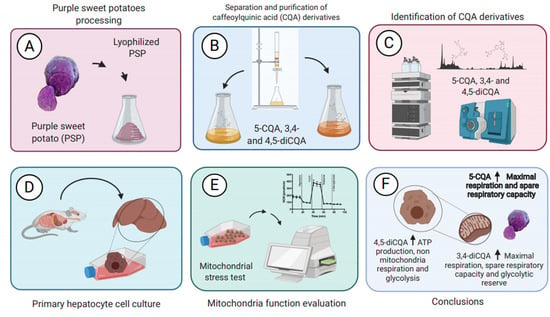
Caffeoylquinic Acid Derivatives of Purple Sweet Potato as Modulators of Mitochondrial Function in Mouse Primary Hepatocytes
Abstract
1. Introduction
2. Results
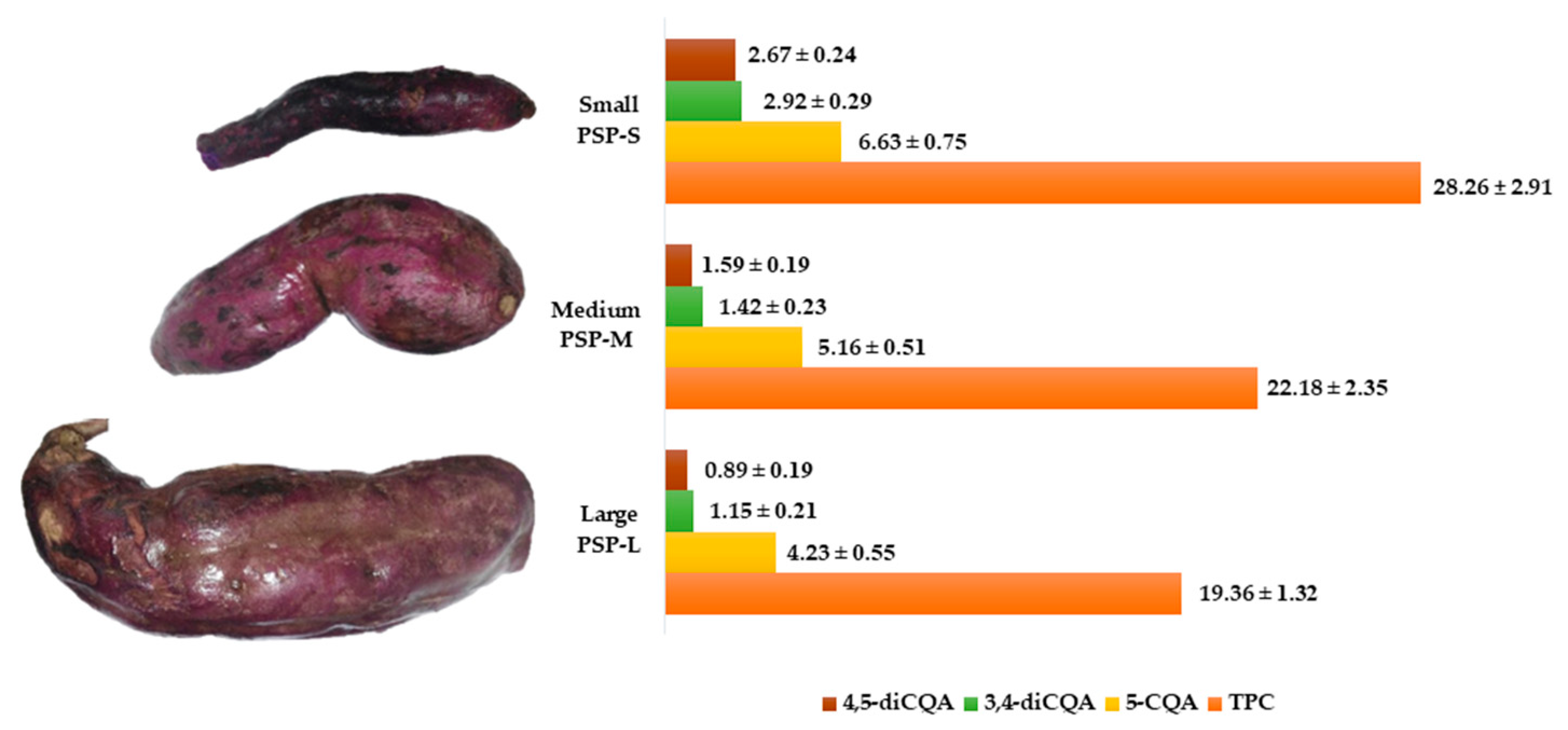
2.1. Identification and Quantification of CQA-Derivatives
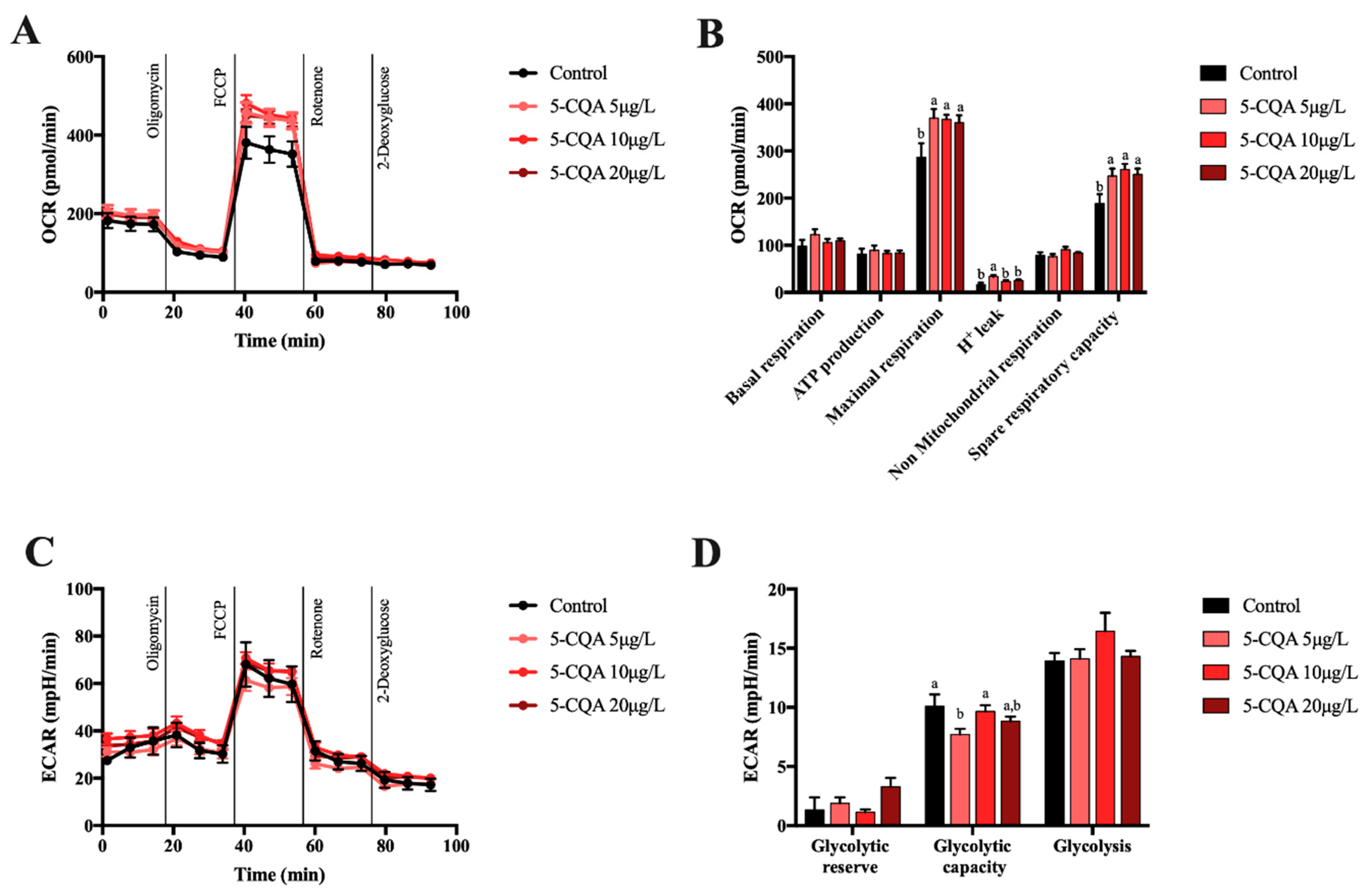
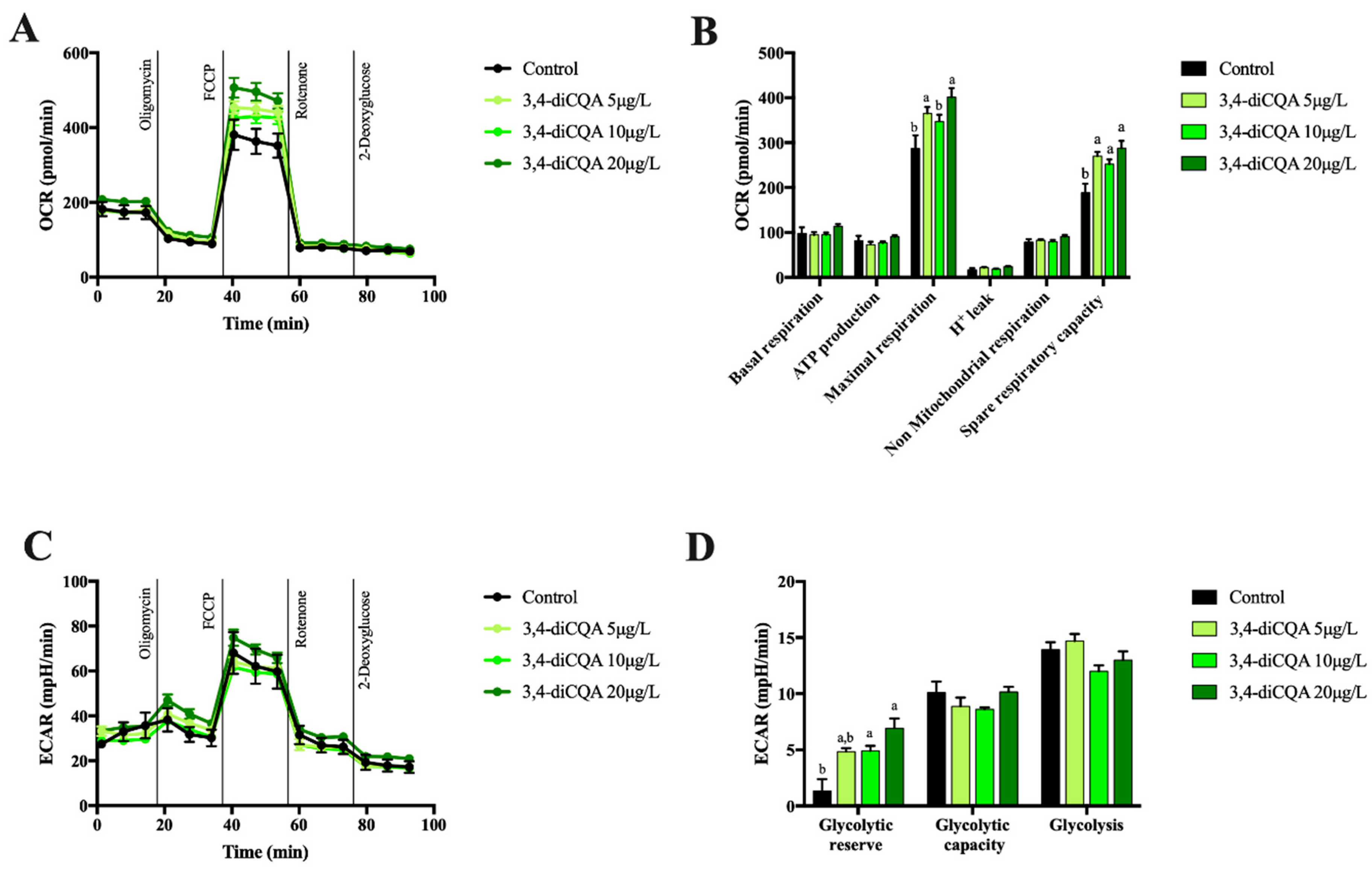
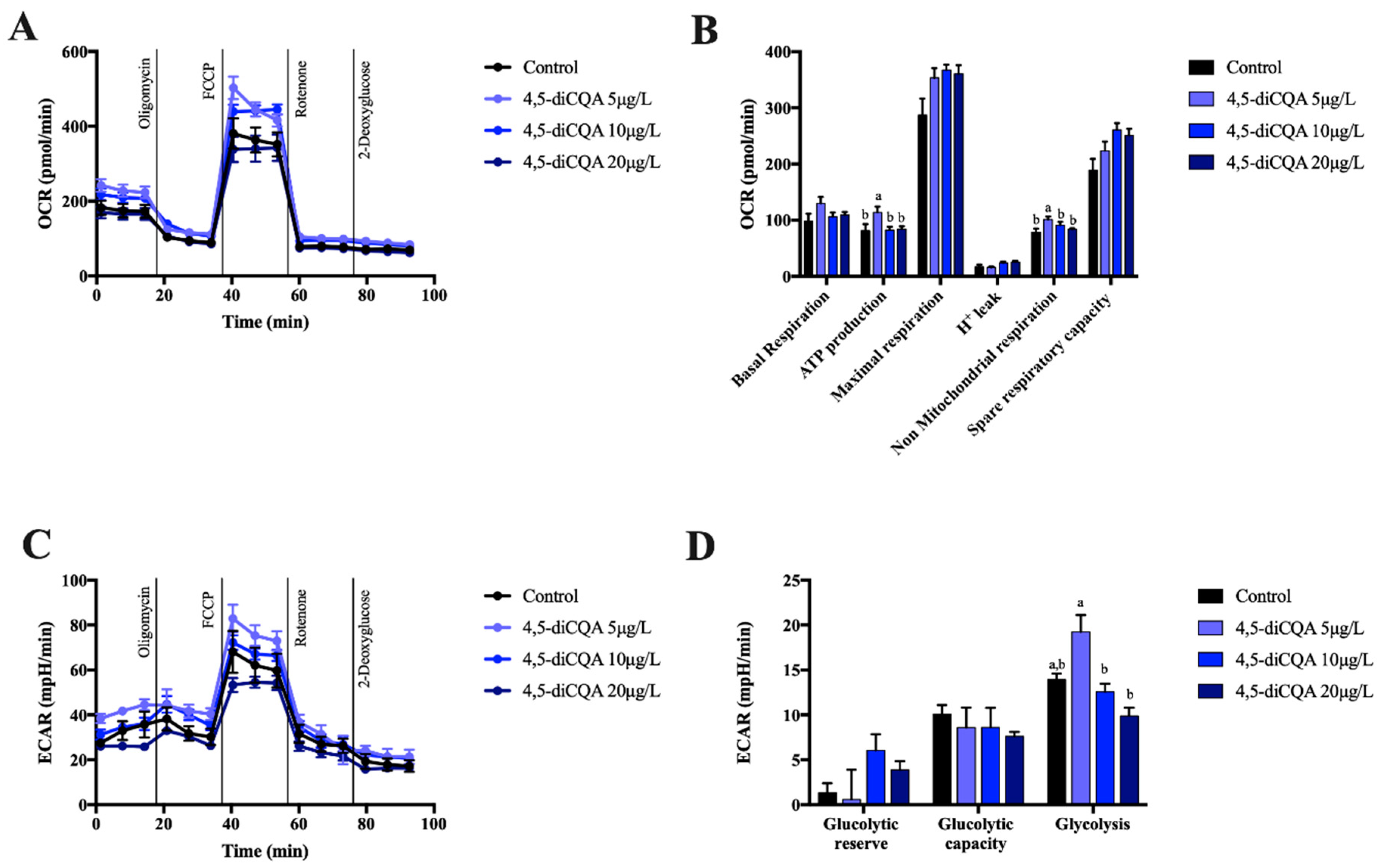
2.2. Effect of 5-CQA, 3,4-diCQA or 4,5-diCQA on Mitochondrial Function on Primary Hepatocytes
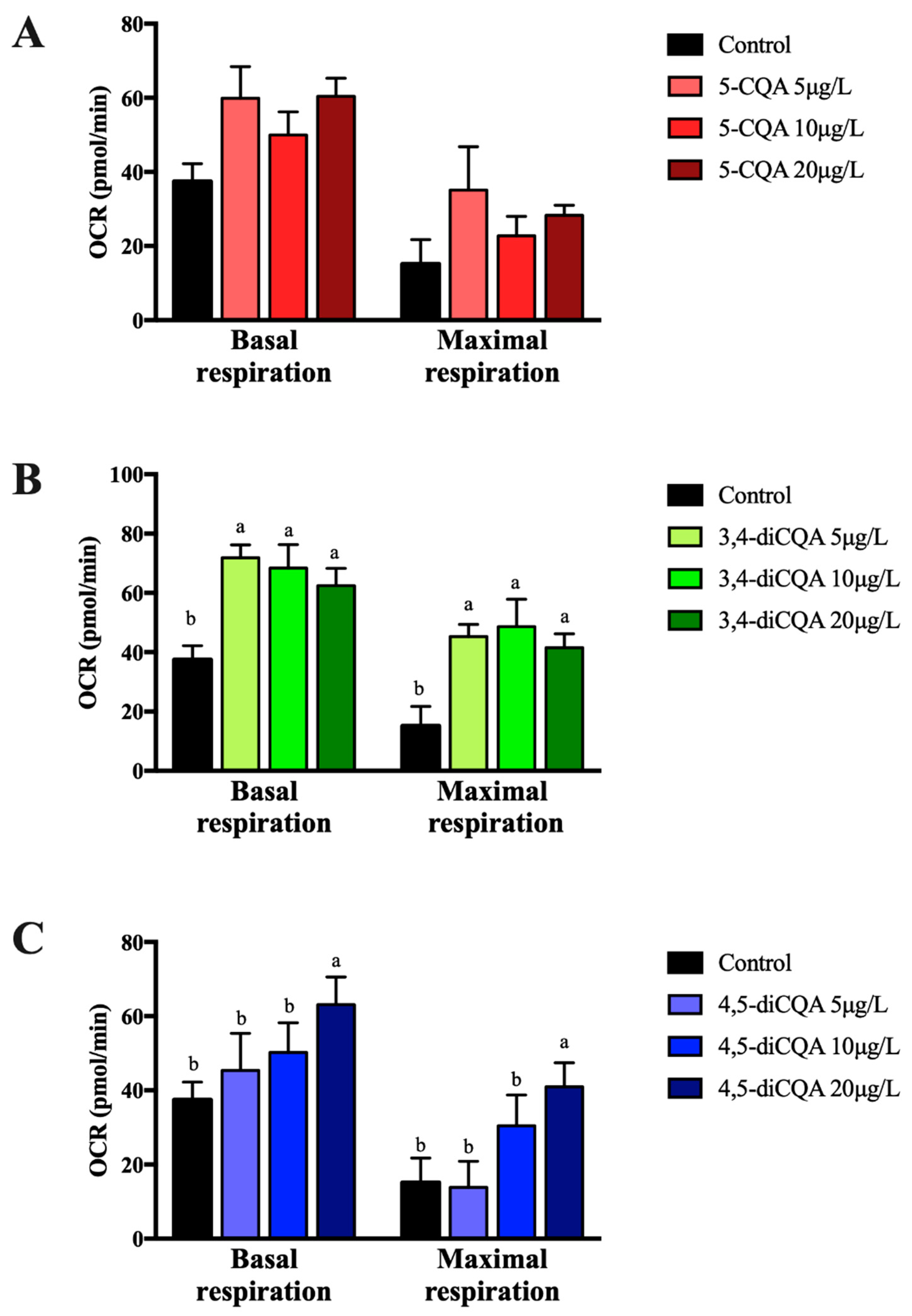
2.3. Effect of 5-CQA, 3,4-diCQA or 4,5-diCQA on Fatty Acid Oxidation on Primary Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Plant Purchase, Processing, and Extraction of Phenolic Content
4.2. Quantification of Total Content of Phenolic Content
4.3. Separation and Purification of CQA-Derivatives
4.4. Identification of CQA-Derivatives
4.5. Quantification of 5-CQA, 3,4- and 4,5-diCQA
4.6. Primary Hepatocyte Cell Culture
4.7. Mitochondria Function Evaluation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Loebenstein, G. Origin, distribution and economic importance. In The Sweetpotato, 1st ed.; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 9–12. [Google Scholar]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.; Sun, H.; Zhang, M.; Wang, C. Chapter 7-Chlorogenic acids from sweet potato. In Sweet Potato Processing Technology, 1st ed.; Mu, T., Sun, H., Zhang, M., Wang, C., Eds.; Academic Press: Cambridge MA, USA, 2017; pp. 357–403. [Google Scholar]
- Zhao, J.G.; Yan, Q.Q.; Xue, R.Y.; Zhang, J.; Zhang, Y.Q. Isolation and identification of colourless caffeoyl compounds in purple sweet potato by HPLC-DAD-ESI/MS and their antioxidant activities. Food Chem. 2014, 161, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Yahara, S.; Okuno, S.; Islam, M.S.; Ishiguro, K.; Yamakawa, O. Antimutagenicity of mono, di-, and tricaffeoylquinic acid derivatives isolated from sweetpotato (Ipomoea batatas L.) leaf. Biosci. Biotechnol. Biochem. 2002, 66, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Moore, P.S.; De Tommasi, N.; De Simone, F.; Colman, S.; Hay, A.J.; Pizza, C. Inhibition of HIV infection by caffeoylquinic acid derivatives. Antivir. Chem. Chemother. 1993, 4, 235–240. [Google Scholar] [CrossRef]
- dos Santos Pereira, A.; de Miranda Pereira, A.F.; Trugo, L.C.; de Aquino Neto, F.R. Distribution of quinic acid derivatives and other phenolic compounds in Brazilian Propolis. Z. Naturforsch. C J. Biosci. 2015, 58, 590–593. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liang, X.; Zhong, Y.; He, W.; Wang, Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food. Agric. 2015, 95, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yao, L.; He, X.; Wang, L.; Li, M.; Yang, Y.; Wan, C. Hypoglycemic activity and constituents analysis of blueberry (Vaccinium corymbosum) fruit extracts. Metab. Syndr. Obes. Targets Ther. 2018, 11, 357–366. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.W.; Wong, C.N.Y.; Pin, W.K.; Wong, M.H.Y.; Kwok, C.Y.; Chan, R.Y.K.; Yu, P.H.F.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; Baardman, J.; de Winther, M.P. Metabolic characterization of polarized M1 and M2 bone marrow-derived macrophages using real-time extracellular flux analysis. J. Vis. Exp. 2015, 28, 53424. [Google Scholar] [CrossRef] [PubMed]
- Leal-Díaz, A.M.; Noriega, L.G.; Torre-Villalvazo, I.; Torres, N.; Alemán-Escondrillas, G.; López-Romero, P.; Sánchez-Tapia, M.; Aguilar-López, M.; Furuzawa-Carballeda, J.; Velázquez-Villegas, L.A.; et al. Aguamiel concentrate from Agave salmiana and its extracted saponins attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice. Sci. Rep. 2016, 6, 34242. [Google Scholar] [CrossRef]
- Palacios-González, B.; Vargas-Castillo, A.; Velázquez-Villegas, L.A.; Vasquez-Reyes, S.; López, P.; Noriega, L.G.; Aleman, G.; Tovar-Palacio, C.; Torre-Villalvazo, I.; Yang, L.J.; et al. Genistein increases the thermogenic program of subcutaneous WAT and increases energy expenditure in mice. J. Nutr. Biochem. 2019, 68, 59–68. [Google Scholar] [CrossRef]
- Meng, X.-j.; Tan, C.; Feng, Y. Solvent extraction and in vitro simulated gastrointestinal digestion of phenolic compounds from purple sweet potato. Int. J. Food Sci. Technol. 2019, 54, 2887–2896. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Q.; Gao, L.; Gong, X.; Qu, Y.; Feng, B. Functional and physicochemical properties of flours and starches from different tuber crops. Int. J. Biol. Macromol. 2020, 148, 324–332. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Tsao, R. Anthocyanin-rich phenolic extracts of purple root vegetables inhibit pro-inflammatory cytokines induced by H2O2 and enhance antioxidant enzyme activities in Caco-2 cells. J. Funct. Foods. 2016, 22, 363–375. [Google Scholar] [CrossRef]
- Kita, A.; Bakowska-Barczak, A.; Hamouz, K.; Kułakowska, K.; Lisińska, G. The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2013, 32, 169–175. [Google Scholar] [CrossRef]
- Scarano, A.; Gerardi, C.; D’Amico, L.; Accogli, R.; Santino, A. Phytochemical analysis and antioxidant properties in colored Tiggiano carrots. Agriculture 2018, 8, 102. [Google Scholar] [CrossRef]
- Burgos, G.; Amoros, W.; Muñoa, L.; Sosa, P.; Cayhualla, E.; Sanchez, C.; Díaz, C.; Bonierbale, M. Total phenolic, total anthocyanin and phenolic acid concentrations and antioxidant activity of purple-fleshed potatoes as affected by boiling. J. Food Compos. Anal. 2013, 30, 6–12. [Google Scholar] [CrossRef]
- Jackson, K.M.P.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural constraints and importance of caffeic acid moiety for anti-hyperglycemic effects of caffeoylquinic acids from chicory. Mol. Nutr. Food Res. 2017, 61, 1601118. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr. Med. 2014, 13, 35–43. [Google Scholar]
- Zhang, X.; Wu, C.; Wu, H.; Sheng, L.; Su, Y.; Zhang, X.; Luan, H.; Sun, G.; Sun, X.; Tian, Y.; et al. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS ONE 2013, 8, e61922. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L., Jr.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-activated protein kinase mediates mitochondrial fission. Science 2016, 351, 275–281. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Zamora-López, K.; Noriega, L.G.; Estanes-Hernández, A.; Escalona-Nández, I.; Tobón-Cornejo, S.; Tovar, A.R.; Barbero-Becerra, V.; Pérez-Monter, C. Punica granatum L.-derived omega-5 nanoemulsion improves hepatic steatosis in mice fed a high fat diet by increasing fatty acid utilization in hepatocytes. Sci. Rep. 2020, 10, 15229. [Google Scholar] [CrossRef]
- Betteridge, D.J. What is oxidative stress? Metab. Clin. Exp. 2020, 49, 3–8. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2002, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.W.; Bai, J.P.; Zhang, Q.; Hu, X.L.; Tian, X.; Zhu, J.; Liu, J.; Meng, W.H.; Zhao, Q.C. Caffeoylquinic Acid Derivatives Protect SH-SY5Y Neuroblastoma Cells from Hydrogen Peroxide-Induced Injury Through Modulating Oxidative Status. Cell Mol. Neurobiol. 2017, 37, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, X.; He, Y.L.; Wang, Y.; Dong, L.; Ma, X.; Zheng, L.; Liu, C.H.; Wang, G.C.; Zheng, J.; et al. In Vivo and In Vitro anti-arthritic effects of cardenolide-rich and caffeoylquinic acid-rich fractions of Periploca forrestii. Molecules 2018, 23, 1988. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, S.; Sarriá, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: Results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur. J. Nutr. 2019, 58, 865–878. [Google Scholar] [CrossRef]
- Rector, R.S.; Thyfault, J.P.; Wei, Y.; Ibdah, J.A. Non-alcoholic fatty liver disease and the metabolic syndrome: An update. World J. Gastroenterol. 2008, 14, 185–192. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Tominaga, K.; Fujimoto, E.; Suzuki, K.; Hayashi, M.; Ichikawa, M.; Inaba, Y. Prevalence of non-alcoholic fatty liver disease in children and relationship to metabolic syndrome, insulin resistance, and waist circumference. Environ. Health Prev. Med. 2009, 14, 142–149. [Google Scholar] [CrossRef]
- Nugroho, A.; Kim, K.H.; Lee, K.R.; Alam, M.B.; Choi, J.S.; Kim, W.B.; Park, H.J. Qualitative and quantitative determination of the caffeoylquinic acids on the Korean mountainous vegetables used for Chwinamul and their peroxynitrite-scavenging effect. Arch. Pharm. Res. 2009, 32, 1361–1367. [Google Scholar] [CrossRef]
- Che, Y.; Wang, Z.; Zhu, Z.; Ma, Y.; Zhang, Y.; Gu, W.; Zhang, J.; Rao, G. Simultaneous qualitation and quantitation of chlorogenic acids in Kuding tea using ultra-high-performance liquid chromatography-diode array detection coupled with linear ion trap-orbitrap mass spectrometer. Molecules 2016, 21, 1728. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Y.; Hu, N.; Sun, Q.; Ding, X.; Li, G.; Zheng, B.; Gu, M.; Huang, F.; Sun, Y.Q.; et al. Extract of kuding tea prevents high-fat diet-induced metabolic disorders in C57bl/6 mice via liver x receptor (LXR) β antagonism. PLoS ONE 2012, 7, e51007. [Google Scholar] [CrossRef]
- Xie, M.; Chen, G.; Wan, P.; Dai, Z.; Zeng, X.; Sun, Y. Effects of Dicaffeoylquinic Acids from Ilex kudingcha on Lipid Metabolism and Intestinal Microbiota in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2019, 67, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Erk, T.; Renouf, M.; Williamson, G.; Melcher, R.; Steiling, H.; Richling, E. Absorption and isomerization of caffeoylquinic acids from different foods using ileostomist volunteers. Eur. J. Nutr. 2014, 53, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Berry, M.N.; Friend, D.S. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell. Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef]
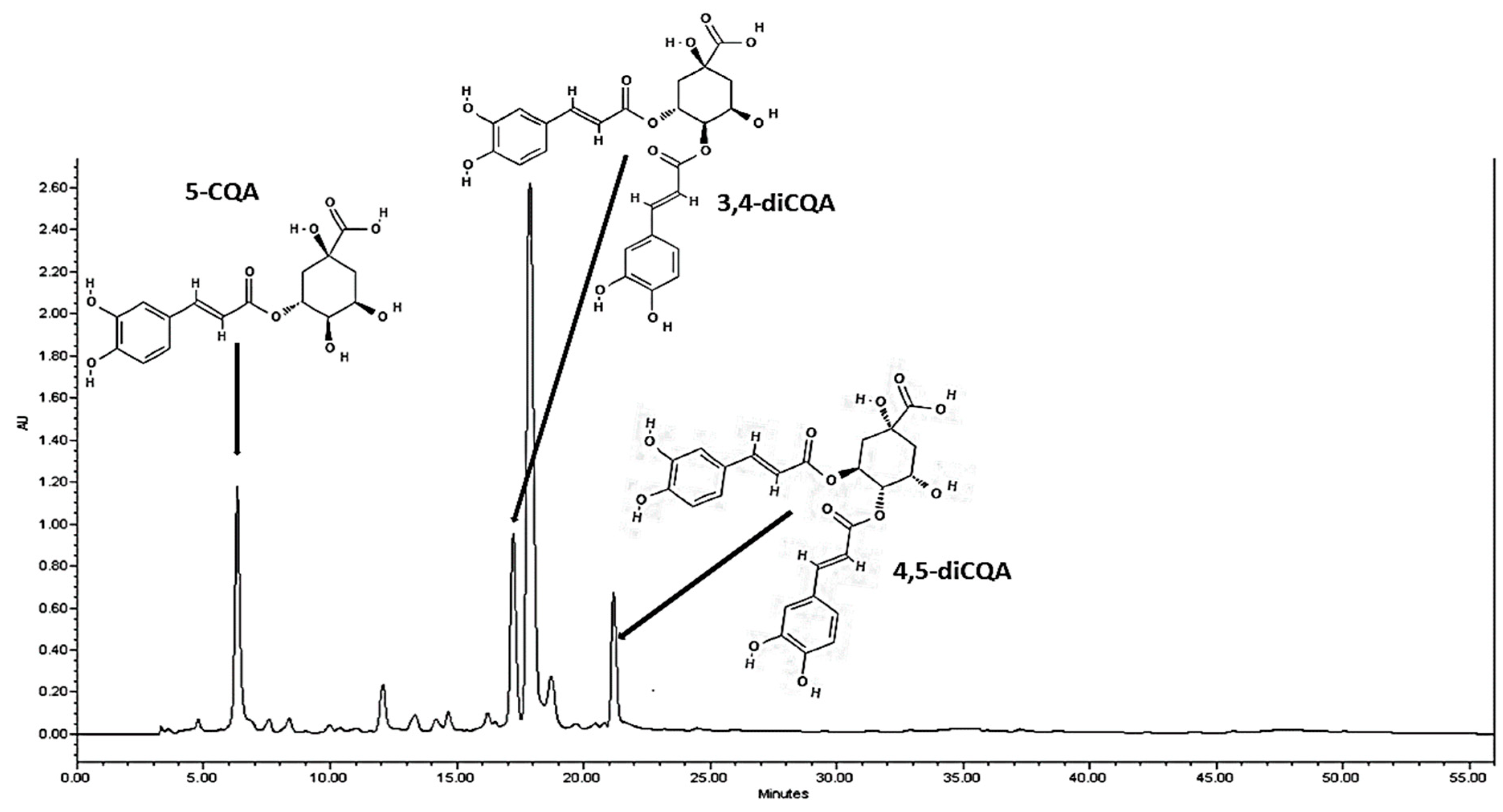
| tR (Min) | Compound | Molecular Formula | Molecular Ion (M-H) (m/z) | Fragments (m/z) | % of Purity |
|---|---|---|---|---|---|
| 6.2 | 5-CQA | C16H18O9 | 353 | 191 | 97 |
| 17.7 | 3,4-diCQA | C25H24O12 | 515 | 353 | 95 |
| 21.5 | 4,5-diCQA | C25H24O12 | 515 | 353 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, A.; Noriega, L.G.; Delgadillo-Puga, C.; Tovar, A.R.; Navarro-Ocaña, A. Caffeoylquinic Acid Derivatives of Purple Sweet Potato as Modulators of Mitochondrial Function in Mouse Primary Hepatocytes. Molecules 2021, 26, 319. https://doi.org/10.3390/molecules26020319
Torres A, Noriega LG, Delgadillo-Puga C, Tovar AR, Navarro-Ocaña A. Caffeoylquinic Acid Derivatives of Purple Sweet Potato as Modulators of Mitochondrial Function in Mouse Primary Hepatocytes. Molecules. 2021; 26(2):319. https://doi.org/10.3390/molecules26020319
Chicago/Turabian StyleTorres, Andrea, Lilia G. Noriega, Claudia Delgadillo-Puga, Armando R. Tovar, and Arturo Navarro-Ocaña. 2021. "Caffeoylquinic Acid Derivatives of Purple Sweet Potato as Modulators of Mitochondrial Function in Mouse Primary Hepatocytes" Molecules 26, no. 2: 319. https://doi.org/10.3390/molecules26020319
APA StyleTorres, A., Noriega, L. G., Delgadillo-Puga, C., Tovar, A. R., & Navarro-Ocaña, A. (2021). Caffeoylquinic Acid Derivatives of Purple Sweet Potato as Modulators of Mitochondrial Function in Mouse Primary Hepatocytes. Molecules, 26(2), 319. https://doi.org/10.3390/molecules26020319