Design, Synthesis, Molecular Docking, and Tumor Resistance Reversal Activity Evaluation of Matrine Derivative with Thiophene Structure
Abstract
1. Introduction
2. Results
2.1. Chemistry
2.2. In Vitro Cytotoxicity
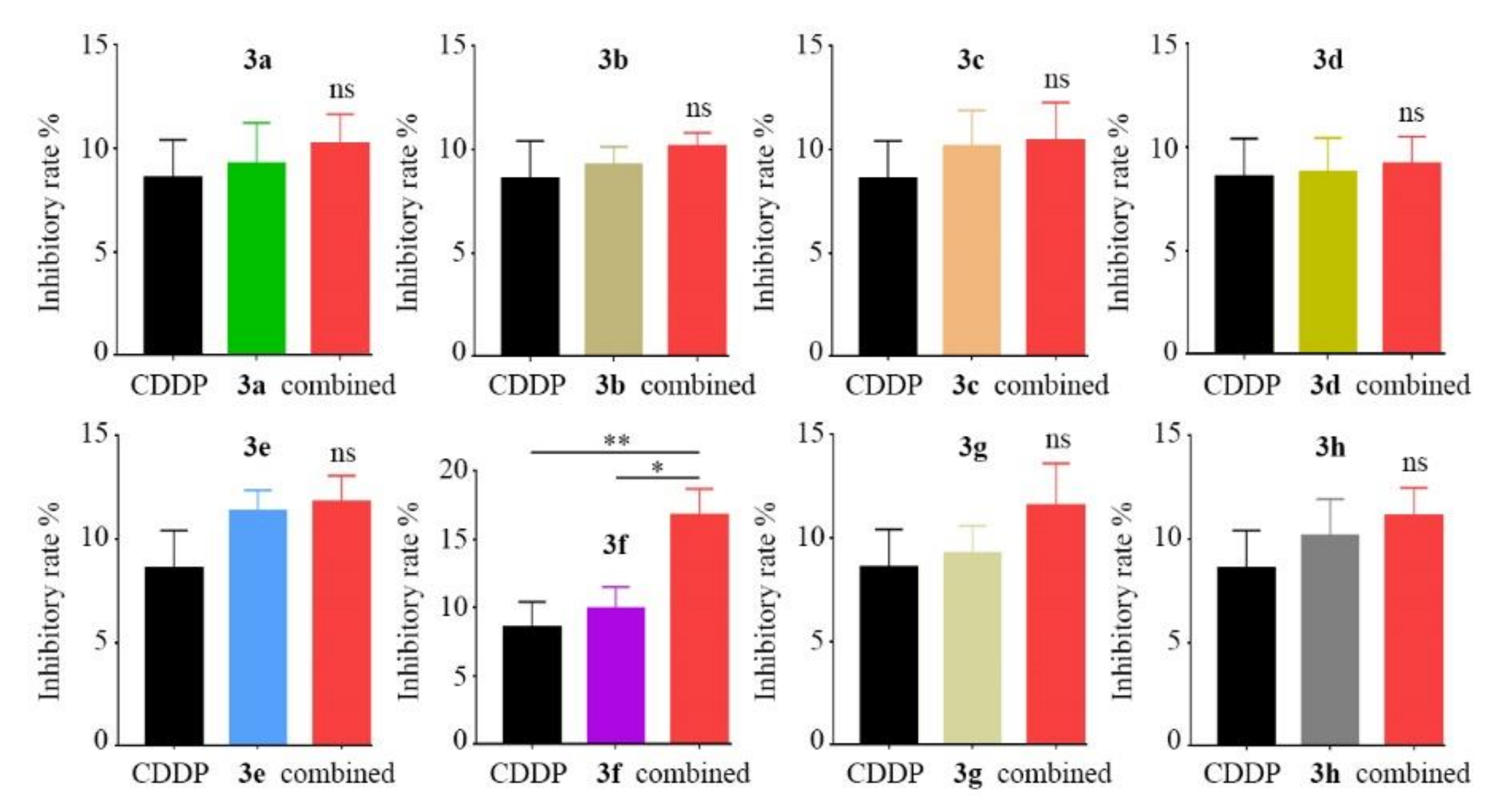
2.3. Combined Inhibitory Effects Evaluation of 8 Derivatives with CDDP
2.4. Synergistic Inhibitory Effect Evaluation of Compound 3f with CDDP
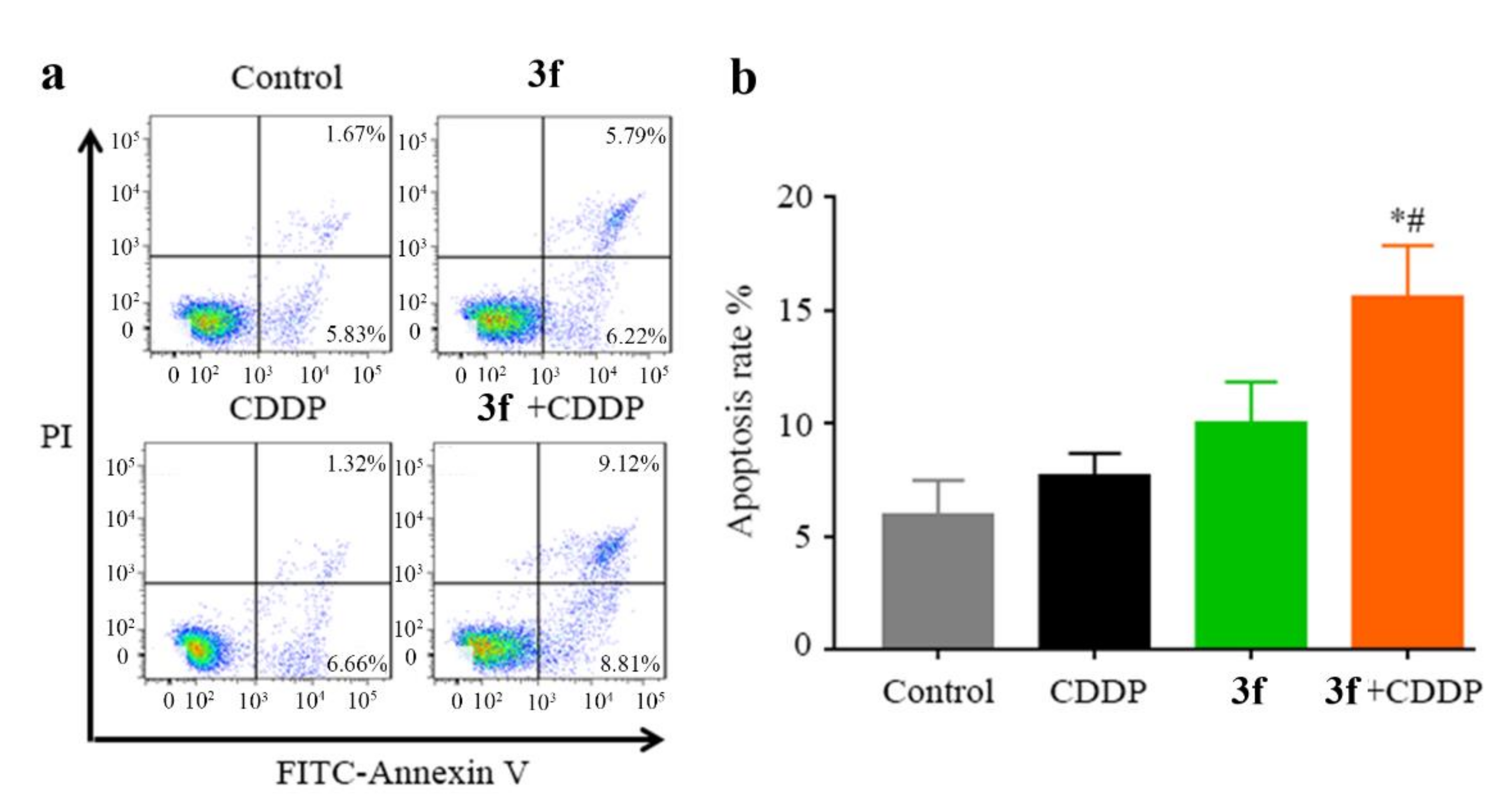
2.5. Combination Treatment of Compound 3f with CDDP Potentiated Apoptosis in CNE2/CDDP Cells
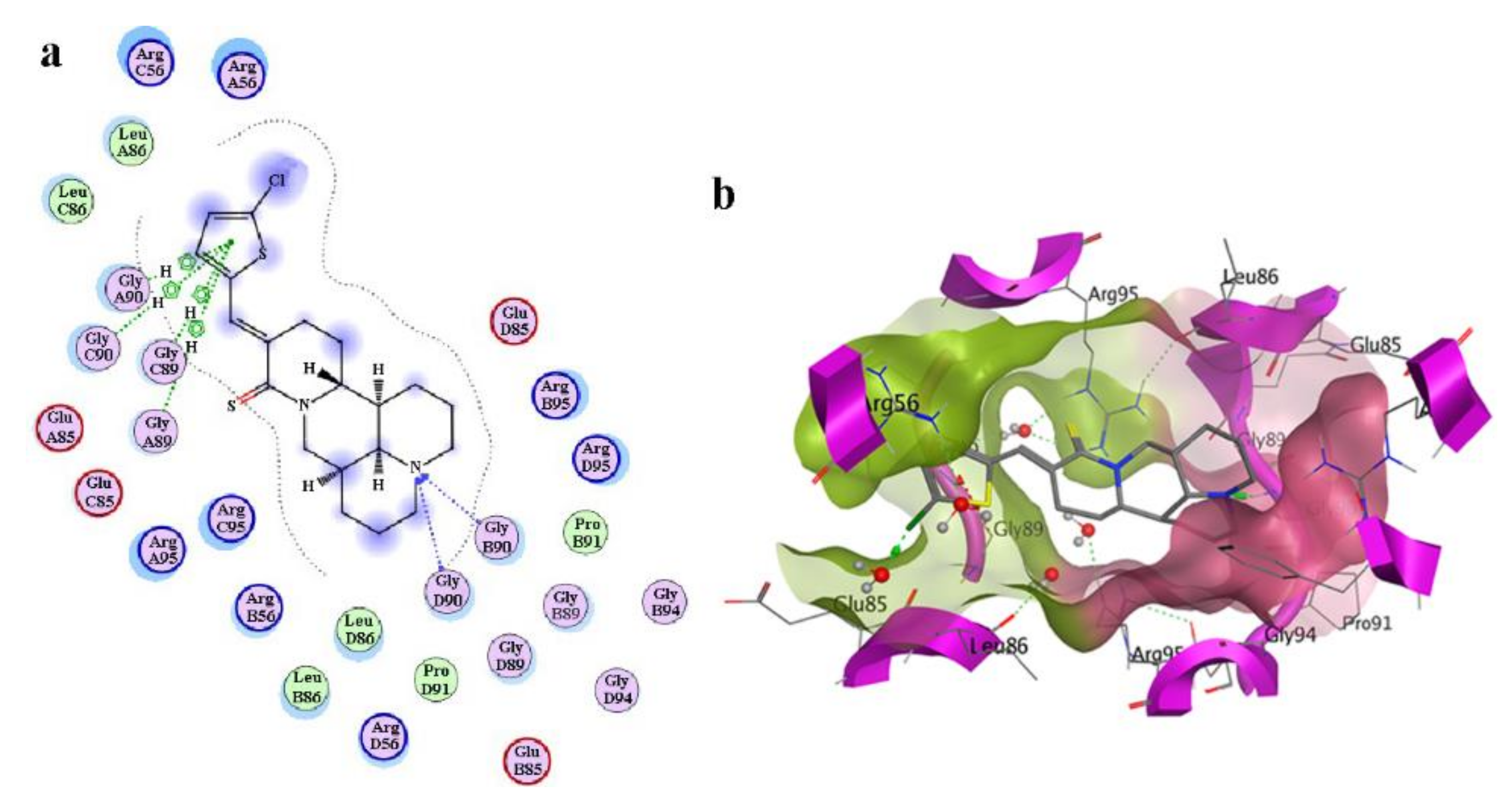
2.6. Molecular Docking of Compound 3f with Anti-Apoptosis Protein Bcl-w
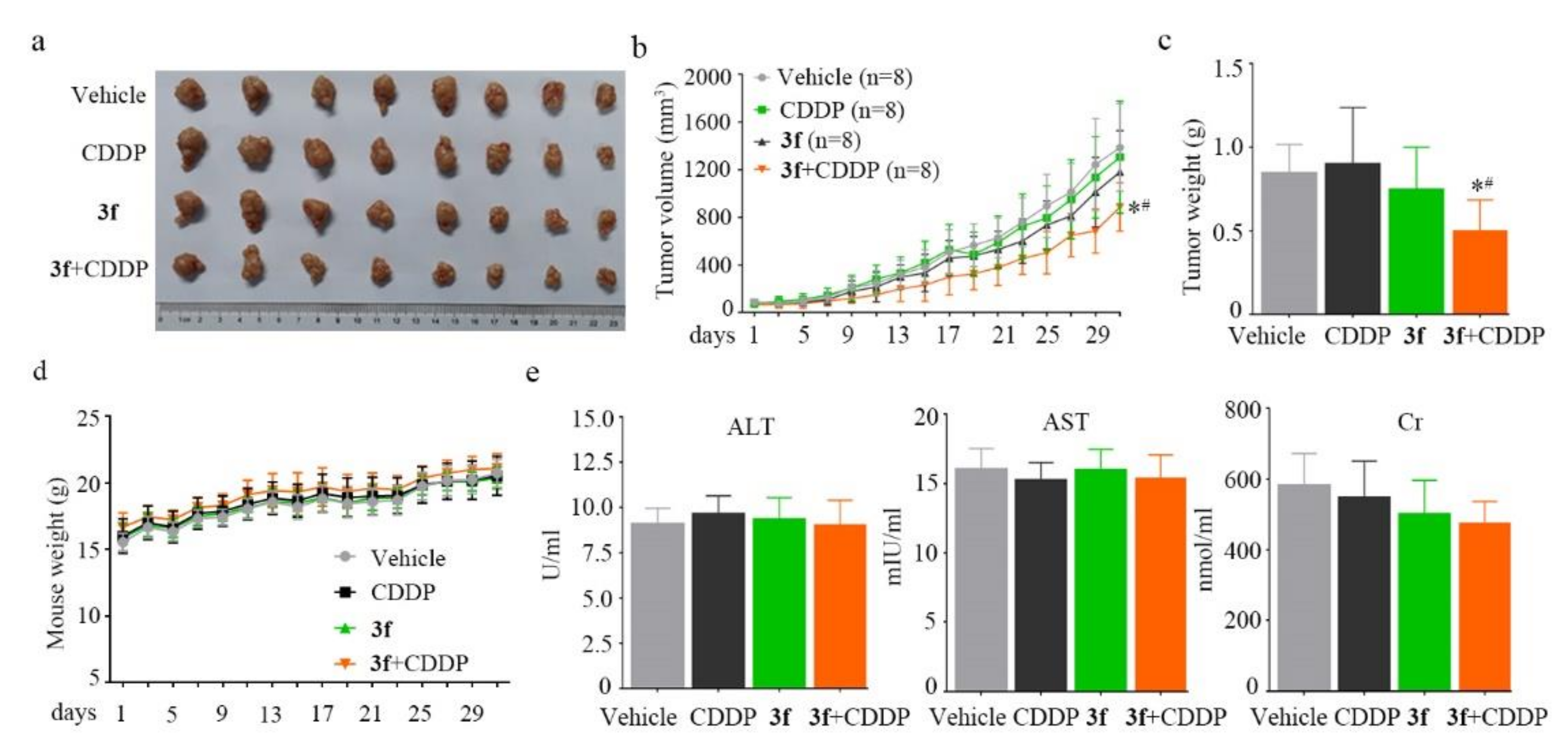
2.7. In Vivo Anticancer Evaluation of Compound 3f
3. Discussion
4. Experimental
4.1. Chemistry Synthesis
4.2. Characterization of 3c~3h
4.3. Cell Viability Assay
4.4. Synergistic Effects Calculation
4.5. Apoptosis Assay
4.6. Molecular Docking
4.7. In Vivo Mouse Model
4.8. ELISA Assays
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| NPC | Nasopharyngeal Carcinoma |
| CDDP | Cisplatin |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| Cr | Creatinine |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide |
| HRMS | High Resolution Mass Spectrometer |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012: Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Scarpati, G.D.V.; Caponigro, F.; Ionna, F.; Longo, F.; Buonopane, S.; Muto, P.; Di Marzo, M.; Pisconti, S.; Solla, R. Management of recurrent nasopharyngeal carcinoma: Current perspectives. OncoTargets Ther. 2019, 12, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Wei, J.; Huang, L.; Wu, L. Chemotherapy and chemo-resistance in nasopharyngeal carcinoma. Eur. J. Med. Chem. 2020, 207, 112758. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Lee, A.; Marguet, S.; Leclercq, J.; Ng, W.T.; Ma, J.; Chan, A.T.; Huang, P.Y.; Benhamou, E.; Zhu, G.; et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015, 16, 645–655. [Google Scholar] [CrossRef]
- Perri, F.; Scarpati, G.D.V.; Buonerba, C.; Di Lorenzo, G.; Longo, F.; Muto, P.; Schiavone, C.; Sandomenico, F.; Caponigro, F. Combined chemo-radiotherapy in locally advanced nasopharyngeal carcinomas. World J. Clin. Oncol. 2013, 4, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Geara, F.B.; Glisson, B.S.; Sanguineti, G.; Tucker, S.L.; Garden, A.S.; Ang, K.K.; Lippman, S.M.; Clayman, G.L.; Goepfert, H.; Peters, L.J.; et al. Induction chemotherapy followed by radiotherapy versus radio-therapy alone in patients with advanced nasopharyngeal carcinoma: Results of a matched cohort study. Cancer 1997, 79, 1279–1286. [Google Scholar] [CrossRef]
- Perri, F.; Bosso, D.; Buonerba, C.; Lorenzo, G.D.; Scarpati, G.D. Locally advanced nasopharyngeal carcinoma: Current and emerging treatment strategies. World J. Clin. Oncol. 2011, 2, 377–383. [Google Scholar] [CrossRef]
- National-Comprehensive-Cancer-Network NCCN Guidelines: Head and Neck Cancer Version 2. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 9 June 2020).
- Zhang, H.; Chen, L.; Sun, X.; Yang, Q.; Wan, L.; Guo, C. Matrine: A Promising Natural Product with Various Pharmacological Activities. Front. Pharmacol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, Y.; Wu, B.; Zhang, Y.; Jiang, H.; He, P. Matrine induces the apoptosis of lung cancer cells through downregulation of inhibitor of apoptosis proteins and the Akt signaling pathway. Oncol. Rep. 2014, 32, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Bai, M.; Suo, Y.; Zhang, G.; Cao, X. Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int. J. Clin. Exp. Pathol. 2015, 8, 14793–14799. [Google Scholar]
- Qin, X.-G.; Hua, Z.; Shuang, W.; Wang, Y.-H.; Cui, Y. Effects of matrine on HepG2 cell proliferation and expression of tumor relevant proteinsin vitro. Pharm. Biol. 2010, 48, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; He, G.; Wang, R.; Shi, S.; Chen, J.; Ye, Y.; Xie, L.; Yi, X.; Tang, A. Matrine-Induced Apoptosis of Human Nasopharyngeal Carcinoma Cells via In Vitro Vascular Endothelial Growth Factor-A/Extracellular Signal-Regulated Kinase1/2 Pathway Inactivation. Horm. Metab. Res. 2014, 46, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, S.; Wei, J.; Li, D.; Liu, X.; Wang, J.; Wang, L. Synthesis and biological evaluation of matrine derivatives as anti-hepatocellular cancer agents. Bioorganic Med. Chem. Lett. 2016, 26, 4267–4271. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Cai, B.; Luo, M.; Huang, M.; Rashid, H.U.; Yang, Y.; Jiang, J.; Wang, L. Design, synthesis, and biological evaluation of thiomatrine derivatives as potential anticancer agents. Med. Chem. Res. 2018, 27, 1941–1955. [Google Scholar] [CrossRef]
- Wu, L.; Wang, G.; Wei, J.; Huang, N.; Zhang, S.; Yang, F.; Li, M.; Zhou, G.; Wang, L. Matrine derivative YF-18 inhibits lung cancer cell proliferation and migration through down-regulating Skp2. Oncotarget 2017, 8, 11729–11738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, B.-G.; Wei, C.-S.; Zhang, S.; Zhang, Z.; Gao, H.-M. Matrine reversed multidrug resistance of breast cancer MCF-7/ADR cells through PI3K/AKT signaling pathway. J. Cell. Biochem. 2018, 119, 3885–3891. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Deng, W.-Y.; Wang, X.; Lü, H.-F.; Han, L.-L.; Chen, B.; Chen, X.-B.; Li, N. Molecular mechanism of indirubin-3′-monoxime and Matrine in the reversal of paclitaxel resistance in NCI-H520/TAX25 cell line. Chin. Med. J. 2013, 126, 925–929. [Google Scholar] [PubMed]
- Liao, X.-Z.; Tao, L.-T.; Liu, J.-H.; Gu, Y.-Y.; Xie, J.; Chen, Y.; Lin, M.-G.; Liu, T.-L.; Wang, D.; Guo, H.-Y.; et al. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 2017, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, A.-Z. [Reversal effect of matrine on drug resistance of human nasopharyngeal carcinoma cell line]. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2012, 35, 1989–1994. [Google Scholar]
- Flemer, S., Jr. Selenol Protecting Groups in Organic Chemistry: Special Emphasis on Selenocysteine Se-Protection in Solid-Phase Peptide Synthesis. Molecules 2011, 16, 3232–3251. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Pan, X.-F.; Wu, F.-Q.; Ma, L.-Y.; Liu, D.-P.; Liu, Y.; Feng, T.-T.; Meng, F.-Y.; Liu, X.-L.; Jiang, Q.-L.; et al. Synergy between Proteasome Inhibitors and Imatinib Mesylate in Chronic Myeloid Leukemia. PLoS ONE 2009, 4, e6257. [Google Scholar] [CrossRef] [PubMed]
- Lomonosova, E.; Chinnadurai, G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene 2008, 27, S2–S19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Nishimura, N.; Okamoto, T.; Wada, K.; Naora, K. Molecular Mechanism of Matrine from Sophora alopecuroides in the Reversing Effect of Multi-Anticancer Drug Resistance in K562/ADR Cells. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gramec, D.; Mašič, L.P.; Dolenc, M.S. Bioactivation Potential of Thiophene-Containing Drugs. Chem. Res. Toxicol. 2014, 27, 1344–1358. [Google Scholar] [CrossRef]
- Joshi, E.M.; Heasley, B.H.; Chordia, M.D.; Macdonald, T.L. In Vitro Metabolism of 2-Acetylbenzothiophene: Relevance to Zileuton Hepatotoxicity†. Chem. Res. Toxicol. 2004, 17, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Lopez-Cara, L.C.; Salvador, M.K.; Preti, D.; Tabrizi, M.A.; Balzarini, J.; Nussbaumer, P.; Bassetto, M.; Brancale, A.; et al. Design, synthesis and biological evaluation of 3,5-disubstituted 2-amino thiophene derivatives as a novel class of antitumor agents. Bioorganic Med. Chem. 2013, 22, 5097–5109. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Bashandy, M.S.; Al-Said, M.S. Novel thiophene derivatives with sulfonamide, isoxazole, benzothiazole, quinoline and anthracene moieties as potential anticancer agents. Acta Pharm. 2014, 64, 419–431. [Google Scholar] [CrossRef]
- Lisboa, T.M.H.; Silva, D.K.F.; Duarte, S.S.; Ferreira, R.; Andrade, C.; Lopes, A.L.D.O.; Ribeiro, J.; Farias, D.F.; Moura, R.O.; Reis, M.; et al. Toxicity and Antitumor Activity of a Thiophene–Acridine Hybrid. Molecules 2019, 25, 64. [Google Scholar] [CrossRef]
- Li, Z.; Luo, M.; Cai, B.; Rashid, H.U.; Huang, M.; Jiang, J.; Wang, L.; Wu, L. Design, synthesis, biological evaluation and structure-activity relationship of sophoridine derivatives bearing pyrrole or indole scaffold as potential antitumor agents. Eur. J. Med. Chem. 2018, 157, 665–682. [Google Scholar] [CrossRef] [PubMed]


| Compound | IC50 (µM) | ||||
|---|---|---|---|---|---|
| CNE2 | HONE1 | HK-1 | CNE2/CDDP | LO2 | |
| 3a | 113.1 ± 7.2 | 152.4 ± 8.4 | 178.8 ± 12.1 | 375.3 ± 18.1 | 212 ± 15.3 |
| 3b | 158.3 ± 11.3 | 125.3 ± 6.3 | 208.2 ± 9.4 | 582.6 ± 15.3 | 156 ± 11.7 |
| 3c | 143.5 ± 10.3 | 182.6 ± 9. 8 | 191.4 ± 11.3 | 675.6 ± 23.8 | 285 ± 20.7 |
| 3d | 92.4 ± 6.5 | 121.3 ± 7.7 | 138.5 ± 9.4 | 293.7 ± 20.1 | 138 ± 9.6 |
| 3e | 147.6 ± 5.6 | 184.6 ± 12.4 | 179.5 ± 7.3 | 451.5 ± 26.8 | 243 ± 12.7 |
| 3f | 35.7 ± 3.4 | 48.9 ± 5. 6 | 51.3 ± 3.5 | 91.4 ± 8.8 | 72 ± 5.8 |
| 3g | 58.3 ± 2.9 | 71.5 ± 4.2 | 90.7 ± 6.6 | 205.7 ± 11.7 | 68 ± 5.1 |
| 3h | 76.2 ± 4.5 | 89.7 ± 6.2 | 103.3 ± 7.8 | 177.7 ± 15.8 | 92 ± 8.6 |
| Matrine | >1000 | >1000 | >1000 | >1000 | >1000 |
| CDDP | 3.77 ± 0.26 | 5.23 ± 0.37 | 6.73 ± 0.42 | 29.53 ± 3.12 | 6.33 ± 0.56 |
| Group | 3f | CDDP | Combination |
|---|---|---|---|
| 1 | 20 µM | 5 µM | 3f (20 µM) + CDDP (5 µM) |
| 2 | 20 µM | 10 µM | 3f (20 µM) + CDDP (10 µM) |
| 3 | 20 µM | 15 µM | 3f (20 µM) + CDDP (15 µM) |
| 4 | 40 µM | 5 µM | 3f (40 µM) + CDDP (5 µM) |
| 5 | 40 µM | 10 µM | 3f (40 µM) + CDDP (10 µM) |
| 6 | 40 µM | 15 µM | 3f (40 µM) + CDDP (15 µM) |
| 7 | 60 µM | 5 µM | 3f (60 µM) + CDDP (5 µM) |
| 8 | 60 µM | 10 µM | 3f (60 µM) + CDDP (10 µM) |
| 9 | 60 µM | 15 µM | 3f (60 µM) + CDDP (15 µM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Liang, Y.; Wu, L. Design, Synthesis, Molecular Docking, and Tumor Resistance Reversal Activity Evaluation of Matrine Derivative with Thiophene Structure. Molecules 2021, 26, 417. https://doi.org/10.3390/molecules26020417
Wei J, Liang Y, Wu L. Design, Synthesis, Molecular Docking, and Tumor Resistance Reversal Activity Evaluation of Matrine Derivative with Thiophene Structure. Molecules. 2021; 26(2):417. https://doi.org/10.3390/molecules26020417
Chicago/Turabian StyleWei, Jinrui, Yuehui Liang, and Lichuan Wu. 2021. "Design, Synthesis, Molecular Docking, and Tumor Resistance Reversal Activity Evaluation of Matrine Derivative with Thiophene Structure" Molecules 26, no. 2: 417. https://doi.org/10.3390/molecules26020417
APA StyleWei, J., Liang, Y., & Wu, L. (2021). Design, Synthesis, Molecular Docking, and Tumor Resistance Reversal Activity Evaluation of Matrine Derivative with Thiophene Structure. Molecules, 26(2), 417. https://doi.org/10.3390/molecules26020417





