Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds
Abstract
1. Introduction
2. Results
2.1. Mineral Content in Leaf Buds
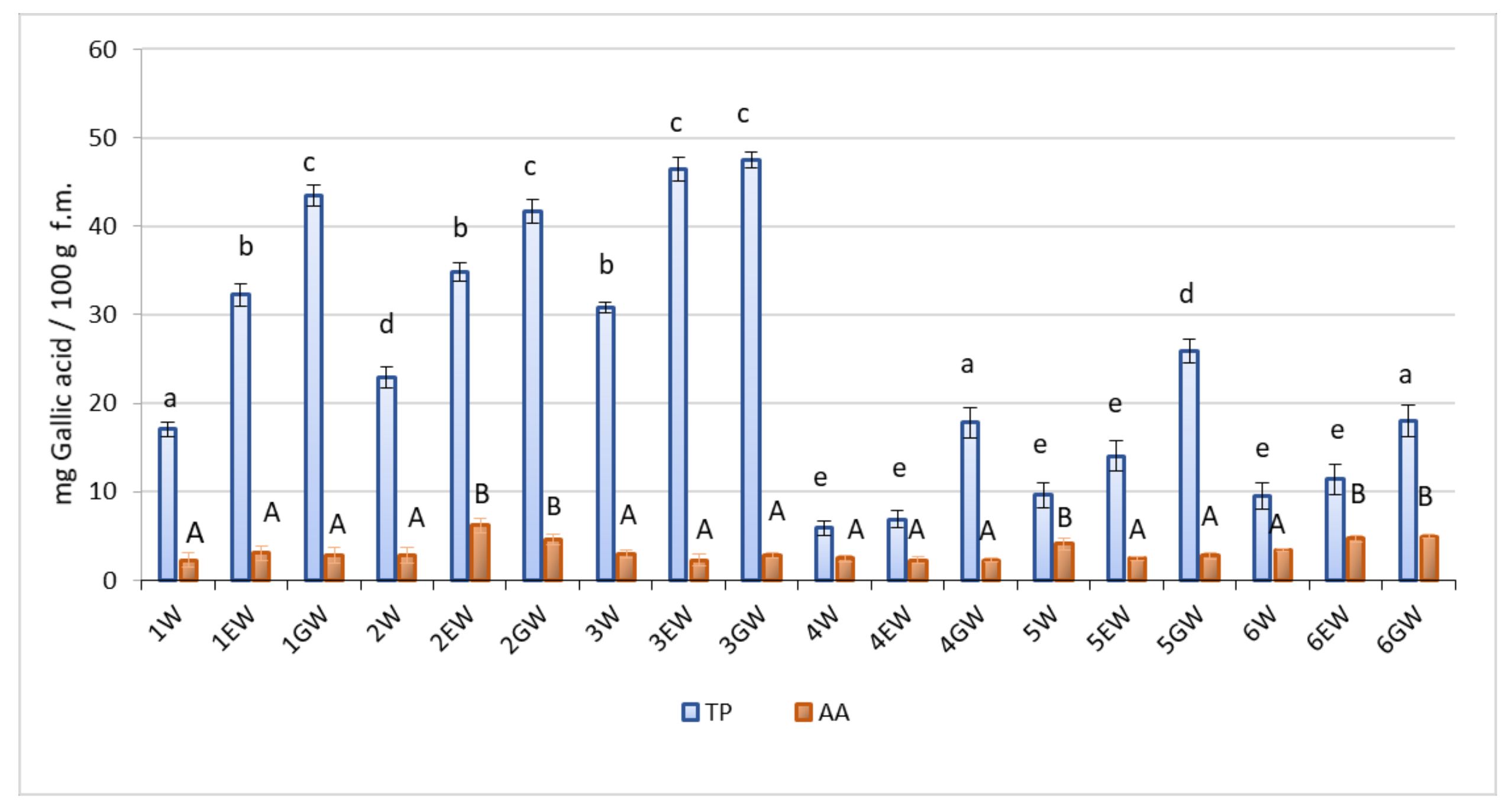
2.2. TP and AA Contents
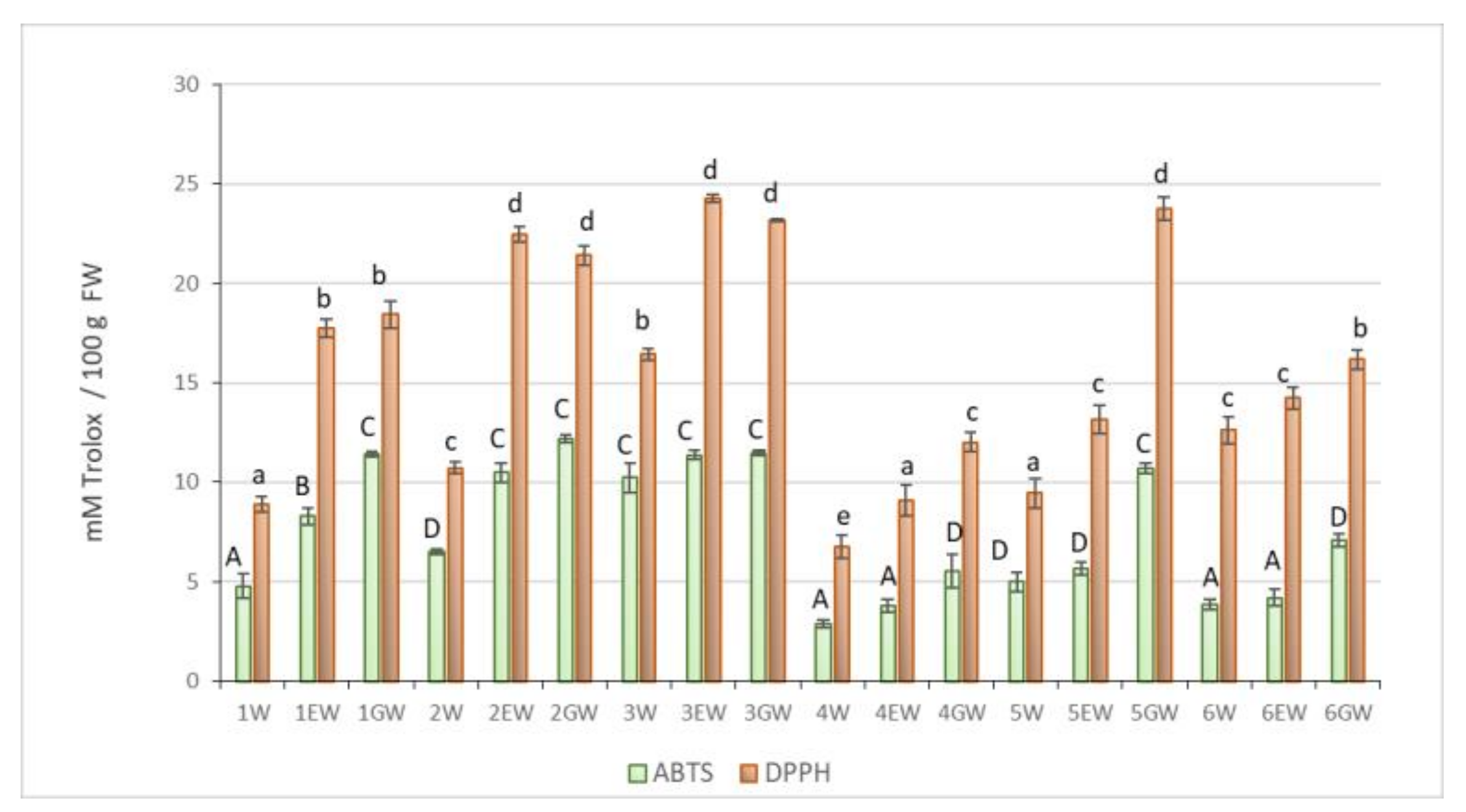
2.3. Total Antioxidant Capacity
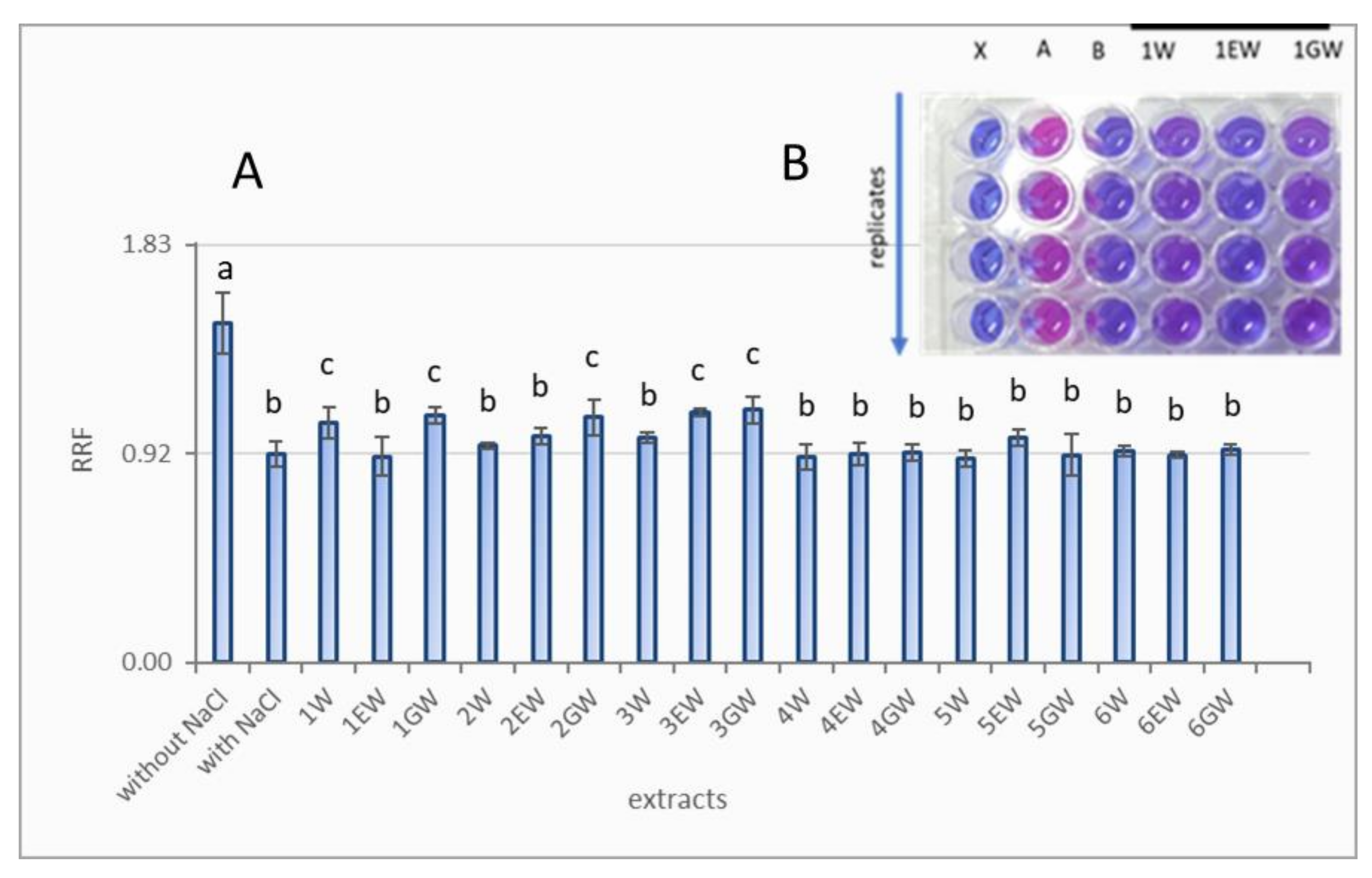
2.4. Antioxidant Yeast Test Based on Growth ∆sod1 Mutant in Hypertonic Medium
2.5. Antibacterial Activity of Leaf Bud Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Microorganisms
4.3. Determination of Mineral Composition
4.4. Extract Preparation
4.5. Determination of Total Antioxidant Capacity (TAC)
4.6. Determination of TP and AA Content
4.7. Antioxidant Yeast Test
4.8. Determination of the Antimicrobial Activity of the Extracts
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Biomolecules and natural medicine preparations: Analysis of new sources of bioactive compounds from Ribes and Rubus spp. buds. Pharmaceuticals 2016, 9, 7. [Google Scholar] [CrossRef]
- Winiarska, J.; Szember, E.; Żmuda, E.; Murawska, D. Comparison of chemical composition of fruit in chosen raspberry cultivars Rubeus idaeus L. Ann. UMCS Sect. EEE Hortic. 2005, XV, 29–33. [Google Scholar]
- Czech, A.; Rusinek, E.; Merska, M. Content of selected bio-elements in berry fruit and juice. Probl. Hig. Epidemiol. 2011, 92, 836–839. (In Polish) [Google Scholar]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101–104. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins, ellagic acid in leaves from Rubus L. species. Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Skwaryło-Bednarz, B.; Święciło, A. Buds, leaves, and seeds of blackcurrant—Source of bioactive substances with pro-health properties. Food Sci. Technol. Qual. 2018, 2, 24–33. (In Polish) [Google Scholar]
- Święciło, A.; Rybczyńska-Tkaczyk, K.; Najda, A.; Krzepiłko, A.; Prażak, R.; Zawiślak, G. Application of growth tests employing a ∆sod1 mutant of Saccharomyces cerevisiae to study the antioxidant activity of berry fruit extracts. LWT Food Sci. Technol. 2018, 92, 96–102. [Google Scholar]
- Świeciło, A.; Rybczyńska-Tkaczyk, K. Resazurin method for evaluation of bioactive compounds from cranberry extracts using the metabolic activity of a Δsod1 mutant of Saccharomyces cerevisiae yeast under severe osmotic stress. J. AOAC Int. 2020, 103, 422–427. [Google Scholar] [CrossRef]
- Sanchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Hargreaves, J.; Adl, M.S.; Warman, P.R.; Rupasinghe, H.P.V. The effects of organic amendments on mineral element uptake and fruit quality of raspberries. Plant Soil 2008, 308, 213–226. [Google Scholar] [CrossRef]
- Koumanov, K.S.; Tsareva, I.; Klev, K.; Kornov, G. Fertigation of primocane-fruiting raspberry—Leaf and soil nutrient content between applications. Acta Hort. 2009, 825, 341–348. [Google Scholar] [CrossRef]
- Horuz, A.; Korkmaz, A.; Karaman, M.R.; Dizman, M.; Turan, M. The evaluation of leaf nutrient contents and element ratios of different raspberry varieties. J. Food Agric. Environ. 2013, 1, 588–593. [Google Scholar]
- Karaklajić-Stajić, Ž.; Glišić, I.S.; Ružić, D.J.; Vujović, T.; Pešaković, M. Microelements content in leaves of raspberry cv. Willamette as affected by foliar nutrition and substrates. Hort. Sci. 2019, 39, 67–73. [Google Scholar]
- Kessel, C. Fertilizing raspberries—Raspberry leaf analysis. Fruit Prod. Recom. 2003, 360, 1–3. [Google Scholar]
- Dresler, S.; Bednarek, W.; Tkaczyk, P.; Hawrylak-Nowak, B. Estimation of the macro- and micronutrient status of raspberries grown in the Lublin region. Folia Hort. 2015, 27, 53–62. [Google Scholar] [CrossRef]
- Heghedus-Mindru, R.C.; Heghedus Mindru, G.; Negrea, P.; Sumalan, R.; Negrea, A.; Stef, D. 2014.The monitorin gofmineral elements content in fruit purchased in supermarkets and food markets in Timisoara, Romania. Ann. Agric. Environ. Med. 2014, 21, 98–105. [Google Scholar]
- Koczka, N.; Stefanovits-Bányai, E.; Prokaj, E. Element composition, total phenolics and antioxidant activity of wild and cultivated Blackberry (Rubus fruticosus L.) fruits and leaves during the harvest time. Not. Bot. Horti. Agrobot. 2018, 46, 563–569. [Google Scholar] [CrossRef]
- Misimović, M.; Lakić, Ž.; Maličević, Z. Effects of different plant extracts on the mineral content of blackberry leaf (R. fruticosus) in organic production. Agric. Forest. 2020, 66, 143–151. [Google Scholar] [CrossRef]
- Bosiacki, M.; Tyksiński, W. Copper, zinc, iron and manganese content in edible parts of some fresh vegetables sold on markets in Poznań. J. Elem. 2009, 14, 13–22. [Google Scholar] [CrossRef]
- Ancuceanu, R.; Dinu, M.; Hovaneţ, M.V.; Anghel, A.I.; Popescu, C.V.; Negreş, A. Survey of plant iron content-a semi-systematic review. Nutrients 2015, 7, 10320–10351. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Sariburun, E.; Sahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, J.; Maksimovic, V.; Nikolic, M.; Bogdanovic, J.; Maletic, R.; Milatovic, D. Chemical and antioxidant properties of cultivated and wild Fragaria and Rubus berries. J. Food Qual. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Fan-Chiang, H.J.; Wrolstad, R.E. Anthocyanin pigment composition of blackberries. J. Food Sci. 2005, 70, 198–202. [Google Scholar] [CrossRef]
- Vatai, T.; Skerget, M.; Knez, Z.; Kareth, S.; Wachowski, M.; Weidner, E. Extraction and formulation of anthocyanin concentrates from grape residues. J. Supercrit. Fluids 2008, 45, 32–36. [Google Scholar] [CrossRef]
- Celant, V.M.; Braga, G.C.; Vorpagel, J.A.; Salibe, A.B. Phenolic composition and antioxidant capacity of aqueous and ethanolic extracts of blackberries. Rev. Bras. Frutic. 2016, 38, e-411. [Google Scholar] [CrossRef][Green Version]
- Pantelidis, G.E.; Vasilakakio, M.; Manganaris, G.A.; Diamantidis, G.R. Antioxidant capacity phenol anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants gooseberries, and cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Cata, A.; Stefanut, M.N.; Pop, R.; Tanasie, C.; Mosoarca, C.; Zamfir, A.D. Evaluation of antioxidant activities of some small fruits containing anthocyanins using electrochemical and chemical methods. Croat. Chem. Acta. 2016, 89, 37–48. [Google Scholar] [CrossRef]
- Stajčić, S.M.; Tepić, A.N.; Djilas, S.M.; Šumić, Z.M.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Vulić, J.J.; Tumbas, V.T. Chemical composition and antioxidant activity of berry fruits. Acta Period. Technol. 2012, 43, 93–105. [Google Scholar] [CrossRef]
- Rimac-Brnčić, S.; Sabolović, B.M.; Žlabur, Š.J.; Jelovečki, M. Colour stability and antioxidant activity of some berry extracts. Croat. J. Food Technol. Biotechnol. Nutr. 2015, 10, 115–119. [Google Scholar]
- Ponder, A.; Hallmann, E. Phenolics and carotenoid contents in the leaves of different organic and conventional raspberry (Rubus idaeus L.) cultivars and their in vitro activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef]
- García-Alonso, F.J.; Navarro-González, O.; Ros, G.; Periago, M.J. Assessment of the antioxidant properties of tomato extracts. A synergistic approach using in vitro chemical tests and cell-based assays. Acta Aliment. 2015, 44, 297–303. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharm. 2014, 1, 35–40. [Google Scholar]
- Bolling, B.W.; Chen, Y.Y.; Chen, C.Y.O. Contributions of phenolics and added vitamin C to the antioxidant capacity of pomegranate and grape juices: Synergism and antagonism among constituents. Int. J. Food Sci. Technol. 2013, 48, 2650–2658. [Google Scholar] [CrossRef]
- Dos Santos, G.; Solidônio, E.; Costa, M.; Melo, R.; De Souza, I.; Silva-Lacerada, G.; Sena, K. Study of the Enterobacteriaceae group CESP (Citrobacter, Enterobacter, Serratia, Providencia, Morganella and Hafnia). In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs, 2nd ed.; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2015; pp. 794–805. [Google Scholar]
- Vogel, M.; Schmitz, R.P.H.; Hagel, S.; Pletz, M.W. Infectious disease consultation for Staphylococcus aureus bacteremia—A systematic review and meta-analysis. J. Infect. 2016, 72, 19–28. [Google Scholar] [CrossRef]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018, 7, 303–309. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac. J. Trop. Med. 2017, 10, 729–743. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Bioph. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.-D.-D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Skroza, D.; Šimat, V.; Možina, S.S.; Katalinić, V.; Boban, N.; Mekinić, I.G. Interactions of resveratrol with other phenolics and activity against food-borne pathogens. Food Sci. Nutr. 2019, 7, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Parekh, J.; Jadeja, D.; Chanda, S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 2005, 29, 203–210. [Google Scholar]
- Vieitez, I.; Maceiras, L.; Jachmanián, I.; Alborés, S. Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. J. Supercrit. Fluids 2018, 133, 58–64. [Google Scholar] [CrossRef]
- PN-EN ISO 6869:2002. Forage. Determine the Content of Calcium, Copper, Iron, Magnesium, Manganese and Zinc-Method by Atomic Absorption Spectrometry; ISO: Warszawa, Poland, 2002; pp. 1–18. (In Polish)
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenyl picrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A. Effect of extraction solvents on the phenolic content and antioxidant properties of two papaya cultivars. J. Med. Plants Res. 2013, 7, 3354–3359. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.P.; Huang, D.; Dng, C.N. Antioxidant activity and profiles of common vegetables in Singapore. Food Chem. 2020, 120, 993–1003. [Google Scholar] [CrossRef]
- Zyracka, E.; Zadrag, R.; Koziol, S.; Krzepilko, A.; Bartosz, G.; Bilinski, T. Ascorbate abolishes auxotrophy caused by the lack of superoxide dismutase in Saccharomyces cerevisiae. Yeast can be a biosensor for antioxidants. J. Biotechnol. 2005, 115, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Koziol, S.; Zagulski, M.; Bilinski, T.; Bartosz, G. Antioxidants protect the yeast Saccharomyces cerevisiae against hypertonic stress. Free Radic. Res. 2005, 39, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.A.; Bailey, S.; Fukuto, J.M.; Valentine, J.S.; Gralla, E.B. Induction of phenotypes resembling CuZn-superoxide dismutase deletion in wild-type yeast cells: An in vivo assay for the role of superoxide in the toxicity of redox-cycling compounds. Chem. Res. Toxicol. 2005, 18, 1279–1286. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Pączka, A.; Małoń, M.; Bartosz, G. Dimethyl sulfoxide induces oxidative stress in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 13, 820–830. [Google Scholar] [CrossRef]
- Osaka, I.; Hefty, P.S. Simple resazurin-based microplate assay for measuring Chlamydia infections. Antimicrob. Agents Chemother. 2013, 57, 2838–2840. [Google Scholar] [CrossRef]
- Castilho, A.L.; Caleffi-Ferracioli, K.R.; Canezin, P.H.; Dias Siqueira, V.L.; de Lima Scodro, R.B.; Cardoso, R.F. Detection of drug susceptibility in rapidly growing mycobacteria by resazurin broth microdilution assay. J. Microbiol. Methods. 2015, 2015. 111, 119–121. [Google Scholar] [CrossRef]
- CSLI. Performers Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 7th ed.; CSLI Document M02-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78, 633–639. [Google Scholar] [CrossRef]
| Forms * | Mineral Content-mg 100 g−1 DW | ||||||
|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Fe | Zn | Mn | Cu | |
| 1 | 609.04 ± 35.21a | 219.97 ± 15.21a | 91.58 ± 2.15a | 23.45 ± 5.43a | 2.03 ± 0.09a | 0.39 ± 0.10a | 0.10 ± 0.04a |
| 2 | 630.32 ± 26.47a | 222.40 ± 12.80a | 95.63 ± 2.49a | 24.02 ± 5.61a | 1.83 ± 0.08b | 0.58 ± 0.01b | 0.09 ± 0.03a |
| 3 | 638.04 ± 17.50a | 188.74 ± 8.29b | 89.16 ± 6.37a | 15.90 ± 1.42b | 2.76 ± 0.31c | 0.72 ± 0.08c | 0.12 ± 0.02a |
| 4 | 687.54 ± 12.21c | 154.48 ± 16.05c | 91.20 ± 4.16a | 11.77 ± 4.48c | 1.85 ± 0.07b | 0.46 ± 0.11ab | 0.18 ± 0.03c |
| 5 | 448.93 ± 14.28b | 195.50 ± 9.32b | 76.27 ± 3.37b | 19.14 ± 1.20a | 1.72 ± 0.11b | 0.62 ± 0.02c | 0.27 ± 0.05b |
| 6 | 662.70 ± 19.34ac | 206.73 ± 10.34ab | 111.15 ± 4.55c | 22.95 ± 4.34a | 1.44 ± 0.12d | 1.05 ± 0.12d | 0.17 ± 0.02c |
| Method of Analysis | AA | TP | ABTS | DPPH |
|---|---|---|---|---|
| AA | 1.00 | |||
| TP | 1.00 | |||
| ABTS | 0.94 | 1.00 | ||
| DPPH | 0.28 | 0.84 | 0.90 | 1.00 |
| Table | Analysis | W | EW | GW | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TP | ABTS | DPPH | TP | ABTS | DPPH | TP | ABTS | DPPH | ||
| W | TP | 1.00 | - | - | - | - | - | - | - | - |
| ABTS | 0.92 | 1.00 | - | - | - | - | - | - | - | |
| DPPH | 0.73 | 0.81 | 1.00 | - | - | - | - | - | - | |
| EW | TP | 0.97 | 0.88 | 0.63 | 1.00 | - | - | - | - | - |
| ABTS | 0.96 | 0.86 | 0.56 | 0.97 | 1.00 | - | - | - | - | |
| DPPH | 0.96 | 0.87 | 0.72 | 0.95 | 0.96 | 1.00 | - | - | - | |
| GW | TP | 0.90 | 0.78 | 0.46 | 0.96 | 0.95 | 0.89 | 1.00 | - | - |
| ABTS | 0.75 | 0.67 | - | 0.81 | 0.84 | 0.80 | 0.87 | 1.00 | - | |
| DPPH | 0.62 | 0.72 | 0.53 | 0.62 | 0.67 | 0.66 | 0.62 | 0.85 | 1.00 | |
| Extract * | The Amount of Antioxidant Substances Per Disc | Microorganism/Zone of Inhibition [mm] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TPC [µg GA] | ABTS [µM trolox] | DPPH [µM trolox] | EA | EC | EF | SA | SE | SM | |
| 1W | 0.19 | 0.05 | 0.24 | 0 | 0 | 4 | 3 | 0 | 0 |
| 1EW | 0.35 | 0.09 | 0.44 | 2 | 0 | 8 | 5 | 0 | 0 |
| 1GW | 0.47 | 0.12 | 0.60 | 0 | 0 | 14.5 | 8 | 0 | 0 |
| 2W | 0.25 | 0.07 | 0.32 | 0 | 0 | 3 | 4.5 | 0 | 0 |
| 2EW | 0.38 | 0.11 | 0.49 | 3.5 | 0 | 4 | 3 | 0 | 0 |
| 2GW | 0.45 | 0.13 | 0.59 | 0 | 0 | 14 | 4 | 0 | 0 |
| 3W | 0.34 | 0.11 | 0.45 | 0 | 0 | 2 | 4.7 | 0 | 0 |
| 3EW | 0.51 | 0.12 | 0.63 | 1.5 | 0 | 6 | 4.5 | 0 | 0 |
| 3GW | 0.52 | 0.12 | 0.64 | 0 | 0 | 10.5 | 5.5 | 0 | 0 |
| 4W | 0.06 | 0.03 | 0.09 | 0 | 0 | 1 | 1.3 | 0 | 0 |
| 4EW | 0.07 | 0.04 | 0.12 | 1.3 | 0 | 4 | 1.5 | 0 | 0 |
| 4GW | 0.19 | 0.06 | 0.25 | 0 | 0 | 8 | 6 | 0 | 0 |
| 5W | 0.10 | 0.05 | 0.16 | 0 | 0 | 2 | 2 | 0 | 0 |
| 5EW | 0.15 | 0.06 | 0.21 | 2.4 | 0 | 1 | 2 | 0 | 0 |
| 5GW | 0.28 | 0.12 | 0.40 | 0 | 0 | 9.5 | 7.4 | 0 | 0 |
| 6W | 0.10 | 0.04 | 0.15 | 0 | 0 | 5 | 6 | 0 | 0 |
| 6EW | 0.12 | 0.05 | 0.17 | 1.3 | 0 | 2 | 4 | 0 | 0 |
| 6GW | 0.20 | 0.07 | 0.27 | 0 | 0 | 7.5 | 7.2 | 0 | 0 |
| Kanamycin | 23 | 23 | 26 | 23.8 | 27 | 26 | |||
| Significant values of correlation coefficients at α = 0.05 between the TP amount and the growth inhibition zone | - | - | 0.68 | 0.51 | - | - | |||
| Significant values of correlation coefficients at α = 0.05 between the ABTS specific antioxidants amount and the growth inhibition zone | - | - | 0.68 | 0.53 | - | - | |||
| Significant values of correlation coefficients at α = 0.05 between the DPPH specific antioxidant amount and the growth inhibition zone | - | - | 0.70 | 0.52 | - | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules 2021, 26, 327. https://doi.org/10.3390/molecules26020327
Krzepiłko A, Prażak R, Święciło A. Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules. 2021; 26(2):327. https://doi.org/10.3390/molecules26020327
Chicago/Turabian StyleKrzepiłko, Anna, Roman Prażak, and Agata Święciło. 2021. "Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds" Molecules 26, no. 2: 327. https://doi.org/10.3390/molecules26020327
APA StyleKrzepiłko, A., Prażak, R., & Święciło, A. (2021). Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules, 26(2), 327. https://doi.org/10.3390/molecules26020327






