Muscari comosum L. Bulb Extracts Modulate Oxidative Stress and Redox Signaling in HepG2 Cells
Abstract
1. Introduction
2. Results
2.1. Total Polyphenol and Flavonoid Content of M. Comosum Extracts (MCE)
2.2. Antioxidant Activity In Vitro
2.3. Effect of M. Comosum Extracts on Cell Viability
2.4. Effect of M. comosum Extracts on Intracellular Reactive Oxygen Species
2.5. Effect of M.Comosum Bulb Extracts on Several Markers Involved in Oxidative Stress
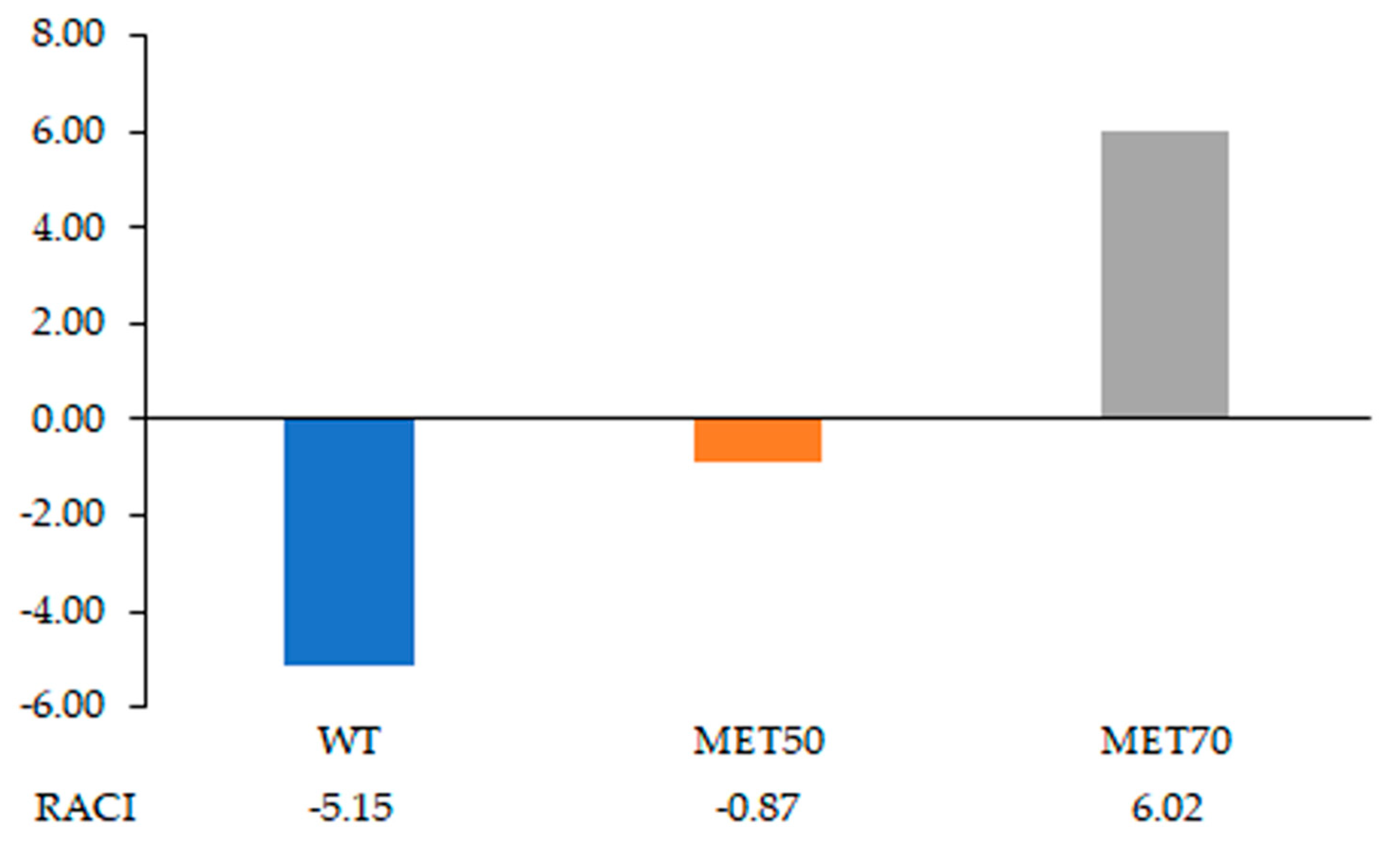
2.6. Effect of M. Comosum Extracts on Metabolite Composition of Cells and Corresponding Culture Media
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bulb Extracts
4.3. Determination of Total Phenol and Flavonoid Contents
4.4. Free Radical Scavenging Activity
4.5. Total Reducing Power
4.6. Cell Culture and Treatments
4.7. Cell Viability Assay
4.8. Western Blot Analysis
4.9. Measurement of the Intracellular Reactive Oxygen Species
4.10. Real-Time Reverse Transcription PCR (qRT-PCR)
4.11. Sample Preparation for NMR Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wildman, R.E.C. Handbook of Nutraceutical and Funcional Foods; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849364099. [Google Scholar]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Waltenberger, B.; Halabalaki, M.; Schwaiger, S.; Adamopoulos, N.; Allouche, N.; Fiebich, B.; Hermans, N.; Jansen-Dürr, P.; Kesternich, V.; Pieters, L.; et al. Novel Natural Products for Healthy Ageing from the Mediterranean Diet and Food Plants of Other Global Sources—The MediHealth Project. Molecules 2018, 23, 1097. [Google Scholar] [CrossRef] [PubMed]
- Hadjichambis, A.C.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; de Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Lietava, J. Medicinal plants in a Middle Paleolithic grave Shanidar IV? J. Ethnopharmacol. 1992, 35, 263–266. [Google Scholar] [CrossRef]
- Forbes, M.C. The Pursuit of Wild Edibles, Present and Past. Exped. Mag. 1976, 19, 12–18. [Google Scholar]
- Casoria, P.; Menale, B.; Muoio, R. Muscari comosum, Liliaceae, in the Food Habits of South Italy. Econ. Bot. 1999, 53, 113–115. [Google Scholar]
- Motti, R.; Antignani, V.; Idolo, M. Traditional plant use in the Phlegraean Fields Regional Park (Campania, Southern Italy). Hum. Ecol. 2009, 37, 775–782. [Google Scholar] [CrossRef]
- Carteni, A.; Maranelli, E. Chemical composition of the bulbs of Muscari comosum, Mill, and changes following cooking. Quad. Nutr. 1943, 9, 181–184. [Google Scholar]
- Adinolfi, M.; Barone, G.; Belardini, M.; Lanzetta, R.; Laonigro, G.; Parrilli, M. Homoisoflavanones from Muscari comosum bulbs. Phytochemistry 1985, 24, 2423–2426. [Google Scholar] [CrossRef]
- Adinolfi, M.; Barone, G.; Corsaro, M.M.; Lanzetta, R.; Mangoni, L.; Parrilli, M. Glycosides from Muscari comosum. 7. Structure of three novel muscarosides. Can. J. Chem. 1987, 65, 2317–2326. [Google Scholar] [CrossRef]
- Pieroni, A.; Quave, C.; Nebel, S.; Heinrich, M. Ethnopharmacy of the ethnic Albanians (Arbëreshë) of northern Basilicata, Italy. Fitoterapia 2002, 73, 217–241. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Pugliese, A.; Bonesi, M.; Solimene, U.; Menichini, F. Chelating, antioxidant and hypoglycaemic potential of Muscari comosum (L.) Mill. bulb extracts. Int. J. Food Sci. Nutr. 2010, 61, 780–791. [Google Scholar] [CrossRef]
- Rao, V.M.; Damu, G.L.V.; Sudhakar, D.; Siddaiah, V.; Rao, C.V. New efficient synthesis and bioactivity of homoisoflavonoids. Arkivoc 2008, 2008, 285–294. [Google Scholar] [CrossRef]
- Grande, F.; Rizzuti, B.; Occhiuzzi, M.A.; Ioele, G.; Casacchia, T.; Gelmini, F.; Guzzi, R.; Garofalo, A. Identification by Molecular Docking of Homoisoflavones from Leopoldia comosa as Ligands of Estrogen Receptors. Molecules 2018, 23, 894. [Google Scholar] [CrossRef] [PubMed]
- Larocca, M.; di Marsico, M.; Riccio, P.; Rossano, R. The in vitro antioxidant properties of Muscari comosum bulbs and their inhibitory activity on enzymes involved in inflammation, post-prandial hyperglycemia, and cognitive/neuromuscular functions. J. Food Biochem. 2018, 42, 1–8. [Google Scholar]
- Casacchia, T.; Sofo, A.; Casaburi, I.; Marrelli, M.; Conforti, F.; Statti, G.A. Antioxidant, enzyme-inhibitory and antitumor activity of the wild dietary plant Muscari comosum (L.) Mill. Int. J. Plant Biol. 2017, 8, 31–35. [Google Scholar] [CrossRef]
- Sun, T.; Tanumihardjo, S.A. An Integrated Approach to Evaluate Food Antioxidant Capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Calabrone, L.; Larocca, M.; Marzocco, S.; Martelli, G.; Rossano, R. Total Phenols and Flavonoids Content, Antioxidant Capacity and Lipase Inhibition of Root and Leaf Horseradish (Armoracia rusticana) Extracts. Food Nutr. Sci. 2015, 6, 64–74. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.W.; Kim, Y.K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Intracellular redox status and oxidative stress: Implications for cell proliferation, apoptosis, and carcinogenesis. Arch. Toxicol. 2008, 82, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef]
- Tang, W. Role of Nrf2 in chronic liver disease. World J. Gastroenterol. 2014, 20, 13079. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Suzuki, H. Inhibitors of human ABCG2: From technical background to recent updates with clinical implications. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Ostuni, A.; Carmosino, M.; Miglionico, R.; Abruzzese, V.; Martinelli, F.; Russo, D.; Laurenzana, I.; Petillo, A.; Bisaccia, F. Inhibition of ABCC6 Transporter Modifies Cytoskeleton and Reduces Motility of HepG2 Cells via Purinergic Pathway. Cells 2020, 9, 1410. [Google Scholar] [CrossRef]
- Martinelli, F.; Cuviello, F.; Pace, M.C.; Armentano, M.F.; Miglionico, R.; Ostuni, A.; Bisaccia, F. Extracellular ATP regulates CD73 and ABCC6 expression in HepG2 cells. Front. Mol. Biosci. 2018, 5. [Google Scholar] [CrossRef]
- Salvia, A.M.; Cuviello, F.; Coluzzi, S.; Nuccorini, R.; Attolico, I.; Pascale, S.P.; Bisaccia, F.; Pizzuti, M.; Ostuni, A. Expression of Some ATP-Binding Cassette Transporters in Acute Myeloid Leukemia. Hematol. Rep. 2017, 9, 137–141. [Google Scholar] [CrossRef]
- Miglionico, R.; Ostuni, A.; Armentano, M.F.; Milella, L.; Crescenzi, E.; Carmosino, M.; Bisaccia, F. ABCC6 knockdown in HepG2 cells induces a senescent-like cell phenotype. Cell. Mol. Biol. Lett. 2017, 22, 7. [Google Scholar] [PubMed]
- Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Chen, Z.S. Multidrug Resistance Proteins (MRPs) and Cancer Therapy. AAPS J. 2015, 17, 802–812. [Google Scholar] [PubMed]
- Higashikuni, Y.; Sainz, J.; Nakamura, K.; Takaoka, M.; Enomoto, S.; Iwata, H.; Tanaka, K.; Sahara, M.; Hirata, Y.; Nagai, R.; et al. The ATP-Binding cassette transporter ABCG2 protects against pressure overload-induced cardiac hypertrophy and heart failure by promoting angiogenesis and antioxidant response. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Aleksunes, L.M.; Klaassen, C.D. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab. Dispos. 2012, 40, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Aftab, O.; Engskog, M.K.R.; Haglöf, J.; Elmsjö, A.; Arvidsson, T.; Pettersson, C.; Hammerling, U.; Gustafsson, M.G. NMR spectroscopy-based metabolic profiling of drug-induced changes in vitro can discriminate between pharmacological classes. J. Chem. Inform. Model. 2014, 54, 3251–3258. [Google Scholar] [CrossRef]






| a Extracts | b Yield (%) | c Phenols (mgGAE/g) | d Flavonoids (mg QE/g) | e DPPH (IC50 µg/mL) | f ˙NO (IC50 µg/mL) | g O2− (IC50 µg/mL) | h Reducing Power (mg GAE/g) |
|---|---|---|---|---|---|---|---|
| WT | a 10.22 ± 0.21 | a 52.66 ± 1.80 | a 14.99 ± 0.70 | a 38.02 ± 1.91 | a 269.21 ± 11.28 | a 51.43 ± 2.97 | a 37.93 ± 1.55 |
| MET50 | b 8.56 ± 0.11 | a 54.01 ± 0.83 | b 19.70 ± 0.96 | b 29.43 ± 2.70 | b 168.52 ± 15.29 | a 50.10 ± 5.29 | b 44.51 ± 2.63 |
| MET70 | c 7.83 ± 0.19 | b 58.72 ± 1.11 | b 20.37 ± 1.24 | c 24.60 ± 2.13 | c 122.94 ± 7.06 | b 36.50 ± 3.84 | b 47.52 ± 2.86 |
| Gallic acid | 5.05 ± 1.85 | ||||||
| Ascorbic acid | 39.66 ± 2.17 | 45.62 ± 3.01 |
| Gene | Accession Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| ABCC6 | NM_001171.5 | 5′-ATCACTGATCCTTCCATCTTG-3′ | 5′-ACCAGCGACACAGAGAAGAGG-3′ |
| ABCG2 | NM_004827.2 | 5′-ATCACTGATCCTTCCATCTTG-3′ | 5′-GCTTAGACATCCTTTTCAGG-3′ |
| β-actin | NM_001101.3 | 5′-CCTGGCACCCAGCACAAT-3′ | 5′-GCCGATCCACACGGAGTACT-3′ |
| Catalase | NM_001752.4 | 5′-ATACCTGTGAACTGTCCCTACCG-3′ | 5′-GTTGAATCTCCGCACTTCTCCAG-3′ |
| GPX1 | NM_000581.4 | 5‘-CAGTCGGTGTATGCCTTCTCG-3′ | 5′-CTCGTTCATCTGGGTGTAGTCC-3′ |
| SOD2 | NM_000636.4 | 5′-CCGACCTGCCCTACGACTAC-3′ | 5′- AACGCCTCCTGGTACTTCTCC-3′ |
| NQO1 | NM_000903 | 5′-GGTGGTGGAGTCGGACCTCTA-3′ | 5′-AGGGTCCTTCAGTTTACCTGTGAT-3′ |
| NRF2 | NM_00114541.3 | 5′-AACTACTCCCAGGTTGCCCA-3′ | 5′-CATTGTCATCTACAAACGGGAA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giglio, F.; Castiglione Morelli, M.A.; Matera, I.; Sinisgalli, C.; Rossano, R.; Ostuni, A. Muscari comosum L. Bulb Extracts Modulate Oxidative Stress and Redox Signaling in HepG2 Cells. Molecules 2021, 26, 416. https://doi.org/10.3390/molecules26020416
Giglio F, Castiglione Morelli MA, Matera I, Sinisgalli C, Rossano R, Ostuni A. Muscari comosum L. Bulb Extracts Modulate Oxidative Stress and Redox Signaling in HepG2 Cells. Molecules. 2021; 26(2):416. https://doi.org/10.3390/molecules26020416
Chicago/Turabian StyleGiglio, Fabiana, Maria Antonietta Castiglione Morelli, Ilenia Matera, Chiara Sinisgalli, Rocco Rossano, and Angela Ostuni. 2021. "Muscari comosum L. Bulb Extracts Modulate Oxidative Stress and Redox Signaling in HepG2 Cells" Molecules 26, no. 2: 416. https://doi.org/10.3390/molecules26020416
APA StyleGiglio, F., Castiglione Morelli, M. A., Matera, I., Sinisgalli, C., Rossano, R., & Ostuni, A. (2021). Muscari comosum L. Bulb Extracts Modulate Oxidative Stress and Redox Signaling in HepG2 Cells. Molecules, 26(2), 416. https://doi.org/10.3390/molecules26020416






