Highly Porous Hydroxyapatite/Graphene Oxide/Chitosan Beads as an Efficient Adsorbent for Dyes and Heavy Metal Ions Removal
Abstract
1. Introduction
2. Results and Discussion
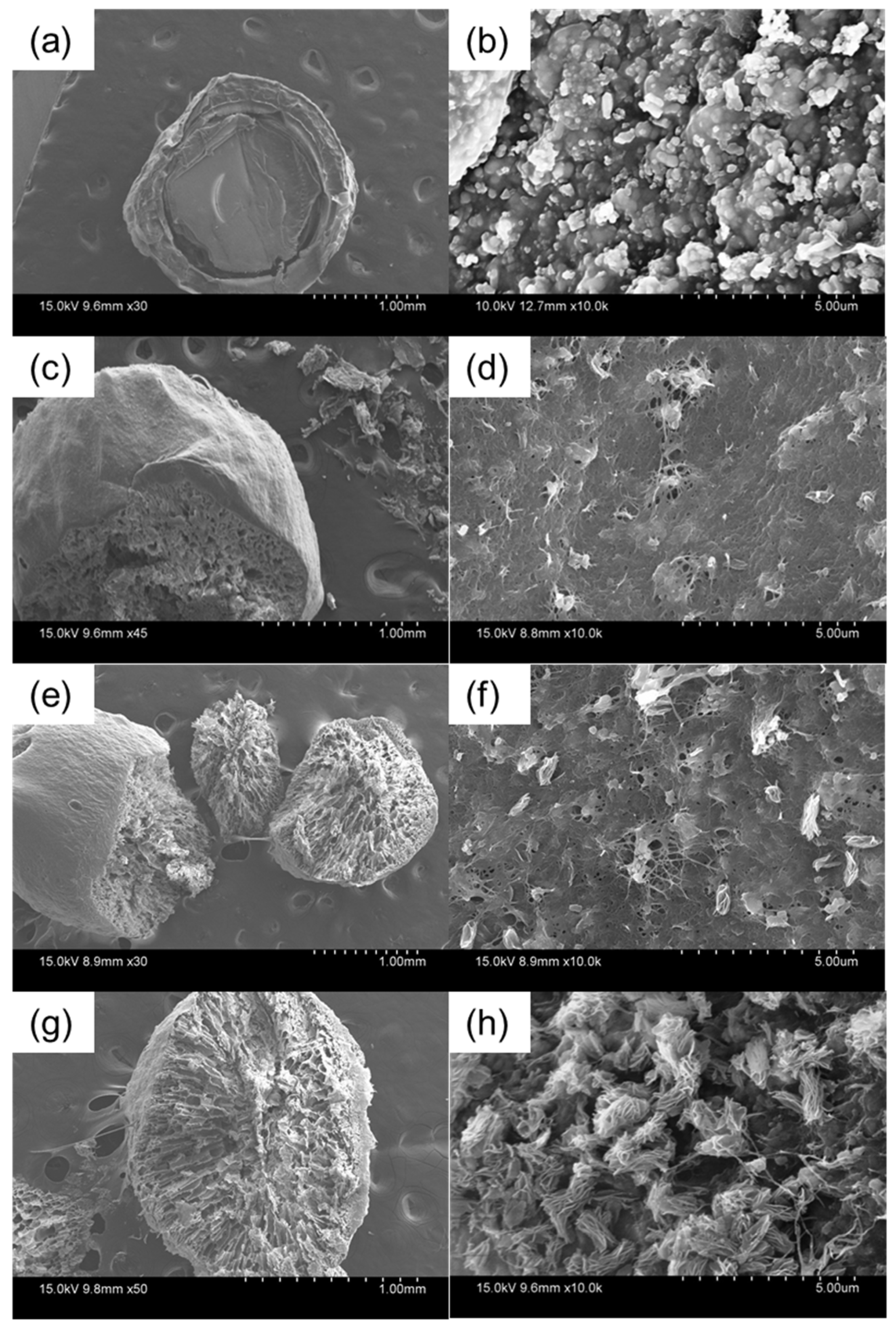
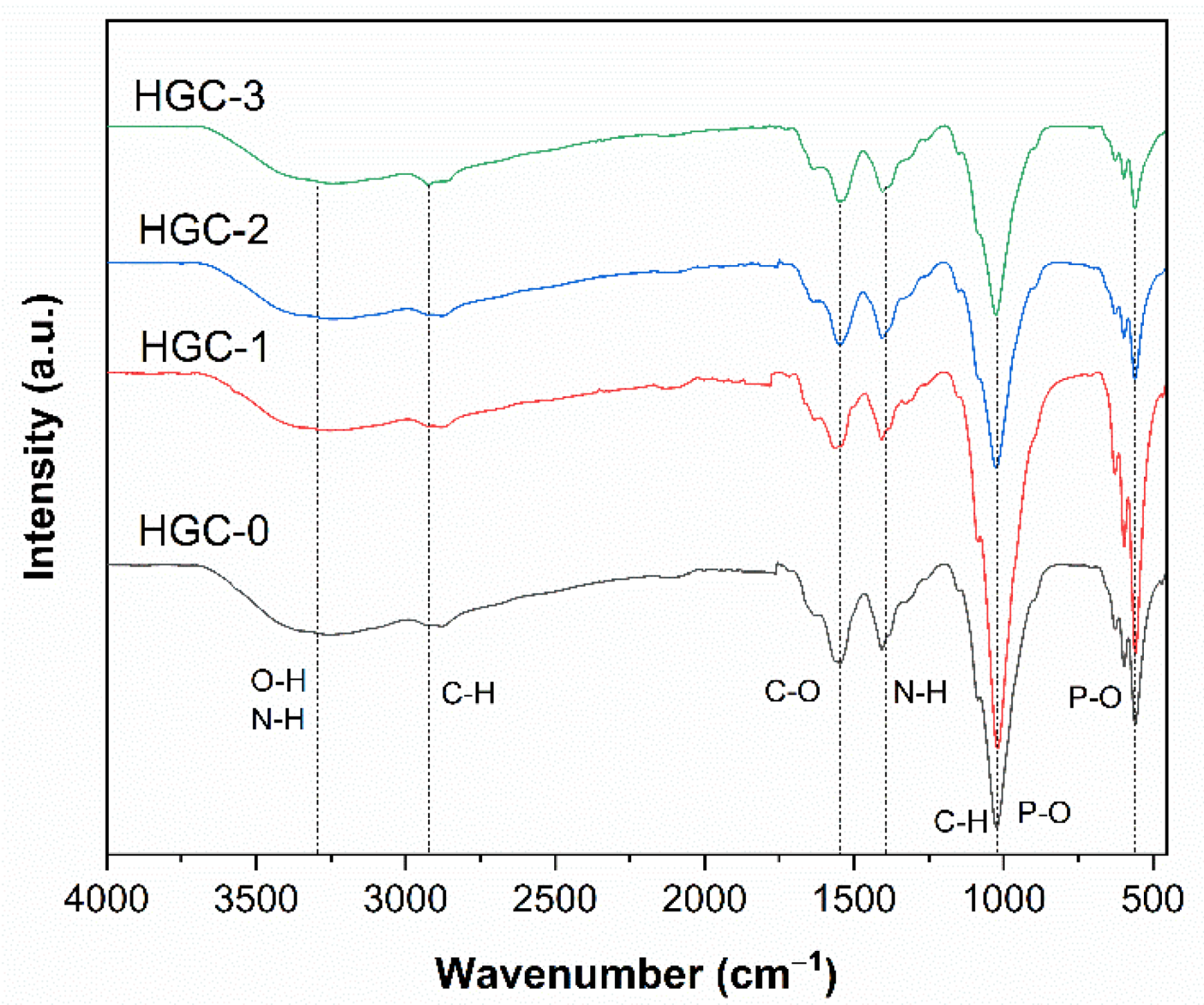
2.1. Characterization of Prepared Beads
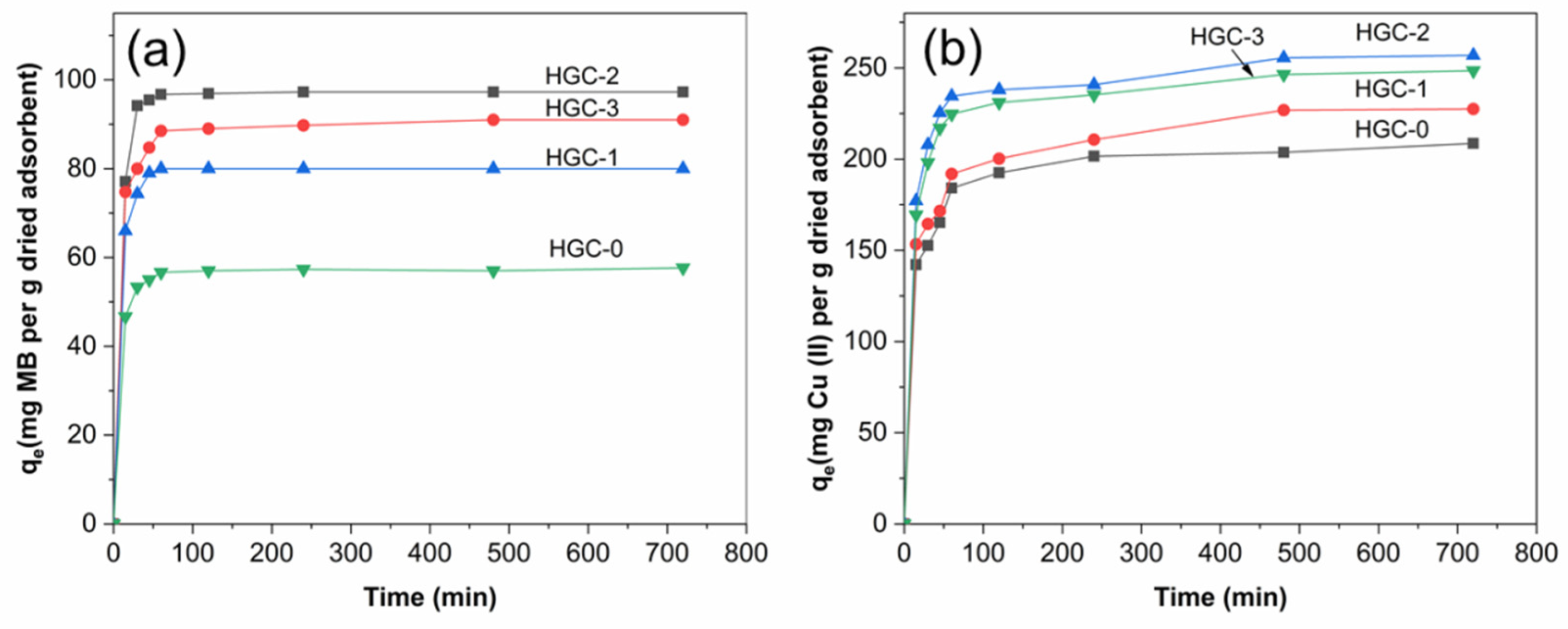
2.2. Effect of Contact Time
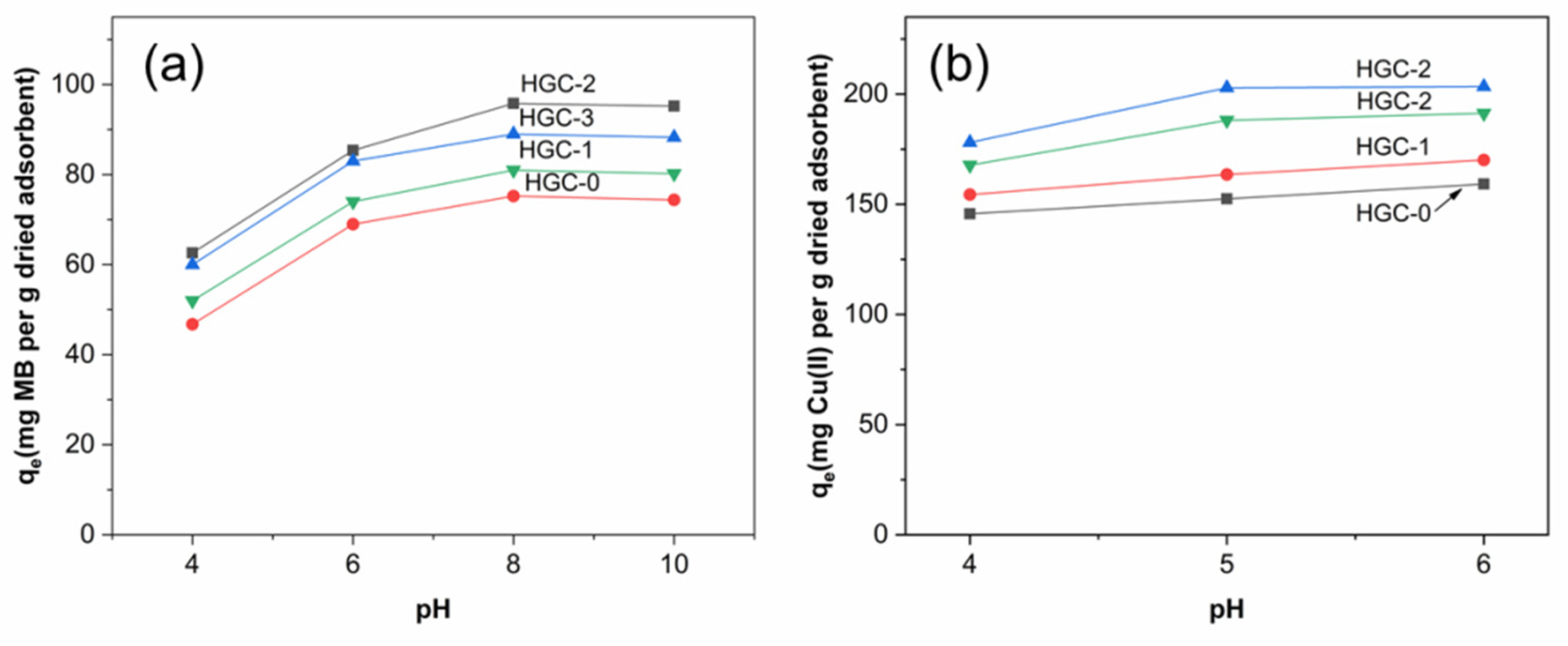
2.3. pH Effect
2.4. Sorption Isotherms
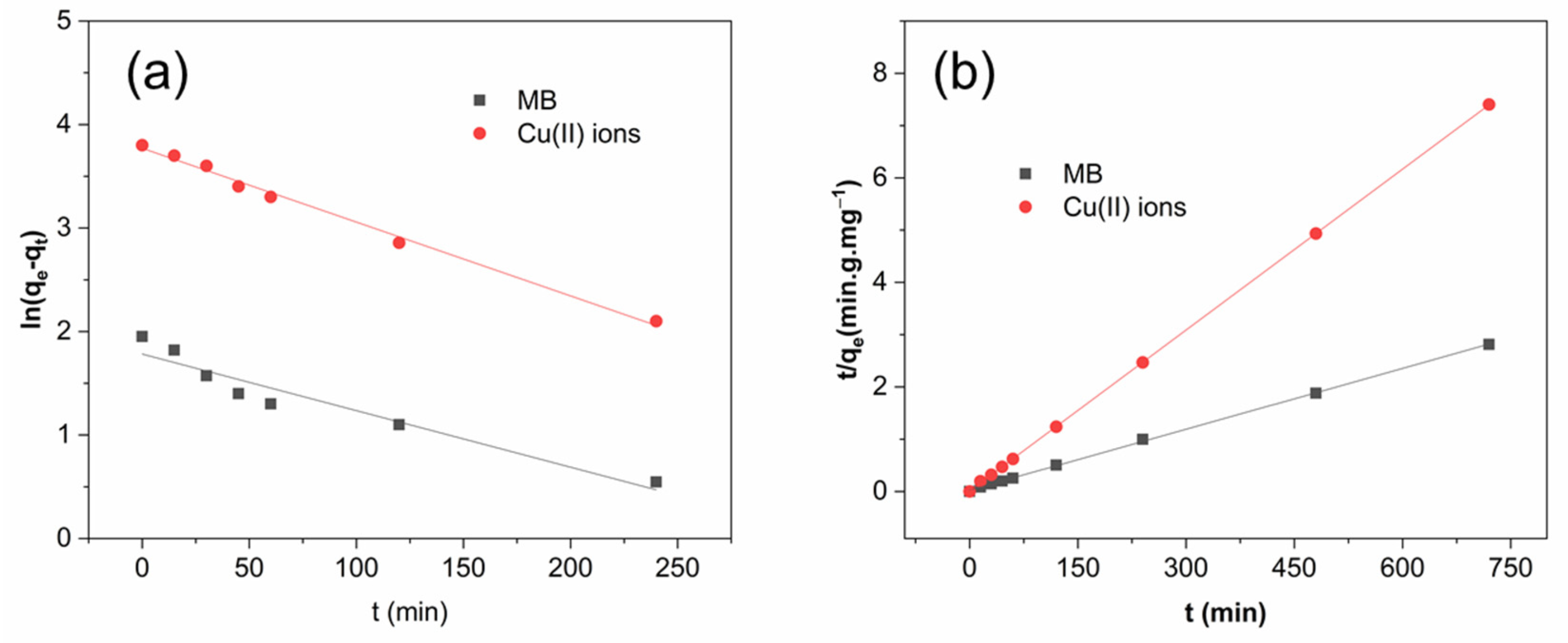
2.5. Adsorption Kinetics
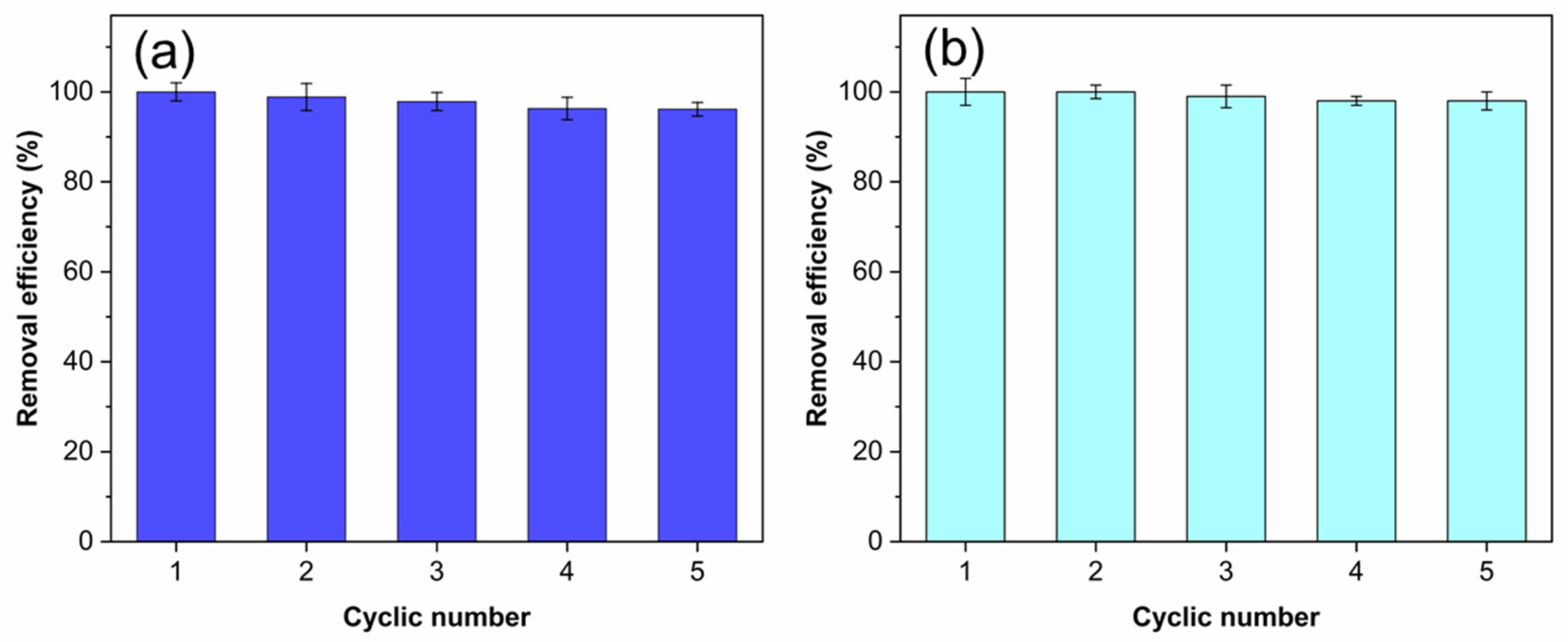
2.6. Cyclic Test
3. Materials and Methods
3.1. Materials
3.2. Preparation of the HGC Beads
3.3. Characterization
3.4. Adsorption Test
3.5. Adsorption Isotherms
3.6. Adsorption Kinetics
3.7. Recycle Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dutt, M.A.; Hanif, M.A.; Nadeem, F.; Bhatti, H.N. A review of advances in engineered composite materials popular for wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 104073. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Xing, H.T.; Chen, J.H.; Sun, X.; Huang, Y.H.; Su, Z.B.; Hu, S.R.; Weng, W.; Li, S.X.; Guo, H.X.; Wu, W.B.; et al. NH2-rich polymer/graphene oxide use as a novel adsorbent for removal of Cu(II) from aqueous solution. Chem. Eng. J. 2015, 263, 280–289. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Adsorptive removal of Pb2+, Co2+ and Ni2+ by hydroxyapatite/chitosan composite from aqueous solution. J. Taiwan Inst. Chem. Eng. 2012, 43, 125–131. [Google Scholar] [CrossRef]
- Candido, I.C.M.; Soares, J.M.D.; Barbosa, J.A.B.; Oliveira, H.P. Adsorption and identification of traces of dyes in aqueous solutions using chemically modified eggshell membranes. Bioresour. Technol. Rep. 2019, 7, 100267–100277. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.S.; Franca, A.S.; Alves, M.T.; Rocha, S.D. Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J. Harz. Mater. 2008, 133, 507–512. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Heidary, F. Effect of surface modification of microfiltration membrane on capture of toxic heavy metal ions. Environ. Technol. 2012, 33, 393–399. [Google Scholar] [CrossRef]
- Wu, Z.C.; Wang, Z.Z.; Liu, J.; Yin, J.H.; Kuang, S.P. A new porous magnetic chitosan modified by melamine for fast and efficient adsorption of Cu(II) ions. Inter. J. Biol. Macromol. 2015, 81, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-T.; Shi, W.; Hu, Z.-J.; Yang, T.; Chen, M.-L.; Zhao, B.; Wang, J.-H. Fabrication of magnetic Fe3O4@metal organic framework@covalent organic framework composite and its selective separation of trace copper. Appl. Surf. Sci. 2020, 530, 147254. [Google Scholar] [CrossRef]
- Bazargan-Lari, R.; Zafarani, H.R.; Bahrololoom, M.E.; Nemati, A. Removal of Cu(II) ions from aqueous solutions by low-cost natural hydroxyapatite/chitosan composite: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2014, 45, 1642–1648. [Google Scholar] [CrossRef]
- Iqbal, J.; Yin, C.; Geng, J.; Li, X.; Li, X.; Du, Y.; Liu, B.X. Ultra-sensitive spectrophotometric determination of nickel after complexation and membrane filtration. Microchim. Acta 2012, 177, 195–200. [Google Scholar] [CrossRef]
- Dolgormaa, A.; Lv, C.; Li, Y.; Yang, J.; Yang, J.-X.; Chen, P.; Wang, H.; Huang, J. Adsorption of Cu(II) and Zn(II) ions from aqueous solution by Gel/PVA-modified super-paramagnetic iron oxide nanoparticles. Molecules 2018, 23, 2982. [Google Scholar] [CrossRef]
- Pica, M. Treatment of wastewaters with zirconium phosphate based materials: A review on efficient systems for the removal of heavy metal and dye water pollutants. Molecules 2021, 26, 2392. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Romero-Pareja, P.M.; Aragon, C.A.; Quiroga, J.M.; Coello, M.D. Evaluation of a biological wastewater treatment system combining an OSA process with ultrasound for sludge reduction. Ultrason. Sonochem. 2017, 36, 336–342. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, K.E.; Dionysiou, D.D. Advanced oxidation processes for water treatment. J. Phys. Chem. Lett. 2012, 3, 2112–2113. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N. Recent modifications of chitosan for adsorption applications: A critical and systematic review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef] [PubMed]
- Mohandes, F.; Salavati-Niasari, M. Freeze-drying synthesis, characterization and in vitro bioactivity of chitosan/grapheme oxide/hydroxyapatite nanocomposite. RSC Adv. 2014, 4, 25993–26001. [Google Scholar] [CrossRef]
- Lia, L.; Iqbal, J.; Zhu, Y.; Zhang, P.; Chen, W.; Bhatnagar, A.; Du, Y. Chitosan/Ag-hydroxyapatite nanocomposite beads as a potential adsorbent for the efficient removal of toxic aquatic pollutants. Inter. J. Biol. Macromol. 2018, 120, 1752–1759. [Google Scholar] [CrossRef]
- Wong, S.M.; Zulkifli, M.Z.A.; Nordin, D.; Teow, Y.H. Synthesis of cellulose/nano-hydroxyapatite composite hydrogel absorbent for removal of heavy metal ions from palm oil mill effluents. J. Polym. Environ. 2021, in press. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Wang, B.; Wang, Y.; Zhang, L.; Jiang, Y.; Zhao, M.; Zeng, J.; Chen, H.; Zhang, Y. A facile yet versatile method for adsorption and relayed fluorescent detection of heavy metal ions. J. Environ. Chem. Eng. 2021, 9, 105737. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, M.; Wang, F.; Ma, J. Experimental and theoretical study on the adsorption mechanism of Amino trimethylphosphate (ATMP) functionalized hydroxyapatite on Pb (II) and Cd (II). Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127029. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, D.; Wang, A. Chitosan-g-poly(acrylic acid) hydrogel with crosslinked polymeric networks for Ni2+ recovery. Anal. Chim. Acta 2011, 687, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.; Artifon, S.E.S.; Cesco, C.T.; Vilela, P.B.; Becegato, V.A.; Paulino, A.T. Chitosan-based hydrogels for the sorption of metals and dyes in water: Isothermal, kinetic, and thermodynamic evaluations. Colloid Polym. Sci. 2021, 299, 649–662. [Google Scholar] [CrossRef]
- Tang, J.; Huang, J.; Zhou, G.; Liu, S. Versatile fabrication of ordered cellular structures double network composite hydrogel and application for cadmium removal. J. Chem. Thermodyn. 2020, 141, 105918. [Google Scholar] [CrossRef]
- Gerhardt, R.; Farias, B.S.; Moura, J.M.; De Almeida, L.S.; Adriano, R.; Dias, D.; Cadaval, T.R.S.; Pinto, L.A.A. Development of chitosan/Spirulina sp. blend films as biosorbents for Cr6+ and Pb2+ removal. Int. J. Biol. Macromol. 2020, 155, 142–152. [Google Scholar] [CrossRef]
- Yang, Z.; Chai, Y.; Zeng, L.; Gao, Z.; Zhang, J.; Ji, H. Efficient removal of copper ion from wastewater using a stable chitosan gel material. Molecules 2019, 24, 4205. [Google Scholar] [CrossRef]
- Alves, D.C.S.; Healy, B.; Pinto, l.A.A.; Cadaval, T.S.A.J.; Breslin, C.B. Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Dotto, G.L.; Vieira, M.L.G.; Pinto, L.A.A. Kinetics and mechanism of tartrazine adsorption onto chitin and chitosan. Ind. Eng. Chem. Res. 2012, 51, 6862–6868. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interf. Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef]
- Bao, S.; Wu, D.; Wang, Q.; Su, T. Functional elastic hydrogel as recyclable membrane for the adsorption and degradation of methylene blue. PLoS ONE 2014, 9, e88802. [Google Scholar] [CrossRef][Green Version]
- Marcano, C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Phuong, P.T.D.; Trung, T.S.; Minh, N.C.; Bao, H.N.D.; Stevens, W.F.; Hoa, N.V. Valorization of heavy waste of modern intensive shrimp farming as a potential source for chitin and chitosan production. Waste Biomass Valor. 2021, in press. [Google Scholar] [CrossRef]
- Nam, P.V.; Hoa, N.V.; Trung, T.S. Properties of hydroxyapatites prepared from different fish bones: A comparative study. Ceram. Inter. 2019, 45, 20141–20147. [Google Scholar] [CrossRef]
- Qiao, L.; Zhao, L.; Liang, C.; Du, K. The construction of porous chitosan microspheres with high specific surface area by using agarose as the pore-forming agent and further functionalized application in bioseparation. J. Mater. Chem. B 2019, 7, 5510–5519. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Wu, S.J.; Lin, F.M. Adsorption of copper(II) ions by a chitosan–oxalatecomplex biosorbent. Inter. J. Biol. Macromol. 2015, 72, 136–144. [Google Scholar] [CrossRef] [PubMed]


| Sample | BET Specific Surface Area (m2 g−1) | BJH Specific Pore Volume (cm3 g−1) | BJH Pore Size (nm) |
|---|---|---|---|
| HGC-0 | 6.86 | 0.078 | 10.11 |
| HGC-1 | 13.62 | 0.042 | 11.91 |
| HGC-2 | 16.87 | 0.041 | 12.20 |
| HGC-3 | 29.74 | 0.046 | 16.17 |
| Isotherm Models | Isotherm Parameters | Adsorbate | |
|---|---|---|---|
| MB | Cu(II) Ions | ||
| Langmuir | R2 | 0.9727 | 0.9368 |
| KL (L mg−1) | 0.0333 | 0.0058 | |
| qmax (mg g−1) | 99.00 | 256.41 | |
| χ2 | 0.0014 | 0.0721 | |
| SD (%) | 3.719 | 13.770 | |
| Freundlich | R2 | 0.9607 | 0.9029 |
| KF (L mg−1) | 5.1364 | 1.9921 | |
| n | 1.5494 | 4.1841 | |
| χ2 | 0.0190 | 0.0012 | |
| SD (%) | 3.519 | 26.845 | |
| Adsorbate | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg g−1) | SD (%) | k2 (g mg−1 min−1) | qe (mg g−1) | SD (%) | |
| MB | 0.0149 | 54.93 | 6.9 | 9.2 × 10−3 | 94.46 | 1.26 |
| Cu(II) Ions | 0.0200 | 5.76 | 7.3 | 0.5 × 10−3 | 256.40 | 1.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoa, N.V.; Minh, N.C.; Cuong, H.N.; Dat, P.A.; Nam, P.V.; Viet, P.H.T.; Phuong, P.T.D.; Trung, T.S. Highly Porous Hydroxyapatite/Graphene Oxide/Chitosan Beads as an Efficient Adsorbent for Dyes and Heavy Metal Ions Removal. Molecules 2021, 26, 6127. https://doi.org/10.3390/molecules26206127
Hoa NV, Minh NC, Cuong HN, Dat PA, Nam PV, Viet PHT, Phuong PTD, Trung TS. Highly Porous Hydroxyapatite/Graphene Oxide/Chitosan Beads as an Efficient Adsorbent for Dyes and Heavy Metal Ions Removal. Molecules. 2021; 26(20):6127. https://doi.org/10.3390/molecules26206127
Chicago/Turabian StyleHoa, Nguyen Van, Nguyen Cong Minh, Hoang Ngoc Cuong, Pham Anh Dat, Pham Viet Nam, Pham Hau Thanh Viet, Pham Thi Dan Phuong, and Trang Si Trung. 2021. "Highly Porous Hydroxyapatite/Graphene Oxide/Chitosan Beads as an Efficient Adsorbent for Dyes and Heavy Metal Ions Removal" Molecules 26, no. 20: 6127. https://doi.org/10.3390/molecules26206127
APA StyleHoa, N. V., Minh, N. C., Cuong, H. N., Dat, P. A., Nam, P. V., Viet, P. H. T., Phuong, P. T. D., & Trung, T. S. (2021). Highly Porous Hydroxyapatite/Graphene Oxide/Chitosan Beads as an Efficient Adsorbent for Dyes and Heavy Metal Ions Removal. Molecules, 26(20), 6127. https://doi.org/10.3390/molecules26206127







