The Natural Compound Hydrophobic Usnic Acid and Hydrophilic Potassium Usnate Derivative: Applications and Comparisons
Abstract
:1. Introduction
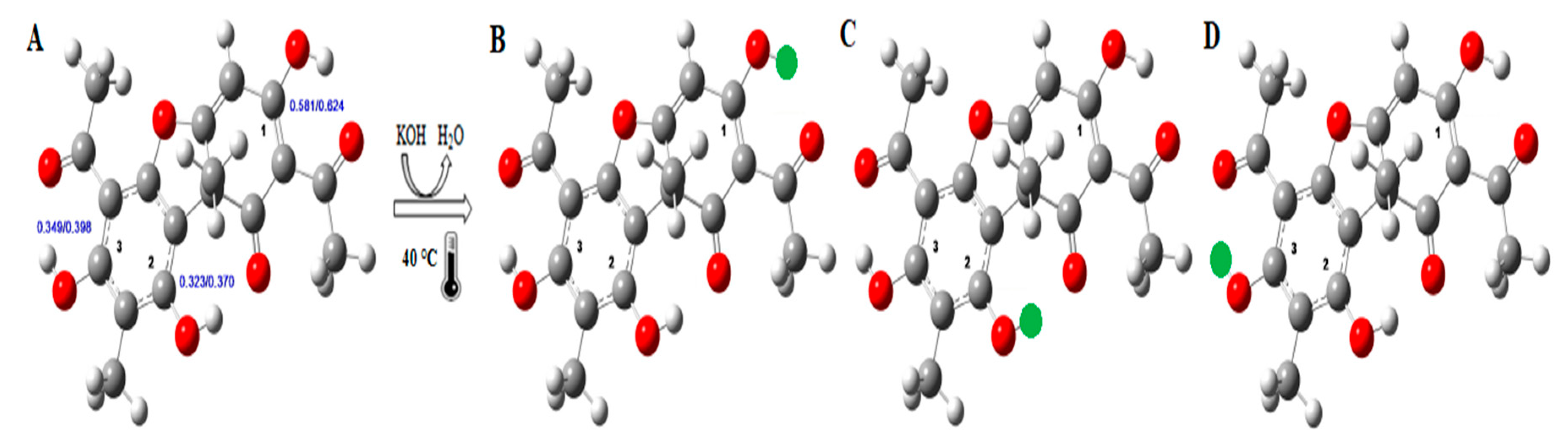
2. Chemical Properties of Usnic Acid and Potassium Usnate
3. Molluscicidal and Antiparasitic Activities
4. Antinociceptive Activity and Cytotoxicity on Peripheral Blood Cells
5. Antitumor Activity and Bioavailability
| Molluscicidal Activity on Biomphalaria glabrata | Reference | |||
|---|---|---|---|---|
| Usnic Acid (μg/mL) | ||||
| Embryonic Stages | LC50 | LC90 | LC100 | |
| Blastula | 1.38 | 1.62 | 2.0 | [26,27] |
| Gastrula | 3.47 | 4.45 | 4.5 | |
| Trocophore | 5.11 | 5.36 | 6.0 | |
| Veliger | 2.93 | 4.49 | 6.0 | |
| Adult | 2.12 | 3.45 | 4.0 | |
| Potassium Usnate (μg/mL) | ||||
| Blastula | 4.97 | 5.41 | 6.0 | [35,36] |
| Gastrula | 3.11 | 3.58 | 4.0 | |
| Trocophore | 3.55 | 4.44 | 4.5 | |
| Veliger | 2.67 | 3.89 | 4.5 | |
| Adult | 0.92 | - | 1.0 | [28] |
| Schistosomicidal Activity on Schistosoma mansoni | ||||
| Usnic Acid (μg/mL) | ||||
| LC50 | LC90 | LC100 | ||
| Cercariae | NI | NI | 10 (90 min) | [47] |
| Adult | NI | NI | 200 (120 h) | [50] |
| Potassium Usnate (μg/mL) | ||||
| Cercariae | 1.98 (60 min) | 4.93 (60 min) | 5 (120 min) | [35,36] |
| Adult | 50 (24 h) | 12.5 (96 h) | 100 (24 h) | [37,39] |
| Antileishmania Activity | ||||
| Phase | Usnic Acid (μM) | Potassium Usnate (μM) | ||
| Promastigote (IC50) | 18.30 | 2.99 | [52] | |
| Antitumor Activity | ||||
| Mean Cytotoxicity of Cancer Cells | ||||
| Lineage | Molecule Analyzed | |||
| Usnic Acid (µM) | Potassium Usnate (µM) | [59] | ||
| HCT116 | 97.4 | 87.0 | ||
| DLD1 | 96.0 | 67.5 | ||
| SW480 | 84.0 | 94.0 | ||
| HT29 | 68.5 | 57.4 | ||
| SW620 | 46.3 | 32.0 | ||
| Caco2 | 38.5 | 25.0 | ||
| CT26 | 38.4 | 35.0 | ||
| COL320 | 94.0 | 59.6 | ||
| Mean Invasive Ability of Cancer Cells after Drug Treatment | ||||
| Usnic Acid (µM) | Potassium Usnate (µM) | |||
| CaCo2 | 73.0 | 65.0 | ||
| HCT116 | 64.0 | 52.0 | ||
| CT26 | 80.5 | 79.5 | ||
6. Behavioral Changes and Toxicity
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahti, T.; Stenroos, S.; Xavier Filho, L. The lichen family Cladoniaceae in Paraíba, Pernambuco and Sergipe, Northeast Brazil. Trop. Biol. 1993, 7, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Ingólfsdóttir, K. Usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Calcott, M.J.; Ackerley, D.F.; Knight, A.; Keyzers, R.A.; Owen, J.G. Secondary metabolism in the lichen symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef] [Green Version]
- Ranković, B.; Kosanić, M. Biotechnological substances in lichens. Natural Bioactive Compounds. 2021, 249–265. [Google Scholar] [CrossRef]
- Cocchietto, M.; Skert, N.; Nimis, P.L.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwissenschaften 2002, 89, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Cazarin, C.A.; Dalmagro, A.P.; Gonçalves, A.E.; Boeing, T.; Silva, L.M.; Corrêa, R.; Klein-Júnior, L.C.; Pinto, B.C.; Lorenzett, T.S.; Sobrinho, T.U.C.; et al. Usnic acid enantiomers restore cognitive deficits and neurochemical alterations induced by Aβ1-42 in mice. Behav. Brain Res. 2021. In Press. [Google Scholar] [CrossRef]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances, 1st ed.; Springer: Heidelberg/Berlin, Germany, 1996; pp. 1–449. [Google Scholar]
- Macedo, D.C.S.; Almeida, F.J.F.; Wanderley, M.S.O.; Ferraz, M.S.; Santos, N.P.S.; López, A.M.Q.; Santos-Magalhães, N.S.; Lira-Nogueira, M.C.B. Usnic acid: From an ancient lichen derivative to promising biological and nanotechnology applications. Phytochem. Rev. 2020, 20, 1–22. [Google Scholar] [CrossRef]
- Bustinza, F. Antibacterial substances from lichens. Econ. Bot. 1952, 6, 402–406. [Google Scholar] [CrossRef]
- Rafanelli, S.; Bacchilega, R.; Stanganelli, I.; Rafanelli, A. Contact dermatitis from usnic acid in vaginal ovules. Contact Dermatitis. 1995, 33, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Rosan, S.; Boehm, K.; Fernández, E.; Hidalgo, M.E.; Quihot, W.; Rubio, C.; Boehm, F.; Piazena, H.; Oltmanns, U. Protection against UVB irradiation by natural filters extracted from lichens. J. Photochem. Photobiol. B. 2002, 68, 133–139. [Google Scholar] [CrossRef]
- Nybakken, L.; Julkunen-Tiitto, R. UV-B induces usnic acid in reindeer lichens. Lichenologist 2006, 38, 477–485. [Google Scholar] [CrossRef]
- Goel, M.; Kalra, R.; Ponnan, P.; Jayaweera, J.A.A.S.; Kumbukgolla, W.W. Inhibition of penicillin-binding protein 2a (PBP2a) in methicillin resistant Staphylococcus aureus (MRSA) by combination of oxacillin and a bioactive compound from Ramalina. roesleri. Microb. Pathog. 2021, 150, 1–5. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, J.P.N.; Singh, R.P. Usnic acid induces apoptosis in human gastric cancer cells through ROS generation and DNA damage and causes up-regulation of DNA-PKcs and γ-H2A.X phosphorylation. Chem. Biol. Interact. 2020, 315. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A.; Firsov, A.M.; Kotova, E.A.; Rokitskaya, T.I.; Khailova, L.S.; Popova, L.B.; Chernyak, B.V.; Antonenko, Y.N. Usnic acid as calcium ionophore and mast cells stimulator. Biochim. Biophys. Acta Biomembr. 2020, 1862, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.N.; Zarubaev, V.V.; Shtro, A.A.; Polovinka, M.P.; Luzina, O.A.; Komarova, N.I.; Salakhutdinov, N.F.; Kiselev, O.I. Anti-viral activity of (-)/(+) usnic acids and their derivatives against influenza virus A(H1N1) 2009. Bioorg. Med. Chem. Lett. 2012, 22, 7060–7064. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ramtekeb, P.W.; Pandeyc, A.C.; Pandey, H. Evaluation of antifungal activity of blended cinnamon oil and usnic acid nanoemulsion using candidiasis and dermatophytosis models. Biocatal. Agric. Biotechnol. 2019, 18, 1–5. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Ha, S.; Chung, H.Y.; Kim, H.; Hur, J.S.; Lee, J. Anti-inflammatory effects of usnic acid in an MPTP-induced mouse model of Parkinson’s disease. Brain Res. 2020, 1730, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Si, K.; Wei, L.; Yu, X.; Wu, F.; Li, X.; Li, C.; Cheng, Y. Data on (+)-usnic acid: A new application to treat toxoplasmosis. Data Brief. 2016, 8, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Chepanova, A.A.; Zafar, A.; et al. Novel tyrosyl-DNA phosphodiesterase 1 inhibitors enhance the therapeutic impact of topotecan on in vivo tumor models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef]
- Kristmundsdóttir, T.; Jónsdóttir, E.; Ogmundsdóttir, H.M.; Ingólfsdóttir, K. Solubilization of poorly soluble lichen metabolites for biological testing on cell lines. Eur. J. Pharm. Sci. 2005, 24, 539–543. [Google Scholar] [CrossRef]
- Jin, J.; Rao, Y.; Bian, X.; Zeng, A.; Yang, G. Solubility of (+)-usnic acid in water, ethanol, acetone, ethyl acetate and n-hexane. J. Solution Chem. 2013, 42, 1018–1027. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Ye, W.; Li, S.; Li, D.; Liu, Z.; Zhang, W. Ochuscins A-G, highly oxygenated usnic acid derivatives from the deep-sea-derived fungus Ochroconis sp. FS449. Tetrahedron. 2020, 76, 1–5. [Google Scholar] [CrossRef]
- Shi, C.J.; Peng, W.; Zhao, J.H.; Yang, H.L.; Qu, L.L.; Wang, C.; Kong, L.Y.; Wang, X.B. Usnic acid derivatives as tau-aggregation and neuroinflammation inhibitors. Eur. J. Med. Chem. 2020, 187. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Silva, L.R.S.; Siqueira, W.N.; Fonseca, C.S.M.; Silva, N.H.; Melo, A.M.M.A.; Martins, M.C.B.; Lima, V.L.M. Toxicity of usnic acid from Cladonia substellata (Lichen) to embryos and adults of Biomphalaria glabrata. Acta Trop. 2018, 179, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Silva, L.R.S.; Siqueira, W.N.; Fonseca, C.S.M.; Silva, N.H.; Melo, A.M.M.A.; Martins, M.C.B.; Lima, V.L.M. Dataset on usnic acid from Cladonia substellata Vainio (Lichen) schistosomiasis mansoni’s vector control and environmental toxicity. Data Brief. 2018, 17, 228–291. [Google Scholar] [CrossRef]
- Martins, M.C.B.; Silva, R.L.; Barbosa, P.S.; Rodrigues, B.R.M.; Albuquerque, A.C.; Falcão, P.S.; Lima, V.L.M.; Silva, N.H.; Pereira, E.C. Effects of usnic, barbatic and fumarprotocetraric acids on survival of Nasutitermes corniger (Isoptera: Termitidae: Nasutitermitinae). Sociobiology. 2018, 65, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.C.B.; Lima, M.J.G.; Santiago, R.; Buril, M.L.L.; Pereira, E.C.; Legaz, M.E.; Vicente, C.; Silva, N.H. New biotechnological methods for producing therapeutic compounds (Usnic, Stictic and Norstictic acids) by cell immobilization of the lichen Cladonia substellata Vainio. Biotechnol. Ind. J. 2017, 13, 1–13. [Google Scholar]
- Santos, F.T.J.; Siqueira, W.N.; Santos, M.L.O.; Silva, H.A.M.F.; Sá, J.L.F.; Fernandes, T.S.; Silva, N.H.; França, E.J.; Silva, E.B.; Melo, A.M.M.A. Radiosensitizer effect of usnic acid on Biomphalaria glabrata embryos. Int. J. Radiat. Biol. 2018, 94, 838–843. [Google Scholar] [CrossRef]
- Santiago, R.; Martins, M.C.B.; Vilaça, M.D.; Barros, L.F.B.; Nascimento, T.; Silva, N.H.; Falcão, E.P.S.; Legaz, M.E.; Vicente, C.; Pereira, E.C. Phytochemical and biological evaluation of metabolites produced by alginate-immobilized bionts isolated from the lichen Cladonia substellata vain. Fitoterapia 2018, 131, 23–34. [Google Scholar] [CrossRef]
- Silva, C.R.; Marinho, K.S.N.; Silva, T.D.S.; Ferreira, D.K.S.; Aguiar, G.M.; Martins, M.C.B.; Santos, K.R.P.; Aguiar Júnior, F.C.A.; Santos, N.P.S.; Pereira, E.C.; et al. Teratogenic effect of usnic acid from Cladonia substellata Vainio during organogenesis. BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luz, J.S.B.; Oliveira, E.B.; Martins, M.C.B.; Silva, N.H.; Alves, L.C.; Santos, F.A.B.; Silva, L.L.S.; Silva, E.C.; Medeiros, P.L. Ultrastructural analysis of Leishmania infantum chagasi promastigotes forms treated in vitro with usnic acid. Sci. World J. 2015, 25, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.C.B.; Silva, M.C.; Silva, L.R.S.; Lima, V.L.M.; Pereira, E.C.; Falcão, E.P.; Melo, A.M.M.A.; Silva, N.H. Usnic acid potassium salt: An alternative for the control of Biomphalaria glabrata (Say, 1818). PLOS ONE 2014, 9, e111102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, H.D.A.; Melo, A.M.M.A.; Siqueira, W.N.; Martins, M.C.B.; Aires, A.L.; Albuquerque, M.C.P.A.; Silva, N.H.; Lima, V.L.M. Potassium usnate toxicity against embryonic stages of the snail Biomphalaria glabrata and Schistosoma mansoni cercariae. Acta Trop. 2018, 188, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Melo, A.M.M.A.; Siqueira, W.N.; Martins, M.C.B.; Aires, A.L.; Albuquerque, M.C.P.A.; Silva, N.H.; Lima, V.L.M. Dataset on schistosomiasis control using potassium usnate against Biomphalaria glabrata at different developmental stage and Schistosoma mansoni cercariae. Data Brief. 2018, 21, 1347–1351. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Aires, A.L.; Soares, C.L.R.; Brito, T.G.S.; Nascimento, W.M.; Martins, M.C.B.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; et al. Usnic acid potassium salt from Cladonia substellata (Lichen): Synthesis, cytotoxicity and in vitro anthelmintic activity and ultrastructural analysis against adult worms of Schistosoma mansoni. Acta Trop. 2019, 192, 1–10. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Silva Júnior, J.G.; Oliveira, J.R.S.; Ribeiro, M.H.M.L.; Martins, M.C.B.; Bezerra, M.A.C.; Aires, A.L.; Albuquerque, M.C.P.A.; Melo-Júnior, M.R.; Pontes Filho, N.T.; et al. Usnic acid potassium salt: Evaluation of the acute toxicity and antinociceptive effect in murine model. Molecules 2019, 24, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Araújo, H.D.A.; Silva, N.H.; Albuquerque, M.C.P.A.; Aires, A.L.; Lima, V.L.M. Potassium usnate, a water-soluble usnic acid salt, shows enhanced activity against Schistosoma mansoni in vitro. Exp. Parasitol. 2020, 208, 1–5. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Santos, V.H.B.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; Albuquerque, M.C.P.A.; Aires, A.L.; Lima, V.L.M. In vitro activity of usnic acid potassium salt against different developmental stages of Schistosoma mansoni: An ultrastructural study. Acta Trop. 2020, 201, 1–11. [Google Scholar] [CrossRef]
- Buemi, G.; Zuccarello, F. Molecular conformations, hydrogen-bond strengths and electronic structure of usnic acid: An AM1 and CNDO/S study. J. Mol. Struc.-Theochem. 1990, 209, 89–99. [Google Scholar] [CrossRef]
- Galasso, V. Probing the molecular and electronic structure of the lichen metabolite usnic acid: A DFT study. Chem. Phys. 2010, 374, 138–145. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Q.; Fang, J.L.; Mei, N.; Ali, A.A.; Lewis, S.M.; Leakey, J.E.; Frankos, V.H. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Health. C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Scholte, R.G.; Gosoniu, L.; Malone, J.B.; Chammartin, F.; Utzinger, J.; Vounatsou, P. Predictive risk mapping of schistosomiasis in Brazil using Bayesian geostatistical models. Acta Trop. 2014, 132, 57–63. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Silva, H.A.M.F.; Siqueira, W.N.; Santos, V.H.B.; Lima, M.V.; Silva Júnior, J.; Silva, N.H.; Albuquerque, M.C.P.A.; Melo, A.M.M.A.; Aires, A.L.; et al. Sublethal concentrations of usnic acid potassium salt impairs physiological parameters of Biomphalaria glabrata (Say, 1818) (Pulmonata: Planorbidae) infected and not infected with Schistosoma mansoni. Acta Trop. 2021, 222, 1–13. [Google Scholar] [CrossRef]
- Famakinde, D.O. Molecular context of Schistosoma mansoni transmission in the molluscan environments: A mini-review. Acta Trop. 2017, 176, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.N.; Melo, A.M.M.A.; Amâncio, F.F.; Araújo, H.D.A.; Silva, H.A.M.F.; Albuquerque, M.C.P.A.; Aires, A.L.; Martins, M.C.B.; Silva, N.H. Avaliação da atividade do ácido úsnico sobre cercárias de Schistosoma mansoni. XXIV Congresso da Sociedade Brasileira de Parasitologia. XXIII Congresso Latinoamericano de Parasitologia. 2015, P 263, 857. [Google Scholar]
- Katz, N. The discovery of schistosomiasis mansoni in Brazil. Acta Trop. 2008, 108, 69–71. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiological Record. Schistosomiasis and Soil-transmitted Helminthiases: Number of People Treated in 2016. 2017. Available online: http://apps.-who.int/iris/bitstream/handle/10665/259593/WER9249.pdf?sequence=1 (accessed on 2 May 2021).
- Salloum, A.I.O.; Lucarini, V.R.; Tozatti, M.G.; Medeiros, J.; Silva, M.L.A.; Magalhães, L.G.; Cunha, W.R. In vitro schistosomicidal activity of Usnea steineri extract and its major constituent (+)-usnic acid against Schistosoma mansoni. Planta Med. 2012, 78, PI304. [Google Scholar] [CrossRef]
- Charyyeva, A.; Çetinkaya, Ü.; Özkan, B.; Şahin, S.; Yaprak, N.; Şahin, I.; Yurchenko, V.; Kostygov, A.Y. Genetic diversity of Leishmania tropica: Unexpectedly complex distribution pattern. Acta Trop. 2021. In Press. [Google Scholar] [CrossRef] [PubMed]
- Luz, J.S.B. Análise Comparativa Da Atividade Leishmanicida In Vitro Do Ácido Úsnico E Do Seu Derivado Usnato De Potássio Isolado Da Cladonia Substellata Vainio. In Ph.D. Thesis; Universidade Federal de Pernambuco: Recife, Brazil, 2019. [Google Scholar]
- DeSantana, J.M.; Perissinotti, D.M.N.; Oliveira Junior, J.O.; Correia, L.M.F.; Oliveira, C.M.; Fonseca, P.R.B. Revised definition of pain after four decades. BrJP. 2020, 3, 197–198. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised international association for the study of pain definition of pain: Concepts, challenges, and compromises. Pain. 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Okuyama, E.; Umeyama, K.; Yamazaki, M.; Kinoshita, Y.; Yamamoto, Y. Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 1995, 61, 113–115. [Google Scholar] [CrossRef]
- Prokopiev, I.A.; Filippov, E.V.; Filippova, G.V.; Gladkina, N.P. Genotoxic effect of usnic acid enantiomers in vitro in human peripheral blood lymphocytes. Tsitologiia. 2017, 59, 13–18. [Google Scholar] [CrossRef]
- O’Connell, E.; Reynolds, I.S.; McNamara, D.A.; Burke, J.P.; Prehn, J.H.M. Resistance to cell death in mucinous colorectal cancer - A review. Cancers 2021, 13, 1389. [Google Scholar] [CrossRef]
- Rubio, J.; Cristóbal, I.; Santos, A.; Caramés, C.; Luque, M.; Sanz-Alvarez, M.; Zazo, S.; Madoz-Gúrpide, J.; Rojo, F.; García-Foncillas, J. Low microRNA-19b expression shows a promising clinical impact in locally advanced rectal cancer. Cancers 2021, 13, 1456. [Google Scholar] [CrossRef]
- Yang, Y.; Bae, W.K.; Lee, J.Y.; Choi, Y.J.; Lee, K.H.; Park, M.S.; Yu, Y.H.; Park, S.Y.; Zhou, R.; Taş, İ.; et al. Potassium usnate, a water-soluble usnic acid salt, shows enhanced bioavailability and inhibits invasion and metastasis in colorectal cancer. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Veras, B.O.; Oliveira, J.R.S.; Lima, V.L.M.; Navarro, D.M.A.F.; Aguiar, J.C.R.O.F.; Moura, G.M.M.; Silva, J.W.; Assis, C.R.D.; Gorlach-Lira, K.; Assis, P.A.C.; et al. The essential oil of the leaves of Verbesina macrophylla (Cass.) S.F.Blake has antimicrobial, anti-inflammatory and antipyretic activities and is toxicologically safe. J. Ethnopharmacol. 2021, 265, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich, Safety Data Sheet. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=BR&language=pt&productNumber=329967&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Fterm%3D7562610%26interface%3DCAS%2520No.%26N%3D0%26mode%3Dpartialmax%26lang%3Dpt%26region%3DBR%26focus%3Dproduct (accessed on 2 May 2021).
- Abo-Khatwa, A.N.; Al-Robai, A.A.; Al-Jawhari, D.A. The uncoupling of oxidative phosphorylation of mouse-liver mitochondria in vivo by usnic acid. JKAU. 2015, 17, 35–45. [Google Scholar] [CrossRef]
- Joseph, A.; Lee, T.; Moland, C.L.; Branham, W.S.; Fuscoe, J.C.; Leakey, J.E.A.; Allaben, W.T.; Lewis, S.M.; Ali, A.A.; Desai, V.G. Effect of (+)-usnic acid on mitochondrial functions as measured by mitochondria-specific oligonucleotide microarray in liver of B6C3F1 mice. Mitochondrion 2009, 9, 149–158. [Google Scholar] [CrossRef]
- Dailey, R.N.; Montgomery, D.L.; Ingram, J.T.; Siemion, R.; Vasquez, M.; Raisbeck, M.F. Toxicity of the lichen secondary metabolite (+)-usnic acid in domestic sheep. Vet. Pathol. 2008, 45, 19–25. [Google Scholar] [CrossRef] [PubMed]
| Antinociceptive Activity | References | ||
| Usnic Acid | |||
| Concentration (mg/kg) | Effect (%) | ||
| 30 | - | [55] | |
| 100 | 60 | ||
| Potassium Usnate | |||
| Concentration (mg/kg) | Effect (%) | ||
| 10 | 69 | [38] | |
| 20 | 78 | ||
| Antitumor Activity and Bioavailability | |||
| Mean of Distribution, in the Tumor, Liver and Blood Plasma | [59] | ||
| Tissue | Molecule Analyzed | ||
| Usnic Acid | Potassium Usnate | ||
| Tumor nmole/g | 0.0 | 1.5 | |
| Liver nmole/g | 0.28 | 2.6 | |
| Plasma µM | 0.2 | 1.7 | |
| Mean Levels of Gene Expression of EMT Markers in Caco2 Cells Treated with the Drugs | |||
| Usnic Acid | Potassium Usnate | ||
| E-cad | 0.98 | 0.78 | |
| N-cad | 0.84 | 0.74 | |
| Snail | 0.49 | 0.53 | |
| Twist | 0.63 | 0.64 | |
| Slug | 0.75 | 0.62 | |
| ZEB1 | 0.95 | 0.74 | |
| ZEB2 | 0.82 | 0.73 | |
| Mean Levels of mRNA Expression of Genes Related to Cell Motility in Caco2 Cells Treated with the Drugs | |||
| Usnic Acid | Potassium Usnate | ||
| CANP1 | 0.95 | 0.74 | |
| CDC42 | 0.90 | 0.55 | |
| CFL1 | 0.78 | 0.70 | |
| IGF1 | 0.77 | 0.70 | |
| WASF1 | 1.03 | 0.71 | |
| WASL | 1.00 | 0.53 | |
| Acute Toxicity | |||
| Usnic Acid | |||
| Animals | Concentration (mg/kg) | LD50(mg/kg) | |
| Mice | NI | 25 | [2] |
| Rats and rabbits | NI | 30 | |
| Dogs | NI | 40 | |
| Sheep | NI | 485 e 647 | [64] |
| Mice | 80–280 | 180 | [62] |
| Mice | NI | 838 | [61] |
| Potassium Usnate | |||
| Mice | 500, 1000 and 2000 | > 2000 | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, H.D.A.d.; Silva, H.A.M.F.; Silva Júnior, J.G.d.; Albuquerque, M.C.P.d.A.; Coelho, L.C.B.B.; Aires, A.d.L. The Natural Compound Hydrophobic Usnic Acid and Hydrophilic Potassium Usnate Derivative: Applications and Comparisons. Molecules 2021, 26, 5995. https://doi.org/10.3390/molecules26195995
Araújo HDAd, Silva HAMF, Silva Júnior JGd, Albuquerque MCPdA, Coelho LCBB, Aires AdL. The Natural Compound Hydrophobic Usnic Acid and Hydrophilic Potassium Usnate Derivative: Applications and Comparisons. Molecules. 2021; 26(19):5995. https://doi.org/10.3390/molecules26195995
Chicago/Turabian StyleAraújo, Hallysson Douglas Andrade de, Hianna Arely Milca Fagundes Silva, José Guedes da Silva Júnior, Mônica Camelo Pessoa de Azevedo Albuquerque, Luana Cassandra Breitenbach Barroso Coelho, and André de Lima Aires. 2021. "The Natural Compound Hydrophobic Usnic Acid and Hydrophilic Potassium Usnate Derivative: Applications and Comparisons" Molecules 26, no. 19: 5995. https://doi.org/10.3390/molecules26195995
APA StyleAraújo, H. D. A. d., Silva, H. A. M. F., Silva Júnior, J. G. d., Albuquerque, M. C. P. d. A., Coelho, L. C. B. B., & Aires, A. d. L. (2021). The Natural Compound Hydrophobic Usnic Acid and Hydrophilic Potassium Usnate Derivative: Applications and Comparisons. Molecules, 26(19), 5995. https://doi.org/10.3390/molecules26195995







