Abstract
Dysregulation of glucose homeostasis followed by chronic hyperglycemia is a hallmark of diabetes mellitus (DM), a disease spreading as a worldwide pandemic for which there is no satisfactory dietary treatment or cure. The development of glucose-controlling drugs that can prevent complications of DM, such as hyperglycemia and oxidative stress, which contribute to the impairment of the key physiological processes in the body, is of grave importance. In pursuit of this goal, this study screened 41 plant extracts for their antidiabetic and antioxidant activities by employing assays to test for α-amylase inhibition and free radical scavenging activity (FRSA) and by measuring glucose uptake in L6-GLUT4myc cells. While extracts of Rhus coriaria, Punica granatum, Olea europaea, Pelargonium spp., Stevia rebaudiana, and Petroselinum crispum demonstrated significant α-amylase inhibition, the extracts of Rhus coriaria and Pelargonium spp. also demonstrated increased FRSA, and the extract of Rhus coriaria stimulated glucose uptake. These natural extracts, which are believed to have fewer side effects because they are prepared from edible plants, interfere with the process in the small intestine that breaks down dietary carbohydrates into monosaccharide and disaccharide derivatives, and thereby suppress increases in diet-induced blood glucose; hence, they may have clinical value for type 2 diabetes management. The Pelargonium spp. and Rhus coriaria extracts demonstrated the highest antidiabetic and antioxidant activities. Both plants may offer valuable medical benefits, especially because they can be taken as dietary supplements by patients with diabetes and can serve as sources of new, natural-based antidiabetic drug candidates. The enhancement of cellular glucose uptake stimulated by Rhus coriaria extract could lead to the development of clinical applications that regulate blood glucose levels from within the circulatory system. Isolating bioactive substances from these plant extracts and testing them in diabetic mice will significantly advance the development of natural drugs that have both antidiabetic and free radical-scavenging properties, likely with lesser side effects.
1. Introduction
Digestible starch, which is the principal source of carbohydrates and accounts for 40–60% of the energy intake in the human diet, gives rise to a prolonged release of glucose moieties in the lumen of the small intestine, which are subsequently absorbed into the bloodstream, causing increased postprandial glycemia [1,2]. As glucose makes up 80% of the total absorbable monosaccharides, high blood levels trigger cells in the pancreas to secrete insulin, a hormone that is important for regulating glucose homeostasis [3]. Insulin then signals adipose tissue to metabolize glucose into triglycerides, and skeletal muscle tissues metabolize it to generate energy; excess glucose is stored in the liver in the form of glycogen [4,5]. This tight regulatory process continuously maintains glucose homeostasis, even under prolonged fasting. Diabetes mellitus (DM) is a condition in which blood hyperglycemia occurs and cannot be controlled due to a lack of insulin activity [5,6,7]. DM is a metabolic disorder affecting hundreds of millions of people throughout the globe [8]; it is caused either by an insulin deficiency (type 1 diabetes) or by insulin resistance (type 2 diabetes) [9,10]. As 80% of the postprandial glucose is transported into muscles by the insulin-stimulated glucose transporter 4 (GLUT4) system, the resistance of muscle to insulin, which is typical in type 2 diabetes, disrupts glucose homeostasis in the whole human body [11,12,13]. Abnormalities in glucose homeostasis cause damage to tissues and organs and eventually lead to diabetic angiopathy, neuropathy, retinopathy, and nephropathy [14]. If this condition is not managed, these hyperglycemic abnormalities can also damage large blood vessels, which can progress to cardiovascular disease and stroke [15,16,17].
Because hyperglycemia can be managed to some extent by a low-carbohydrate diet, researchers and pharmaceutical companies seek methods to control it by modulating starch digestion [18,19]. However, although it can control blood glycemia, making starch resistant to digestion is not recommended due to safety concerns [18,19,20,21]. Inhibition of the activity of starch-digesting enzymes within the intestine, which delays carbohydrate digestion, reduces glucose absorption, and thereby decreases postprandial blood glucose levels, has become a widely accepted approach [22,23]. α-Amylase is a key player in dietary starch digestion, and because its inhibition plays an important role in reducing and regulating postprandial hyperglycemia [23,24,25,26], it is considered a desirable biological target for DM treatment [23]. The enzyme catalyzes carbohydrate digestion inside the small intestine by hydrolyzing 1, 4-glycosidic linkages and converting polysaccharides (starch) to disaccharides. The drug acarbose is a pseudotetrasaccharide containing a nonhydrolyzable, nitrogen-linked bond, which binds α-amylase and suppresses its activity through competitive, reversible inhibition. It is one of the second-line drugs in the treatment of diabetes after metformin [27,28]. However, its application is limited due to the cost of production and the side effects associated with its synthetic nature [29]. Therefore, in recent years, many studies have investigated the inhibition of α-amylase by natural products, such as plant extracts [30,31], and found that plants constitute a rich source for antidiabetic drug candidates acting via α-amylase inhibition [32,33]. Due to their natural characteristics and long process of evolutionary selection, plant extracts may induce fewer side effects when compared to synthetic drugs. In general, natural-based medicines have been proven safer [34,35,36] and are inherently better tolerated than synthetic drugs, and thus may be the best source of future medicines [37,38]. Specifically, many plants are used in the treatment of DM patients [39]; e.g., Rhus coriaria, a traditional Middle Eastern table spice, is recommended for the treatment of hyperlipidemia in diabetic patients [39,40].
In addition, there is a large body of evidence showing that oxidative stress is primarily induced by hyperglycemia and is associated with a key process in the initiation and progression of diabetic complications [41,42]; however, the precise mechanisms by which oxidative stress accelerates the development of diabetic complications are only partly known [43,44]. Inflammatory mediators are generated, and these are stimulated by hyperglycemia-induced oxidative stress, which leads to the production of free radical species [42,45]. Free radicals are an unstable species of molecule that pair up their odd free electrons by attacking healthy cells, causing loss of cell structure and/or function [46]. These damaged cells contribute greatly to degenerative diseases such as cancer, inflammation, immune system weakening, liver disease, brain dysfunction, cardiovascular conditions, diabetic renal failure, and others [47]. Plant antioxidants are free radical-scavenging agents that can control the detrimental effect of these unstable species on the human body [48] and may be useful in treating some diabetic complications. This work aimed to perform a large-scale screening of plant extracts that exhibit both inhibitory effects on α-amylase and free radical-scavenging properties with minimal side effects. To this end, 41 extracts from plants widely used by people in the Middle East as food and medication were screened for such activities. Once a plant extract that can act as both an antidiabetic and antioxidant is identified, it can be further studied to isolate its bioactive compounds.
2. Results
2.1. Screening Plant Extracts for Their Antidiabetic and Free Radical Scavenging Activities
Both the lack of effective drugs for the treatment of type 2 diabetes and the increasing prevalence of the disease have created an urgent need to improve drug therapies. Some studies have shown that natural, plant-derived products have safe characteristics, with minimal side effects, unlike many widely used synthetic drugs [37]. The use of natural drugs for the treatment of diabetes and its associated complications is expanding worldwide, and many plant-based products exhibit antidiabetic effects [37,49].
Our initial objective was to examine the antidiabetic and antioxidant effects of the extracts of 41 medicinal/edible local plants (Table 1). All methanolic extracts of these plants were screened for α-amylase inhibitory activity at a concentration of 2 mg/mL. Two of the extracts demonstrated high α-amylase inhibitory activity, suggesting antidiabetic activity; these were from Pelargonium spp. and Rhus coriaria, which exhibited 100 and 82% inhibition, respectively. We performed evaluations of the free radical scavenging activity (FRSA) of the extracts in parallel. Of the 41, Cinnamomum aromaticum extract showed the lowest EC50 for FRSA, and Ceratonia siliqua extract showed the highest (Table 1). Most importantly, Pelargonium spp. and Rhus coriaria, which had a strong inhibitory effect on α-amylase activity, also exhibited high levels of scavenging activity, indicating a dual function.

Table 1.
Medicinal/edible plant extract-driven inhibition of α-amylase activity and the EC50 values for free radical-scavenging activity. Active extracts that inhibit α-amylase in a ratio above 25% are labeled in bold.
2.2. In Vitro α-Amylase Inhibition
Table 1 shows that Pelargonium spp. and Rhus coriaria display particularly high α-amylase inhibition and are as potent as acarbose (EC50 = 30 μg/mL [50]). Although the inhibition is clear, little is known about the mechanism by which it occurs. Further studies will shed light on the mechanism and help us to more effectively validate the use of the extract to treat type 2 diabetes. Furthermore, kinetic analyses are crucial to determining the type of inhibition exerted on α-amylase; the pattern of inhibition will suggest whether the active site of the enzyme is directly involved in the mechanism of action. In addition, the plant extract most likely contains some compounds that could serve as substrate analogs that compete for the active site of α-amylase.
The effects of increasing the concentrations of the plant extracts of Pelargonium spp. (Figure 1A) and Rhus coriaria (Figure 1B) to inhibit α-amylase activity were assessed, aiming to evaluate the values of EC50 (acarbose at concentration of 1.25 mM was used as a positive control). The EC50s of Pelargonium spp. and of Rhus coriaria were 0.60 and 1.78 mg/mL, respectively. The strong α-amylase inhibitory activity indicates the presence of active compounds that could inhibit the breakdown of complex carbohydrates into oligosaccharides, which could diminish the effects of carbohydrate consumption on postprandial hyperglycemia.
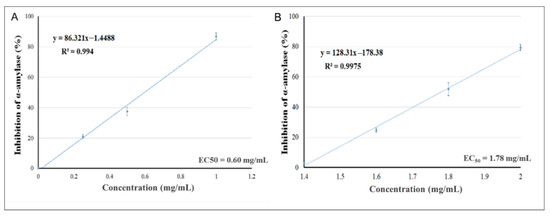
Figure 1.
Inhibitory activity of Pelargonium spp. (A) and Rhus coriaria (B) against α-amylase.
An in vivo study to further confirm the results is required to validate and fractionate the active natural material to be developed for type 2 diabetes treatment.
Pelargonium spp. and Rhus coriaria may carry valuable medical benefits, especially because these substances may be used as nutritional supplements for people with diabetes.
2.3. Effects of the Plant Extracts on Free Radical Scavenging
The generation of reactive oxygen species (ROS) (or free radicals) plays an important role in the pathogenesis of diabetes, resulting in increased oxidative damage at the molecular and cellular levels. Excessive production of ROS in diabetic patients has been targeted with antioxidants in an effort to prevent and suppress the development of oxidative damage to DNA, proteins, and lipids within cells and tissues.
Plant products are the main source of natural antioxidant molecules that can eradicate or neutralize the damage caused by ROS [51]. They are currently available in many pharmaceutical venues and are used to manage diseases and health conditions, including to counteract ROS. Curcumin, a natural polyphenol derived from the rhizome of turmeric, and quercetin, an antioxidant derived from fruits and vegetables, have been shown to have potent FRSA and antidiabetic activity [52]. Polysaccharides from the leaves of Guava (GLP) [53], Lepidium Meyenii (MECA) [54], and Olive [55] have been evaluated for their antioxidant activity in vitro and have been found to display good FRSA.
The concentrations of the 41 plant extracts were increased, and the impacts on scavenging (antioxidative) activity were assessed using the in vitro 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. This is a reliable assay commonly used for the large-scale screening of plant extract samples for in vitro antioxidant activity. The results show that Pelargonium spp. and Rhus coriaria both have a low EC50 for FRSA (Table 2).

Table 2.
The inhibition of α-amylase and free radical scavenging by Pelargonium spp. and Rhus coriaria, the two most effective plant extracts with dual functionality.
2.4. Correlation Analysis between α-Amylase Inhibition and Free Radical Scavenging Activity
Verifying the correlation between α-amylase inhibition and free radical scavenging activity will advance the subsequent screening of plant extracts by highlighting those with advantageous dual antidiabetic and antioxidant functions. The inhibition of α-amylase was first verified by screening all of the extracts at a concentration of 2 mg/mL (Table 1). The extracts that displayed a percentage of inhibition above 50% were then tested at lower concentrations to determine their IC50 values (Table 2). Two extracts were found to have IC50 values < 2 mg/mL—Pelargonium spp. and Rhus coriaria. Their IC50 values, as measured by dose-response experiments, were 0.60 and 1.78 mg/mL, respectively. Treatment with 2 mg/mL of Olea europaea, Petroselinum crispum, Stevia rebaudiana, and Punica granatum inhibited α-amylase by 42.6, 37.5, 35.7 and 27.4%, respectively (Table 1).
Rules-based analysis using Matthews correlation coefficient (MCC) scores and enrichment factors as criteria for the evaluation of the efficiency of the model revealed that plant extracts with a free radical-scavenging EC50 ≤ 10 µg/ml showed somewhat better inhibition of α-amylase (see Table 3). The values for the enrichment factor, the MCC, accuracy, and precision were 1.7, 0.286, 0.61, and 0.25, respectively. These findings are of great importance for screening projects. Identifying extracts with a 1.7 order of enrichment in inhibiting α-amylase from among plant extracts with an EC50 of FRSA ≤ 10 µg/mL could save time and money in high-throughput screening. It is worth noting that, taking these results together, we might be deceived and conclude that there is no correlation between the inhibition of α-amylase and the free radical scavenging activities of the screened extracts (as shown in Figure 2, R2 = 0.0471). For the six most active plant extracts, there was a low correlation, with R2 = 0.3006 (shown in Figure 3). Five of the six active plant extracts (i.e., Pelargonium spp., Punica granatum, Olea europaea, Rhus coriaria, and Stevia rebaudiana) showed relatively high FRSA, while the activity for Petroselinum crispum was relatively low.

Table 3.
EC50 cutoffs for free radical scavenging activity (FRSA).
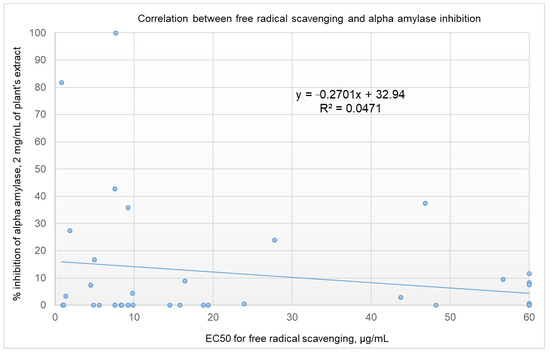
Figure 2.
Correlation between the percentage of inhibition of α-amylase by 2 mg/mL of plant extract and the EC50 for FRSA. A free radical scavenging EC50 > 60 µg/mL is set at 60 µg/mL.
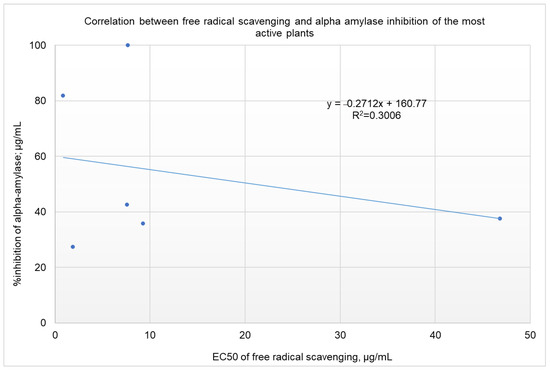
Figure 3.
Correlation between the percentage of inhibition of α-amylase and the EC50 for FRSA for the six most active plant extracts.
Figure 4 presents the enrichment plot, and Figure 5 shows the receiver operating characteristic (ROC) plot for the α-amylase inhibition/free radical-scavenging correlation model. The area under the curve (AUC) that was obtained for the current model was 0.723 (Figure 5), indicating a very weak correlation between α-amylase inhibition and FRSA. The enrichment plot (Figure 4) illustrates how quickly the active extracts of plants can be identified when they are sorted according to their FRSA. In-depth analysis reveals that the shape seen in Figure 4 fits well with the conclusions drawn from the detailed analysis of Table 3, which shows that the plant extracts with an EC50 for FRSA ≤ 10 µg/mL exhibit strong α-amylase inhibition of a 1.7 order of magnitude.
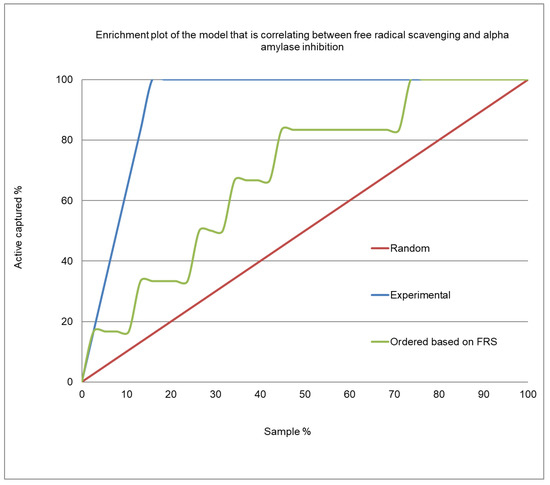
Figure 4.
Enrichment plot of the prediction model for α-amylase inhibition by the plant extracts, based on their FRSA.

Figure 5.
A receiver operating characteristic (ROC) curve showing the performance of the FRSA-α-amylase inhibition correlation model.
In Table 3, the Matthews correlation coefficient (MCC) scores and enrichment factors are utilized as criteria in the evaluation of the models. The calculations were based on the assumption that a 250 µg/mL-plant extract that produced α-amylase inhibition ≥25% was active (a true positive); extracts with inhibition below 25% were considered inactive. Six out of the 41 tested plant extracts showed an inhibition of α-amylase ≥25%.
Figure 4 shows the enrichment plot, and Figure 5 shows the ROC plot, of the FRSA-α-amylase inhibition correlation model. The enrichment plot illustrates how quickly the active extracts of plants can be identified when they are sorted according to their FRSA. In the enrichment plot, a close-to-perfect curve reflects the high prioritization power of the proposed model.
According to the results of the above analysis, there is, to some extent, a correlation between α-amylase inhibition and the FRSA of the tested plant extracts. Plant extracts with FRSA EC50 ≤ 10 μg/mL have a higher chance (a 1.7 order of magnitude) of inhibiting α-amylase enzyme than plant extracts with FRSA EC50 > 10 μg/mL. This piece of information is significant because it helps to reduce the number of plant extracts that have to be included in subsequent investigational screenings and highlights natural products that have dual antidiabetic and antioxidant properties.
2.5. Effects of the Plant Extracts on Glucose Uptake in Skeletal Muscles
Pelargonium spp. and Rhus coriaria extracts were further investigated for their capacity to stimulate glucose uptake by the L6-GLUT4myc skeletal muscle cell line. First, the concentration ranges of the substances at which the muscle cells preserve their viability were determined.
2.5.1. Effects of the Plant Extracts on Cell Viability
Pelargonium spp. at concentrations ranging between 0.006 and 0.705 mg/mL had no effect on cell viability, while higher concentrations resulted in 85–95% cell viability (Figure 6A). Exposure of cells to Rhus coriaria extract at concentrations between 0.0078125 and 0.5 mg/mL had no effect on cell viability, while higher concentrations resulted in decreased cell viability (up to 15%) compared to the control (Figure 6B). Viability above 100% (the control), observed at some extract concentrations, can be explained by the absorption of some components within the extracts that had not been washed out.
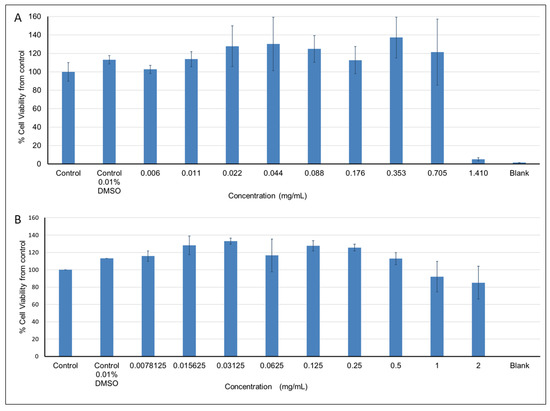
Figure 6.
The effects of increasing concentrations of the plant extracts on the viability of L6-GLUT4myc cells: (A) Pelargonium spp. and (B) Rhus coriaria extracts.
2.5.2. Effects of Pelargonium spp. and Rhus coriaria Extracts on GLUT4 Translocation
The effects of Pelargonium spp. and Rhus coriaria extracts on GLUT4 translocation in L6-GLUT4myc cells were assessed by exposing cells to the extracts at three concentrations that were found to inhibit α-amylase and show FRSA. The solvent in which the extract was dissolved was used as a negative control. Insulin was used as a positive control, and solvent without extract was used as a negative control. No effect of the Pelargonium spp. extract on GLUT4 translocation was observed (Figure 7A). In contrast, treatment of cells with 0.5, 1, or 2 mg/mL of Rhus coriaria extract increased the translocation of GLUT4 by 111% (1.11-fold, p < 0.05), 119% (1.19-fold, p < 0.05), and 139% (1.39-fold, p < 0.01), respectively, as opposed to the control (with 0% concentration of the extract).
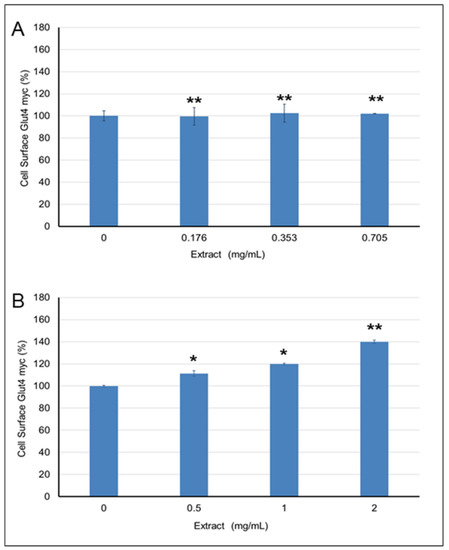
Figure 7.
The effect of: (A) Pelargonium spp. and (B) Rhus coriaria extracts on GLUT4 translocation. The results represent a mean ± standard deviation of three experiments (n = 3). One asterisk (*) indicates p < 0.05. Two asterisks (**) indicate p < 0.001.
These results are consistent with those of studies reporting increased glucose uptake as a result of enhanced GLUT4 levels in L6 cells [56,57,58]. Previous studies have also showed decreased GLUT4 expression in skeletal muscle and adipose tissue in GLUT4 +/− heterozygous mice and, as a result, a decrease in glucose uptake in muscle cells and an increase in peripheral insulin resistance, followed by an increase in blood glucose levels [59,60]. However, when the transgenic mice overexpressed GLUT4, increased sensitivity to insulin was observed [61].
Therefore, deficiencies in glucose uptake in skeletal muscle cells are mostly linked to defects in the insulin signaling pathway and are probably caused by changes in GLUT4 transporter translocation to the cell surface [62]. Therefore, extracts such as Rhus coriaria (Figure 7B), which mobilize normal GLUT4 transporter levels to the cell surface, may be useful in the development of drugs for treating type 2 diabetes. Rhus coriaria extract also exhibited increased anti-α-amylase, as well as FRSA. This higher activity might be due to the presence of supportive phytochemicals that need to be isolated and chemically identified, after which the biological activity of the individual phytochemicals, along with the mechanisms involved, should be verified.
3. Materials and Methods
3.1. Materials
All of the plants used in this study were purchased from Al Alim Ltd. (Medicinal Herb Center, Zippori, Israel). DPPH and the solvents were purchased from Sigma, Israel. Acarbose, starch, glucose, and α-amylase were all purchased from Sigma-Aldrich, Saint Louis, MO, USA.
3.2. Preparation of Plant Extracts
To produce each extract sample, approximately 1 g of dried plant material was packed in a tube and soaked in methanol (10 mL for each gram of material), sonicated for 75 min at 40 °C, then left for 3 h to cool for complete extraction. The methanolic extracts were then filtered through grade-1 Whatman paper, and the filtered solution was concentrated by evaporation to dryness in a vacuum, dissolved in DMSO to a concentration of 100 mg/mL, and stored at 4 °C for future activity testing.
3.3. α-Amylase Activity
α-Amylase inhibitory activity was tested using a standard assay, with minor modifications [13]. Reaction mixtures containing 20 μL of extracts at different concentrations (8, 4, 2, 1, 0.5 mg/mL), 50 μL phosphate buffer (100 mM, pH = 6.8), and 10 μL of α-amylase (2 U/mL) were incubated for 5 min at 37 °C, in 96-well plates. Thereafter, 20 μL of 1% soluble starch (100 mM phosphate buffer, pH 6.8) was added, and the mixture was incubated for 5 min, at 37 °C; the buffer did not include magnesium or calcium and served as the substrate for the reaction. Afterward, 100 μL of DNS color reagent was added, and the mixture was boiled for 10 min, after which the absorbance was measured at 540 nm with a microplate reader. Acarbose was used as a positive control, and each experiment was carried out in quadruplicate. The percentage of α-amylase inhibition was calculated according to Equation (1):
3.4. Free Radical Scavenging Activity (FRSA)
The FRSA of the methanolic extracts of the various plants was measured using the DPPH assay protocol, with minor modifications [14]. The DPPH was serially diluted in pasteurized water, and the tests were carried out in 96-well cell culture plates. Diluted DPPH solution (200 ppm, 100 μL) was added to 100 μL of the methanolic plant extracts. The mixture was shaken and allowed to stand for 30 min in the dark at room temperature. Then the absorbance of the solution was measured at 517 nm and converted into a percentage of FRSA, using Equation (2):
where Asample is the absorbance of the plant extract and the DPPH mixture, Ablank1 is the absorbance of the plant extract, Acontrol is the absorbance of the ethanolic solution of DPPH, and Ablank2 is the absorbance of ethanol.
FRSA is expressed in terms of the half-maximal effective concentration (EC50), i.e., the amount of antioxidants necessary to decrease the initial DPPH absorbance by 50%. The EC50 value for each plant extract was determined by calculating the value using the equation of the linear part of the graph. Gallic acid (100 µg/mL) was used as a positive control.
3.5. Cell Viability
The viability of cells exposed to different concentrations of the plant extracts was assessed using the Cell Proliferation Kit (XTT based) (Biological Industries, Beit Haemek, Israel). The assay is based on the ability of the metabolically active cells to reduce the amount of applied dye, whose color changes to orange, the intensity of which is proportionate to the number of cells. The intensity of the color was measured with a spectrophotometer at a wavelength of 450 nm.
L6-GLUT4myc cells (2 × 104/well) were seeded in 100 μL of growth medium in 96-well plates and incubated for 24 h. The Pelargonium spp. and Rhus coriaria extracts were then added at various concentrations, and the plates were incubated for 20 h at 37 °C. The next day, XTT reagents were added to the plate (medium was added to the control wells) and incubated for 2 h at 37 °C. The control samples were treated with 0.01% DMSO (the solvent in which the plant extracts were dissolved). Cell viability was evaluated according to the manufacturer’s instructions, using a Thermo Scientific Varioskan LUX Multimode Microplate Reader at 450 nm. The measured absorbance was subtracted from the reference absorbance (620 nm), and each experiment was repeated independently three times.
3.6. Glucose Uptake
Glucose uptake into skeletal muscle is mostly performed via GLUT4, which is recruited from the cytosol to the cell surface, a process that is stimulated by insulin or other antidiabetic molecules [11,12,13,63]. GLUT4 content on cell surfaces has been documented to be positively correlated with glucose uptake activity in skeletal muscle cells [63]. L6-GLUT4myc cells are the only cellular model available for investigating glucose uptake and GLUT4 translocation without requiring cell permeabilization or fractionation. Cell-surface GLUT4-myc levels were quantitated using a previously published protocol [63]. In brief, rat L6 muscle cell-line cells stably expressing myc-tagged GLUT4 were cultured (5% CO2, 37 °C) in a complete growth medium composed of α-MEM, 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. The cells (2 × 105/well) were seeded onto 24-well plates and cultured for 24 h, then treated with natural products and plant extracts for 20 h. Next, they were incubated with serum-free medium for 3 h and then treated with 1 µM of insulin (as a positive control) or with plant extracts at 37 °C for 20 min. The cells were then washed twice with ice-cold PBS, fixed with 3% paraformaldehyde (PFA) (10 min), incubated with 0.1 M glycine, blocked with 3% (v/v) goat serum for 30 min, and reacted with polyclonal anti-myc antibody for 1 h at 4 °C. After that, the cells were washed 5 times with wash buffer and reacted with anti-rabbit IgG for 1 h at 4 °C, then washed another 5 times with wash buffer. Following this, 0.2 mL of 3,3′,5,5′-tetramethylbenzidine (TMB) was added to each well, and the cells were incubated in the dark at RT for 5–10 min, after which 3N hydrochloric acid (HCl) (0.2 mL/well) was added to terminate the reaction. Finally, the absorbance of the supernatants was measured using a Thermo Scientific Varioskan LUX Multimode Microplate Reader at 492 nm.
3.7. Model Assessments
Parameters, such as the Matthews correlation coefficient (MCC), accuracy, the precision enrichment factor, and the area under the ROC curve (AUC), were calculated to assess the quality of the free radical scavenging α-amylase activity correlation models (see Equations (3)–(6)).
Equation (3). Matthews correlation coefficient (MCC)
where P, N, Pf, and Nf are the number of true positive, true negative, false positive, and false-negative predictions, respectively. A perfect prediction yields MCC = 1.0, while a random performance yields MCC = 0.0 and MCC = −1.0 indicates a completely erroneous prediction.
Equation (4). Accuracy
Accuracy = (P + N)/(P + N + Pf + Nf)
Equation (5). Precision
Precision = P/(P + Pf)
Equation (6). Enrichment factor
where TFRS is the percentage of actives when using the FRS threshold criterion, and TRS is the percentage of actives by random selection.
EF = TFRS/TRS,
3.8. Statistical Analyses
Statistical analyses were performed using SPSS software, and means were compared using the two-tailed parametric test (Student’s t-test). Statistical significance levels were set at * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Conclusions
Since ROS are abundant and massively produced in diabetic patients, we aimed to draw a correlation between the inhibition of α-amylase and the FRSA of plant extracts, with the ultimate goals of identifying plant extracts possessing both antioxidant and antidiabetic activity and facilitating the screening process for identifying antidiabetic natural products, preferably those exhibiting both antidiabetic and antioxidant activity. To this end, the FRSA of several plant extracts was assessed using the DPPH assay, while α-amylase inhibition was measured using the DNS assay. Pelargonium spp., Punica granatum, Olea europaea, Rhus coriaria, Stevia rebaudiana, and Petroselinum crispum plant extracts were found to display some anti-α-amylase activity. An in-depth analysis of possible correlations between FRSA and α-amylase inhibition revealed that the plant extracts which exhibited an FRSA EC50 ≤ 10 μg/mL showed some degree of enrichment for anti-α-amylase activity (at a 1.7 order of magnitude). These findings are of great importance as they may significantly shorten screening processes, saving both time and money.
The Rhus coriaria extract exhibited α-amylase inhibition, a high level of free radical scavenging, and increased GLUT4 translocation onto cell membranes in a dose-dependent manner. Hence, the extract of Rhus coriaria may be useful as a nutraceutical or as a source of drugs to treat type 2 diabetes disease. Its high activity might be due to the presence of supportive phytochemicals, which will need to be isolated and chemically identified, after which the biological activity of the individual phytochemicals, along with their mechanisms, should be verified.
Due to these considerations, our next study will use a novel in-house bioassay-guided fractionation approach [64,65,66,67] and in silico-based modeling techniques [68,69,70,71,72] to isolate substances from bioactive plant extracts that exhibit antidiabetic activity. We suggest that subsequent studies validate the conclusions reached in the current study by testing other antidiabetic parameters. In addition, the biological activity of the isolated substances should be tested in diabetic mice. Preventing oxidative stress and balancing blood glucose levels in diabetic mice by orally administered natural products will significantly advance the development of natural drugs exhibiting both antidiabetic and antioxidant functions.
Author Contributions
All authors contributed extensively to this work. M.G., B.A.-F., M.R. and R.M. ran the experiments and performed the analysis. A.B., M.R., R.M., S.S., A.R. and M.F. interpreted the data and wrote up the conclusions. M.F. and A.R. wrote the first draft of the manuscript. M.F. and A.R. conceived the study and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Ministry for the Development of the Periphery, the Negev and the Galilee, Israel.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This work was supported by the Ministry for the Development of the Periphery, the Negev and the Galilee, Israel. A.R. acknowledges the Al-Qasemi Research Foundation for partially supporting this work. The L6-GLUT4myc cells were provided by Arie-Lev Gruzman, Faculty of Exact Sciences, Bar-Ilan University, Israel.
Conflicts of Interest
The authors declare no conflict of interest and that the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; nor in the decision to publish the results.
Sample Availability
Samples of the compounds/plants are available from the corresponding authors.
References
- Miao, M.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Slowly digestible starch—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Warren, F.; Zhang, B.; Waltzer, G.; Gidley, M.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M. Carbohydrate: Digestion, Absorption and Metabolism. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 643–650. [Google Scholar]
- Lebovitz, H.E. Chapter 42-Hyperglycemia Secondary to Nondiabetic Conditions and Therapies. In Endocrinology: Adult and Pediatric, 7th ed.; Jameson, J.L., De Groot, L.J., de Kretser, D.M., Giudice, L.C., Grossman, A.B., Melmed, S., Potts, J.T., Weir, G.C., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 737–751.e6. [Google Scholar]
- Brockman, R.P. Roles of glucagon and insulin in the regulation of metabolism in ruminants. A review. Can. Vet. J. 1978, 19, 55–62. [Google Scholar] [PubMed]
- Alatrach, M.; Agyin, C.; Mehta, R.; Adams, J.; DeFronzo, R.A.; Abdul-Ghani, M. Glucose-Mediated Glucose Disposal at Baseline Insulin Is Impaired in IFG. J. Clin. Endocrinol. Metab. 2019, 104, 163–171. [Google Scholar] [CrossRef]
- Liu, A.; Liu, J.; Chen, X.; Lu, B.; Zeng, C.; Ye, H. A novel crustacean hyperglycemic hormone (CHH) from the mud crab Scylla paramamosain regulating carbohydrate metabolism. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 231, 49–55. [Google Scholar] [CrossRef]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int. J. Health Sci. (Qassim) 2007, 1, V–VIII. [Google Scholar]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle--A focus on the molecular mechanisms of insulin resistance. Mol. Cell Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef]
- Lund, S.; Holman, G.D.; Schmitz, O.; Pedersen, O. Glut 4 content in the plasma membrane of rat skeletal muscle: Comparative studies of the subcellular fractionation method and the exofacial photolabelling technique using ATB-BMPA. FEBS Lett. 1993, 330, 312–318. [Google Scholar] [CrossRef]
- Lund, S.; Holman, G.D.; Zierath, J.R.; Rincon, J.; Nolte, L.A.; Clark, A.E.; Schmitz, O.; Pedersen, O.; Wallberg-Henriksson, H. Effect of insulin on GLUT4 cell surface content and turnover rate in human skeletal muscle as measured by the exofacial bis-mannose photolabeling technique. Diabetes 1997, 46, 1965–1969. [Google Scholar] [CrossRef]
- Wilson, C.M.; Cushman, S.W. Insulin stimulation of glucose transport activity in rat skeletal muscle: Increase in cell surface GLUT4 as assessed by photolabelling. Biochem. J. 1994, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Jandeleit-Dahm, K. The pathobiology of diabetic vascular complications--cardiovascular and kidney disease. J. Mol. Med. (Berl.) 2014, 92, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Parim, B.; Sathibabu Uddandrao, V.V.; Saravanan, G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019, 24, 279–299. [Google Scholar] [CrossRef] [PubMed]
- van Sloten, T.T.; Sedaghat, S.; Carnethon, M.R.; Launer, L.J.; Stehouwer, C.D.A. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020, 8, 325–336. [Google Scholar] [CrossRef]
- Tian, J.; Ogawa, Y.; Shi, J.; Chen, S.; Zhang, H.; Liu, D.; Ye, X. The microstructure of starchy food modulates its digestibility. Crit. Rev. Food Sci. Nutr. 2019, 59, 3117–3128. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, W.; McClements, D. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Robertson, M.D. Dietary-resistant starch and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 362–367. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Li, Y.; Cui, S.W.; Zhang, T. Structure elucidation of catechins for modulation of starch digestion. LWT Food Sci.Technol. 2014, 57, 188–193. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Jiang, H.; Zhang, T.; Li, X. Interaction mechanism between green tea extract and human alpha-amylase for reducing starch digestion. Food Chem. 2015, 186, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of alpha-amylase, alpha-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Jiang, H.; Jiang, B.; Zhang, T.; Cui, S.W.; Jin, Z. Phytonutrients for controlling starch digestion: Evaluation of grape skin extract. Food Chem. 2014, 145, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Jayathilake, C.; Liyanage, R. A simple microplate-based method for the determination of alpha-amylase activity using the glucose assay kit (GOD method). Food Chem. 2016, 211, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Shi, J.; Tang, Z.; Sawhney, M.; Hu, H.; Shi, L.; Fonseca, V.; Dong, H. Comparison of glucose lowering effect of metformin and acarbose in type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2015, 10, e0126704. [Google Scholar] [CrossRef]
- Dileep, K.V.; Nithiyanandan, K.; Remya, C. Binding of acarbose, an anti-diabetic drug to lysozyme: A combined structural and thermodynamic study. J. Biomol. Struct. Dyn. 2018, 36, 3354–3361. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K. Phytochemical profiling, GC-MS analysis and alpha-amylase inhibitory potential of ethanolic ectract of cocos nucifera Linn endocarp. Endocr. Metab. Immune Disord. Drug. Targets 2019, 19, 419–442. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G. Characterization and Comparison of Protein and Peptide Profiles and their Biological Activities of Improved Common Bean Cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum. Nutr. 2015, 70, 105–112. [Google Scholar] [CrossRef]
- Mattio, L.M.; Marengo, M.; Parravicini, C.; Eberini, I.; Dallavalle, S.; Bonomi, F.; Iametti, S.; Pinto, A. Inhibition of Pancreatic alpha-amylase by Resveratrol Derivatives: Biological Activity and Molecular Modelling Evidence for Cooperativity between Viniferin Enantiomers. Molecules 2019, 24, 3225. [Google Scholar] [CrossRef]
- Parizad, P.A.; Capraro, J.; Scarafoni, A.; Bonomi, F.; Blandino, M.; Marengo, M.; Giordano, D.; Carpen, A.; Iametti, S. The Bio-Functional Properties of Pigmented Cereals may Involve Synergies among Different Bioactive Species. Plant Foods Hum. Nutr. 2019, 74, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Tajaldini, M.; Samadi, F.; Khosravi, A.; Ghasemnejad, A.; Asadi, J. Protective and anticancer effects of orange peel extract and naringin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse model. Biomed. Pharmacother. 2020, 121, 109594. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Dombe, S.; Shirote, P. Nanosponges Encapsulated Phytochemicals for Targeting Cancer: A Review. Curr. Drug Targets 2020. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzyme inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef]
- Zaid, H.; Raiyn, J.; Nasser, A.; Saad, B.; Rayan, A. Physicochemical Properties of Natural Based Products versus Synthetic Chemicals. Open Nutraceuticals J. 2010, 3, 194–202. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of alpha-amylase and alpha-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. World Health Organ. 1985, 63, 965–981. [Google Scholar] [CrossRef]
- Mohammadi, S.; Montasser Kouhsari, S.; Monavar Feshani, A. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. Daru 2010, 18, 270–275. [Google Scholar]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, Oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Perrone, S.; Santacroce, A.; Longini, M.; Proietti, F.; Bazzini, F.; Buonocore, G. The Free Radical Diseases of Prematurity: From Cellular Mechanisms to Bedside. Oxid. Med. Cell Longev. 2018, 2018, 7483062. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Yang, S.C.; Hsu, C.Y.; Chou, W.L.; Fang, J.Y.; Chuang, S.Y. Bioactive agent discovery from the natural compounds for the treatment type 2 diabetes rat model. Molecules 2020, 25, 5713. [Google Scholar] [CrossRef]
- Boojar, F.M.A.; Aghaei, R.; Mashhadi Akbar Boojar, M. Data on possible in vitro anti-diabetic effects of verticinone on β-TC6 pancreatic and C2C12 skeletal muscle cells. Data Brief 2020, 28, 104828. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef]
- Caicai, K.; Limin, H.; Liming, Z.; Zhiqiang, Z.; Yongwu, Y. Isolation, purification and antioxidant activity of polysaccharides from the leaves of maca (Lepidium Meyenii). Int. J. Biol. Macromol. 2018, 107, 2611–2619. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Taha, C.; Rose, E.; Marcusohn, J.; Klip, A.; Skolnik, E.Y. The inability of phosphatidylinositol 3-kinase activation to stimulate GLUT4 translocation indicates additional signaling pathways are required for insulin-stimulated glucose uptake. Proc. Natl. Acad. Sci. USA 1995, 92, 10247–10251. [Google Scholar] [CrossRef]
- Kumar, P.M.; Venkataranganna, M.V.; Manjunath, K.; Viswanatha, G.L.; Ashok, G. Methanolic extract of Momordica cymbalaria enhances glucose uptake in L6 myotubes in vitro by up-regulating PPAR-γ and GLUT-4. Chin. J. Nat. Med. 2014, 12, 895–900. [Google Scholar] [CrossRef][Green Version]
- Zhao, P.; Ming, Q.; Qiu, J.; Tian, D.; Liu, J.; Shen, J.; Liu, Q.H.; Yang, X. Ethanolic extract of Folium Sennae Mediates the glucose uptake of L6 cells by GLUT4 and Ca2+. Molecules 2018, 23, 2934. [Google Scholar] [CrossRef]
- Li, J.; Houseknecht, K.L.; Stenbit, A.E.; Katz, E.B.; Charron, M.J. Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice. FASEB J. 2000, 14, 1117–1125. [Google Scholar] [CrossRef]
- Stenbit, A.E.; Tsao, T.S.; Li, J.; Burcelin, R.; Geenen, D.L.; Factor, S.M.; Houseknecht, K.; Katz, E.B.; Charron, M.J. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 1997, 3, 1096–1101. [Google Scholar] [CrossRef]
- Tsao, T.S.; Li, J.; Chang, K.S.; Stenbit, A.E.; Galuska, D.; Anderson, J.E.; Zierath, J.R.; McCarter, R.J.; Charron, M.J. Metabolic adaptations in skeletal muscle overexpressing GLUT4: Effects on muscle and physical activity. FASEB J. 2001, 15, 958–969. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotech. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016, 196, 1066–1074. [Google Scholar] [CrossRef]
- Kacergius, T.; Abu-Lafi, S.; Kirkliauskiene, A.; Gabe, V.; Adawi, A.; Rayan, M.; Qutob, M.; Stukas, R.; Utkus, A.; Zeidan, M.; et al. Inhibitory capacity of Rhus coriaria L. extract and its major component methyl gallate on Streptococcus mutans biofilm formation by optical profilometry: Potential applications for oral health. Mol. Med. Rep. 2017, 16, 949–956. [Google Scholar] [CrossRef]
- Frank, A.; Abu-Lafi, S.; Adawi, A.; Schwed, J.S.; Stark, H.; Rayan, A. From medicinal plant extracts to defined chemical compounds targeting the histamine H4 receptor: Curcuma longa in the treatment of inflammation. Inflamm. Res. 2017, 66, 923–929. [Google Scholar] [CrossRef]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Zeidan, M.; Abu-Farich, B.; Austys, D.; Masalha, M.; Rayan, A. Suppressive Effects of Octyl Gallate on Streptococcus mutans Biofilm Formation, Acidogenicity, and Gene Expression. Molecules 2019, 24, 3170. [Google Scholar] [CrossRef]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Kalesinskas, P.; Masalha, M.; Falah, M.; Abu-Farich, B.; Melninkaitis, A.; Zeidan, M.; Rayan, A. Inhibitory Effects of Ethyl Gallate on Streptococcus mutans Biofilm Formation by Optical Profilometry and Gene Expression Analysis. Molecules 2019, 24, 529. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Rayan, M.; Abdallah, Z.; Abu-Lafi, S.; Masalha, M.; Rayan, A. Indexing natural products for their antifungal activity by filters-based approach: Disclosure of discriminative properties. Curr. Comput. Aided Drug Des. 2019, 15, 235–242. [Google Scholar] [CrossRef]
- Masalha, M.; Rayan, M.; Adawi, A.; Abdallah, Z.; Rayan, A. Capturing antibacterial natural products with in silico techniques. Mol. Med. Rep. 2018, 18, 763–770. [Google Scholar] [CrossRef]
- Aswad, M.; Rayan, M.; Abu-Lafi, S.; Falah, M.; Raiyn, J.; Abdallah, Z.; Rayan, A. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Inflamm. Res. 2018, 67, 67–75. [Google Scholar] [CrossRef]
- Zeidan, M.; Rayan, M.; Zeidan, N.; Falah, M.; Rayan, A. Indexing Natural Products for Their Potential Anti-Diabetic Activity: Filtering and Mapping Discriminative Physicochemical Properties. Molecules 2017, 22, 1563. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).

