Abstract
Based on the structure of the natural product cysteine, a series of thiazolidine-4-carboxylic acids were designed and synthesized. All target compounds bearing thiazolidine-4-carboxylic acid were characterized by 1H-NMR, 13C-NMR, and HRMS techniques. The antiviral and antifungal activities of cysteine and its derivatives were evaluated in vitro and in vivo. The results of anti-TMV activity revealed that all compounds exhibited moderate to excellent activities against tobacco mosaic virus (TMV) at the concentration of 500 μg/mL. The compounds cysteine (1), 3–4, 7, 10, 13, 20, 23, and 24 displayed higher anti-TMV activities than the commercial plant virucide ribavirin (inhibitory rate: 40, 40, and 38% at 500 μg/mL for inactivation, curative, and protection activity in vivo, respectively), especially compound 3 (inhibitory rate: 51%, 47%, and 49% at 500 μg/mL for inactivation, curative, and protection activity in vivo, respectively) with excellent antiviral activity emerged as a new antiviral candidate. Antiviral mechanism research by TEM exhibited that compound 3 could inhibit virus assembly by aggregated the 20S protein disk. Molecular docking results revealed that compound 3 with higher antiviral activities than that of compound 24 did show stronger interaction with TMV CP. Further fungicidal activity tests against 14 kinds of phytopathogenic fungi revealed that these cysteine derivatives displayed broad-spectrum fungicidal activities. Compound 16 exhibited higher antifungal activities against Cercospora arachidicola Hori and Alternaria solani than commercial fungicides carbendazim and chlorothalonil, which emerged as a new candidate for fungicidal research.
1. Introduction
Fungal and viral pathogens can induce various of plant diseases such as those causing brown spots on peanut leaves, ring rots on stem and fruit of apples, gray mold on cucumbers and grapes, leading to huge losses to agriculture and horticulture production and threatening food security [1]. Tobacco mosaic virus (TMV), known as “plant cancer”, not only can seriously harm tobacco, but also can infect potato, pepper, tomato, eggplant, nightshade, and more than 400 other plant species [2,3]. The long-term, large-scale use of traditional high-toxic pesticides not only forces bacteria and viruses to develop resistance, but also poses a threat to human health. The development of new antifungal and antiviral agents with unique mode of action is becoming more and more urgent for plant protection and agricultural production [4,5,6].
Amino acids are substances that exist widely in Nature. Cysteine is the only sulfur-containing amino acid with the ability to form disulfide bonds. Due to the bigger atomic radius of sulfur and the lower dissociation energy of the S−H bond, the thiol group of cysteine possesses the ability to perform both nucleophilic and redox-active functions which are unfeasible for the other natural amino acids [7,8,9]. Cysteine, with its easily modified molecular structure, has attracted attention from biological and chemical scientists. A series of biological activities of cysteine and its derivatives have been reported, such as cytotoxicity [10], neurotoxicity [11,12,13], oxidant activity [14], accelerated DNA oxidative damage [15], and so on.
Heterocycles are an important framework for the development of new drugs, especially S-containing heterocycles which have been found to have the ability to induce apoptosis of various cells [16]. Thiazolidine drugs containing N and S atoms can exert drug effects through various mechanisms of action, such as inhibiting neuraminidase of influenza A virus [17], inhibiting protein synthesis [18], accelerating cell apoptosis [16,19], enhancing antioxidant capacity [7], immune stimulation [20], etc. Some thiazolidine-4-carboxylic acid derivatives can also oxidatively cleave DNA and interact with metal ions [21]. Some thiazolidine derivatives have been used in a variety of synthetic modifications due to their simple structure, diverse biological characteristics, and excellent environmental compatibility [22,23].
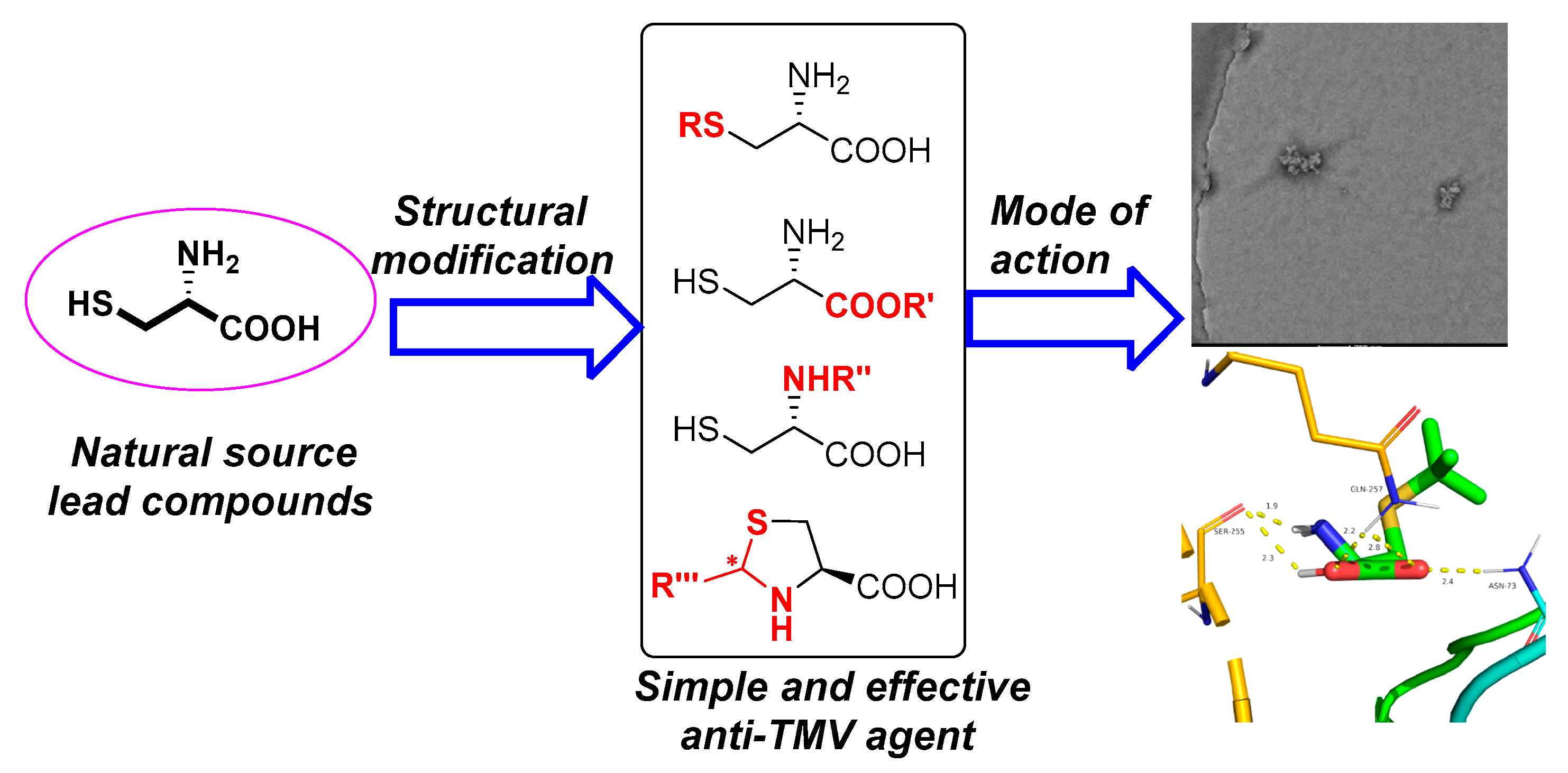
It is an important research direction for us to find effective antiviral candidates based on natural products [24]. In our previous work, a series of amino acid gossypol Schiff bases were designed and synthesized. The structure-activity relationship revealed that both the carboxy group and substituents in amino acids had significant effect on anti-TMV [25]. Combined with our existing work experience and the above findings, we made systematic research on cysteine and its derivatives (Figure 1) in the present work. All synthetic compounds were characterized by 1H-NMR, 13C-NMR, and HRMS. The antiviral and antifungal activities of these compounds and the structure-activity relationship were evaluated systematically.
Figure 1.
Design of cysteine derivatives.
2. Results
2.1. Chemistry
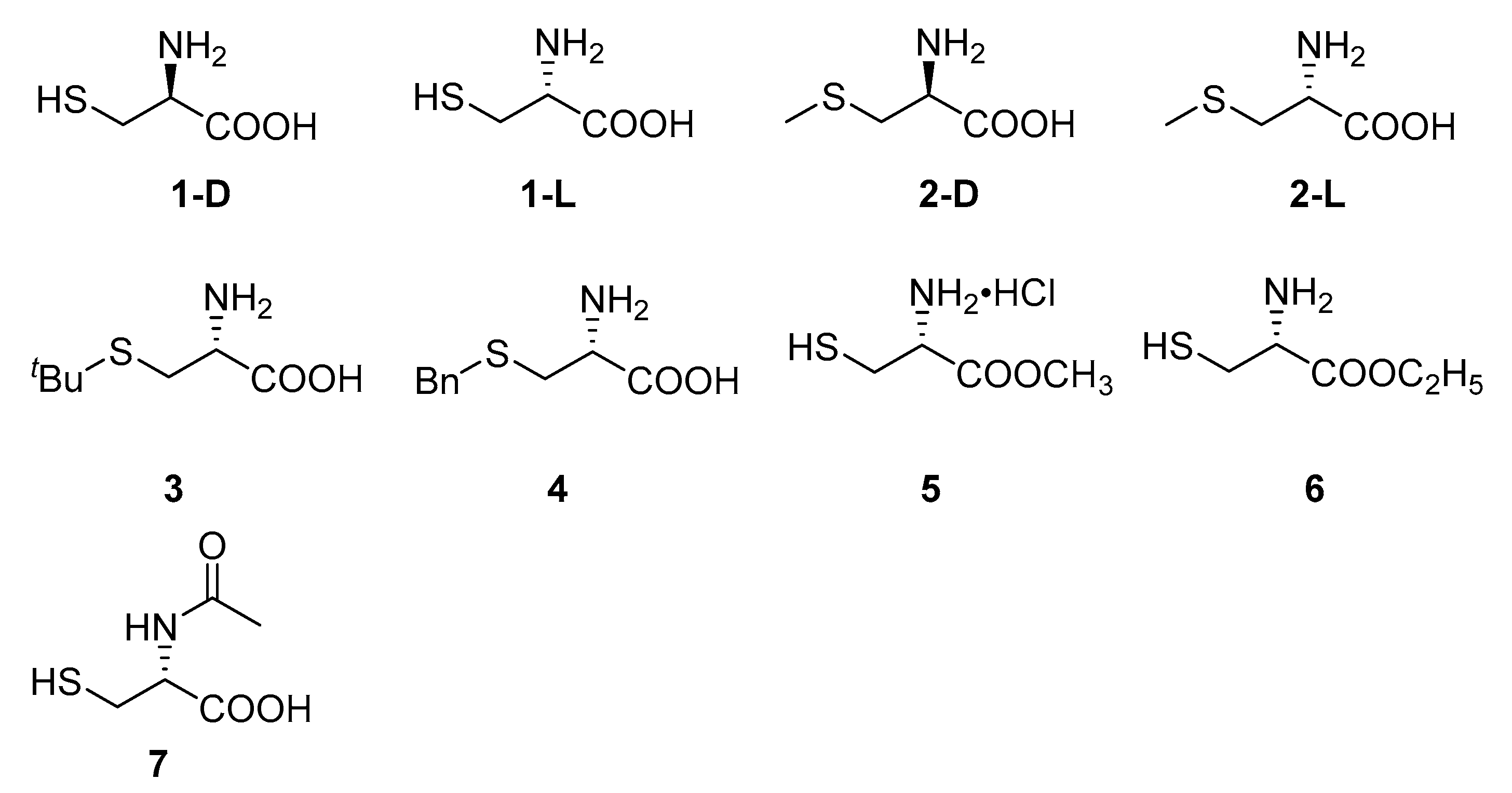
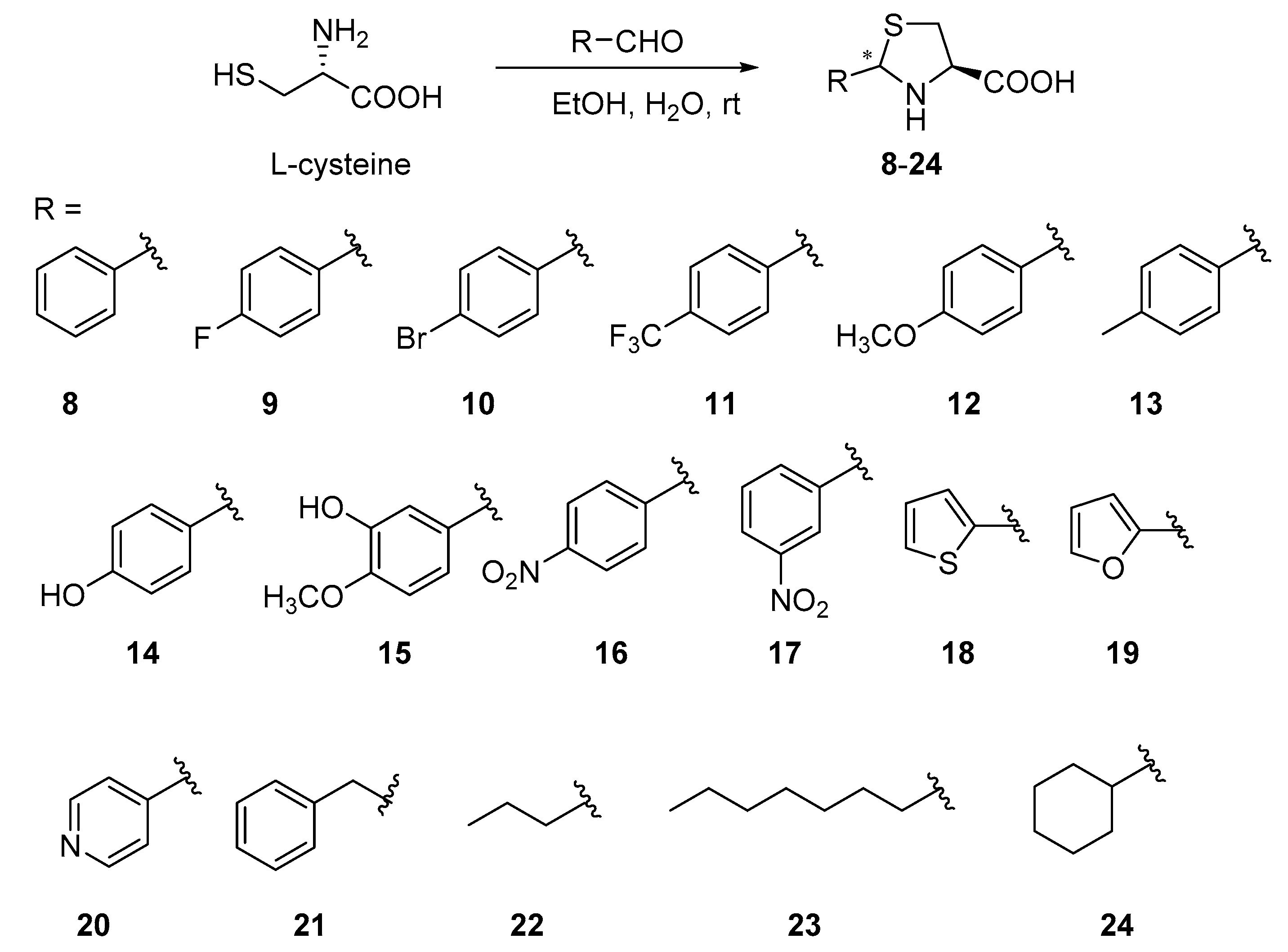
Cysteine and its derivatives 1–7 (Figure 2) were purchased directly. A series of thiazole ring containing L-cysteine derivatives 8–24 were designed and synthesized from L-cysteine and different substituted aldehydes (Scheme 1) by a one-pot method [7,26].
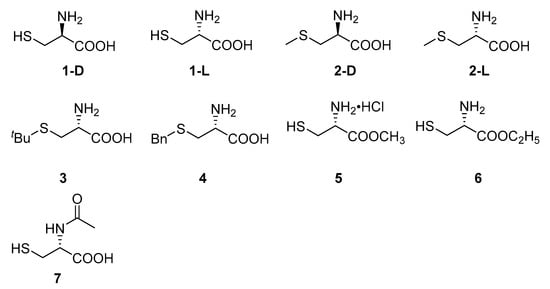
Figure 2.
Structures of compounds 1–7.
Scheme 1.
Synthesis of 8–24.
2.2. Phytotoxic Activity
The phytotoxicity-activity tests revealed that cysteine and its derivatives were safe for testing on plants at 500 μg/mL. The detailed test procedures can be seen in our previous reports [5,27]. The detailed test procedures can also be found in the Supplementary Materials.
2.3. Antiviral Activity
2.3.1. In Vitro Anti-TMV Activity
The anti-TMV activities of cysteine and its derivatives 1–24 are listed in Table 1 with the commercial drugs ribavirin and ningnanmycin as controls. Compounds 1–7 had better anti-TMV activities than ribavirin. Among compounds 1–7, compounds 3 (inhibitory rate: 48% at 500 μg/mL) and 4 (inhibitory rate: 45% at 500 μg/mL) showed better anti-TMV activities than the others. At the concentration of 100 μg/mL, compounds 3 (inhibitory rate: 13%) and 4 (inhibitory rate: 12%) also displayed better anti-TMV activities than the commercial plant virucide ribavirin (inhibitory rate: 7%). Most of the cysteine derivatives 8–24 with thiazolidine structure exhibited better anti-TMV activities than that of the commercial plant virucide ribavirin. Especially, compounds 23 (inhibitory rate: 46% at 500 μg/mL) and 24 (inhibitory rate: 45% at 500 μg/mL) showed 10% higher anti-TMV activity than ribavirin (inhibitory rate: 35% at 500 μg/mL).

Table 1.
In vitro antiviral activity of compounds 1–24 against TMV.
2.3.2. In Vivo Anti-TMV Activity
In vivo anti-TMV activity includes three test modes: inactivation, curative, and protection. As shown in Table 2, most of the compounds also displayed higher in vivo activities than ribavirin. Compound 3 displayed the best anti-TMV activity at 500 μg/mL (inactivation activity, 51%; curative activity, 47%; protection activity, 49%), which is significantly higher than that of ribavirin (inactivation activity, 40%; curative activity, 40%; protection activity, 38%).

Table 2.
In Vivo antiviral activity of compounds 1–24 against TMV.
2.4. Mode of Action Studies
2.4.1. Preliminary Mode of Action
Preliminary mode of action revealed that these compounds can inhibit the assembly of TMV. The detailed method was described in the literature [24] and also can be found in the Supporting Materials.
2.4.2. Docking Studies
To further study the mechanism of the interaction between cysteines and TMV CP, we chose AutoDock Vina 1.1.2 for molecular docking [28]. The docking poses are ranked according to their docking sites and the lowest binding energy of macromolecule-ligand complex is considered being the best. It can be proved H-bond interaction and strong binding affinity between cysteines and TMV CP.
2.5. Fungicidal Activity
2.5.1. In Vitro Fungicidal Activity
We further tested the inhibitory effects of compounds 1–24 on 14 common agricultural pathogens at a concentration of 50 μg/mL using fungicidal growth rate assay [27,29] with commercial fungicidal agents chlorothalonil and carbendazim as controls. All compounds showed broad-spectrum fungicidal activities (Table 3). Compound 16 exhibited higher antifungal activities against Cercospora arachidicola Hori (inhibitory rate: 71%) and Alternaria solani (inhibitory rate: 58%) than commercial fungicides carbendazim and chlorothalonil. Compound 16 had an inhibitory rate of 83% against Physalospora piricola.

Table 3.
In vitro fungicidal activities of compounds 1–24 against 14 kinds of fungi.
2.5.2. In Vivo Fungicidal Activity
Compounds 1–24 were further tested in vivo fungicidal activity at a concentration of 200 μg/mL using standard methods [29] with azoxystrobin as a control against Blumeria graminis f.sp. tritici, Sclerotinia sclerotiorum, Botrytis cinereal, Rhizoctonia solani, Corynespora cassiicola, and Phytophthora capsica 6 kinds of pathogenic fungi. As shown in Table 4, most of these compounds also displayed broad-spectrum in vivo fungicidal activities. Compounds 12 and 18 showed 20% inhibition rate against Rhizoctonia solani. Compound 12 exhibited higher activity than other compounds against Botrytis cinereal. The inhibition rate of compound 18 is more than 20% against Corynespora cassiicola.

Table 4.
In vivo fungicidal activities of compounds 1–24 against six kinds of fungi.
3. Discussion
3.1. Synthesis
Compounds 8–24 bearing a thiazolidine ring based on L-cysteine were synthesized under basic conditions by a one-pot method in high to nearly quantitative yields. The cyclization of aldehydes with cysteine has been reported in the literature under conditions using a water/ethanol mixture (50:50, v:v) [7,26]. To promote the precipitation of the product from the reaction system and improve the yield of the reaction, the amount of ethanol was reduced based on references. The yields of compounds 8–24 ranged from 82% to 99%. Two nucleophilic attacks of the aldehyde produced the closed ring structure and led to the generation of a new uncontrolled chiral center. Thus, compounds 8–24 are obtained as diastereomeric mixtures [7]. Although the 2R, 4R and 2S,4R isomers were mixed, the distinctive singlet around 5.5 ppm of the hydrogen on C-2 gave a clearly distinguishable ratio of the isomers [7].
3.2. Phytotoxic Activity
Healthy growing 5–6 leaf stage tobaccos (Nicotiana tabacum var Xanthi nc) were slected for phytotoxic activity tests. The fresh solutions (500 μg/mL) of compounds 1–24 were gently smeared on the leaves. One hour after treatment, all the treated tobacco leaves were observed intact. The growth of tobacco leaves was continuously observed and calculated the weight after 3, 7, and 10 days, respectively. Encouragingly, none of the compounds were toxic to tobacco leaves.
3.3. Structure-Activity Relationship of the Antiviral Activity
As the results of Table 1, compounds 1–7 had better anti-TMV activities than ribavirin. Chirality has no obvious effect on the antiviral activities of these compounds (inhibitory effect: 1-D ≈ 1-L, 2-D ≈ 2-L). Therefore, in the follow-up study, we only used L-cysteine derivatives to explore the effect of different substituents. The antiviral activity was increased slightly when the S atom and N atom of cysteine had substituents (antiviral activity: 3 > 4 > 7 > 1, 2, 5, 6). Cyclization is an important method to improve molecular stability and biological activity. A series of thiazolidine-4-carboxylic acid-containing compounds 8–24 were designed and synthesized. As shown in Table 1, most of the designed compounds displayed better antiviral activities than ribavirin. For the substituted benzene compounds 8–17, the electron-withdrawing group substitution at the para position of the benzene ring is beneficial to the improvement of biological activity (inhibitory effect: 11 > 16 > 10, 17 > 9 > 8). When introduction of electron-donating groups such as CH3O (12), CH3 (13) into the para position of the benzene ring, the anti-TMV activity is also improved (inhibitory effect: 13 > 12 > 15 > 8, 17 > 9 > 8). However, the activity is sharply declined after the introduction of -OH at the para-position of the benzene ring (14). When the 2-position of thiazolidine structure is heterocyclic groups, such as thiophene (18), furan (19), pyridine (20), only compound 20 displayed better anti-TMV activity. Compounds 21–24 with the aliphatic groups at 2-position of thiazolidine exhibited moderate to excellent anti-TMV activities, the introduction of long-chain fat groups (23), and cyclohexyl groups (24) at 2-position of thiazolidine can lead to an increase in activity (23 > 24 > 35 > 22).
Just like the antiviral activity in vitro, compounds 2−24 displayed moderate to good in vivo activities (Table 2), and the activities of compounds 3, 4, 7, 13, 23, 24 are significantly higher than that of cysteine. In particular, compound 3 showed excellent activity against TMV (inhibitory rate: 51%, 47%, and 49% at 500 μg/mL for inactivation, curative, and protection activity in vivo, respectively). Compounds 4, 7, 10, 13, 20, 23, and 24 displayed higher anti-TMV activities than the commercial plant virucide ribavirin (inhibitory rate: 40, 40, and 38% at 500 μg/mL for inactivation, curative, and protection activity in vivo, respectively). Compounds 2, 10, 12, 16 exhibited approximate anti-TMV activities as cysteine. The structure-activity relationship revealed that the substitutions of S atom and N atom had a great influence on the anti-TMV activity.
3.4. Study on the Mechanism of Anti-TMV Activity
3.4.1. Preliminary Mode of Action
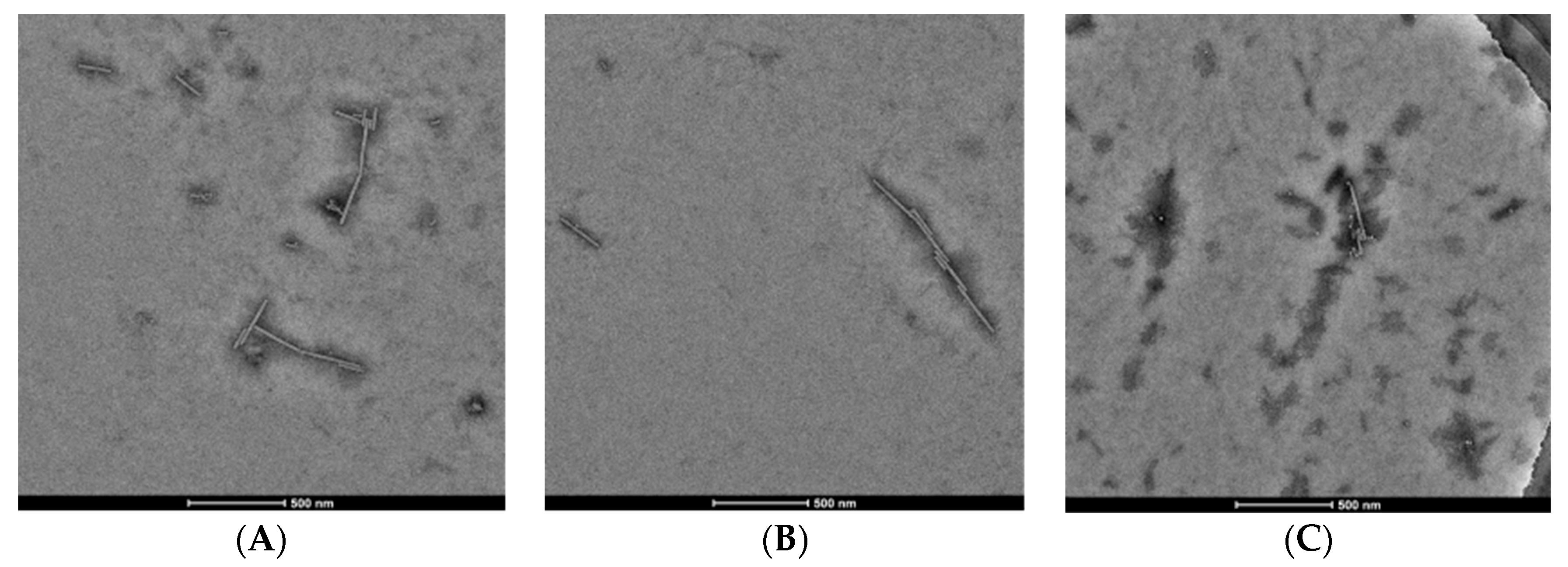
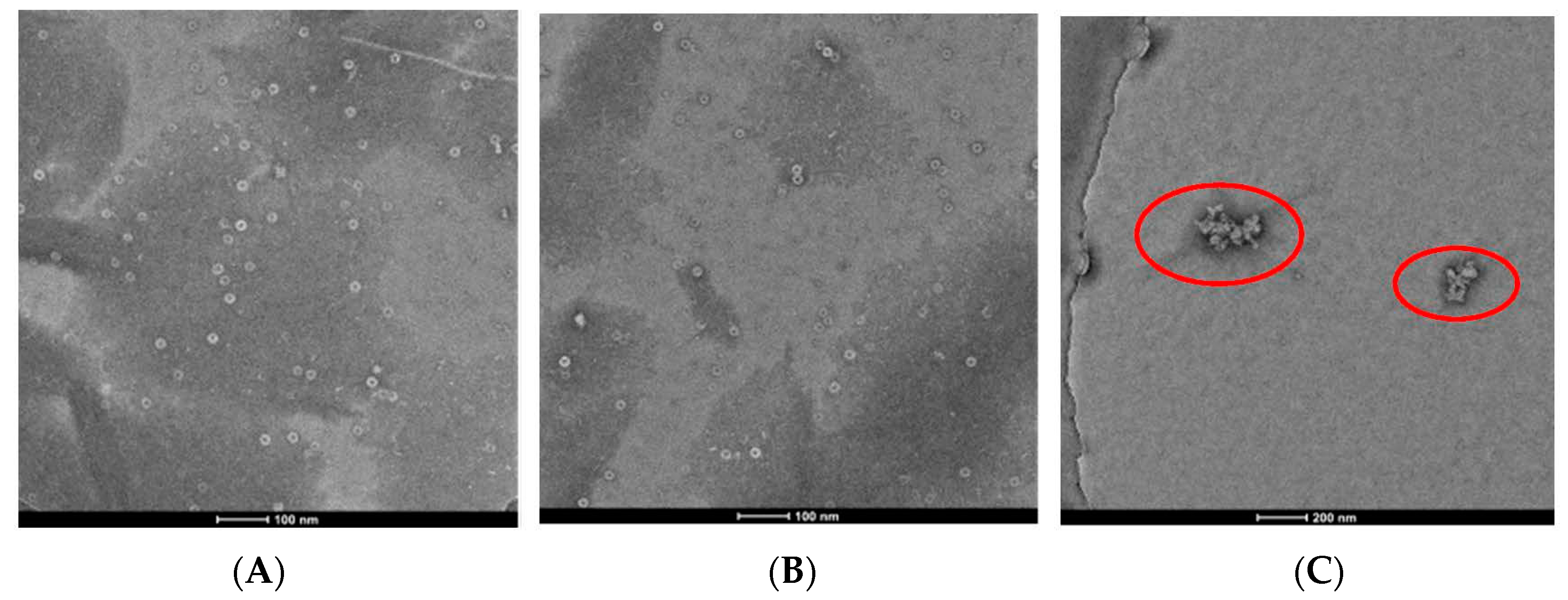
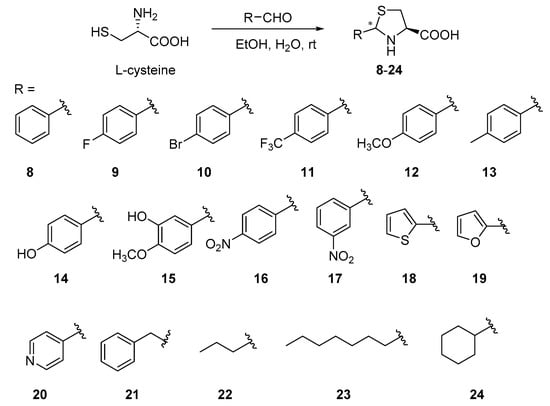
Considering structural features and biological activity, compound 3 was chosen to study the mechanism of anti-TMV activity. The results showed that 20S CP and TMV RNA could assembled into TMV particles of about 300 nm in length, and dimethyl sulfoxide (DMSO) had no effect on the assembly (panels A and B of Figure 3). Compound 3 can cause a reduction in the length and number of TMV particles, indicating that it can inhibit the assembly of the viruses (panel C of Figure 3). The interaction experiment between compound 3 and 20S CP was also designed. The TMV protein can form the homogeneous-dispersed disc structure (Figure 4A), and a small amount of DMSO has no effect on the formation (Figure 4B). As seen in Figure 4C, compound 3 can lead to polymerization of TMV CP.
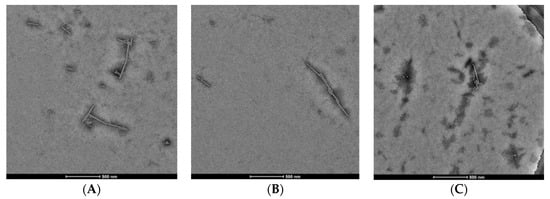
Figure 3.
TMV rod assembly inhibition of compound 3 and NK0209: (A) 20S CP disk + RNA (500 nm scale bar), (B) 20S CP disk + RNA + 1/100 DMSO (500 nm scale bar), (C) 20S CP disk + RNA + 10 μM 3 (500 nm scale bar).
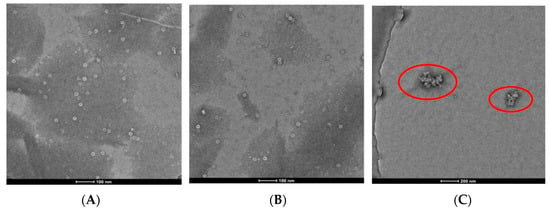
Figure 4.
20S CP disk assembly inhibition of compound 3 (100 nm scale bar): (A) CP, (B) CP + 1/100 DMSO (100 nm scale bar), (C) CP + 10 μM compound 3 (200 nm scale bar).
3.4.2. Molecular Docking Study
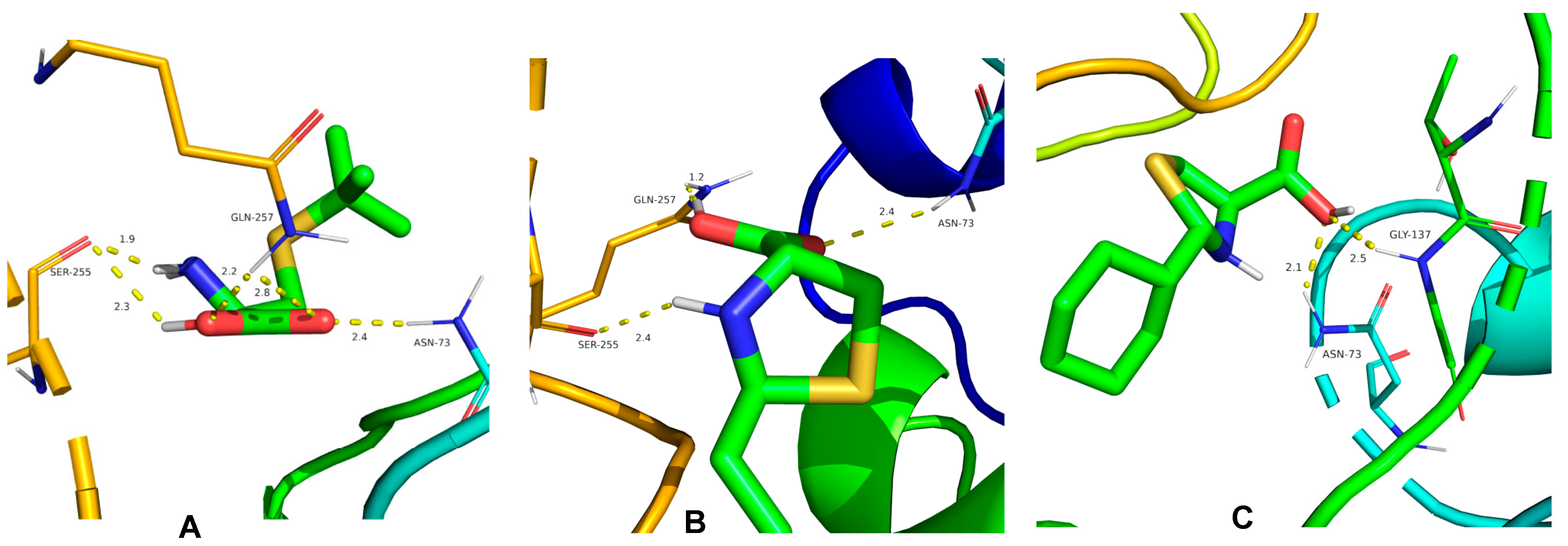
Molecular docking studies were performed to explore the binding sites of cysteine derivatives on TMV CP. Compounds 3, 23, and 24 were selected for molecular docking with TMV CP (PDB code 1EI7). The results showed that compound 3 has lain into the TMV CP activity pocket of SER 255, ASN 73, and GLN 257 (Figure 5A). Compound 3 forms five conventional hydrogen bonds with the active site of SER 255 (1.9 Å and 2.3 Å), GLN 257 (2.2 Å and 2.8 Å), and ASN 73 (2.4 Å) (Figure 5A). Compound 23 forms three conventional hydrogen bonds with the active site of SER 255 (2.4 Å), GLN 257 (1.2 Å), and ASN 73 (2.4 Å) (Figure 5B). As seen in Figure 5C, compound 24 forms two conventional hydrogen bonds with ASN 73 (2.1 Å) and GLY 137 (2.5 Å). The molecular docking results indicate that these compounds interact with CP through hydrogen bonding. The results of molecular docking also showed that compound 3 had more binding sites with TMV CP and shorter hydrogen bond distance. The stronger the interaction with TMV CP, the greater influence on the assembly of TMV, and the higher the inhibition rate. This result is consistent with the activity test.
Figure 5.
Molecule docking results of (A) compound 3, (B) compound 23, (C) compound 24 with TMV CP.
3.5. Structure-Activity Relationship of the Fungicidal Activity
As the results showed in Table 3, all compounds exhibited broad-spectrum fungicidal activities in vitro. Kapachery et al. [30] reported that N-acetylcysteine (NAC, compound 7) affected four kinds of bacteria (Aeromonas hydrophila, Pseudomonas putida, Stenotrophomonas sp., and Serratia marcescens) at the concentration of 1.5 mg/mL which were isolated from polluted reverse osmosis membrane. Perez-Giraldo et al. [31] confirmed that NAC could control bacterial biofilm formation on medical catheters. NAC has been widely used as a mucolytic agent in the treatment of chronic bronchitis [32]. However, the activity of cysteine and its derivatives on plant phytopathogenic fungi was not significant. The inhibitory effect of NAC on 14 plant pathogens was less than 50%. Changing the substituents of N, S, and O atoms in cysteine can slightly raise its anti-plant pathogen activity. Encouraged by the antifungal activities of compound 16, compounds 1–24 were further evaluated in vivo fungicidal activity. The modification of cysteine had some effect on its fungicidal activity in vivo. It can be seen from the results of fungicidal activity that cysteine and its derivatives have broad-spectrum activity, but with a low to moderate degree of activity.
4. Materials and Methods
4.1. General Procedures
4.1.1. Instruments
The melting points of the products were determined on an X-4 binocular microscope (Gongyi Yuhua Instrument Co., Gongyi, China) and are not corrected. NMR spectra were acquired with a 400 MHz (100 MHz for 13C) instrument (Bruker, Billerica, MA, USA) at room temperature. Chemical shifts were measured relative to residual solvent peaks of DMSO-d6 as internal standards (1H: δ = 2.5 and 3.3 ppm; 13C: δ = 39.9 ppm). The following abbreviations are used to designate chemical shift multiplicities: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, m = multiplet, and brs = broad singlet. HRMS data were recorded with a QFT-ESI instrument (Varian, Palo Alto, CA, USA). All reagents were of analytical reagent (AR) grade or chemically pure (CR). Compounds 1–7 (AR) were purchased from Shanghai Bidepharm Co., Ltd. (Shanghai, China).
4.1.2. Synthesis of Compounds 8–24
l-Cysteine (3.63 g, 30 mmol) was dissolved in a mixed solvent of water (50 mL) and EtOH (6 mL). Then the solution of corresponding aldehydes (1.0 equiv.) in EtOH (15 mL) was added. The mixture was stirred at 25 °C for 6 h, filtered, washed with water, and dried to afford compounds 8–24 [7,26].
(2RS,4R)-2-Phenyl-1,3-thiazolidine-4-carboxylic acid (8). White solid, 93% yield, m.p. 155−157 °C (lit. [33] 158−159 °C); 1H-NMR (DMSO-d6): 7.25–7.52 (m, 5H, Ar-H), 5.67 (s, 0.5H, Ar-CH), 5.50 (s, 0.5H, Ar-CH), 4.24 (dd, J = 4.4 and 6.8 Hz, 0.5H, CH2CH), 3.90 (dd, J = 7.6 and 8.4 Hz, 0.5H, CH2CH), 3.38 (dd, J = 7.2 and 10.0 Hz, 0.5H, CH2), 3.30 (dd, J = 7.2 and 10.4 Hz, 0.5H, CH2), 3.14 (dd, J = 4.8 and 10.4 Hz, 0.5H, CH2), 3.08 (t, J = 8.8 Hz, 0.5H, CH2); 13C-NMR (DMSO-d6): 172.9, 172.2, 141.2, 138.9, 128.5, 128.3, 127.6, 127.2, 126.9, 71.7, 71.1, 65.4, 64.8, 38.4, 37.9; HRMS (ESI) m/z calc’d for C10H11NO2S [M + H]+: 209.0510, found 209.0503.
(2RS,4R)-2-(4-Fluorophenyl)-1,3-thiazolidine-4-carboxylic acid (9). White solid, 82% yield, m.p. 153–155 °C (lit. [34] 166 °C); 1H-NMR (DMSO-d6): 7.48–7.60 (m, 2H, Ar-H), 7.13–7.22 (m, 2H, Ar-H), 5.67 (s, 0.6H, Ar-CH), 5.51 (s, 0.4H, Ar-CH), 4.21 (dd, J = 4.8 and 6.8 Hz, 0.6H, CH2CH), 3.89 (t, J = 7.6 Hz, 0.4H, CH2CH), 3.36 (dd, J = 7.2 and 10.0 Hz, 0.5H, CH2), 3.30 (dd, J = 7.2 and 10.0 Hz, 0.7H, CH2), 3.13 (dd, J = 4.4 and 10.0 Hz, 0.6H, CH2), 3.09 (t, J = 9.2 Hz, 0.5H, CH2); 13C-NMR (DMSO-d6): 173.4, 172.6, 163.5, 163.1, 161.1, 160.7, 138.0, 135.8, 130.0, 129.9, 129.4, 115.8, 115.6, 115.5, 115.3, 71.4, 70.7, 66.0, 65.3, 38.8, 38.4; HRMS (ESI) m/z calc’d for C10H10FNO2S [M + H]+: 227.0416, found 227.0421.
(2RS,4R)-2-(4-Bromophenyl)-1,3-thiazolidine-4-carboxylic acid (10). White solid, 87% yield, m.p. 158–161 °C (lit. [34] 165–166 °C); 1H-NMR (DMSO-d6): 7.38–7.58 (m, 4H, Ar-H), 5.67 (s, 0.6H, Ar-CH), 5.49 (s, 0.4H, Ar-CH), 4.17 (dd, J = 4.8 and 6.8 Hz, 0.6H, CH2CH), 3.90 (dd, J = 7.2 and 8.8 Hz, 0.4H, CH2CH), 3.35 (dd, J = 6.8 and 10.0 Hz, 0.6H, CH2), 3.29 (dd, J = 7.2 and 10.4 Hz, 0.7H, CH2), 3.12 (t, J = 5.4 Hz, 0.6H, CH2), 3.08 (t, J = 7.2 Hz, 0.4H, CH2); 13C-NMR (DMSO-d6): 173.3, 172.5, 141.6, 139.1, 131.8, 131.6, 130.1, 129.6, 130.0, 71.3, 70.6, 66.0, 65.3, 38.8, 38.5; HRMS (ESI) m/z calc’d for C10H10BrNO2S [M + H]+: 288.9616, found 288.9614.
(2RS,4R)-2-(4-Trifluoromethylphenyl)-1,3-thiazolidine-4-carboxylic acid (11). White solid, 99% yield, m.p. 145–147 °C; 1H-NMR (DMSO-d6): 7.63–7.77 (m, 4H, Ar-H), 5.81 (s, 0.6H, Ar-CH), 5.61 (s, 0.4H, Ar-CH), 4.15 (t, J = 6.0 Hz, 0.6H, CH2CH), 3.95 (dd, J = 7.2 and 8.4 Hz, 0.4H, CH2CH), 3.37 (dd, J = 6.8 and 10.0 Hz, 0.4H, CH2), 3.31 (dd, J = 7.2 and 10.4 Hz, 0.6H, CH2), 3.08–3.12 (m, 1H, CH2); 13C-NMR (DMSO-d6): 173.2, 172.5, 147.3, 128.7, 128.0, 125.8, 125.7, 125.6, 71.1, 70.4, 66.1, 65.3, 38.6, 38.5; HRMS (ESI) m/z calc’d for C11H10F3NO2S [M +H]+: 277.0384, found 277.0383.
(2RS,4R)-2-(4-Methoxyphenyl)-1,3-thiazolidine-4-carboxylic acid (12). White solid, 93% yield, m.p. 153–156 °C (lit. [33] 163–164 °C); 1H-NMR (DMSO-d6): 7.44 (d, J = 8.8 Hz, 1H, Ar-H), 7.37 (d, J = 8.8 Hz, 1H, Ar-H), 6.92 (d, J = 8.8 Hz, 1H, Ar-H), 6.88 (d, J = 8.4 Hz, 1H, Ar-H), 5.60 (s, 0.5H, Ar-CH), 5.45 (s, 0.5H, Ar-CH), 4.25 (dd, J = 4.0 and 6.8 Hz, 0.5H, CH2CH), 3.87 (dd, J = 7.2 and 8.8 Hz, 0.5H, CH2CH), 3.75 (s, 1.5H, CH3), 3.74 (s, 1.5H, CH3), 3.36 (dd, J = 7.2 and 10.0 Hz, 0.5H, CH2), 3.28 (dd, J = 7.2 and 10.0 Hz, 0.5H, CH2), 3.15 (dd, J = 4.0 and 10.4 Hz, 0.5H, CH2), 3.07 (t, J = 8.8 Hz, 0.5H, CH2); 13C-NMR (DMSO-d6): 173.1, 172.3, 159.2, 158.7, 132.8, 130.7, 128.5, 128.3, 114.5, 113.8, 113.6, 71.5, 70.9, 65.4, 64.8, 55.1, 55.0, 38.5, 37.9; HRMS (ESI) m/z calc’d for C11H13NO3S [M + H]+: 239.0616, found 239.0612.
(2RS,4R)-2-(4-Methylphenyl)-1,3-thiazolidine-4-carboxylic acid (13). White solid, 99% yield, m.p. 146–148 °C (lit. [34] 163.2–163.7 °C); 1H-NMR (DMSO-d6): 7.39 (d, J = 8.0 Hz, 1H, Ar-H), 7.32 (d, J = 8.0 Hz, 1H, Ar-H), 7.17 (d, J = 7.6 Hz, 1H, Ar-H), 7.13 (d, J = 7.6 Hz, 1H, Ar-H), 5.61 (s, 0.5H, Ar-CH), 5.46 (s, 0.5H, Ar-CH), 4.24 (dd, J = 4.4 and 6.8 Hz, 0.5H, CH2CH), 3.87 (dd, J = 7.6 and 8.8 Hz, 0.5H, CH2CH), 3.36 (dd, J = 6.8 and 10.0 Hz, 0.5H, CH2), 3.28 (dd, J = 6.8 and 10.0 Hz, 0.5H, CH2), 3.14 (dd, J = 4.0 and 10.0 Hz, 0.5H, CH2), 3.07 (t, J = 8.8 Hz, 0.5H, CH2), 2.30 (s, 1.5H, CH3), 2.28 (s, 1.5H, CH3); 13C-NMR (DMSO-d6): 173.5, 172.8, 138.5, 138.1, 137.3, 136.3, 130.2, 130.1, 129.4, 129.2, 127.6, 127.4, 72.2, 71.6, 65.9, 65.3, 38.9, 38.4, 21.2, 21.1; HRMS (ESI) m/z calc’d for C11H13NO2S [M + H]+: 223.0667, found 223.0673.
(2RS,4R)-2-(4-Hydroxyphenyl)-1,3-thiazolidine-4-carboxylic acid (14). White solid, 81% yield, m.p. 161–164 °C (lit. [35] 167–1169 °C); 1H-NMR (DMSO-d6): 9.51 (br s, 1H, OH), 7.33 (d, J = 8.8 Hz, 1H, Ar-H), 7.26 (d, J = 8.0 Hz, 1H, Ar-H), 6.75 (d, J = 8.0 Hz, 1H, Ar-H), 6.72 (d, J = 8.8 Hz, 1H, Ar-H), 5.55 (s, 0.5H, Ar-CH), 5.41 (s, 0.5H, Ar-CH), 4.26 (dd, J = 3.6 and 6.8 Hz, 0.5H, CH2CH), 3.86 (t, J = 7.6 Hz, 0.5H, CH2CH), 3.36 (dd, J = 6.8 and 10.0 Hz, 0.5H, CH2), 3.28 (dd, J = 6.8 and 10.0 Hz, 0.5H, CH2), 3.16 (dd, J = 3.6 and 10.4 Hz, 0.5H, CH2), 3.06 (t, J = 9.2 Hz, 0.5H, CH2); 13C-NMR (DMSO-d6): 173.1, 172.3, 157.4, 156.9, 130.7, 128.8, 128.5, 128.3, 115.1, 114.9, 71.8, 71.2, 65.2, 64.7, 38.5, 37.8; HRMS (ESI) m/z calc’d for C10H11NO3S [M + H]+: 225.0460, found 225.0463.
(2RS,4R)-2-(3-Hydroxy-4-methoxyphenyl)-1,3-thiazolidine-4-carboxylic acid (15). White solid, 93% yield, m.p. 170–173 °C; 1H-NMR (DMSO-d6): 9.03 (br s, 1H, OH), 7.12 (s, 0.5H, Ar-H), 7.11 (s, 0.5H, Ar-H), 6.83–6.90 (m, 1H, Ar-H), 6.69–6.74 (m, 1H, Ar-H), 5.53 (s, 0.5H, Ar-CH), 5.40 (s, 0.5H, Ar-CH), 4.29 (dd, J = 3.6 and 6.8 Hz, 0.5H, CH2CH), 3.83 (dd, J = 7.2 and 8.8 Hz, 0.5H, CH2CH), 3.77 (s, 1.5H), 3.76 (s, 0.5H, CH2CH), 3.33 (dd, J = 7.2 and 10.0 Hz, 0.5H, CH2), 3.28 (dd, J = 7.2 and 10.0 Hz, 0.5H, CH2), 3.16 (dd, J = 3.6 and 10.4 Hz, 0.5H, CH2), 3.06 (t, J = 9.2 Hz, 0.5H, CH2); 13C-NMR (DMSO-d6): 173.7, 172.8, 148.0, 147.8, 147.1, 146.6, 131.7, 129.9, 120.4, 120.1, 115.6, 115.4, 111.9, 111.7, 72.6, 72.0, 66.0, 65.3, 56.1, 56.0, 38.9, 38.3; HRMS (ESI) m/z calc’d for C11H13NO4S [M + H]+: 255.0565, found 255.0573.
(2RS,4R)-2-(4-Nitrophenyl)-1,3-thiazolidine-4-carboxylic acid (16). White solid, 99% yield, m.p. 94–97 °C (lit. [34] 95–97 °C); 1H-NMR (DMSO-d6): 8.17–8.23 (m, 2H, Ar-H), 7.80 (d, J = 8.8 Hz, 0.8H, Ar-H), 7.67 (d, J = 8.8 Hz, 1H, Ar-H), 5.87 (s, 0.6H, Ar-CH), 5.66 (s, 0.4H, Ar-CH), 4.12 (t, J = 6.4 Hz, 0.7H, CH2CH), 3.97 (dd, J = 6.8 and 9.2 Hz, 0.5H, CH2CH), 3.37 (dd, J = 6.8 and 10.0 Hz, 0.7H, CH2), 3.32 (dd, J = 6.8 and 10.0 Hz, 0.8H, CH2), 3.06–3.13 (m, 1H, CH2); 13C-NMR (DMSO-d6): 173.1, 172.5, 150.6, 147.7, 147.1, 129.1, 128.3, 124.0 123.9, 70.6, 69.9, 66.2, 65.4, 38.6, 38.5; HRMS (ESI) m/z calc’d for C10H10N2O4S [M + H]+: 254.0361, found 254.0367.
(2RS,4R)-2-(3-Nitrophenyl)-1,3-thiazolidine-4-carboxylic acid (17). White solid, 99% yield, m.p. 90–93 °C; 1H-NMR (DMSO-d6): 8.46 (s, 0.4H, Ar-H), 8.29 (s, 0.6H, Ar-H), 8.17 (dd, J = 1.6 and 8.0 Hz, 0.4H, Ar-H), 8.12 (dd, J = 1.6 and 8.4 Hz, 0.6H, Ar-H), 7.96 (d, J = 8.0 Hz, 0.4H, Ar-H), 7.87 (d, J = 7.6 Hz, 0.6H, Ar-H), 7.60–7.67 (m, 1H, Ar-H), 5.86 (s, 0.6H, Ar-CH), 5.67 (s, 0.4H, Ar-CH), 4.14 (t, J = 6.0 Hz, 0.6H), 3.95 (dd, J = 7.2 and 8.4 Hz, 0.5H, CH2CH), 3.31–3.38 (m, 1H, CH2), 3.09–3.15 (m, 1H, CH2); 13C-NMR (DMSO-d6): 173.1, 172.4, 148.2, 148.1, 145.1, 142.5, 134.8, 134.3, 130.4, 130.3, 70.6, 69.9, 66.3, 65.2, 38.6, 38.5; HRMS (ESI) m/z calc’d for C10H10N2O4S [M + H]+: 254.0361, found 254.0365.
(2RS,4R)-2-(Thiophen-2-yl)thiazolidine-4-carboxylic acid (18). White solid, 99% yield, m.p. 144–147 °C (lit. [36] 146–147 °C); 1H-NMR (DMSO-d6): 7.51 (dd, J = 0.8 and 4.8 Hz, 0.3H, Ar-H), 7.42 (dd, J = 0.8 and 5.2 Hz, 0.6H, Ar-H), 7.20 (d, J = 3.2 Hz, 0.4H, Ar-H), 7.06 (d, J = 3.6 Hz, 0.6H, Ar-H), 6.99 (dd, J = 3.2 and 4.8 Hz, 0.3H, Ar-H), 6.95 (dd, J = 3.6 and 4.2 Hz, 0.6H, Ar-H), 5.94 (s, 0.7H, Ar-CH), 5.75 (s, 0.4H, Ar-CH), 4.07 (t, J = 6.4 Hz, 0.7H, CH2CH), 3.91 (dd, J = 7.2 and 8.8 Hz, 0.4H, CH2CH), 3.31–3.39 (m, 1H, CH2), 3.03–3.11 (m, 1H, CH2); 13C-NMR (DMSO-d6): 172.6, 172.2, 147.1, 142.8, 126.8, 126.7, 126.2, 125.4, 125.2, 66.6, 66.1, 65.4, 64.5, 38.5, 38.0; HRMS (ESI) m/z calc’d for C8H9NO2S2 [M + H]+: 215.0075, found 215.0079.
(2RS,4R)-2-(Furan-2-yl)thiazolidine-4-carboxylic acid (19). White solid, 99% yield, m.p. 137–140 °C (lit. [33] 137–138 °C); 1H-NMR (DMSO-d6): 7.65–7.66 (m, 0.3H, Ar-H), 7.58–7.59 (m, 0.6H, Ar-H), 6.50 (d, J = 3.2 Hz, 0.3H, Ar-H), 6.43–6.44 (m, 0.3H, Ar-H), 6.37–6.38 (m, 0.6H, Ar-H), 6.34–6.35 (m, 0.6H, Ar-H), 5.74 (s, 0.7H, Ar-CH), 5.61 (s, 0.4H, Ar-CH), 4.11 (t, J = 6.4 Hz, 0.7H, CH2CH), 3.87 (dd, J = 6.8 and 8.8 Hz, 0.4H, CH2CH), 3.27–3.37 (m, 1H, CH2), 2.97–3.01 (m, 1H, CH2); 13C-NMR (DMSO-d6): 173.0, 172.7, 154.9, 151.8, 143.4, 142.9, 111.1, 110.8, 108.0, 106.8, 65.9, 65.3, 64.7, 64.4, 38.6, 38.3; HRMS (ESI) m/z calc’d for C8H9NO3S [M + H]+: 119.0303, found 119.0305.
(2RS,4R)-2-(Pyridin-4-yl)thiazolidine-4-carboxylic acid (20). White solid, 93% yield, m.p. 163–165 °C (lit. [34] 175–176 °C); 1H-NMR (DMSO-d6): 8.55 (d, J = 6.0 Hz, 0.4H, Ar-H), 8.50 (d, J = 6.1 Hz, 1.6H, Ar-H), 7.52 (d, J = 6.0 Hz, 0.4H, Ar-H), 7.40 (d, J = 6.0 Hz, 1.6H, Ar-H), 5.75 (s, 0.8H, Ar-CH), 5.54 (s, 0.2H, Ar-CH), 4.10 (t, J = 6.3 Hz, 0.8H, CH2CH), 3.97 (dd, J = 6.9 and 8.8 Hz, 0.2H, CH2CH), 3.36 (dd, J = 6.9 and 10.0 Hz, 0.2H, CH2), 3.30 (dd, J = 6.8 and 10.2 Hz, 0.8H, CH2), 3.04–3.10 (m, 1H, CH2); 13C-NMR (DMSO-d6): 173.0, 172.5, 151.9, 151.1, 150.1, 149.8, 122.3, 122.1, 70.2, 69.5, 66.2, 65.3, 38.6, 38.4; HRMS (ESI) m/z calc’d for C9H10N2O2S [M + H]+: 210.0463, found 210.0460.
(2RS,4R)-2-Benzylthiazolidine-4-carboxylic acid (21). White solid, 93% yield, m.p. 152–155 °C (lit. [35] 165–166 °C); 1H-NMR (DMSO-d6): 7.18–7.30 (m, 5H, Ar-H), 4.81 (t, J = 7.0 Hz, 0.7H, SCH), 4.65 (t, J = 6.8 Hz, 0.3H, SCH), 4.15 (dd, J = 5.6 and 6.7 Hz, 0.7H, CH2CH), 3.72 (dd, J = 6.9 and 9.2 Hz, 0.3H, CH2CH), 3.07–3.27 (m, 2H, CH2CH and Ar-CH2), 3.00 (dd, J = 7.3 and 13.7 Hz, 0.3H, CH2CH), 2.92 (dd, J = 5.4 and 10.2 Hz, 0.7H, CH2CH), 2.75–2.85 (m, 1H, Ar-CH2); 13C-NMR (DMSO-d6): 173.3, 172.7, 139.5, 139.3, 129.6, 129.4, 128.7, 128.6, 126.9, 126.7, 72.4, 71.8, 65.7, 64.6, 43.3, 41.1, 37.8, 37.6; HRMS (ESI) m/z calc’d for C11H13NO2S [M + H]+: 223.0667, found 223.0662.
(2RS,4R)-2-Propylthiazolidine-4-carboxylic acid (22). White solid, 93% yield, m.p. 190–192 °C; 1H-NMR (DMSO-d6): 4.56 (t, J = 6.6 Hz, 0.5H, SCH), 4.42 (t, J = 7.2 Hz, 0.5H, SCH), 4.07 (dd, J = 6.7 Hz and 5.4 Hz, 0.5H, SCH2CH), 3.71 (dd, J = 7.0 and 9.0 Hz, 0.5H, SCH2CH), 3.20 (dd, J = 7.0 and 9.9 Hz, 0.5H, SCH2), 3.09 (dd, J = 7.0 and 10.2 Hz, 0.5H, SCH2), 2.94 (dd, J = 5.1 and 10.2 Hz, 0.5H, SCH2), 2.76 (t, J = 9.4 Hz, 0.5H, SCH2), 1.85–1.93 (m, 0.5H, CH2CH2CH), 1.65–1.77 (m, 1H, CH2CH2CH), 1.49–1.56 (m, 0.5H, CH2CH2CH), 1.35–1.43 (m, 2H, CH2CH2), 0.86–0.91 (m, 3H, CH3); 13C-NMR (DMSO-d6): 173.3, 172.8, 71.2, 70.4, 65.6, 64.6, 39.3, 39.2, 37.4, 37.0, 21.4, 21.1, 14.3, 14.2; HRMS (ESI) m/z calc’d for C7H13NO2S [M + H]+: 175.0667, found 175.0670.
(2RS,4R)-2-Heptylthiazolidine-4-carboxylic acid (23). White solid, 93% yield, m.p. 136–139 °C (lit. [37] 159–160 °C); 1H-NMR (DMSO-d6): 4.54 (t, J = 6.6 Hz, 0.5H, SCH), 4.40 (t, J = 6.7 Hz, 0.5H, SCH), 4.06 (t, J = 6.1 Hz, 0.5H, SCH2CH), 3.70 (t, J = 8.5 Hz, 0.5H, SCH2CH), 3.19 (t, J = 9.6 Hz, 0.5H, SCH2), 3.08 (t, J = 9.8 Hz, 0.5H, SCH2), 2.93 (dd, J = 4.9 and 9.8 Hz, 0.5H, SCH2), 2.75 (t, J = 9.6 Hz, 0.5H, SCH2), 1.85–1.92 (m, 0.5H, CH2CH2CH), 1.69–1.76 (m, 1H, CH2CH2CH), 1.50–1.67 (m, 0.5H, CH2CH2CH), 1.24–1.48 (m, 10H, CH2CH2), 0.84–0.86 (m, 3H, CH3); 13C-NMR (DMSO-d6): 173.3, 172.8, 71.5, 70.8, 65.7, 64.6, 37.4, 37.1, 37.0, 35.3, 31.7, 29.3, 29.2, 29.1, 29.0, 28.1, 27.9, 22.5, 14.4; HRMS (ESI) m/z calc’d for C11H21NO2S [M + H]+: 231.1293, found 231.1294.
(2RS,4R)-2-Cyclohexylthiazolidine-4-carboxylic acid (24). White solid, 82% yield, m.p.184–187 °C; 1H-NMR (DMSO-d6): 4.01–4.02 (m, 0.4H, SCH), 3.65–3.69 (m, 0.6H, SCH), 3.13–3.16 (m, 0.6H, SCH2CH), 2.98–3.01 (m, 0.4H, SCH2CH), 2.87–2.89 (m, 0.4H, SCH2CH), 2.65–2.68 (m, 0.6H, SCH2CH), 1.95–1.97 (m, 1H, CH2CHCH2), 1.40–1.66 (m, 4H, CH2CH2CH), 0.98–1.15 (m, 6H, CH2CH2CH2); 13C-NMR (DMSO-d6): 173.4, 172.9, 77.4, 76.6, 65.7, 64.8, 44.1, 43.1, 36.7, 31.7, 31.6, 30.1, 26.4, 26.0, 25.9, 25.8; HRMS (ESI) m/z calc’d for C10H17NO2S [M + H]+: 215.0980, found 215.0978.
4.2. Biological Assays
Each test was repeated three times at 25 ± 1 °C. Active effect expressed in percentage scale of 0–100 (0: no activity; 100: total inhibited). Specific test methods for the anti-TMV and fungicidal activities were carried out by the literature method [5,27], Detailed bioassay procedures for the anti-TMV and fungicidal activities were described in the literature and can be seen in the Supplementary Materials.
5. Conclusions
Based on the structure of natural product cysteine, a series of cysteine and its derivatives were designed, synthesized, and evaluated for their antiviral and antifungal activities in vitro and in vivo. By studying the influence of O, N, and S atom substituents of cysteine, it was found that some compounds had excellent anti-TMV activity. The preliminary mode of action studies exhibited that compound 3 can hold back virus assembly by aggregating the 20S protein disk. We further study the binding sites of the interaction between cysteines and TMV CP by molecular docking. Further fungicidal activity tests against 14 kinds of phytopathogenic fungi revealed that these cysteine derivatives displayed broad-spectrum fungicidal activities. In this work, cysteine and its derivatives are found to be potential inhibitors against plant viruses and plant pathogens.
Supplementary Materials
The following are available online, Supplementary data (Detailed bio-assay procedures for the anti-TMV and fungicidal activities; mode of action studies; copies of 1H & 13C-NMR spectra) can be found in the online version.
Author Contributions
Project administration, supervision, Z.W. and A.L.; writing—original draft, A.L., chemical methodology, S.Y. and T.W., biological methodology, L.S. and Y.Z.; docking studies, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Fundation of Hebei Province (B2020202028).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors also acknowledge the State Key Laboratory of Elemento-Organic Chemistry (Nankai University) for biological activity test.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 8–24 are available from the authors.
References
- Wilson, R.A.; Talbot, N.J. Fungal physiology—A future perspective. Microbiology 2009, 155, 3810–3815. [Google Scholar] [CrossRef]
- Ji, X.F.; Guo, J.C.; Liu, Y.X.; Lu, A.D.; Wang, Z.W.; Li, Y.Q.; Yang, S.X.; Wang, Q.M. Marine-natural-product development: First discovery of nortopsentin alkaloids as novel antiviral, anti-phytopathogenic-fungus, and insecticidal agents. J. Agric. Food Chem. 2018, 66, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.C.; Hao, Y.Y.; Ji, X.F.; Wang, Z.W.; Liu, Y.X.; Ma, D.J.; Li, Y.Q.; Pang, H.L.; Ni, J.P.; Wang, Q.M. Optimization, structure−activity relationship, and mode of action of nortopsentin analogues containing thiazole and oxazole moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, R.; Li, Y.A.; Li, S.Y.; Yu, J.; Zhao, B.F.; Liao, A.C.; Wang, Y.; Wang, Z.W.; Lu, A.D.; et al. Discovery of pimprinine alkaloids as novel agents against a plant virus. J. Agric. Food Chem. 2019, 67, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.W.; Zhu, H.H.; Wang, P.Y.; Zeng, D.; Wu, Y.Y.; Liu, L.W.; Wu, Z.B.; Li, Z.; Yang, S. Synthesis of thiazolium-labeled 1,3,4-oxadiazole thioethers as prospective antimicrobials: In vitro and in vivo bioactivity and mechanism of action. J. Agric. Food Chem. 2019, 67, 12696–12708. [Google Scholar] [CrossRef] [PubMed]
- Önen Bayram, F.E.; Sipahi, H.; Acar, E.T.; Kahveci Ulugöl, R.; Buran, K.; Akgün, H. The cysteine releasing pattern of some antioxidant thiazolidine-4-carboxylic acids. Eur. J. Med. Chem. 2016, 114, 334–337. [Google Scholar] [CrossRef]
- Postma, T.M.; Albericio, F. Disulfide formation strategies in peptide synthesis. Eur. J. Org. Chem. 2014, 17, 3519–3530. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Álvarez, M.; Albericio, F. Amino acid-protecting groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [CrossRef]
- White, J.D.; Xu, Q.; Lee, C.S.; Valeriote, F.A. Total synthesis and biological evaluation of (+)-kalkitoxin, a cytotoxic metabolite of the cyanobacterium Lyngbya majuscule. Org. Biomol. Chem. 2004, 2, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Puka-Sundvall, M.; Eriksson, P.; Nilsson, P.; Sandberg, M.; Lehmann, A. Neurotoxicity of cysteine: Interaction with glutamate. Brain Res. 1995, 705, 65–70. [Google Scholar] [CrossRef]
- Janáky, R.; Varga, V.; Hermann, A.; Saransaari, P.; Oja, S.S. Mechanisms of L-cysteine neurotoxicity. Neurochem. Res. 2000, 25, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Lee, C.S.; Xu, Q. Total synthesis of (+)-kalkitoxin. Chem. Commun. 2003, 34, 2012–2013. [Google Scholar] [CrossRef]
- Nagy, P.; Ashby, M.T. Reactive sulfur species: Kinetics and mechanisms of the oxidation of cysteine by hypohalous acid to give cysteine sulfenic acid. J. Am. Chem. Soc. 2007, 129, 14082–14091. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Imlay, J.A. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 2003, 185, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Guo, X.F.; Fan, Z.J.; Chen, L.; Ma, L.Y.; Wang, H.X.; Wei, Y.; Xu, X.M.; Lin, J.P.; Bakulev, V.A. Approach to thiazole containing thiazole-containing tetrahydropyridines via Aza–Rauhut–Currier reaction and their potent biological fungicidal and insecticidal activity. RSC Adv. 2016, 6, 112704–112711. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, F.B.; Xu, Y.Y.; Xie, Y.C.; Shi, F.Y.; Fang, H.; Li, M.Y.; Xu, W.F. Design, synthesis and biological activity of thiazolidine-4-carboxylic acid derivatives as novel influenza neuraminidase inhibitors. Bioorg. Med. Chem. 2011, 19, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, A.B.A.; Gaafar, A.M.; Aly, A.S. Design, synthesis, and preliminary evaluation as antimicrobial activity of novel spiro-1,3-thiazolidine C-acyclic nucleoside analogs. J. Sulfur Chem. 2008, 29, 549–558. [Google Scholar] [CrossRef]
- Onen-Bayram, F.E.; Durmaz, I.; Scherman, D.; Herscovici, J.; Cetin-Atalay, R. A novel thiazolidine compound induces caspase-9 dependent apoptosis in cancer cells. Bioorg. Med. Chem. 2012, 20, 5094–5102. [Google Scholar] [CrossRef]
- Chen, H.; Yin, Q.M.; Li, C.X.; Wang, E.K.; Gao, F.; Zhang, X.B.; Yin, Z.; Wei, S.N.; Li, X.L.; Meng, M.; et al. Synthesis of C-pseudonucleosides bearing thiazolidin-4-one as a novel potential immunostimulating agent. ACS Med. Chem. Lett. 2011, 2, 845–848. [Google Scholar] [CrossRef]
- Thalamuthu, S.; Annaraj, B.; Vasudevan, S.; Sengupta, S.; Neelakantan, M.A. DNA binding, nuclease, and colon cancer cell inhibitory activity of a Cu(II) complex of a thiazolidine-4-carboxylic acid derivative. J. Coord. Chem. 2013, 66, 1805–1820. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, B.; Fan, Z.J.; Hu, M.X.; Li, Q.; Hu, W.H.; Li, J.W.; Zhang, J.L. Discovery of novel isothiazole, 1,2,3-thiadiazole, and thiazole-based cinnamamides as fungicidal candidates. J. Agric. Food Chem. 2019, 67, 12357–12365. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, Y.J.; Fan, Z.J.; Guo, X.F.; Zhang, Z.M.; Xu, J.H.; Song, Y.Q.; Yurievich, M.Y.; Belskaya, N.P.; Bakulev, V.A. Synthesis of 1,2,3-thiadiazole and thiazole-based strobilurins as potent fungicide candidates. J. Agric. Food Chem. 2017, 65, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.N.; Yang, S.; Li, H.Y.; Lu, A.D.; Wang, Z.W.; Yao, Y.W.; Wang, Q.M. Discovery, structural optimization, and mode of Action of essramycin alkaloid and its derivatives as anti-tobacco mosaic virus and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 471–484. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Liu, Y.X.; Wang, Q.M. Antiviral mechanism study of gossypol and its Schiff base derivatives based on reactive oxygen species (ROS). RSC Adv. 2016, 6, 87637–87648. [Google Scholar] [CrossRef]
- Chen, P.H.; Horton, L.B.; Mikulski, R.L.; Deng, L.S.; Sundriyal, S.; Palzkill, T.; Song, Y.C. 2-Substituted 4,5-dihydrothiazole-4-carboxylic acids are novel inhibitors of metallo-β-lactamases. Bioorg. Med. Chem. Lett. 2012, 22, 6229–6232. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.D.; Wang, T.N.; Hui, H.; Wei, X.Y.; Cui, W.H.; Zhou, C.L.; Li, H.Y.; Wang, Z.W.; Guo, J.C.; Ma, D.Y.; et al. Natural products for drug discovery: Discovery of gramines as novel agents against a plant virus. J. Agric. Food Chem. 2019, 67, 2148–2156. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Zhao, H.P.; Liu, Y.X.; Cui, Z.P.; Beattie, D.; Gu, Y.C.; Wang, Q.M. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef] [PubMed]
- Kappachery, S.; Paul, D.; Kweon, J.H. Effect of N-acetylcysteine against biofouling of reverse osmosis membrane. Desalination 2012, 285, 184–187. [Google Scholar] [CrossRef]
- Perez-Giraldo, C.; Rodriguez-Benito, A.; Moran, F.J.; Hurtado, C.; Blanco, M.T.; Gomez-Garcia, A.C. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J. Antimicrob. Chemother. 1997, 39, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Stey, C.; Steurer, J.; Bachmann, S.; Medici, T.C.; Tramer, M.R. The effect of oral N-acetylcysteine in chronic bronchitis: A quantitative systematic review. Eur. Respir. J. 2000, 16, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Appelt, H.R.; Schneider, P.H.; Rodrigues, O.E.D.; Silveira, C.C.S.; Wessjohann, L.A. New C2-symmetric chiral disulfide ligands derived from (R)-cysteine. Tetrahedron 2001, 57, 3291–3295. [Google Scholar] [CrossRef]
- Györgydeák, Z.; Kajtár-Peredy, M.; Kajtár, J.; Kajtár, M. Synthesis and chiroptical properties of N-acetyl-2-aryl-4-thiazolidinecarboxylic acids. Liebigs Ann. Chem. 1987, 11, 927–934. [Google Scholar] [CrossRef]
- Soloway, H.; Kipnis, F.; Ornfelt, J.; Spoerri, P.E. 2-Substituted-thiazolidine-4-carboxylic acids. J. Am. Chem. Soc. 1948, 70, 1667–1668. [Google Scholar] [CrossRef]
- Sutcliffe, O.B.; Storr, R.C.; Gilchrist, T.L.; Rafferty, P. Azafulvenium methides: New extended dipolar systems. J. Chem. Soc. Perkin. Trans. 1 2001, 15, 1795–1806. [Google Scholar] [CrossRef]
- Schmolka, I.R.; Spoerri, P.E. Thiazolidine Chemistry. Ⅱ. The preparation of 2-substituted thiazolidine-4-carboxylic acids. J. Org. Chem. 1957, 22, 943–946. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).