Curcumin Analogue L48H37 Suppresses Human Osteosarcoma U2OS and MG-63 Cells’ Migration and Invasion in Culture by Inhibition of uPA via the JAK/STAT Signaling Pathway
Abstract
1. Introduction
2. Results
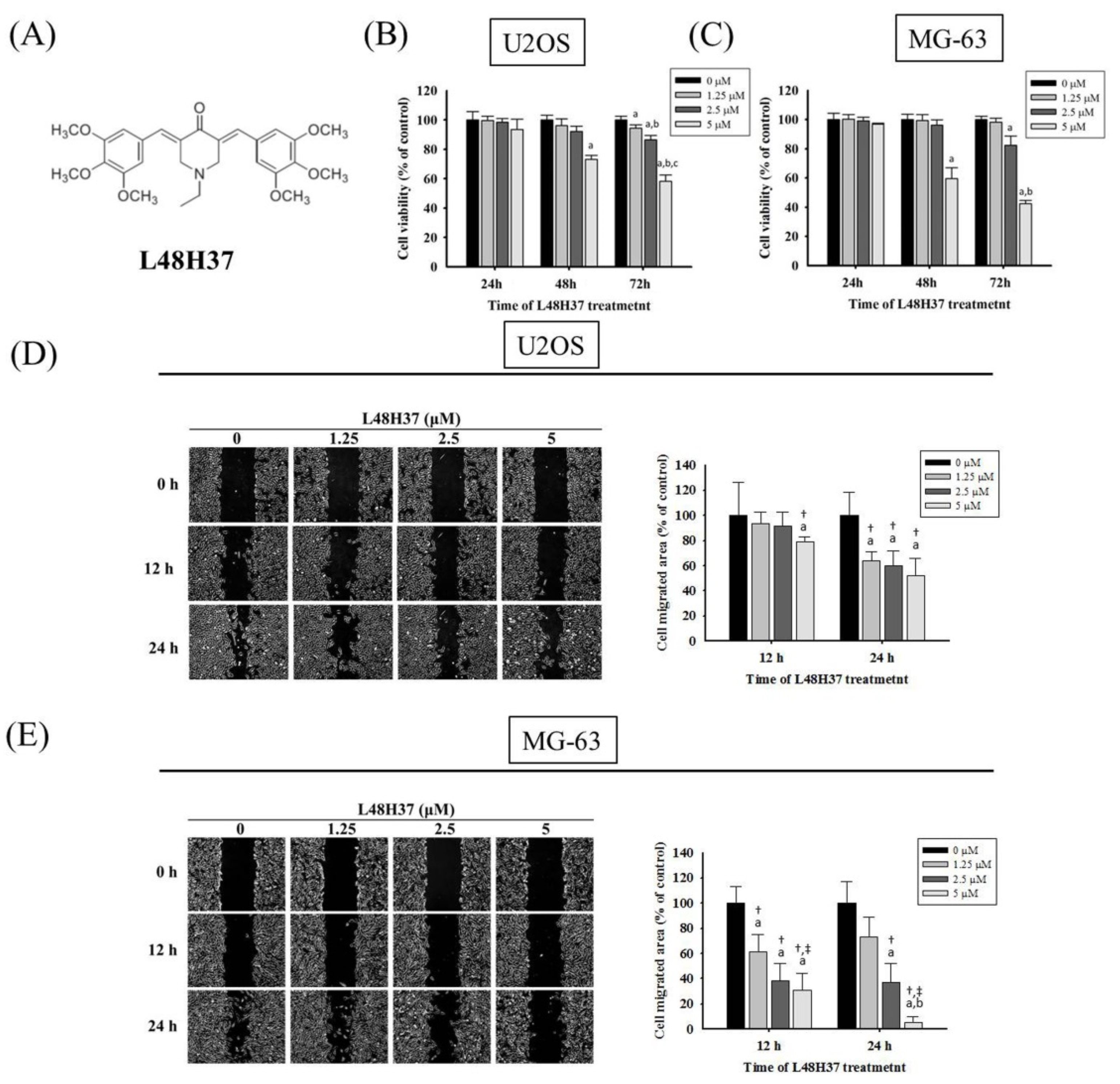
2.1. Cytotoxicity of L48H37 in Osteosarcoma U2OS and MG-63 Cells
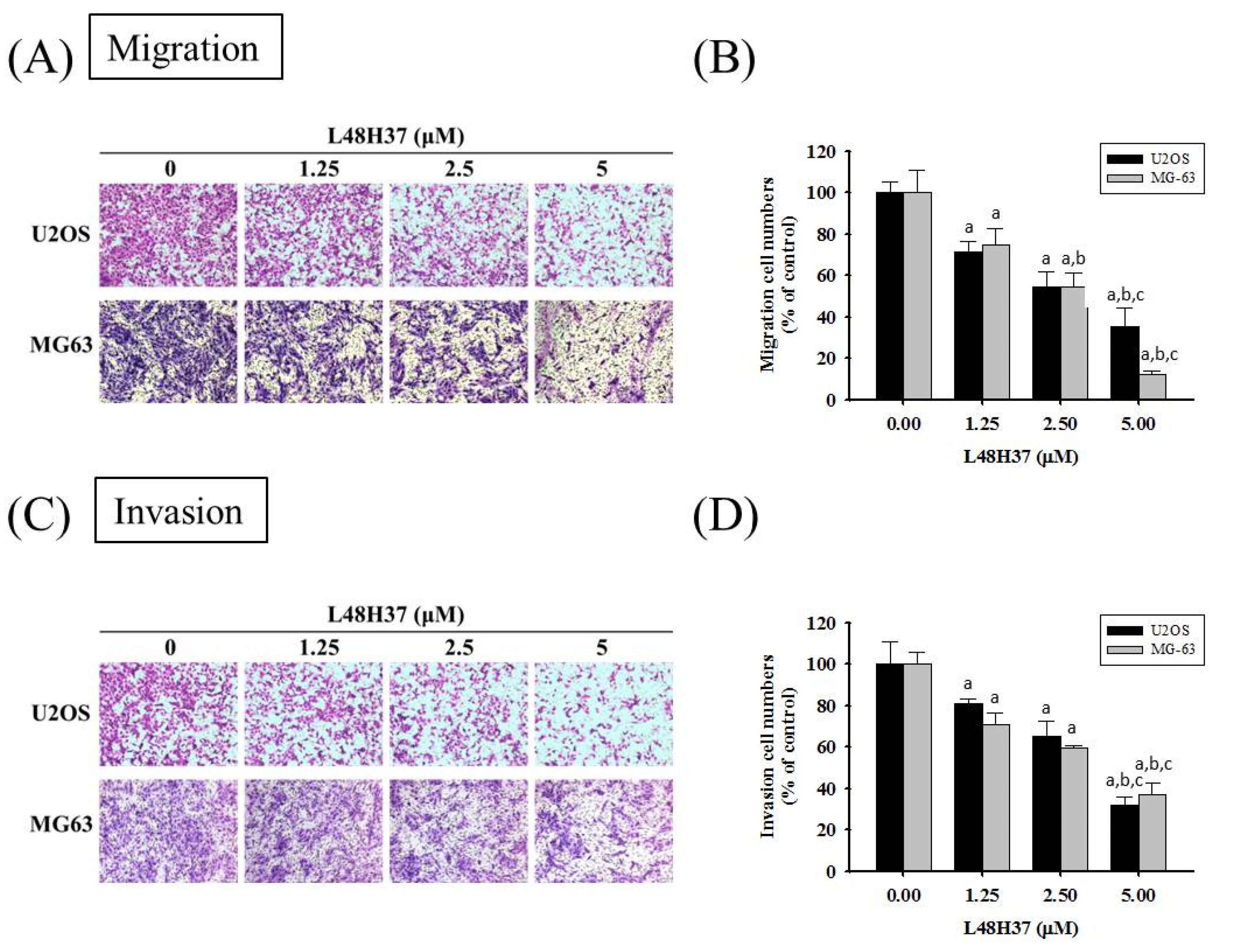
2.2. L48H37 Represses U2OS and MG-63 Cells’ Motility, Migration, and Invasion
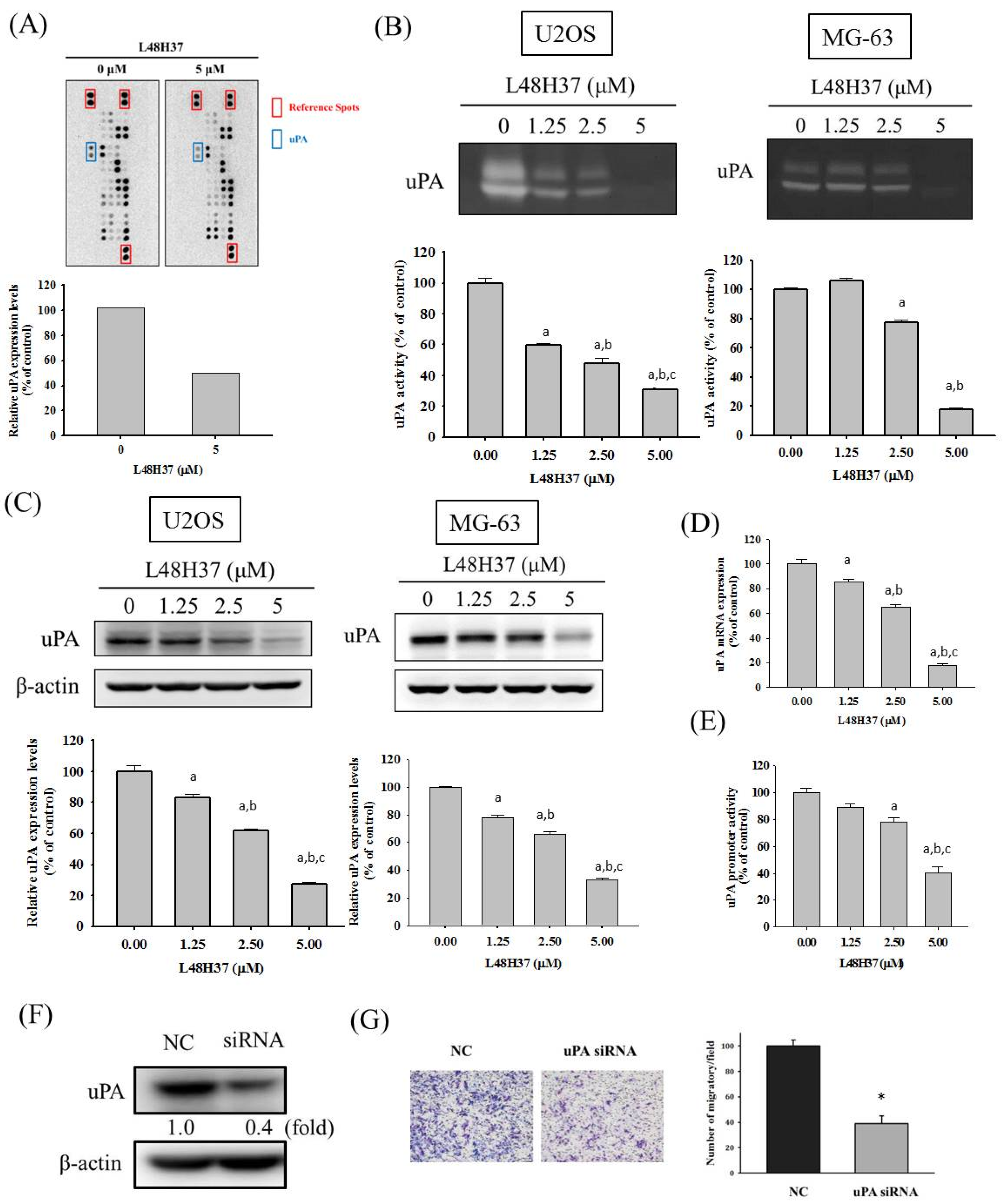
2.3. L48H37 Reduces the Level, Protein and mRNA Expression, and Promoter Activity of uPA in U2OS Cells
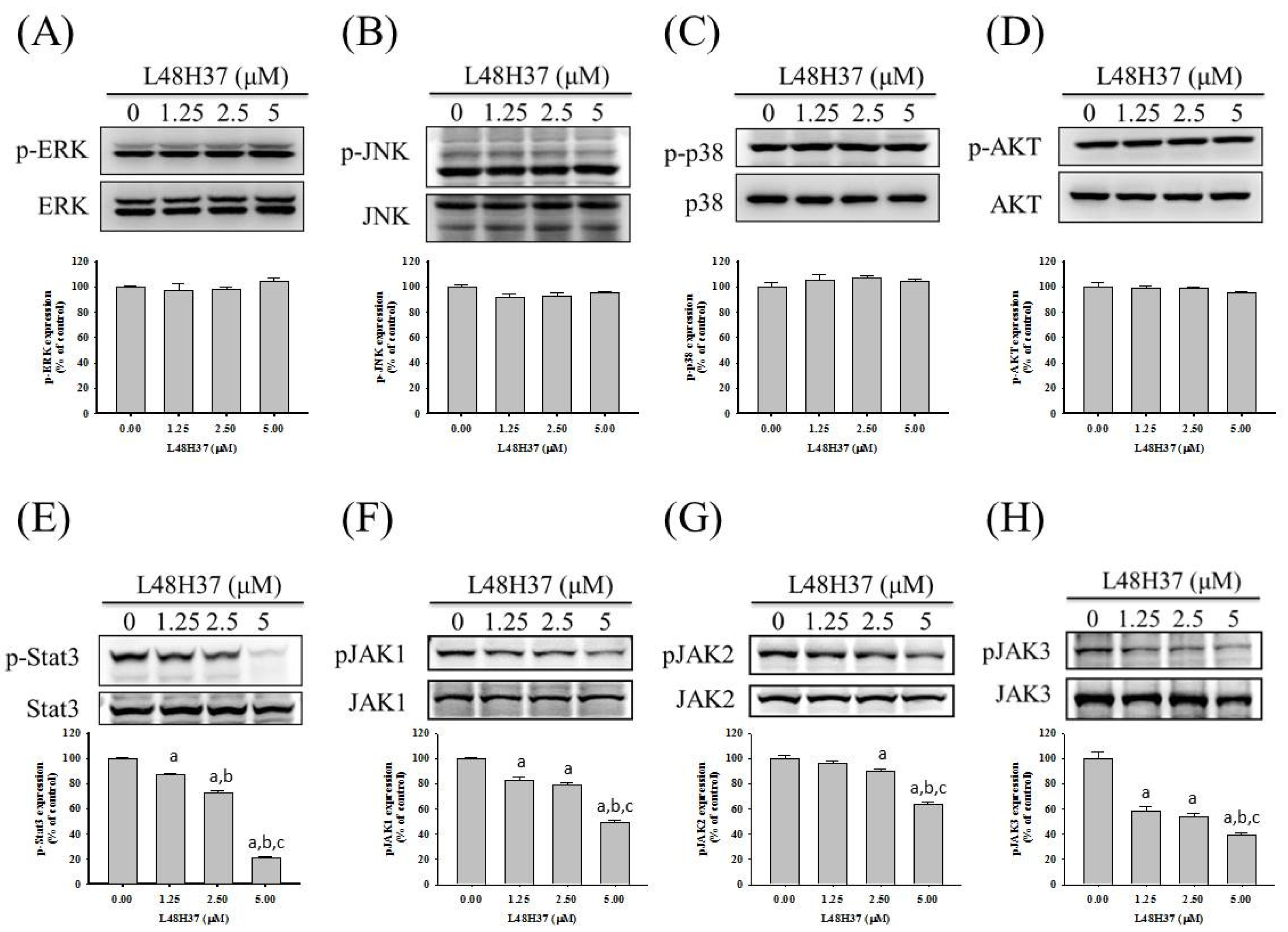
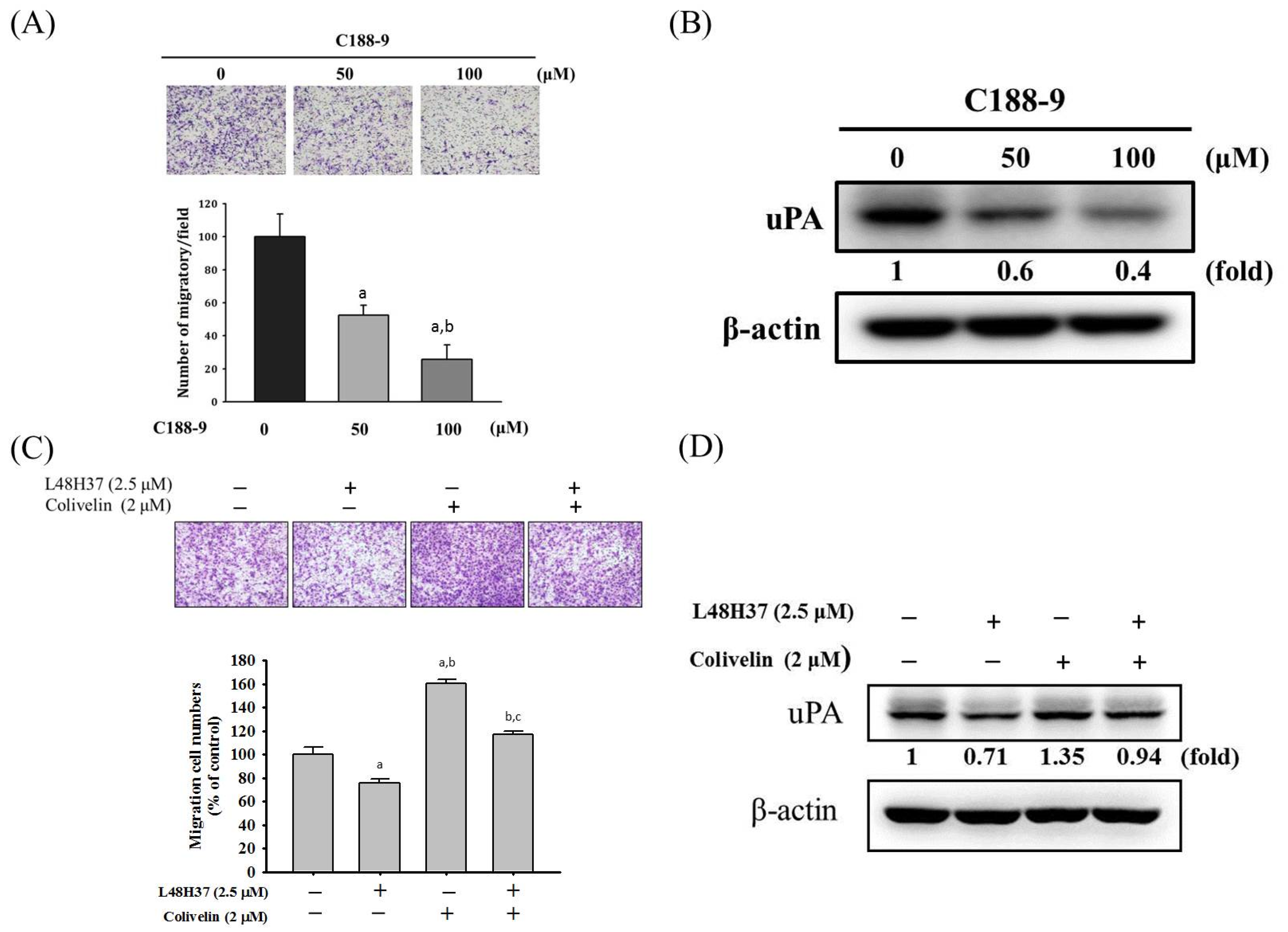
2.4. L48H37 Suppresses the JAK/STAT Pathway in U2OS Cells
2.5. L48H37 Inhibits Cellular Migration and uPA Expression via the JAK/STAT Pathway in U2OS Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and L48H37 Treatment
4.3. MTT Assay
4.4. Wound-Healing Assay
4.5. Cell Migration and Invasion Assays
4.6. Protease Array Analysis
4.7. Preparation of Cell Lysates and Western Blotting Analysis
4.8. Real-Time Polymerase Chain Reaction (PCR)
4.9. uPA Promoter-Driven Luciferase Reporter Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Arndt, C.A.; Rose, P.S.; Folpe, A.L.; Laack, N.N. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin. Proc. 2012, 87, 475–487. [Google Scholar] [CrossRef]
- Lu, K.H.; Lin, C.W.; Hsieh, Y.H.; Su, S.C.; Reiter, R.J.; Yang, S.F. New insights into antimetastatic signaling pathways of melatonin in skeletomuscular sarcoma of childhood and adolescence. Cancer Metastasis Rev. 2020, 39, 303–320. [Google Scholar] [CrossRef]
- Lu, K.H.; Lu, E.W.; Lin, C.W.; Yang, J.S.; Yang, S.F. New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma. Pharmacol. Ther. 2020, 107611. [Google Scholar] [CrossRef]
- Arndt, C.A.; Crist, W.M. Common musculoskeletal tumors of childhood and adolescence. N. Engl. J. Med. 1999, 341, 342–352. [Google Scholar] [CrossRef]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar]
- Oertel, S.; Blattmann, C.; Rieken, S.; Jensen, A.; Combs, S.E.; Huber, P.E.; Bischof, M.; Kulozik, A.; Debus, J.; Schulz-Ertner, D. Radiotherapy in the treatment of primary osteosarcoma--a single center experience. Tumori 2010, 96, 582–588. [Google Scholar] [CrossRef]
- Lu, K.H.; Lin, R.C.; Yang, J.S.; Yang, W.E.; Reiter, R.J.; Yang, S.F. Molecular and cellular mechanisms of melatonin in osteosarcoma. Cells 2019, 8, 1618. [Google Scholar] [CrossRef]
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017, 62, e12370. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Yoon, S.O.; Park, S.J.; Yun, C.H.; Chung, A.S. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J. Biochem. Mol. Biol. 2003, 36, 128–137. [Google Scholar] [CrossRef]
- Mackay, A.R.; Corbitt, R.H.; Hartzler, J.L.; Thorgeirsson, U.P. Basement membrane type iv collagen degradation: Evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res. 1990, 50, 5997–6001. [Google Scholar]
- Nelson, A.R.; Fingleton, B.; Rothenberg, M.L.; Matrisian, L.M. Matrix metalloproteinases: Biologic activity and clinical implications. J. Clin. Oncol. 2000, 18, 1135–1149. [Google Scholar] [CrossRef]
- Cheng, H.L.; Hsieh, M.J.; Yang, J.S.; Lin, C.W.; Lue, K.H.; Lu, K.H.; Yang, S.F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking erk and jnk-mediated mmps expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef]
- Cheng, H.L.; Lin, C.W.; Yang, J.S.; Hsieh, M.J.; Yang, S.F.; Lu, K.H. Zoledronate blocks geranylgeranylation not farnesylation to suppress human osteosarcoma u2os cells metastasis by emt via rho a activation and fak-inhibited jnk and p38 pathways. Oncotarget 2016, 7, 9742–9758. [Google Scholar] [CrossRef]
- Lu, J.; Song, G.; Tang, Q.; Zou, C.; Han, F.; Zhao, Z.; Yong, B.; Yin, J.; Xu, H.; Xie, X.; et al. Irx1 hypomethylation promotes osteosarcoma metastasis via induction of cxcl14/nf-kappab signaling. J. Clin. Investig. 2015, 125, 1839–1856. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Wang, Y.; He, A.; Hu, H.; Zhang, J.; Han, M.; Huang, Y. Curcumin inhibits the proliferation and invasion of mg-63 cells through inactivation of the p-jak2/p-stat3 pathway. Onco. Targets 2019, 12, 2011–2021. [Google Scholar] [CrossRef]
- Lestari, M.L.; Indrayanto, G. Curcumin. Profiles Drug Subst Excip Relat Methodol 2014, 39, 113–204. [Google Scholar]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Seo, J.A.; Kim, B.; Dhanasekaran, D.N.; Tsang, B.K.; Song, Y.S. Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum ca2+ atpase activity in ovarian cancer cells. Cancer Lett. 2016, 371, 30–37. [Google Scholar] [CrossRef]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with folfox chemotherapy. Cancer Lett. 2015, 364, 135–141. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar] [PubMed]
- Wu, J.; Zhang, Y.; Cai, Y.; Wang, J.; Weng, B.; Tang, Q.; Chen, X.; Pan, Z.; Liang, G.; Yang, S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogues of curcumin as anti-inflammatory agents. Bioorg. Med. Chem. 2013, 21, 3058–3065. [Google Scholar] [CrossRef]
- Feng, C.; Xia, Y.; Zou, P.; Shen, M.; Hu, J.; Ying, S.; Pan, J.; Liu, Z.; Dai, X.; Zhuge, W.; et al. Curcumin analogue l48h37 induces apoptosis through ros-mediated endoplasmic reticulum stress and stat3 pathways in human lung cancer cells. Mol. Carcinogenes 2017, 56, 1765–1777. [Google Scholar] [CrossRef]
- Li, S.S.; Jiang, W.L.; Xiao, W.Q.; Li, K.; Zhang, Y.F.; Guo, X.Y.; Dai, Y.Q.; Zhao, Q.Y.; Jiang, M.J.; Lu, Z.J.; et al. Kmt2d deficiency enhances the anti-cancer activity of l48h37 in pancreatic ductal adenocarcinoma. World J. Gastrointest Oncol. 2019, 11, 599–621. [Google Scholar] [CrossRef]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol Oncol. 2017, 10, 10. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Chang, P.Y.; Hsieh, M.J.; Hsieh, Y.S.; Chen, P.N.; Yang, J.S.; Lo, F.C.; Yang, S.F.; Lu, K.H. Tricetin inhibits human osteosarcoma cells metastasis by transcriptionally repressing mmp-9 via p38 and akt pathways. Environ. Toxicol. 2017, 32, 2032–2040. [Google Scholar] [CrossRef]
- Lu, K.H.; Chen, P.N.; Hsieh, Y.H.; Lin, C.Y.; Cheng, F.Y.; Chiu, P.C.; Chu, S.C.; Hsieh, Y.S. 3-hydroxyflavone inhibits human osteosarcoma u2os and 143b cells metastasis by affecting emt and repressing u-pa/mmp-2 via fak-src to mek/erk and rhoa/mlc2 pathways and reduces 143b tumor growth in vivo. Food Chem. Toxicol. 2016, 97, 177–186. [Google Scholar] [CrossRef]
- Yang, J.S.; Lin, C.W.; Hsieh, Y.S.; Cheng, H.L.; Lue, K.H.; Yang, S.F.; Lu, K.H. Selaginella tamariscina (beauv.) possesses antimetastatic effects on human osteosarcoma cells by decreasing mmp-2 and mmp-9 secretions via p38 and akt signaling pathways. Food Chem. Toxicol. 2013, 59, 801–807. [Google Scholar] [CrossRef]
- Reddy, K.B.; Nabha, S.M.; Atanaskova, N. Role of map kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003, 22, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Chu, S.C.; Yang, S.F.; Chen, P.N.; Liu, Y.C.; Lu, K.H. Silibinin suppresses human osteosarcoma mg-63 cell invasion by inhibiting the erk-dependent c-jun/ap-1 induction of mmp-2. Carcinogenesis 2007, 28, 977–987. [Google Scholar] [CrossRef]
- Lu, K.H.; Su, S.C.; Lin, C.W.; Hsieh, Y.H.; Lin, Y.C.; Chien, M.H.; Reiter, R.J.; Yang, S.F. Melatonin attenuates osteosarcoma cell invasion by suppression of c-c motif chemokine ligand 24 through inhibition of the c-jun n-terminal kinase pathway. J. Pineal. Res. 2018, 65, e12507. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Yin, F.; Lin, B.; Wang, Z.; Xu, J.; Wang, H.; Zuo, D.; Wang, G.; Hua, Y. , et al. Toosendanin demonstrates promising antitumor efficacy in osteosarcoma by targeting stat3. Oncogene 2017, 36, 6627–6639. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Yang, J.S.; Lin, R.C.; Hsieh, Y.H.; Yang, S.F.; Chang, H.R.; Lu, K.H. Tomatidine represses invasion and migration of human osteosarcoma u2os and hos cells by suppression of presenilin 1 and c-raf-mek-erk pathway. Molecules 2020, 25, 326. [Google Scholar] [CrossRef]
- Fossey, S.L.; Bear, M.D.; Lin, J.; Li, C.; Schwartz, E.B.; Li, P.K.; Fuchs, J.R.; Fenger, J.; Kisseberth, W.C.; London, C.A. The novel curcumin analogue flll32 decreases stat3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer 2011, 11, 112. [Google Scholar] [CrossRef]
- Lu, K.H.; Yang, H.W.; Su, C.W.; Lue, K.H.; Yang, S.F.; Hsieh, Y.S. Phyllanthus urinaria suppresses human osteosarcoma cell invasion and migration by transcriptionally inhibiting u-pa via erk and akt signaling pathways. Food Chem. Toxicol. 2013, 52, 193–199. [Google Scholar] [CrossRef]
- Chu, S.C.; Yang, S.F.; Liu, S.J.; Kuo, W.H.; Chang, Y.Z.; Hsieh, Y.S. In vitro and in vivo antimetastatic effects of terminalia catappa l. Leaves on lung cancer cells. Food Chem. Toxicol. 2007, 45, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Lin, C.W.; Yang, S.F.; Chen, M.K.; Chiou, H.L. Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of nf-κb and ap-1 activity in human liver cancer cells. Br. J. Pharm. 2014, 171, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.-H.; Wu, H.-H.; Lin, R.-C.; Lin, Y.-C.; Lu, P.W.-A.; Yang, S.-F.; Yang, J.-S. Curcumin Analogue L48H37 Suppresses Human Osteosarcoma U2OS and MG-63 Cells’ Migration and Invasion in Culture by Inhibition of uPA via the JAK/STAT Signaling Pathway. Molecules 2021, 26, 30. https://doi.org/10.3390/molecules26010030
Lu K-H, Wu H-H, Lin R-C, Lin Y-C, Lu PW-A, Yang S-F, Yang J-S. Curcumin Analogue L48H37 Suppresses Human Osteosarcoma U2OS and MG-63 Cells’ Migration and Invasion in Culture by Inhibition of uPA via the JAK/STAT Signaling Pathway. Molecules. 2021; 26(1):30. https://doi.org/10.3390/molecules26010030
Chicago/Turabian StyleLu, Ko-Hsiu, Heng-Hsiung Wu, Renn-Chia Lin, Ya-Chiu Lin, Peace Wun-Ang Lu, Shun-Fa Yang, and Jia-Sin Yang. 2021. "Curcumin Analogue L48H37 Suppresses Human Osteosarcoma U2OS and MG-63 Cells’ Migration and Invasion in Culture by Inhibition of uPA via the JAK/STAT Signaling Pathway" Molecules 26, no. 1: 30. https://doi.org/10.3390/molecules26010030
APA StyleLu, K.-H., Wu, H.-H., Lin, R.-C., Lin, Y.-C., Lu, P. W.-A., Yang, S.-F., & Yang, J.-S. (2021). Curcumin Analogue L48H37 Suppresses Human Osteosarcoma U2OS and MG-63 Cells’ Migration and Invasion in Culture by Inhibition of uPA via the JAK/STAT Signaling Pathway. Molecules, 26(1), 30. https://doi.org/10.3390/molecules26010030






