist, O.; Chen, H.; Rastelli, G. On the integration of in silico drug design methods for drug repurposing. Front. Pharmacol. 2017, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Pei, J.; Lai, L. Computational multitarget drug design. J. Chem. Inf. Model. 2017, 57, 403–412. [Google Scholar] [CrossRef]
- Youssef, M.I.; Zhou, Y.; Eissa, I.H.; Wang, Y.; Zhang, J.; Jiang, L.; Hu, W.; Qi, J.; Chen, Z. Tetradecyl 2,3-dihydroxybenzoate alleviates oligodendrocyte damage following chronic cerebral hypoperfusion through IGF-1 receptor. Neurochem. Int. 2020, 138, 104749. [Google Scholar] [CrossRef]
- Zhong, F.; Xing, J.; Li, X.; Liu, X.; Fu, Z.; Xiong, Z.; Lu, D.; Wu, X.; Zhao, J.; Tan, X. Artificial intelligence in drug design. Sci. China Life Sci. 2018, 61, 1191–1204. [Google Scholar] [CrossRef]
- Hagras, M.; El Deeb, M.A.; Elzahabi, H.S.; Elkaeed, E.B.; Mehany, A.B.; Eissa, I.H. Discovery of new quinolines as potent colchicine binding site inhibitors: Design, synthesis, docking studies, and anti-proliferative evaluation. J. Enzym. Inhib. Med. Chem. 2021, 36, 640–658. [Google Scholar] [CrossRef]
- Eissa, I.H.; Dahab, M.A.; Ibrahim, M.K.; Alsaif, N.A.; Alanazi, A.; Eissa, S.I.; Mehany, A.B.; Beauchemin, A.M. Design and discovery of new antiproliferative 1,2,4-triazin-3 (2H)-ones as tubulin polymerization inhibitors targeting colchicine binding site. Bioorg. Chem. 2021, 112, 104965. [Google Scholar] [CrossRef]
- Eissa, I.H.; El-Helby, A.-G.A.; Mahdy, H.A.; Khalifa, M.M.; Elnagar, H.A.; Mehany, A.B.; Metwaly, A.M.; Elhendawy, M.A.; Radwan, M.M.; ElSohly, M.A.; et al. Discovery of new quinazolin-4 (3H)-ones as VEGFR-2 inhibitors: Design, synthesis, and anti-proliferative evaluation. Bioorg. Chem. 2020, 105, 104380. [Google Scholar] [CrossRef]
- El-Adl, K.; El-Helby, A.-G.A.; Ayyad, R.R.; Mahdy, H.A.; Khalifa, M.M.; Elnagar, H.A.; Mehany, A.B.; Metwaly, A.M.; Elhendawy, M.A.; Radwan, M.M.; et al. Design, synthesis, and anti-proliferative evaluation of new quinazolin-4 (3H)-ones as potential VEGFR-2 inhibitors. Bioorg. Med. Chem. 2021, 29, 115872. [Google Scholar] [CrossRef]
- Hopfinger, A. Computer-assisted drug design. J. Med. Chem. 1985, 28, 1133–1139. [Google Scholar] [CrossRef]
- Jalmakhanbetova, R.I.; Suleimen, Y.M.; Oyama, M.; Elkaeed, E.B.; Eissa, I.; Suleimen, R.N.; Metwaly, A.M.; Ishmuratova, M.Y. Isolation and In Silico Anti-COVID-19 Main Protease (Mpro) Activities of Flavonoids and a Sesquiterpene Lactone from Artemisia sublessingiana. J. Chem. 2021, 2021, 13. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs Against SARS-CoV-2 Targeting the hACE2 Receptor. Front. Chem. 2021, 9, 661230. [Google Scholar] [CrossRef]
- Alesawy, M.S.; Abdallah, A.E.; Taghour, M.S.; Elkaeed, E.B.; Eissa, I.H.; Metwaly, A.M. In Silico Studies of Some Isoflavonoids as Potential Candidates against COVID-19 Targeting Human ACE2 (hACE2) and Viral Main Protease (Mpro). Molecules 2021, 26, 2806. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Metwaly, A.M.; Hassan, A.; El-Aziz, A.; Mohamed, T.; Elkaeed, E.B.; Eissa, I.H.; Arafa, R.K.; Stockand, J.D. Comprehensive virtual screening of the antiviral potentialities of marine polycyclic guanidine alkaloids against SARS-CoV-2 (COVID-19). Biomolecules 2021, 11, 460. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Ghoneim, M.M.; Eissa, I.H.; Elsehemy, I.A.; Mostafa, A.E.; Hegazy, M.M.; Afifi, W.M.; Dou, D. Traditional ancient Egyptian medicine: A review. Saudi J. Biol. Sci. 2021, 28, 5823–5832. [Google Scholar] [CrossRef]
- Han, X.; Yang, Y.; Metwaly, A.M.; Xue, Y.; Shi, Y.; Dou, D. The Chinese herbal formulae (Yitangkang) exerts an antidiabetic effect through the regulation of substance metabolism and energy metabolism in type 2 diabetic rats. J. Ethnopharmacol. 2019, 239, 111942. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Zhu, L.; Huang, L.; Dou, D. Black ginseng and its saponins: Preparation, phytochemistry and pharmacological effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-M.; Ran, X.-K.; Riaz, M.; Yu, M.; Cai, Q.; Dou, D.-Q.; Metwaly, A.M.; Kang, T.-G.; Cai, D.-C. Chemical constituents of stems and leaves of Tagetespatula L. and its fingerprint. Molecules 2019, 24, 3911. [Google Scholar] [CrossRef] [Green Version]
- Metwaly, A. Comparative biological evaluation of four endophytic fungi isolated from nigella sativa seeds. Al-Azhar J. Pharm. Sci. 2019, 59, 123–136. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Wanas, A.S.; Radwan, M.M.; Ross, S.A.; ElSohly, M.A. New α-Pyrone derivatives from the endophytic fungus Embellisia sp. Med. Chem. Res. 2017, 26, 1796–1800. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Kadry, H.A.; Atef, A.; Mohammad, A.-E.I.; Ma, G.; Cutler, S.J.; Ross, S.A. Nigrosphaerin A a new isochromene derivative from the endophytic fungus Nigrospora sphaerica. Phytochem. Lett. 2014, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Metwaly, A.M.; Fronczek, F.R.; Ma, G.; Kadry, H.A.; Atef, A.; Mohammad, A.-E.I.; Cutler, S.J.; Ross, S.A. Antileukemic α-pyrone derivatives from the endophytic fungus Alternaria phragmospora. Tetrahedron Lett. 2014, 55, 3478–3481. [Google Scholar] [CrossRef] [Green Version]
- Imieje, V.O.; Zaki, A.A.; Metwaly, A.M.; Mostafa, A.E.; Elkaeed, E.B.; Falodun, A. Comprehensive In Silico Screening of the Antiviral Potentialities of a New Humulene Glucoside from Asteriscus hierochunticus against SARS-CoV-2. J. Chem. 2021, 2021, 5541876. [Google Scholar] [CrossRef]
- Zhanzhaxina, A.; Suleimen, Y.; Metwaly, A.M.; Eissa, I.H.; Elkaeed, E.B.; Suleimen, R.; Ishmuratova, M.; Akatan, K.; Luyten, W. In Vitro and In Silico Cytotoxic and Antibacterial Activities of a Diterpene from Cousinia alata Schrenk. J. Chem. 2021, 2021, 5542455. [Google Scholar] [CrossRef]
- Imieje, V.O.; Zaki, A.A.; Metwaly, A.M.; Eissa, I.H.; Elkaeed, E.B.; Ali, Z.; Khan, I.A.; Falodun, A. Antileishmanial Derivatives of Humulene from Asteriscus hierochunticus with in silico Tubulin Inhibition Potential. Rec. Nat. Prod. 2021, 16, 150–171. [Google Scholar]
- Jalmakhanbetova, R.; Elkaeed, E.B.; Eissa, I.H.; Metwaly, A.M.; Suleimen, Y.M. Synthesis and Molecular Docking of Some Grossgemin Amino Derivatives as Tubulin Inhibitors Targeting Colchicine Binding Site. J. Chem. 2021, 2021, 5586515. [Google Scholar] [CrossRef]
- Suleimen, Y.M.; Metwaly, A.M.; Mostafa, A.E.; Elkaeed, E.B.; Liu, H.-W.; Basnet, B.B.; Suleimen, R.N.; Ishmuratova, M.Y.; Turdybekov, K.M.; Van Heсke, K. Isolation, Crystal Structure, and In Silico Aromatase Inhibition Activity of Ergosta-5,22-dien-3β-ol from the Fungus Gyromitra esculenta. J. Chem. 2021, 2021, 5529786. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; Afifi, W.M.; Ibrahim, M.; Elagawany, M.; Khayat, M.T.; Aboutaleb, M.H.; Metwaly, A.M. Biological evaluation and molecular docking study of metabolites from Salvadora persica L. Growing in Egypt. Pharmacogn. Mag. 2019, 15, 232. [Google Scholar]
- Liu, L.; Luo, S.; Yu, M.; Metwaly, A.M.; Ran, X.; Ma, C.; Dou, D.; Cai, D. Chemical Constituents of Tagetes patula and Their Neuroprotecting Action. Nat. Prod. Commun. 2020, 15, 1934578X20974507. [Google Scholar]
- Metwaly, A.M.; Ghoneim, M.M.; Musa, A. Two new antileishmanial diketopiperazine alkaloids from the endophytic fungus Trichosporum sp. Derpharmachemica 2015, 7, 322–327. [Google Scholar]
- Yassin, A.M.; El-Deeb, N.M.; Metwaly, A.M.; El Fawal, G.F.; Radwan, M.M.; Hafez, E.E. Induction of apoptosis in human cancer cells through extrinsic and intrinsic pathways by Balanites aegyptiaca furostanol saponins and saponin-coated silvernanoparticles. Appl. Biochem. Biotechnol. 2017, 182, 1675–1693. [Google Scholar] [CrossRef]
- Sharaf, M.H.; El-Sherbiny, G.M.; Moghannem, S.A.; Abdelmonem, M.; Elsehemy, I.A.; Metwaly, A.M.; Kalaba, M.H. New combination approaches to combat methicillin-resistant Staphylococcus aureus (MRSA). Sci. Rep. 2021, 11, 4240. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, H.; Chen, Z.; Yang, F.; Ye, F.; Zheng, Y.; Yang, J.; Lin, X.; Sun, H.; Wang, L.; et al. Crystal structure of SARS-CoV-2 nsp10 bound to nsp14-ExoN domain reveals an exoribonuclease with both structural and functional integrity. Nucleic Acids Res. 2021, 49, 5382–5392. [Google Scholar] [CrossRef]
- Lin, S.; Chen, H.; Ye, F.; Chen, Z.; Yang, F.; Zheng, Y.; Cao, Y.; Qiao, J.; Yang, S.; Lu, G. Crystal structure of SARS-CoV-2 nsp10/nsp16 2′-O-methylase and its implication on antiviral drug design. Signal Transduct. Target. Ther. 2020, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Tazikeh-Lemeski, E.; Moradi, S.; Raoufi, R.; Shahlaei, M.; Janlou, M.A.M.; Zolghadri, S. Targeting SARS-COV-2 non-structural protein 16: A virtual drug repurposing study. J. Biomol. Struct. Dyn. 2020, 39, 4633–4646. [Google Scholar] [CrossRef]
- Yadav, R.; Parihar, R.D.; Dhiman, U.; Dhamija, P.; Kumar, S. Docking of fda approved drugs targeting nsp-16, n-protein and main protease of sars-cov-2 as dual inhibitors. Biointerface Res. Appl. Chem. 2020, 11, 9848–9861. [Google Scholar]
- Parida, P.K.; Paul, D.; Chakravorty, D. Nature’s therapy for COVID-19: Targeting the vital non-structural proteins (NSP) from SARS-CoV-2 with phytochemicals from Indian medicinal plants. Phytomed. Plus 2021, 1, 100002. [Google Scholar] [CrossRef]
- Nasser, M.; Salim, N.; Hamza, H.; Saeed, F.; Rabiu, I. Improved deep learning based method for molecular similarity searching using stack of deep belief networks. Molecules 2021, 26, 128. [Google Scholar] [CrossRef]
- Turchi, M.; Cai, Q.; Lian, G. An evaluation of in-silico methods for predicting solute partition in multiphase complex fluids–A case study of octanol/water partition coefficient. Chem. Eng. Sci. 2019, 197, 150–158. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Enoch, S.J.; Ezendam, J.; Sewald, K.; Roggen, E.L.; Cochrane, S. An adverse outcome pathway for sensitization of the respiratory tract by low-molecular-weight chemicals: Building evidence to support the utility of in vitro and in silico methods in a regulatory context. Applied In Vitro Toxicology 2017, 3, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Altamash, T.; Amhamed, A.; Aparicio, S.; Atilhan, M. Effect of hydrogen bond donors and acceptors on CO2 absorption by deep eutectic solvents. Processes 2020, 8, 1533. [Google Scholar] [CrossRef]
- Wan, Y.; Tian, Y.; Wang, W.; Gu, S.; Ju, X.; Liu, G. In silico studies of diarylpyridine derivatives as novel HIV-1 NNRTIs using docking-based 3D-QSAR, molecular dynamics, and pharmacophore modeling approaches. RSC Adv. 2018, 8, 40529–40543. [Google Scholar] [CrossRef] [Green Version]
- Escamilla-Gutiérrez, A.; Ribas-Aparicio, R.M.; Córdova-Espinoza, M.G.; Castelán-Vega, J.A. In silico strategies for modeling RNA aptamers and predicting binding sites of their molecular targets. Nucleosides Nucleotides Nucleic Acids 2021, 40, 798–807. [Google Scholar] [CrossRef]
- Kaushik, A.C.; Kumar, A.; Bharadwaj, S.; Chaudhary, R.; Sahi, S. Ligand-Based Approach for In-silico Drug Designing. In Bioinformatics Techniques for Drug Discovery; Springer: Berlin, Germany, 2018; pp. 11–19. [Google Scholar]
- Zhang, H.; Ren, J.-X.; Ma, J.-X.; Ding, L. Development of an in silico prediction model for chemical-induced urinary tract toxicity by using naïve Bayes classifier. Mol. Divers. 2019, 23, 381–392. [Google Scholar] [CrossRef]
- Willett, P. Similarity-based virtual screening using 2D fingerprints. Drug Discov. Today 2006, 11, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- Ieritano, C.; Campbell, J.L.; Hopkins, W.S. Predicting differential ion mobility behaviour in silico using machine learning. Analyst 2021, 146, 4737–4743. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Ali, M.; Rashid, U.; Imran, S.; Uddin, N.; Khan, K.M. Molecular hybridization conceded exceptionally potent quinolinyl-oxadiazole hybrids through phenyl linked thiosemicarbazide antileishmanial scaffolds: In silico validation and SAR studies. Bioorg. Chem. 2017, 71, 192–200. [Google Scholar] [CrossRef]
- Chu, H.; He, Q.-X.; Wang, J.; Hu, Y.; Wang, Y.-Q.; Lin, Z.-H. In silico design of novel benzohydroxamate-based compounds as inhibitors of histone deacetylase 6 based on 3D-QSAR, molecular docking, and molecular dynamics simulations. New J. Chem. 2020, 44, 21201–21210. [Google Scholar] [CrossRef]
- Opo, F.A.D.M.; Rahman, M.M.; Ahammad, F.; Ahmed, I.; Bhuiyan, M.A.; Asiri, A.M. Structure based pharmacophore modeling, virtual screening, molecular docking and ADMET approaches for identification of natural anti-cancer agents targeting XIAP protein. Sci. Rep. 2021, 11, 4049. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- El-Nahass, M.; Kamel, M.; El-Deeb, A.; Atta, A.; Huthaily, S. Ab initio HF, DFT and experimental (FT-IR) investigation of vibrational spectroscopy of PN, N-dimethylaminobenzylidenemalononitrile (DBM). Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 79, 443–450. [Google Scholar] [CrossRef]
- Suhasini, M.; Sailatha, E.; Gunasekaran, S.; Ramkumaar, G. Vibrational and electronic investigations, thermodynamic parameters, HOMO and LUMO analysis on Lornoxicam by density functional theory. J. Mol. Struct. 2015, 1100, 116–128. [Google Scholar] [CrossRef]
- Bitencourt-Ferreira, G.; de Azevedo Junior, W.F. Electrostatic Potential Energy in Protein-Drug Complexes. Curr. Med. Chem. 2021, 28, 4954–4971. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.M.; Hasan, M.S.; Uzzaman, M.; Bhuiyan, M.M.H.; Kibria, S.M.; Hossain, M.E.; Roshid, M.H. Synthesis, spectroscopic characterization, molecular docking, and ADMET studies of mannopyranoside esters as antimicrobial agents. J. Mol. Struct. 2020, 1222, 128821. [Google Scholar] [CrossRef]
- Whitley, R.J.; Alford, C.A.; Hirsch, M.S.; Schooley, R.T.; Luby, J.P.; Aoki, F.Y.; Hanley, D.; Nahmias, A.J.; Soong, S.-J.; NIAID Collaborative Antiviral Study Group. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N. Engl. J. Med. 1986, 314, 144–149. [Google Scholar] [CrossRef]
- Whitley, R.J.; Nahmias, A.J.; Soong, S.-J.; Galasso, G.G.; Fleming, C.L.; Alford, C.A.; Connor, J.; Bryson, Y.; Linnemann, C. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics 1980, 66, 495–501. [Google Scholar] [PubMed]
- Pollard, R.B.; Smith, J.L.; Neal, E.A.; Gregory, P.B.; Merigan, T.C.; Robinson, W.S. Effect of vidarabine on chronic hepatitis B virus infection. JAMA 1978, 239, 1648–1650. [Google Scholar] [CrossRef] [PubMed]
- Miwa, N.; Kurosaki, K.; Yoshida, Y.; Kurokawa, M.; Saito, S.; Shiraki, K. Comparative efficacy of acyclovir and vidarabine on the replication of varicella-zoster virus. Antivir. Res. 2005, 65, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.; Flower, A.; Durrant, S. The use of vidarabine in the treatment of human polyomavirus associated acute haemorrhagic cystitis. Bone Marrow Transplant. 1991, 7, 481–483. [Google Scholar] [PubMed]
- Kurosaki, K.; Miwa, N.; Yoshida, Y.; Kurokawa, M.; Kurimoto, M.; Endo, S.; Shiraki, K. Therapeutic basis of vidarabine on adenovirus-induced haemorrhagic cystitis. Antivir. Chem. Chemother. 2004, 15, 281–285. [Google Scholar] [CrossRef]
- Kimura, H.; Morita, M.; Tsuge, I.; Hoshino, Y.; Tanaka, N.; Ito, Y.; Morishima, T. Vidarabine therapy for severe chronic active Epstein–Barr virus infection. J. Pediatric Hematol. Oncol. 2001, 23, 294–299. [Google Scholar] [CrossRef]
- Yousef, R.; Sakr, H.; Eissa, I.; Mehany, A.; Metwaly, A.; Elhendawy, M.A.; Radwan, M.; ElSohly, M.A.; Abulkhair, H.S.; El-Adl, K. New quinoxaline-2 (1H)-ones as potential VEGFR-2 inhibitors: Design, synthesis, molecular docking, ADMET profile and anti-proliferative evaluations. New J. Chem. 2021, 45, 16949–16964. [Google Scholar] [CrossRef]
- Amer, H.H.; Alotaibi, S.H.; Trawneh, A.H.; Metwaly, A.M.; Eissa, I.H. Anticancer activity, spectroscopic and molecular docking of some new synthesized sugar hydrazones, Arylidene and α-Aminophosphonate derivatives. Arab. J. Chem. 2021, 14, 103348. [Google Scholar] [CrossRef]
- Alesawy, M.S.; Al-Karmalawy, A.A.; Elkaeed, E.B.; Alswah, M.; Belal, A.; Taghour, M.S.; Eissa, I.H. Design and discovery of new 1,2,4-triazolo [4,3-c] quinazolines as potential DNA intercalators and topoisomerase II inhibitors. Arch. Pharm. 2021, 354, 2000237. [Google Scholar] [CrossRef]
- Parmar, D.R.; Soni, J.Y.; Guduru, R.; Rayani, R.H.; Kusurkar, R.V.; Vala, A.G.; Talukdar, S.N.; Eissa, I.H.; Metwaly, A.M.; Khalil, A.; et al. Discovery of new anticancer thiourea-azetidine hybrids: Design, synthesis, in vitro antiproliferative, SAR, in silico molecular docking against VEGFR-2, ADMET, toxicity, and DFT studies. Bioorg. Chem. 2021, 115, 105206. [Google Scholar] [CrossRef]
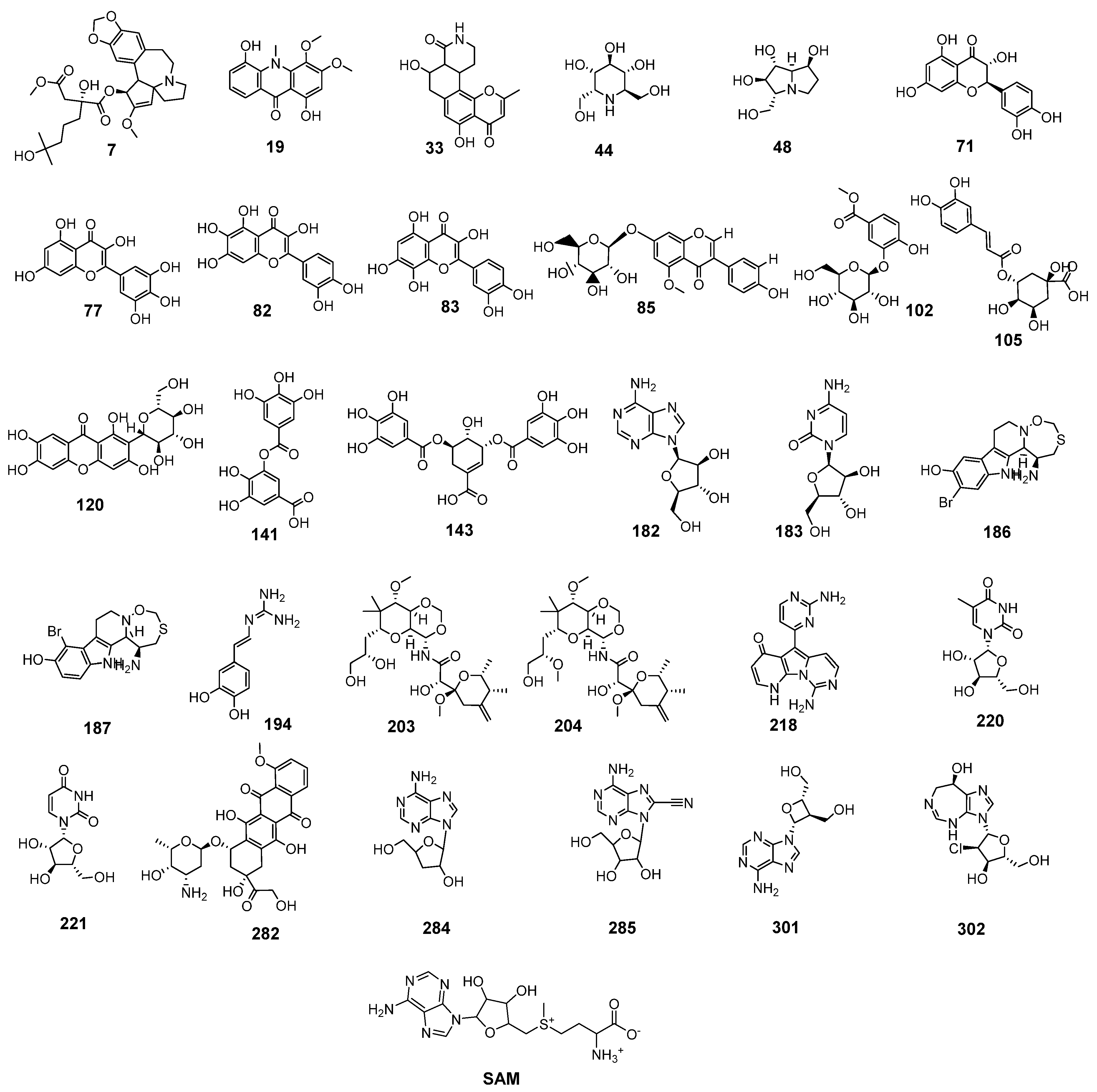


| Candidate | ALog p 1 | M. Wt 2 | HBA 3 | HBD 4 | Rotatable Bonds | Rings | Aromatic Rings | MFPSA 5 | Minimum Distance |
|---|---|---|---|---|---|---|---|---|---|
| 7 | 0.857 | 546.629 | 9 | 3 | 11 | 5 | 1 | 0.223 | 1.272 |
| 19 | 2.643 | 301.294 | 6 | 2 | 2 | 3 | 2 | 0.261 | 1.441 |
| 33 | 0.674 | 315.321 | 5 | 3 | 0 | 4 | 1 | 0.333 | 1.472 |
| 44 | −4.182 | 194.206 | 5 | 6 | 2 | 1 | 0 | 0.597 | 1.379 |
| 48 | −3.556 | 190.217 | 4 | 5 | 1 | 2 | 0 | 0.466 | 1.454 |
| 71 | 1.479 | 304.252 | 7 | 5 | 1 | 3 | 2 | 0.467 | 1.491 |
| 77 | 1.388 | 318.235 | 8 | 6 | 1 | 3 | 2 | 0.526 | 1.477 |
| 82 | 1.388 | 318.235 | 8 | 6 | 1 | 3 | 2 | 0.526 | 1.477 |
| 83 | 1.388 | 318.235 | 8 | 6 | 1 | 3 | 2 | 0.526 | 1.477 |
| 85 | 0.436 | 446.404 | 10 | 5 | 5 | 4 | 2 | 0.373 | 1.093 |
| 102 | −0.729 | 330.287 | 9 | 5 | 5 | 2 | 1 | 0.457 | 0.491 |
| 105 | −1.814 | 353.301 | 9 | 5 | 5 | 2 | 1 | 0.489 | 0.375 |
| 120 | −0.396 | 422.34 | 11 | 8 | 2 | 4 | 2 | 0.533 | 0.652 |
| 141 | 0.207 | 321.216 | 9 | 5 | 4 | 2 | 2 | 0.563 | 0.632 |
| 143 | 0.007 | 477.352 | 13 | 7 | 7 | 3 | 2 | 0.536 | 0.565 |
| 182 | −1.881 | 267.241 | 8 | 4 | 2 | 3 | 2 | 0.539 | 0.489 |
| 183 | −2.396 | 243.217 | 7 | 4 | 2 | 2 | 0 | 0.545 | 0.747 |
| 186 | 1.045 | 371.273 | 4 | 3 | 0 | 4 | 2 | 0.339 | 1.039 |
| 187 | 1.045 | 371.273 | 4 | 3 | 0 | 4 | 2 | 0.339 | 1.039 |
| 194 | 0.253 | 193.203 | 5 | 4 | 2 | 1 | 1 | 0.501 | 0.964 |
| 203 | −0.499 | 503.583 | 10 | 4 | 8 | 3 | 0 | 0.268 | 1.113 |
| 204 | −0.091 | 517.61 | 10 | 3 | 9 | 3 | 0 | 0.237 | 1.229 |
| 218 | 0.536 | 293.283 | 7 | 3 | 1 | 4 | 3 | 0.444 | 0.903 |
| 220 | −2.005 | 258.228 | 6 | 4 | 2 | 2 | 0 | 0.479 | 0.877 |
| 221 | −2.451 | 244.201 | 6 | 4 | 2 | 2 | 0 | 0.525 | 0.876 |
| 282 | −1.049 | 544.527 | 11 | 6 | 5 | 5 | 2 | 0.403 | 0.534 |
| 284 | −1.308 | 251.242 | 7 | 3 | 2 | 3 | 2 | 0.482 | 0.406 |
| 285 | −1.595 | 292.251 | 9 | 4 | 2 | 3 | 2 | 0.57 | 0.432 |
| 301 | −1.614 | 251.242 | 7 | 3 | 3 | 3 | 2 | 0.48 | 0.364 |
| 302 | −1.526 | 302.714 | 7 | 4 | 2 | 3 | 1 | 0.401 | 0.510 |
| SAM | −4.254 | 399.445 | 9 | 4 | 7 | 3 | 2 | 0.483 |
| Comp. | Similarity | SA | SB | SC |
|---|---|---|---|---|
| SAM | 1 | 237 | 0 | 0 |
| 44 | 0.503 | 159 | 79 | 78 |
| 48 | 0.423 | 110 | 23 | 127 |
| 85 | 0.423 | 200 | 236 | 37 |
| 102 | 0.497 | 149 | 63 | 88 |
| 105 | 0.529 | 165 | 75 | 72 |
| 182 | 0.717 | 160 | −14 | 77 |
| 220 | 0.475 | 135 | 47 | 102 |
| 221 | 0.458 | 125 | 36 | 112 |
| 282 | 0.443 | 250 | 327 | −13 |
| 284 | 0.685 | 150 | −18 | 87 |
| 285 | 0.671 | 159 | 0 | 78 |
| 301 | 0.642 | 145 | −11 | 92 |
| 302 | 0.552 | 139 | 15 | 98 |
| Comp. | ∆G [Kcal/mol] | Comp. | ∆G [Kcal/mol] |
|---|---|---|---|
| 44 | −18.65 | 221 | −20.09 |
| 48 | −21.15 | 282 | −19.85 |
| 85 | −19.32 | 284 | −20.07 |
| 102 | −18.98 | 285 | −19.02 |
| 105 | −20.01 | 301 | −18.72 |
| 182 | −21.10 | 302 | −16.96 |
| 220 | −21.17 | SAM | −22.05 |
| Comp. | FDA Rodent Carcinogenicity (Mouse-Female) | Carcinogenic Potency TD50 (Mouse) mg/kg Body Weight/Day | Rat Maximum Tolerated Dose (Feed) a | Rat Oral LD50 a | Rat Chronic LOAEL a | Ocular Irritancy | Skin Irritancy |
|---|---|---|---|---|---|---|---|
| 48 | Non-Carcinogen | 9.295 | 0.191 | 0.778 | 0.018 | Severe | Mild |
| 182 | Non-Carcinogen | 4.245 | 0.175 | 1.119 | 0.010 | Moderate | Mild |
| 220 | Single-Carcinogen | 67.851 | 0.095 | 6.173 | 0.009 | Moderate | Mild |
| 221 | Single-Carcinogen | 55.437 | 0.094 | 4.343 | 0.006 | Moderate | Mild |
| 284 | Multi-Carcinogen | 6.402 | 0.155 | 1.213 | 0.004 | Moderate | Mild |
| Ribavirin | Non-Carcinogen | 13.111 | 0.154 | 0.750 | 0.013 | Mild | Mild |
| Name | Total Energy * | Binding Energy * | HOMO Energy * | LUMO Energy * | Dipole Mag | Band Gap Energy * |
|---|---|---|---|---|---|---|
| 48 | −664.379 | −4.841 | −0.366 | −0.156 | 1.391 | 0.210 |
| 182 | −955.658 | −6.102 | −0.195 | −0.068 | 1.396 | 0.128 |
| SAM | −1675.931 | −8.815 | −0.270 | −0.174 | 3.631 | 0.097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eissa, I.H.; Khalifa, M.M.; Elkaeed, E.B.; Hafez, E.E.; Alsfouk, A.A.; Metwaly, A.M. In Silico Exploration of Potential Natural Inhibitors against SARS-Cov-2 nsp10. Molecules 2021, 26, 6151. https://doi.org/10.3390/molecules26206151
Eissa IH, Khalifa MM, Elkaeed EB, Hafez EE, Alsfouk AA, Metwaly AM. In Silico Exploration of Potential Natural Inhibitors against SARS-Cov-2 nsp10. Molecules. 2021; 26(20):6151. https://doi.org/10.3390/molecules26206151
Chicago/Turabian StyleEissa, Ibrahim H., Mohamed M. Khalifa, Eslam B. Elkaeed, Elsayed E. Hafez, Aisha A. Alsfouk, and Ahmed M. Metwaly. 2021. "In Silico Exploration of Potential Natural Inhibitors against SARS-Cov-2 nsp10" Molecules 26, no. 20: 6151. https://doi.org/10.3390/molecules26206151
APA StyleEissa, I. H., Khalifa, M. M., Elkaeed, E. B., Hafez, E. E., Alsfouk, A. A., & Metwaly, A. M. (2021). In Silico Exploration of Potential Natural Inhibitors against SARS-Cov-2 nsp10. Molecules, 26(20), 6151. https://doi.org/10.3390/molecules26206151









