Short-Chained Platinum Complex Catalyzed Hydrosilylation under Thermomorphic Conditions: Heterogeneous Phase Separation at Ice Temperature
Abstract
1. Introduction
2. Results and Discussion
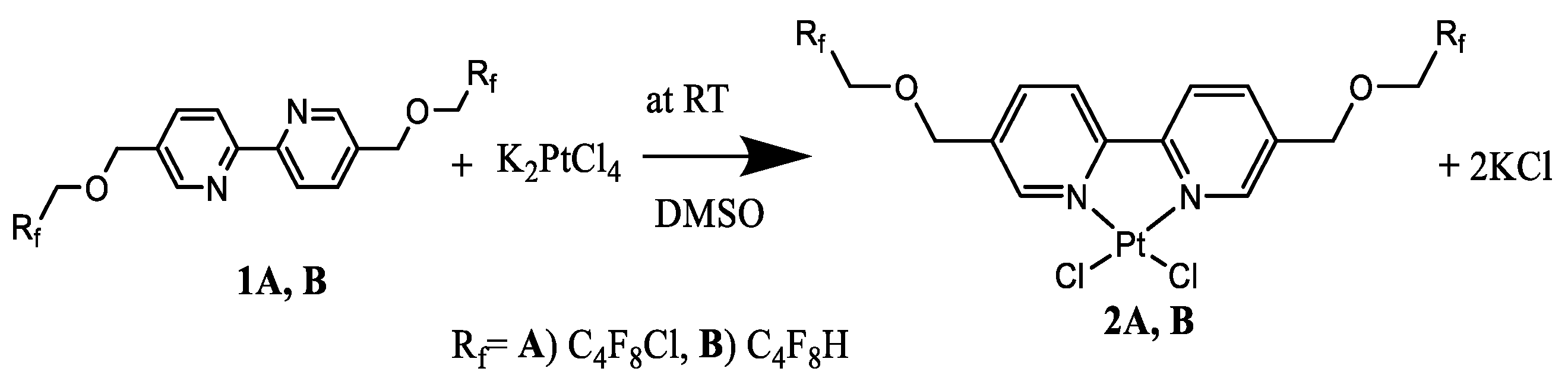
2.1. Catalyst Synthesis
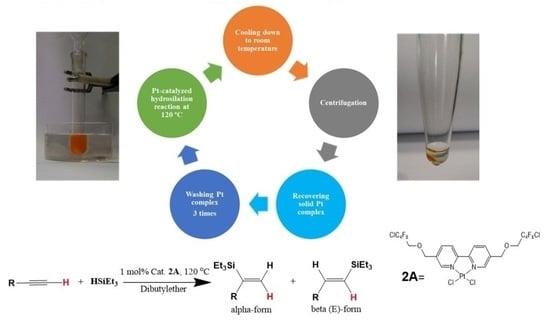
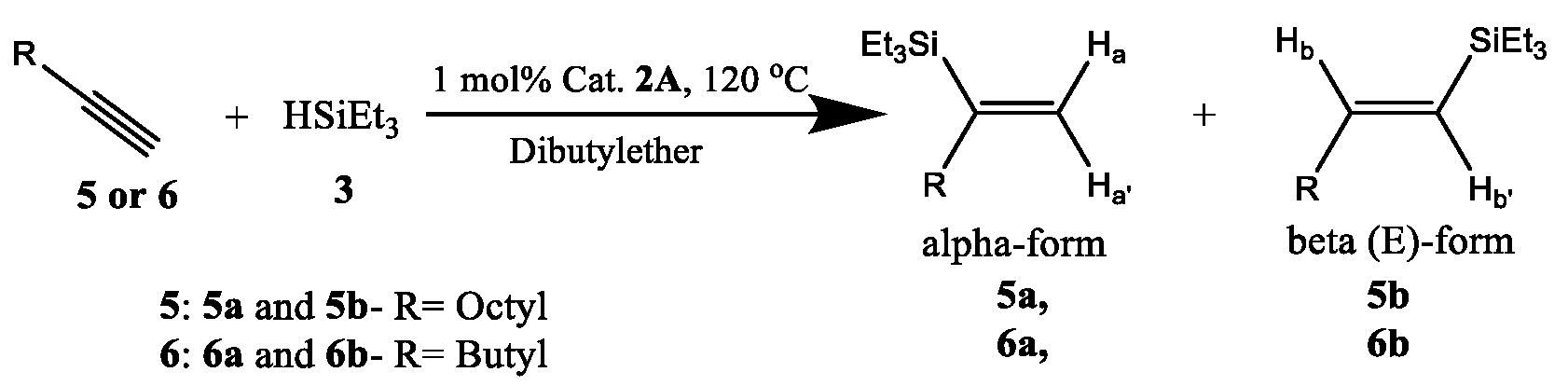
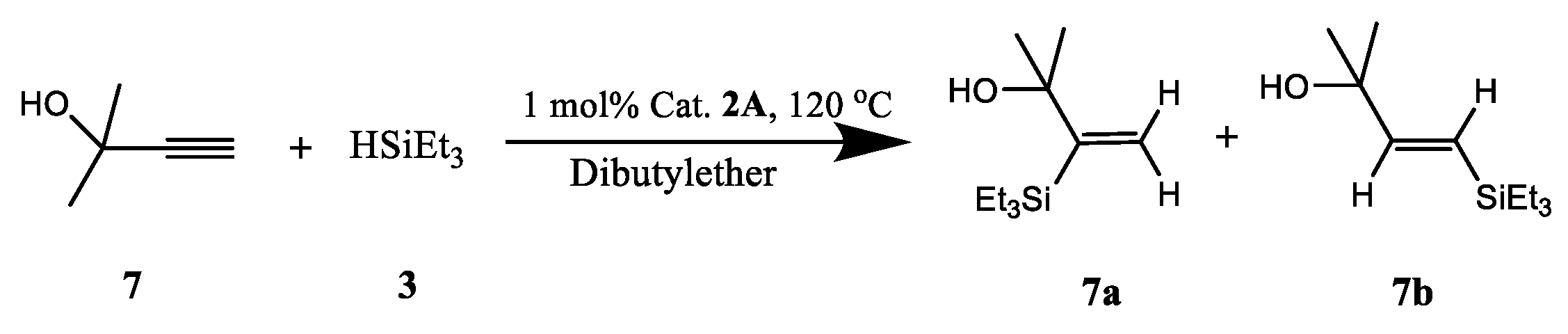
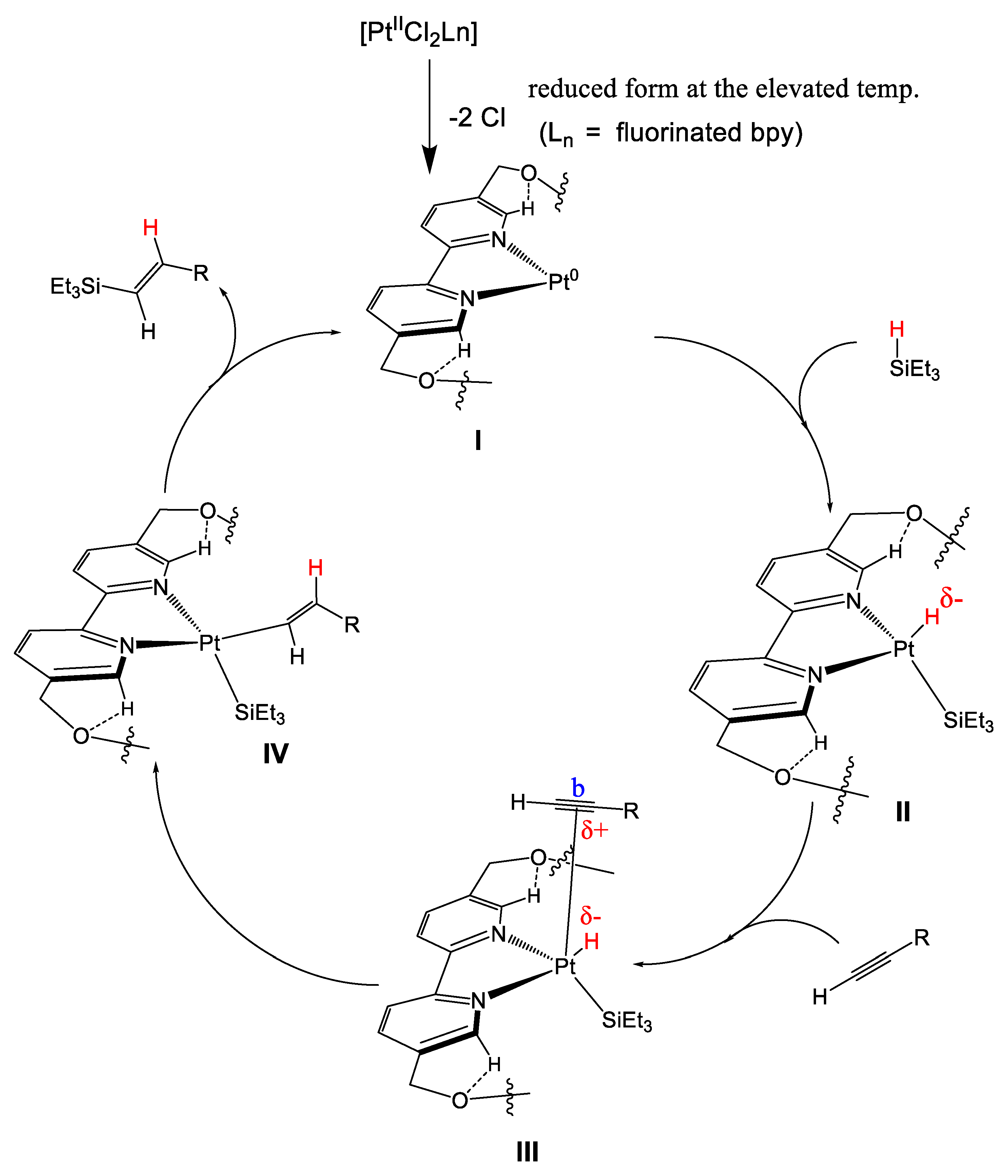
2.2. Recoverable Pt Complex-Catalyzed Hydrosilylation of Alkynes
2.3. Detection of Metal Recovery by ICP-MS
3. Experimental Section
3.1. General Procedures
3.2. General Procedures in Catalytic Hydrosilylation Reaction and Recovery
3.3. Starting Materials
3.4. Preparation of Platinum Complexes
3.5. Analytical Data for Two Ligands and Their Platinum Metal Complexes (1A, 1B, 2A, 2B)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dioumaev, V.K.; Bullock, R.M. A recyclable catalyst that precipitates at the end of the reaction. Nature 2003, 424, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Jankowska-Wajda, M.; Bartlewicz, O.; Walczak, A.; Stefankiewicz, A.R.; Maciejewski, H. Highly efficient hydrosilylation catalysts based on chloroplatinate “ionic liquids”. J. Catal. 2019, 374, 266–275. [Google Scholar] [CrossRef]
- Pospiech, P.; Chojnowski, J.; Mizerska, U.; Cempura, G. Platinum catalyst on polysiloxane microspheres with N-chelating groups. J. Mol. Catal. A Chem. 2016, 424, 402–411. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Li, T.; Yang, C. Synthesis of a fumed silica-supported poly-3-(2-aminoethylamino) propylsiloxane platinum complex and its catalytic behavior in the hydrosilylation of olefins with triethoxysilane. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 728–733. [Google Scholar] [CrossRef]
- Curran, D.P.; Luo, Z. Fluorous synthesis with fewer fluorines (light fluorous synthesis): Separation of tagged from untagged products by solid-phase extraction with fluorous reverse-phase silica gel. J. Am. Chem. Soc. 1999, 121, 9069–9072. [Google Scholar] [CrossRef]
- Dreimann, J.M.; Hoffmann, F.; Skiborowski, M.; Behr, A.; Vorholt, A.J. Merging thermomorphic solvent systems and organic solvent nanofiltration for hybrid catalyst recovery in a hydroformylation process. Ind. Eng. Chem. Res. 2017, 56, 1354–1359. [Google Scholar] [CrossRef]
- Horváth, I.T. Fluorous Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 308. [Google Scholar]
- Horváth, I.T.; Rábai, J. Facile catalyst separation without water: Fluorous biphase hydroformylation of olefins. Science 1994, 266, 72–75. [Google Scholar] [CrossRef]
- Zhang, W. Green chemistry aspects of fluorous techniques—Opportunities and challenges for small-scale organic synthesis. Green Chem. 2009, 11, 911–920. [Google Scholar] [CrossRef]
- Vincent, J.-M.; Contel, M.; Pozzi, G.; Fish, R.H. How the Horváth paradigm, Fluorous Biphasic Catalysis, affected oxidation chemistry: Successes, challenges, and a sustainable future. Coord. Chem. Rev. 2019, 380, 584–599. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Z.; Curran, D.P. Separation of “light fluorous” reagents and catalysts by fluorous solid-phase extraction: Synthesis and study of a family of triarylphosphines bearing linear and branched fluorous tags. J. Org. Chem. 2000, 65, 8866–8873. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Osburn, P.L.; Wilson, A.; Sink, E.M. Palladium-catalyzed C−C coupling under thermomorphic conditions. J. Am. Chem. Soc. 2000, 122, 9058–9064. [Google Scholar] [CrossRef]
- Zielinski, W.; Kukawka, R.; Maciejewski, H.; Smiglak, M. Ionic Liquids as Solvents for Rhodium and Platinum Catalysts Used in Hydrosilylation Reaction. Molecules 2016, 21, 1115. [Google Scholar] [CrossRef] [PubMed]
- Dinh, L.V.; Gladysz, J. “Catalyst-on-a-Tape”—Teflon: A New Delivery and Recovery Method for Homogeneous Fluorous Catalysts. Angew. Chem. Int. Ed. 2005, 44, 4095–4097. [Google Scholar] [CrossRef]
- Wende, M.; Gladysz, J. Fluorous catalysis under homogeneous conditions without fluorous solvents: A “greener” catalyst recycling protocol based upon temperature-dependent solubilities and liquid/solid phase separation. J. Am. Chem. Soc. 2003, 125, 5861–5872. [Google Scholar] [CrossRef]
- Wende, M.; Meier, R.; Gladysz, J. Fluorous catalysis without fluorous solvents: A friendlier catalyst recovery/recycling protocol based upon thermomorphic properties and liquid/solid phase separation. J. Am. Chem. Soc. 2001, 123, 11490–11491. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Ma, W.; Theyssen, N.; Chen, C.; Hou, Z. Temperature-responsive ionic liquids: Fundamental behaviors and catalytic applications. Chem. Rev. 2017, 117, 6881–6928. [Google Scholar] [CrossRef]
- Lu, N.; Chen, S.-C.; Chen, T.-C.; Liu, L.-K. Palladium-catalyzed Heck reaction under thermomorphic mode. Tetrahedron Lett. 2008, 49, 371–375. [Google Scholar] [CrossRef]
- Lu, N.; Chung, W.-C.; Chiang, H.-F.; Fang, Y.-C.; Liu, L.-K. Recoverable platinum bis (fluoro-ponytailed) bipyridine complex as catalyst for hydrosilylation of alkynes under thermomorphic condition. Tetrahedron 2016, 72, 8508–8515. [Google Scholar] [CrossRef]
- Diederich, F.; Stang, P.J. Metal-Catalyzed Cross-Coupling Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Mori, A.; Takahisa, E.; Kajiro, H.; Hirabayashi, K.; Nishihara, Y.; Hiyama, T. RhCl (PPh3)3/NaI Catalyst System for Hydrosilylation of 1-Alkynes: Stereodivergent Syntheses of E-and Z-Alkenylsilanes with Heteroatom Substituents on Silicon. Chem. Lett. 1998, 27, 443–444. [Google Scholar] [CrossRef]
- Mori, A.; Takahisa, E.; Kajiro, H.; Nishihara, Y.; Hiyama, T. Regio-and stereocontrolled hydrosilylation polyaddition catalyzed by RhI(PPh3)3. Syntheses of polymers containing (E)-or (Z)-alkenylsilane moieties. Macromolecules 2000, 33, 1115–1116. [Google Scholar] [CrossRef]
- Sanada, T.; Kato, T.; Mitani, M.; Mori, A. Rhodium-Catalyzed Hydrosilylation of Internal Alkynes with Silane Reagents bearing Heteroatom Substituents. Studies on the Regio-/Stereochemistry and Transformation of the Produced Alkenylsilanes by Rhodium-Catalyzed Conjugate Addition. Adv. Synth. Catal. 2006, 348, 51–54. [Google Scholar] [CrossRef]
- Mori, A.; Takahisa, E.; Nishihara, Y.; Hiyama, T. Isomerization of (Z)-alkenylsilanes to (E)-isomers with hydrosilane and RhI (PPh3)3. Can. J. Chem. 2001, 79, 1522–1524. [Google Scholar] [CrossRef]
- Mori, A.; Kato, T. unsaturated carbonyl compounds. Synlett 2002, 7, 1167–1169. [Google Scholar] [CrossRef]
- Ojima, I.; Li, Z.; Zhu, J. The Chemistry of Organic Silicon Compounds; Patai, S., Rappoport, Z., Eds.; John Wiley and Son: New York, NY, USA, 1989; p. 1479. [Google Scholar]
- Hiyama, T.T. Kusumoto in Comprehensive Organic Synthesis; Pergamon: Oxford, UK, 1991; Volume 8. [Google Scholar]
- Trost, B.M.; Ball, Z.T.; Jöge, T. A chemoselective reduction of alkynes to (E)-alkenes. J. Am. Chem. Soc. 2002, 124, 7922–7923. [Google Scholar] [CrossRef]
- Denmark, S.E.; Pan, W. Intramolecular anti-hydrosilylation and silicon-assisted cross-coupling: Highly regio-and stereoselective synthesis of trisubstituted homoallylic alcohols. Org. Lett. 2002, 4, 4163–4166. [Google Scholar] [CrossRef]
- Lewis, L.N.; Sy, K.G.; Bryant, G.L., Jr.; Donahue, P.E. Platinum-catalyzed hydrosilylation of alkynes. Organometallics 1991, 10, 3750–3759. [Google Scholar] [CrossRef]
- De Bo, G.; Berthon-Gelloz, G.; Tinant, B.; Markó, I.E. Hydrosilylation of alkynes mediated by N-heterocyclic carbene platinum (0) complexes. Organometallics 2006, 25, 1881–1890. [Google Scholar] [CrossRef]
- Wu, C.; Teo, W.J.; Ge, S. Cobalt-catalyzed (e)-selective anti-Markovnikov hydrosilylation of terminal alkynes. ACS Catal. 2018, 8, 5896–5900. [Google Scholar] [CrossRef]
- Silbestri, G.F.; Flores, J.C.; De Jesús, E. Water-soluble N-heterocyclic carbene platinum (0) complexes: Recyclable catalysts for the hydrosilylation of alkynes in water at room temperature. Organometallics 2012, 31, 3355–3360. [Google Scholar] [CrossRef]
- Hitchcock, P.B.; Lappert, M.F.; Warhurst, N.J. Synthesis and Structure of a rac-Tris (divinyldisiloxane) diplatinum (0) Complex and its Reaction with Maleic Anhydride. Angew. Chem. Int. Ed. Engl. 1991, 30, 438–440. [Google Scholar] [CrossRef]
- Mollar Cuni, A.; Borja, M.P.; Guisado-Barrios, G.; Mata Martínez, J.A. A Platinum molecular complex immobilised on the surface of graphene as active catalyst in alkyne hydrosilylation. EurJIC 2020, 45, 4254–4262. [Google Scholar]
- Chauhan, B.P.; Sarkar, A. Functionalized vinylsilanes via highly efficient and recyclable Pt-nanoparticle catalysed hydrosilylation of alkynes. Dalton Trans. 2017, 46, 8709–8715. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Huang, B.; Yan, T.; Cai, M. A recyclable and reusable K 2PtCl4/Xphos-SO3Na/PEG-400/H2O system for highly regio-and stereoselective hydrosilylation of terminal alkynes. Green Chem. 2018, 20, 391–397. [Google Scholar] [CrossRef]
- Rivero-Crespo, M.A.; Leyva-Pérez, A.; Corma, A. A Ligand-Free Pt3 Cluster Catalyzes the Markovnikov Hydrosilylation of Alkynes with up to 106 Turnover Frequencies. Chem. A Eur. J. 2017, 23, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Ball, Z.T. Markovnikov alkyne hydrosilylation catalyzed by ruthenium complexes. J. Am. Chem. Soc. 2001, 123, 12726–12727. [Google Scholar] [CrossRef]
- Zuo, Z.; Yang, J.; Huang, Z. Cobalt-catalyzed alkyne hydrosilylation and sequential vinylsilane hydroboration with markovnikov selectivity. Angew. Chem. Int. Ed. 2016, 55, 10839–10843. [Google Scholar] [CrossRef]
- Mori, A.; Takahisa, E.; Yamamura, Y.; Kato, T.; Mudalige, A.P.; Kajiro, H.; Hirabayashi, K.; Nishihara, Y.; Hiyama, T. Stereodivergent syntheses of (Z)-and (E)-alkenylsilanes via hydrosilylation of terminal alkynes catalyzed by rhodium (I) iodide complexes and application to silicon-containing polymer syntheses. Organometallics 2004, 23, 1755–1765. [Google Scholar] [CrossRef]
- Ubeda, M.A.; Dembinski, R. Fluorous compounds and their role in separation chemistry. J. Chem. Educ. 2006, 83, 84–92. [Google Scholar] [CrossRef]
- Lu, N.; Ou, Y.-M.; Feng, T.-Y.; Cheng, W.-J.; Tu, W.-H.; Su, H.-C.; Wang, X.; Liu, L.; Hennek, M.D.; Sayler, T.S. Synthesis and characterization of polyfluorinated 2, 2′-bipyridines and their palladium and platinum complexes,[MX2 (bis (RfCH2OCH2)-2, 2′-bpy)](X = Cl, Br). J. Fluor. Chem. 2012, 137, 54–63. [Google Scholar] [CrossRef]
- Alonso, F.; Buitrago, R.; Moglie, Y.; Ruiz-Martínez, J.; Sepúlveda-Escribano, A.; Yus, M. Hydrosilylation of alkynes catalysed by platinum on titania. J. Organomet. Chem. 2011, 696, 368–372. [Google Scholar] [CrossRef]
- Meister, T.K.; Riener, K.; Gigler, P.; Stohrer, J.R.; Herrmann, W.A.; Kühn, F.E. Platinum Catalysis Revisited: Unraveling Principles of Catalytic Olefin Hydrosilylation. ACS Catal. 2016, 6, 1274–1284. [Google Scholar] [CrossRef]
- Cano, R.; Yus, M.; Ramón, D.J. Impregnated platinum on magnetite as an efficient, fast, and recyclable catalyst for the hydrosilylation of alkynes. ACS Catal. 2012, 2, 1070–1078. [Google Scholar] [CrossRef]
- Lu, N.; Zheng, J.H.; Lin, L.C.; Liu, L.K.; Chiang, H.F.; Li, T.Y.; Wen, Y.S.; Yang, C.K.; Chen, S.W.; Thrasher, J.S. Studies of two different types of intramolecular C–H···F–C interactions from polyfluorinated diiodometal (II) diimine complexes. J. Chin. Chem. Soc. 2019, 66, 31–40. [Google Scholar] [CrossRef]
- Lu, N.; Lu, Y.J.; Huang, J.Y.; Chiang, H.F.; Kung, C.C.; Wen, Y.S.; Liu, L.K. The Evolution Aspect in the Crystallization Process of [5,5′-(HCF2CF2CH2OCH2)2-2,2′-bpy]MX2 (M = Pt, Pd; X = I, Br): Role of the Intramolecular CF2─HX (─M) Hydrogen Bonds. J. Chin. Chem. Soc. 2018, 65, 613–627. [Google Scholar] [CrossRef]
- Islamova, R.M.; Dobrynin, M.V.; Ivanov, D.M.; Vlasov, A.V.; Kaganova, E.V.; Grigoryan, G.V.; Kukushkin, V.Y. bis-Nitrile and bis-dialkylcyanamide platinum (II) complexes as efficient catalysts for hydrosilylation cross-linking of siloxane polymers. Molecules 2016, 21, 311. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.L.; Lu, N.; Friesen, C.M. New derivatives of poly-hexafluoropropylene oxide from the corresponding alcohol. J. Fluor. Chem. 2005, 126, 281–288. [Google Scholar] [CrossRef]
- Lu, N.; Tu, W.H.; Hou, H.C.; Lin, C.T.; Li, C.K.; Liu, L.K. Synthesis, structure and spectroelectrochemical property of (2,2′-bipyridine)–metal (M = Pt, Pd) dichloride with 4,4ʹ-bis (fluorous-ponytail) on bipyridine. Polyhedron 2010, 29, 1123–1129. [Google Scholar] [CrossRef]
- Li, C.-K.; Ghalwadkar, A.; Lu, N. Recoverable cationic Pd-catalyzed Heck reaction under thermomorphic mode. J. Organomet. Chem. 2011, 696, 3637–3642. [Google Scholar] [CrossRef]
- Periyanagounder, D.; Wei, T.C.; Li, T.Y.; Lin, C.H.; Gonçalves, T.P.; Fu, H.C.; Tsai, D.S.; Ke, J.J.; Kuo, H.W.; Huang, K.W.; et al. Fast-Response, Highly Air-Stable, and Water-Resistant Organic Photodetectors Based on a Single-Crystal Pt Complex. Adv. Mater. 2020, 32, 1904634. [Google Scholar] [CrossRef]


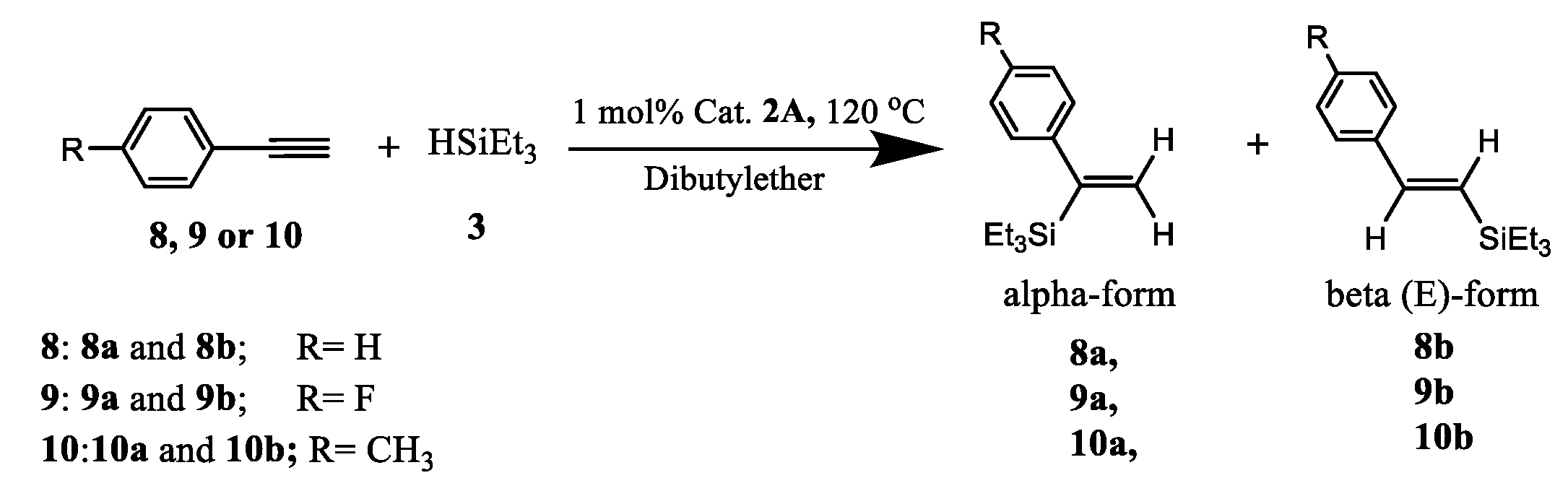
| 55-8FCl-PtCl2 (2A) | 55-8FH-PtCl2 (2B) | |||
|---|---|---|---|---|
| Run | Time (h) | Yield (%) a | Time (h) | Yield (%) a |
| 1 | 2 | 100 | 2 | 100 |
| 2 | 2 | 99 (89) | 2 | 100 |
| 3 | 2 | 100 (94) | 2 | 94 |
| 4 | 2 | 100 | 2 | 100 (91) |
| 5 | 2 | 100 | 2 | 100 (94) |
| 6 | 2 | 100 | 2 | 90 |
| 7 | 2 | 100 | 2 | 100 |
| 8 | 2 | 100 | 2 | 96 |
| Cycle | Time (h) | Yield (%) a |
|---|---|---|
| 1 | 1 | 100 |
| 2 | 1 | 98 |
| 3 | 1 | 100 (97) |
| 4 | 1 | 100 |
| 5 | 1 | 100 |
| 6 | 1 | 100 |
| 7 | 1 | 100 (97) |
| 8 | 1 | 100 |
| Cycle | Time (h) | 5a:5b | Yield (%) a |
|---|---|---|---|
| 1 | 1 | 1:1.58 | 100 |
| 2 | 1 | 1:1.67 | 100 (96) |
| 3 | 1 | 1:1.48 | 100 (92) |
| 4 | 1 | 1:1.77 | 100 |
| 5 | 1 | 1:1.82 | 100 (92) |
| 6 | 1 | 1:1.74 | 100 |
| 7 | 1 | 1:1.40 | 100 (99) |
| 8 | 1 | 1:1.58 | 100 (91) |
| Cycle | Time (h) | 7a:7b | Yield (%) a |
|---|---|---|---|
| 1 | 1 | 1:2.1 | 100 (96) |
| 2 | 1 | 1:2.1 | 99 |
| 3 | 1 | 1:2.0 | 99 (92) |
| 4 | 1 | 1:2.2 | 99 |
| 5 | 1 | 1:2.1 | 98 |
| 6 | 1 | 1:1.9 | 100 |
| 7 | 1 | 1:2.0 | 100 |
| 8 | 1 | 1:2.0 | 99 |
| Cycle | Time (h) | 8a:8b | Yield (%) a |
|---|---|---|---|
| 1 | 2 | 2.02:1 | 99 (92) |
| 2 | 2 | 2.25:1 | 99 (96) |
| 3 | 2 | 2.26:1 | 99 |
| 4 | 2 | 2.25:1 | 100 (98) |
| 5 | 2 | 2.21:1 | 100 |
| 6 | 2 | 2.20:1 | 100 |
| 7 | 2 | 1.86:1 | 100 |
| 8 | 2 | 1.80:1 | 100 |
| Table (& Cycle) | Catalyst | Ppb a | Leaching Level (10–4) b | Recovery (%) |
|---|---|---|---|---|
| 1–2 | 2A | 517.5 | 6.63 | 99.93 |
| 1–3 | 2A | 444.7 | 5.70 | 99.94 |
| 1–7 | 2A | 137.1 | 1.76 | 99.98 |
| 1–8 | 2A | 41.6 | 0.53 | 99.99 |
| 1–2 | 2b | 21.7 | 0.28 | 99.99 |
| 1–3 | 2B | 31.3 | 0.40 | 99.99 |
| 1–7 | 2B | 56.6 | 0.72 | 99.99 |
| 1–8 | 2B | 43.6 | 0.56 | 99.99 |
| 2–4 | 2A | 29.9 | 0.38 | 99.99 |
| 3–2 | 2A | 107.5 | 1.38 | 99.98 |
| 3–4 | 2A | 79.4 | 1.02 | 99.98 |
| 3–6 | 2A | 60.7 | 0.78 | 99.99 |
| S1–4 c | 2A | 98.0 | 1.26 | 99.98 |
| S1–7 c | 2A | 1753.0 | 22.50 | 99.77 |
| S1–8 c | 2A | 2347.0 | 30.10 | 99.69 |
| 4–4 | 2A | 276.5 | 3.54 | 99.96 |
| 5–4 | 2A | 805.7 | 10.30 | 99.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-F.; Ho, J.-H.; Tessema, E.; Lu, Y.; Shen, C.-R.; Lin, C.-W.; Lu, N. Short-Chained Platinum Complex Catalyzed Hydrosilylation under Thermomorphic Conditions: Heterogeneous Phase Separation at Ice Temperature. Molecules 2021, 26, 378. https://doi.org/10.3390/molecules26020378
Chiu C-F, Ho J-H, Tessema E, Lu Y, Shen C-R, Lin C-W, Lu N. Short-Chained Platinum Complex Catalyzed Hydrosilylation under Thermomorphic Conditions: Heterogeneous Phase Separation at Ice Temperature. Molecules. 2021; 26(2):378. https://doi.org/10.3390/molecules26020378
Chicago/Turabian StyleChiu, Chiao-Fan, Jinn-Hsuan Ho, Eskedar Tessema, Yijing Lu, Chia-Rui Shen, Chang-Wei Lin, and Norman Lu. 2021. "Short-Chained Platinum Complex Catalyzed Hydrosilylation under Thermomorphic Conditions: Heterogeneous Phase Separation at Ice Temperature" Molecules 26, no. 2: 378. https://doi.org/10.3390/molecules26020378
APA StyleChiu, C.-F., Ho, J.-H., Tessema, E., Lu, Y., Shen, C.-R., Lin, C.-W., & Lu, N. (2021). Short-Chained Platinum Complex Catalyzed Hydrosilylation under Thermomorphic Conditions: Heterogeneous Phase Separation at Ice Temperature. Molecules, 26(2), 378. https://doi.org/10.3390/molecules26020378