Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites
Abstract
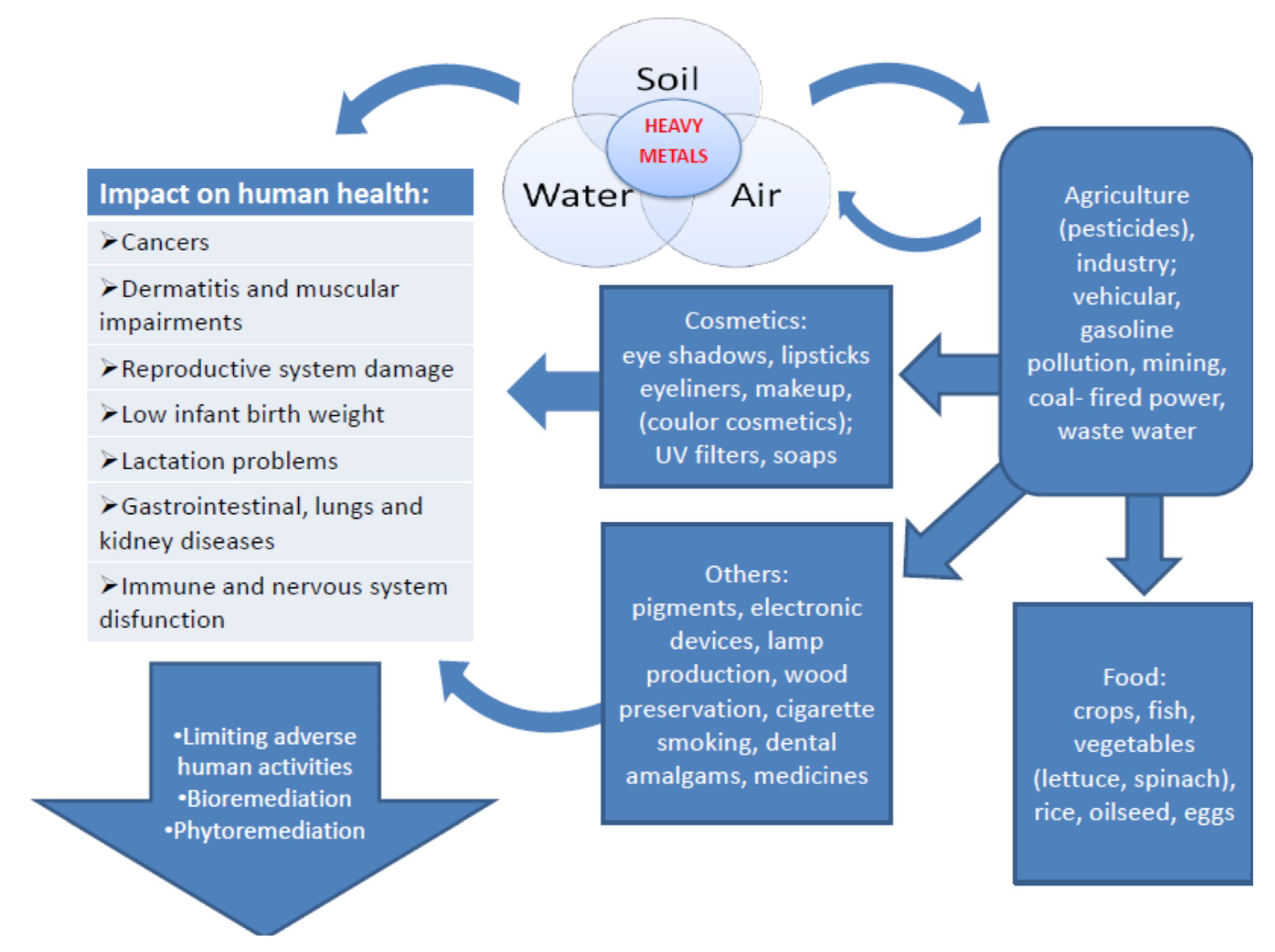
1. Introduction
2. Heavy Metals in Food and Water
3. Heavy Metals in Cosmetics
4. The Impact of Toxic Metals on Human Enzymatic Pathways
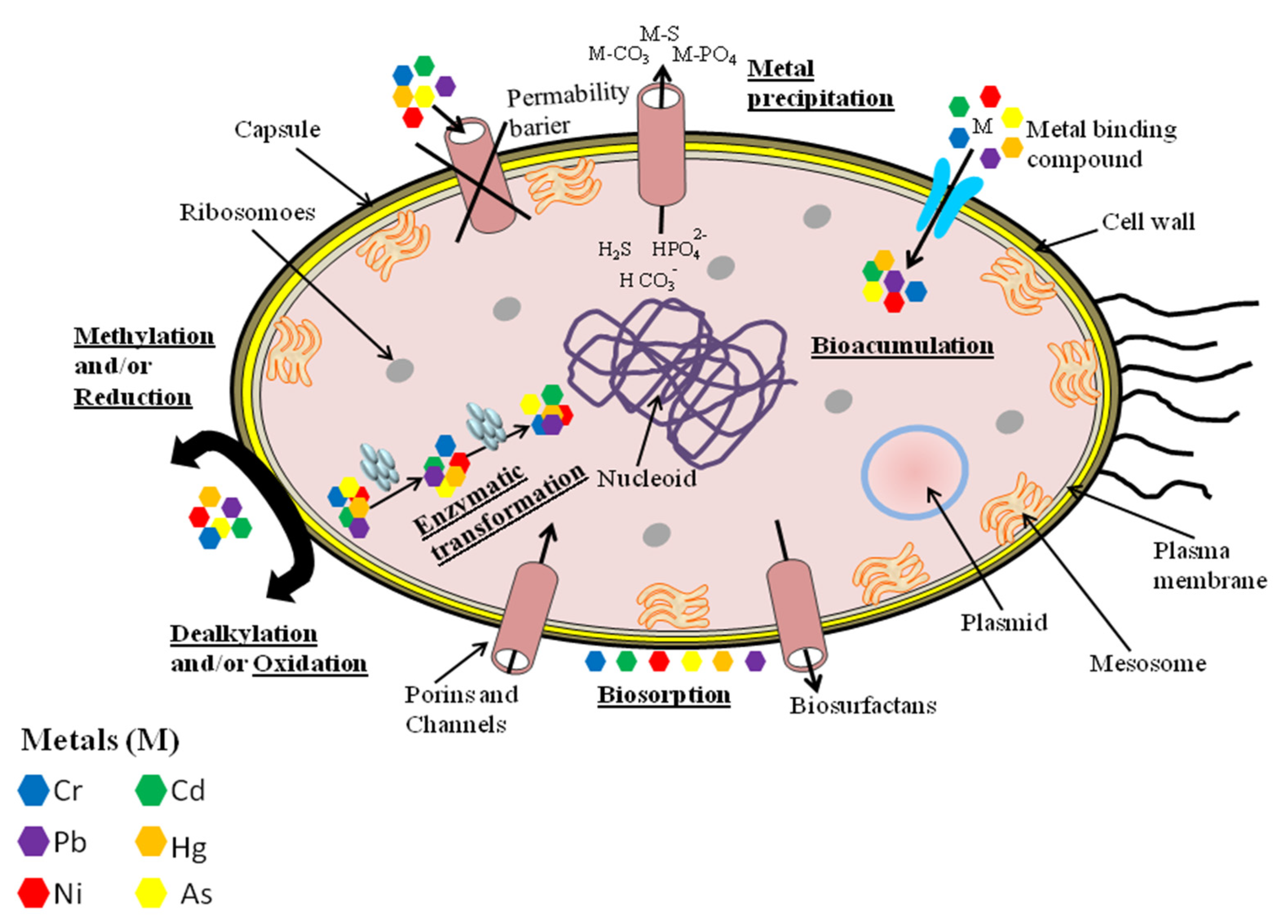
5. Methods of Bioremediation
6. Conclusions
Funding
Conflicts of Interest
References
- Marchetti, C. Role of calcium channels in heavy metal toxicity. ISRN Toxicol. 2013, 2013, 184360. [Google Scholar] [CrossRef] [PubMed]
- Potocki, S.; Rowinska-Zyrek, M.; Witkowska, D.; Pyrkosz, M.; Szebesczyk, A.; Krzywoszynska, K.; Kozlowski, H. Metal transport and homeostasis within the human body: Toxicity associated with transport abnormalities. Curr. Med. Chem. 2012, 19, 2738–2759. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J. Bioinformatics of metalloproteins and metalloproteomes. Molecules 2020, 25, 3366. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 2008, 13, 1205–1218. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson, L.; Kazantzis, G. Chapter 23: Diagnosis and treatment of metal poisoning: General aspects. In Handbook on the Toxicology of Metals, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 487–505. [Google Scholar] [CrossRef]
- Garza-Lombo, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, M.E.; Franco, R. Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure: Redox Signaling and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 1669–1703. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk, A.; Lyndin, M.; Sikora, V.; Lyndina, Y.; Romaniuk, S.; Sikora, K. Heavy metals effect on breast cancer progression. J. Occup. Med. Toxicol. 2017, 12, 32. [Google Scholar] [CrossRef]
- Pietrzak, S.; Wójcik, J.; Baszuk, P.; Marciniak, W.; Wojtyś, M.; Dębniak, T.; Cybulski, C.; Gronwald, J.; Alchimowicz, J.; Masojć, B.; et al. Influence of the levels of arsenic, cadmium, mercury and lead on overall survival in lung cancer. Biomolecules 2021, 11, 1160. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An overview of carcinogenic heavy metal: Molecular toxicity mechanism and prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Carver, A.; Gallicchio, V.S. Chapter 1: Heavy metals and cancer. In Cancer Causing Substances; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Wu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Nickel carcinogenesis mechanism: DNA damage. Int. J. Mol. Sci. 2019, 20, 4690. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, V.K.; Kumar, A.; Sharma, B. Synergistic effects of heavy metals and pesticides in living systems. Front. Chem. 2017, 5, 70. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- World Health Organization (WHO). 10 Chemicals of Public Health Concern. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 6 October 2021).
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniatti-Brazaca, S.G. Heavy metals in vegetables and potential risk for human health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef]
- Li, Y. Environmental contamination and risk assessment of mercury from a historic mercury mine located in southwestern China. Environ. Geochem. Health 2013, 35, 27–36. [Google Scholar] [CrossRef]
- Li, R.; Wu, H.; Ding, J.; Fu, W.; Gan, L. Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Sci. Rep. 2017, 7, 46545. [Google Scholar] [CrossRef] [PubMed]
- Moriarity, R.J.; Liberda, E.N.; Tsuji, L.J.S. Subsistence fishing in the Eeyou Istchee (James Bay, Quebec, Canada): A regional investigation of fish consumption as a route of exposure to methylmercury. Chemosphere 2020, 258, 127413. [Google Scholar] [CrossRef] [PubMed]
- Burger, J. Food chain differences affect heavy metals in bird eggs in Barnegat Bay, New Jersey. Environ. Res. 2002, 90, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-Y.; Li, M.-M.; Wang, J.; Yan, J.; Zhou, C.-C.; Yan, C.-H. Blood mercury concentration, fish consumption and anthropometry in Chinese children: A national study. Environ. Int. 2018, 110, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.R.; Clickner, R.P.; Bodurow, C.C. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ. Health Perspect. 2004, 112, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Perelló, G.; Martí-Cid, R.; Llobet, J.M.; Domingo, J.L. Effects of various cooking processes on the concentrations of arsenic, cadmium, mercury, and lead in foods. J. Agric. Food Chem. 2008, 56, 11262–11269. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, O.; Amyot, M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ. Res. 2011, 11, 1064–1069. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Park, J.-D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef]
- Choy, C.M.Y.; Lam, C.W.K.; Cheung, L.T.F.; Briton-Jones, C.M.; Cheung, L.P.; Haines, C.J. Infertility, blood mercury concentrations and dietary seafood consumption: A case–control study. BJOG Int. J. Obstet. Gynaecol. 2003, 109, 1121–1125. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Sasikala, M.; Ramesh, R. Lead poisoning—An overview. Int. J. Pharmacol. Toxicol. 2012, 2, 70–82. [Google Scholar]
- Mañay, N.; Cousillas, A.Z.; Alvarez, C.; Heller, T. Lead contamination in Uruguay: The “La Teja” neighborhood case. Rev. Environ. Contam. Toxicol. 2008, 195, 93–115. [Google Scholar] [PubMed]
- El-Sharif, N.; Fischbein, A.; Konijn, A.; Gorodetsky, R.; El-Sharif, H.; Kaul, B.; Hershko, C.; Grauer, F.; Foner, H.; Al-Baba, A.; et al. Re-emergence of lead poisoning from contaminated flour in a West Bank Palestinian Village. Int. J. Occup. Environ. Health 2000, 6, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.A.; Brown, M.J. The CDC blood lead reference value for children: Time for a change. Environ. Health 2019, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huo, X.; Zhang, Y.; Cheng, Z.; Chen, S.; Xu, X. Increased intestinal permeability with elevated peripheral blood endotoxin and inflammatory indices for e-waste lead exposure in children. Chemosphere 2021, 279, 130862. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fagerberg, B.; Sallsten, G.; Borné, Y.; Hedblad, B.; Engström, G.; Barregard, L.; Andersson, E.M. Smoking-induced risk of future cardiovascular disease is partly mediated by cadmium in tobacco: Malmö Diet and Cancer Cohort study. Environ. Health 2019, 18, 56. [Google Scholar] [CrossRef]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, A.; Wang, T.; Qu, G.; Shao, J.; Yuan, B.; Wang, Y.; Jiang, G. Influence of e-waste dismantling and its regulations: Temporal trend, spatial distribution of heavy metals in rice grains, and its potential health risk. Environ. Sci. Technol. 2013, 47, 7437–7445. [Google Scholar] [CrossRef]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef]
- Mead, M.N. Cadmium confusion: Do consumers need protection? Environ. Health Perspect. 2010, 118, 528–534. [Google Scholar] [CrossRef]
- Spungen, J.H. Children’s exposures to lead and cadmium: FDA total diet study 2014–16. Food Addit. Contam. Part A 2019, 36, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Rawal, N.; Mathew, B.B. The characteristics, toxicity and effects of cadmium. Int. J. Nanotechnol. Nanosci. 2015, 3, 1–9. [Google Scholar]
- Meltzer, H.M.; Brantsaeter, A.L.; Borch-Iohnsen, B.; Ellingsen, D.G.; Alexander, J.; Thomassen, Y.; Stigum, H.; Ydersbond, T.A. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ. Res. 2010, 110, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.M.; Chen, J.J.; Kovach, J.S. The relationship between body iron stores and blood and urine cadmium concentrations in US never-smoking, non-pregnant women aged 20–49 years. Environ. Res. 2011, 11, 702–707. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S. Iron deficiency increases blood concentrations of neurotoxic metals in children. Korean J. Pediatr. 2014, 57, 345–350. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Hu, F.B. Role of chromium in human health and in diabetes, diabetes care. Diabetes Care 2004, 27, 2741–2751. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Sharma, P.; Bihari, V.; Agarwal, S.K.; Verma, V.; Kesavachandran, C.N.; Pangtey, B.S.; Mathur, N.; Singh, K.P.; Srivastava, M.; Goel, S.K. Groundwater contaminated with hexavalent chromium [Cr (VI)]: A health survey and clinical examination of community inhabitants (Kanpur, India). PLoS ONE 2012, 7, e47877. [Google Scholar] [CrossRef]
- Hopenhayn, C.; Ferreccio, C.; Browning, S.R.; Huang, B.; Peralta, C.; Gibb, H.; Hertz-Picciotto, I. Arsenic exposure from drinking water and birth weight. Epidemiology 2013, 14, 593–602. [Google Scholar] [CrossRef]
- Ashmore, E.; Molyneux, S.; Watson, S.; Miles, G.; Pearson, A. Inorganic arsenic in rice and rice products in New Zealand and Australia. Food Addit. Contam. Part B Surveill. 2019, 12, 275–279. [Google Scholar] [CrossRef]
- Singh, S.K.; Ghosh, A.K. Entry of arsenic into food material—A case study. World Appl. Sci. J. 2011, 13, 385–390. [Google Scholar]
- Orisakwe, O.E.; Nduka, J.K.; Amadi, C.N.; Dike, D.N.; Bede, O. Heavy metals health risk assessment for population via consumption of food crops and fruits in Owerri, south eastern, Nigeria. Chem. Cent. J. 2012, 6, 77. [Google Scholar] [CrossRef]
- Salihaj, M.; Bani, A. The nickel content in honey derived from serpentine and non-serpentine areas of Kosovo. Albanian J. Agric. Sci. Spec. Ed. 2017, 557–563. [Google Scholar]
- Hseu, Z.Y.; Lai, Y.J. Nickel accumulation in paddy rice on serpentine soils containing high geogenic nickel contents in Taiwan. Environ. Geochem. Health 2017, 39, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.I.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities-a review. Environ. Sci. Pollut. Res. Int. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- Squadrone, S.; Crescio, I.; Brizio, P.; Dutto, D.; Bocca, R.; Stella, C.; Colombero, G.; Rizzi, M.; Pederiva, S.; Ingravalle, F.; et al. Nickel occurrence in a livestock food chain (northwestern Italy). Water Air Soil Pollut. 2020, 231, 265. [Google Scholar] [CrossRef]
- Zeinali, T.; Salmani, F.; Naseri, K. Dietary intake of cadmium, chromium, copper, nickel, and lead through the consumption of meat, liver, and kidney and assessment of human health risk in Birjand, southeast of Iran. Biol. Trace Elem. Res. 2019, 191, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Nuapia, Y.; Chimuka, L.; Cukrowska, E. Assessment of heavy metals in raw food samples from open markets in two African cities. Chemosphere 2018, 196, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Mounicou, S.; Szpunar, J.; Andrey, D.; Blake, C.; Lobinski, R. Concentrations and bioavailability of cadmium and lead in cocoa powder and related products. Food Addit. Contam. 2003, 20, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Garcia, W.J.; Blessin, C.W.; Inglett, G.I. Heavy metals in food products from corn. Cereal Chem. 1974, 51, 779. [Google Scholar]
- Pedersen, G.A.; Mortensen, G.K.; Larsen, E.H. Beverages as a source of toxic trace element intake. Food Addit. Contam. 1993, 11, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Hwang, J.Y.; Lee, H.E.; Choi, J.D.; Kang, G.J. Comparative analysis of lead content during food processing. Food Sci. Biotechnol. 2020, 29, 1063–1069. [Google Scholar] [CrossRef]
- Łodyga-Chruścińska, E.; Sykuła, A.; Więdłocha, M. Hidden metals in several brands of lipstick and face powder present on Polish market. Cosmetics 2018, 5, 57. [Google Scholar] [CrossRef]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: A status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef]
- Borowska, S.; Brzóska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Gondal, M.A.; Seddiqi, Z.S.; Nasr, M.M.; Gondal, B. Spectroscopic detection of health hazardous contaminants in lipstick using laser induced breakdown spectroscopy. J. Hazard. Mater. 2010, 175, 726–732. [Google Scholar] [CrossRef]
- Khalid, A.; Bukhari, I.H.; Riaz, M.; Rehman, G.; Ain, Q.U.; Bokhari, T.H.; Rasool, N.; Zubair, M.; Munir, S. Determination of lead, cadmium, chromium and nickel in different brands of lipsticks. IJBPAS 2013, 2, 1003–1009. [Google Scholar]
- Alam, M.F.; Akhter, M.; Mazumder, B.; Ferdous, A.; Hossain, M.D.; Dafader, N.C.; Ahmed, F.T.; Kundu, S.K.; Taheri, T.; Ullah, A.K.M.A. Assessment of some heavy metals in selected cosmetics commonly used in Bangladesh and human health risk. J. Anal. Sci. Technol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of The European Parliament and of the Council. Off. J. Eur. Union 2009, 342, 59–209. Available online: http://en.cirs-ck.com/Uploads/file/20171207/1512633085_11535.pdf (accessed on 6 October 2021).
- Yebpella, G.G.; Magomya, A.M.; Lawal, U.; Gauje, B.; Oko, O.J. Assessment of trace metals in imported cosmetics marketed in Nigeria. J. Nat. Sci. Res. 2014, 4, 11–15. [Google Scholar]
- Mousavi, Z.; Ziarati, P.; Shariatdoost, A. Determination and safety assessment of lead and cadmium in eye shadows purchased in local market in Tehran. J. Environ. Anal. Toxicol. 2013, 3, 3–7. [Google Scholar]
- Navarro-Tapia, E.; Serra-Delgado, M.; Fernández-López, L.; Meseguer-Gilabert, M.; Falcón, M.; Sebastiani, G.; Sailer, S.; Garcia-Algar, O.; Andreu-Fernández, V. Toxic elements in traditional kohl-based eye cosmetics in Spanish and German markets. Int. J. Environ. Res. Public Health 2021, 18, 6109. [Google Scholar] [CrossRef]
- FDA’s U.S. Food and Drug Administration. Available online: https://www.fda.gov/cosmetics/potential-contaminants-cosmetics/fdas-testing-cosmetics-arsenic-cadmium-chromium-cobalt-lead-mercury-and-nickel-content#limits (accessed on 6 October 2021).
- Naujokas, M.F.; Anderson, B.; Ahsan, H. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Abir, T.; Rahman, B.; D’Este, C.; Farooq, A.; Milton, A.H. The association between chronic arsenic exposure and hypertension: A meta-analysis. J. Toxicol. 2012, 2012, 198793. [Google Scholar] [CrossRef] [PubMed]
- Saidalavi, R.; Hashim, A.; Kishor, K.B.; Leena, P.K.; Adake, P. Analysis of lead and arsenic in cosmetics and assessment of students awareness about cosmetic toxicity. Int. J. Basic Clin. Pharmacol. 2017, 6, 1426–1430. [Google Scholar] [CrossRef][Green Version]
- Hamann, C.R.; Boonchai, W.; Wen, L.; Sakanashi, E.N.; Chu, C.-Y.; Hamann, K.; Hamann, C.P.; Sinniah, K.; Hamann, D. Spectrometric analysis of mercury content in 549 skin-lightening products: Is mercury toxicity a hidden global health hazard? J. Am. Acad. Dermatol. 2014, 70, 281–287. [Google Scholar] [CrossRef]
- Mohammed, T.; Mohammed, E.; Bascombe, S. The evaluation of total mercury and arsenic in skin bleaching creams commonly used in Trinidad and Tobago and their potential risk to the people of the Caribbean. J. Public Health Res. 2017, 6, 1097. [Google Scholar] [CrossRef]
- Peregrino, C.P.; Moreno, M.V.; Miranda, S.V.; Rubio, A.D.; Leal, L.O. Mercury levels in locally manufactured Mexican skin-lightening creams. Int. J. Environ. Res. Public Health 2011, 8, 2516–2523. [Google Scholar] [CrossRef]
- Agorku, E.S.; Kwaansa-Ansah, E.E.; Voegborlo, R.B.; Amegbletor, P.; Opoku, F. Mercury and hydroquinone content of skin toning creams and cosmetic soaps, and the potential risks to the health of Ghanaian women. SpringerPlus 2016, 5, 319. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.R.; Subish, P. Fair skin in South Asia: An obsession? J. Pak. Assoc. Dermatol. 2016, 17, 100–104. [Google Scholar]
- CDC. Mercury exposure among household users and nonusers of skin-lightening creams produced in Mexico-California and Virginia, 2010. Morb. Mortal. Wkly. Rep. 2012, 61, 33. [Google Scholar]
- Ladizinski, B.; Mistry, N.; Kundu, R.V. Widespread use of toxic skin lightening compounds: Medical and psychosocial aspects. Dermatol. Clin. 2011, 29, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Lara-Torres, S.; Figueiredo, D.; Paz, S.; Gutiérrez, A.J.; Rubio, C.; González-Weller, D.; Revert, C.; Hardisson, A. Determination and risk assessment of toxic metals in lipsticks from Europe and China. J. Trace Elem. Med. Biol. 2021, 67, 126792. [Google Scholar] [CrossRef] [PubMed]
- Benz, M.R.; Lee, S.H.; Kellner, R.; Döhlemann, C.; Berweck, S. Hyperintense lesions in brain MRI after exposure to a mercuric chloride-containing skin whitening cream. Eur. J. Pediatr. 2011, 170, 747–750. [Google Scholar] [CrossRef]
- Dickenson, C.A.; Woodruff, T.J.; Stotland, N.E.; Dobraca, D.; Das, R. Elevated mercury levels in pregnant woman linked to skin cream from Mexico. Am. J. Obstet. Gynecol. 2013, 209, e4–e5. [Google Scholar] [CrossRef]
- Dlova, N.C.; Hendricks, N.E.; Martincgh, B.S. Skin lightening creams used in Durban, South Africa. Int. J. Dermatol. 2012, 51, 51–53. [Google Scholar] [CrossRef]
- Copan, L.; Fowles, J.; Barreau, T.; McGee, N. Mercury toxicity and contamination of households from the use of skin creams adulterated with mercurous chloride (calomel). Int. J. Environ. Res. Public Health 2015, 12, 10943–10954. [Google Scholar] [CrossRef] [PubMed]
- Faghihian, H.; Nourmoradi, H.; Shokouhi, M. Performance of silica aerogels modified with amino functional groups in PB(II) and CD(II) removal from aqueous solutions. Pol. J. Chem. Technol. 2012, 14, 50–56. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Foroghi, M.; Farhadkhani, M. Assessment of lead and cadmium levels in frequently used cosmetic products in Iran. J. Environ. Public Health 2013, 2013, 962727. [Google Scholar] [CrossRef]
- Deveci, S.; Deveci, E. Histopathological changes in incisive teeth of the newborn pups of cadmium-applied female rats during pregnancy. Int. J. Morphol. 2010, 28, 1131–1134. [Google Scholar] [CrossRef]
- Sah, R.C. Poisonous Cosmetics, the Problem of Lead in Lipstick in Nepal; Center for Public Health and Environmental Development, 2012; pp. 1–18. Available online: http://library.nhrc.gov.np:8080/nhrc/handle/123456789/503 (accessed on 6 October 2021).
- Orisakwe, O.E.; Otaraku, J.O. Metal concentrations in cosmetics commonly used in Nigeria. Sci. World J. 2013, 5, 959637. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Gay, M.B.; Abubakar, A.F. Determination of some heavy metals in selected cosmetic products sold in kano metropolis, Nigeria. Toxicol. Rep. 2016, 3, 866–869. [Google Scholar] [CrossRef]
- Smith, A.M. Chapter 1: Interaction of metal ions with proteins as a source of inspiration for biomimetic materials. In Functional Metallosupramolecular Materials; Royal Society of Chemistry: London, UK, 2015; pp. 1–31. [Google Scholar] [CrossRef]
- Migliorini, C.; Witkowska, D.; Valensin, D.; Kamysz, W.; Kozlowski, H. Competition between histamine-like and poly-imidazole coordination sites for Cu2+ and Zn2+ ions in zebra-fish peptide of prion-like protein. Dalton Trans. 2010, 39, 8663–8670. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Shaanan, B.; Zarivach, R. Transition metal binding selectivity in proteins and its correlation with the phylogenomic classification of the cation diffusion facilitator protein family. Sci. Rep. 2017, 7, 16381. [Google Scholar] [CrossRef]
- Young, T.R.; Xiao, Z. Principles and practice of determining metal–protein affinities. Biochem. J. 2021, 478, 1085–1116. [Google Scholar] [CrossRef]
- Srivastava, P.; Kowshik, M. Mechanisms of metal resistance and homeostasis in haloarchaea. Archaea 2013, 2013, 732864. [Google Scholar] [CrossRef]
- Günther, V.; Lindert, U.; Schaffner, W. The taste of heavy metals: Gene regulation by MTF-1. Biochim. Biophys. Acta 2012, 823, 1416–1425. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, S.K.; Bhardwaj, N.; Paul, A.; Kumar, P.; Kim, K.-H.; Deep, A. Progress in the biosensing techniques for trace-level heavy metals. Biotechnol. Adv. 2016, 34, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jeong, J. Synergistic cellular responses to heavy metal exposure: A minireview. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1584–1591. [Google Scholar] [CrossRef]
- Kern, M.; Wisniewski, M.; Cabell, L.; Audesirk, G. Inorganic lead and calcium interact positively in activation of calmodulin. Neurotoxicology 2000, 21, 353–363. [Google Scholar] [PubMed]
- Sun, X.Y.; Tian, X.T.; Tomsig, J.L.; Suszkiw, J.B. Analysis of differential effects of Pb2+ on protein kinase C isozymes. Toxicol. Appl. Pharmacol. 1999, 156, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.D.; Marchetti, P.S.; Strang, P.; Potter, J.D. Cadmium-substituted skeletal troponin-c metal-binding investigations and sequence assignment of the CD-113 resonances. J. Biol. Chem. 1988, 263, 10284–10288. [Google Scholar] [CrossRef]
- Jacobson, K.B.; Turner, J.E. The interaction of cadmium and certain other metal-ions with proteins and nucleic-acids. Toxicology 1980, 16, 1–37. [Google Scholar] [CrossRef]
- Gu, J.; Dai, S.; Liu, Y.; Liu, H.; Zhang, Y.; Ji, X.; Yu, F.; Zhou, Y.; Chen, L.; Tse, W.K.F.; et al. Activation of Ca2+-sensing receptor as a protective pathway to reduce Cadmium-induced cytotoxicity in renal proximal tubular cells. Sci. Rep. 2018, 8, 1092. [Google Scholar] [CrossRef]
- Kosiba, A.A.; Wang, Y.; Chen, D.; Wong, C.K.C.; Gu, J.; Shi, H. The roles of calcium-sensing receptor (CaSR) in heavy metals-induced nephrotoxicity. Life Sci. 2020, 242, 117183. [Google Scholar] [CrossRef]
- So, K.-Y.; Lee, B.-H.; Oh, S.-H. The critical role of autophagy in cadmium-induced immunosuppression regulated by endoplasmic reticulum stress-mediated calpain activation in RAW264.7 mouse monocytes. Toxicology 2018, 393, 15–25. [Google Scholar] [CrossRef]
- Siewit, C.L.; Gengler, B.; Vegas, E.; Puckett, R.; Louie, M.C. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between ER alpha and c-Jun. Mol. Endocrinol. 2010, 24, 981–992. [Google Scholar] [CrossRef]
- Visser, G.J.; Peters, P.H.J.; Theuvenet, A.P.R. Cadmium ion is a noncompetitive inhibitor of red-cell CA2+-ATPASE activity. Biochim. Biophys. Acta 1993, 1152, 26–34. [Google Scholar] [CrossRef]
- Audesirk, G.; Audesirk, T. The effects of inorganic lead on voltage-sensitive calcium channels differ among cell-types and among channel subtypes. Neurotoxicology 1993, 14, 259–266. [Google Scholar]
- Neal, A.P.; Worley, P.F.; Guilarte, T.R. Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology 2011, 32, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Senut, M.-C.; Cingolani, P.; Sen, A.; Kruger, A.; Shaik, A.; Hirsch, H.; Suhr, S.T.; Ruden, D. Epigenetics of early-life lead exposure and effects on brain development. Epigenomics 2012, 4, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Aimo, L.; Oteiza, P.I. Zinc deficiency increases the susceptibility of human neuroblastoma cells to lead-induced activator protein-1 activation. Toxicol. Sci. 2006, 91, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, X.; Li, F.; Li, J.; Chang, W.; Yuan, J.; Chen, J. Cyclosporin A protects against lead neurotoxicity through inhibiting mitochondrial permeability transition pore opening in nerve cells. Neurotoxicology 2016, 57, 203–213. [Google Scholar] [CrossRef]
- Panel, M.; Ghaleh, B.; Morin, D. Mitochondria and aging: A role for the mitochondrial transition pore? Aging Cell 2018, 17, e12793. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chang, C.; Liu, B.; Li, Z.; Li, H.; Cai, N.; Wang, H.-H. Copper (II) ions activate ligand-independent Receptor Tyrosine Kinase (RTK) signaling pathway. BioMed Res. Int. 2019, 2019, 4158415. [Google Scholar] [CrossRef]
- Bijoor, A.R.; Sudha, S.; Venkatesh, T. Neurochemical and neurobehavioral effects of low lead exposure on the developing brain. Indian J. Clin. Biochem. 2012, 27, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, J.-Y.; Dong, J.-X.; Xiao, Q.; Zhao, J.; Jiang, F.-L. Toxicity of Pb2+ on rat liver mitochondria induced by oxidative stress and mitochondrial permeability transition. Toxicol. Res. 2017, 6, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Sreeshma, J.; Sudandiradoss, C. Identification of metal binding motifs in protein frameworks to develop novel remediation strategies for Hg2+ and Cr(VI). Biometals 2021, 34, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, Y.; Ran, M.; Li, Q.; Ruan, Z.; Jin, N. Inhibition of tyrosinase by mercury chloride: Spectroscopic and docking studies. Front. Pharmacol. 2020, 11, 81. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Kern, J.K.; Geier, D.A.; Homme, K.G.; Geier, M.R. Examining the evidence that ethylmercury crosses the blood-brain barrier. Environ. Toxicol. Pharmacol. 2020, 74, 103312. [Google Scholar] [CrossRef]
- Dórea, J.G.; Farina, M.; Rocha, J.B.T. Toxicity of ethylmercury (and Thimerosal): A comparison with methylmercury. J. Appl. Toxicol. 2013, 33, 700–711. [Google Scholar] [CrossRef]
- Abdollahzade, N.; Majidinia, M.; Babri, S. Melatonin: A pleiotropic hormone as a novel potent therapeutic candidate in arsenic toxicity. Mol. Biol. Rep. 2021, 48, 6603–6618. [Google Scholar] [CrossRef]
- Kuivenhoven, M.; Mason, K. Arsenic toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shen, S.; Li, X.F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic binding to proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef]
- Doerge, D.R.; Twaddle, N.C.; Churchwell, M.I.; Beland, F.A. Reduction by, ligand exchange among, and covalent binding to glutathione and cellular thiols link metabolism and disposition of dietary arsenic species with toxicity. Environ. Int. 2020, 144, 106086. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Luo, J.-H.; Qiu, Z.-Q.; Zhang, L.; Shu, W.-Q. Arsenite exposure altered the expression of NMDA receptor and postsynaptic signaling proteins in rat hippocampus. Toxicol. Lett. 2012, 211, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Morgan, L.G.; Speizer, F.E. Cancers of the lung and nasal sinuses in nickel workers. Br. J. Cancer 1970, 24, 623–632. [Google Scholar] [CrossRef]
- Witkowska, D.; Rowińska-Żyrek, M.; Valensin, G.; Kozłowski, H. Specific poly-histidyl and poly-cysteil protein sites involved in Ni2+ homeostasis in Helicobacter pylori. Impact of Bi3+ ions on Ni2+ binding to proteins. Structural and thermodynamic aspects. Coord. Chem. Rev. 2012, 256, 133–148. [Google Scholar] [CrossRef]
- Eaton, K.A.; Brooks, C.L.; Morgan, D.R.; Krakowka, S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 1991, 59, 2470–2475. [Google Scholar] [CrossRef]
- Mennè, T.; Christopherson, J.; Green, A. Epidemiology of nickel dermatitis. In Nickel and the Skin: Immunologyand Toxicology; Maibach, H.I., Mennè, T., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 109–115. [Google Scholar]
- Jiménez-Lamana, J.; Godin, S.; Aragonès, G.; Bladé, C.; Szpunar, J.; Łobinski, R. Nickel nanoparticles induce the synthesis of a tumor-related polypeptide in human epidermal keratinocytes. Nanomaterials 2020, 10, 992. [Google Scholar] [CrossRef]
- Rosen, T.; Nolan, E.M. Metal sequestration and antimicrobial activity of human calprotectin are pH-dependent. Biochemistry 2020, 59, 2468–2478. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for Europe. Available online: https://www.euro.who.int/__data/assets/pdf_file/0003/97446/4.4.pdf (accessed on 6 October 2021).
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- Tytła, M. Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in Poland—Case study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tripathi, S.; Chandra, R. Highly efficient phytoremediation potential of metal and metalloids from the pulp paper industry waste employing Eclipta alba (L.) and Alternanthera philoxeroide (L.): Biosorption and pollution reduction. Bioresour. Technol. 2020, 319, 124147. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tripathi, S.; Chaturvedi, P.; Chaurasia, D.; Chandra, R. Newly isolated Bacillus sp. PS-6 assisted phytoremediation of heavy metals using Phragmites communis: Potential application in wastewater treatment. Bioresour. Technol. 2020, 320, 124353. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pandey, A.K.; Udayan, A.; Kumar, S. Role of microbial community and metal-binding proteins in phytoremediation of heavy metals from industrial wastewater. Bioresour. Technol. 2021, 326, 124750. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Gunasundari, E. Bioremediation of heavy metals. In Bioremediation: Applications for Environmental Protection and Management; Varjani, S.J., Agarwal, A.K., Gnansounou, E., Gurunathan, B., Eds.; Springer: Singapore, 2018; pp. 165–195. [Google Scholar]
- Huang, C.C.; Liang, C.M.; Yang, T.I.; Chen, J.L.; Wang, W.K. Shift of bacterial communities in heavy metal-contaminated agricultural land during a remediation process. PLoS ONE 2021, 16, e0255137. [Google Scholar] [CrossRef]
- Iqbal, J.; Javed, A.; Baig, M.A. Heavy metals removal from dumpsite leachate by algae and cyanobacteria. Bioremediation J. 2021, 1–13. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. https://doi.org/10.3390/molecules26196060
Witkowska D, Słowik J, Chilicka K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules. 2021; 26(19):6060. https://doi.org/10.3390/molecules26196060
Chicago/Turabian StyleWitkowska, Danuta, Joanna Słowik, and Karolina Chilicka. 2021. "Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites" Molecules 26, no. 19: 6060. https://doi.org/10.3390/molecules26196060
APA StyleWitkowska, D., Słowik, J., & Chilicka, K. (2021). Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules, 26(19), 6060. https://doi.org/10.3390/molecules26196060






