1H NMR Study of the HCa2Nb3O10 Photocatalyst with Different Hydration Levels
Abstract
1. Introduction
2. Results and Discussion
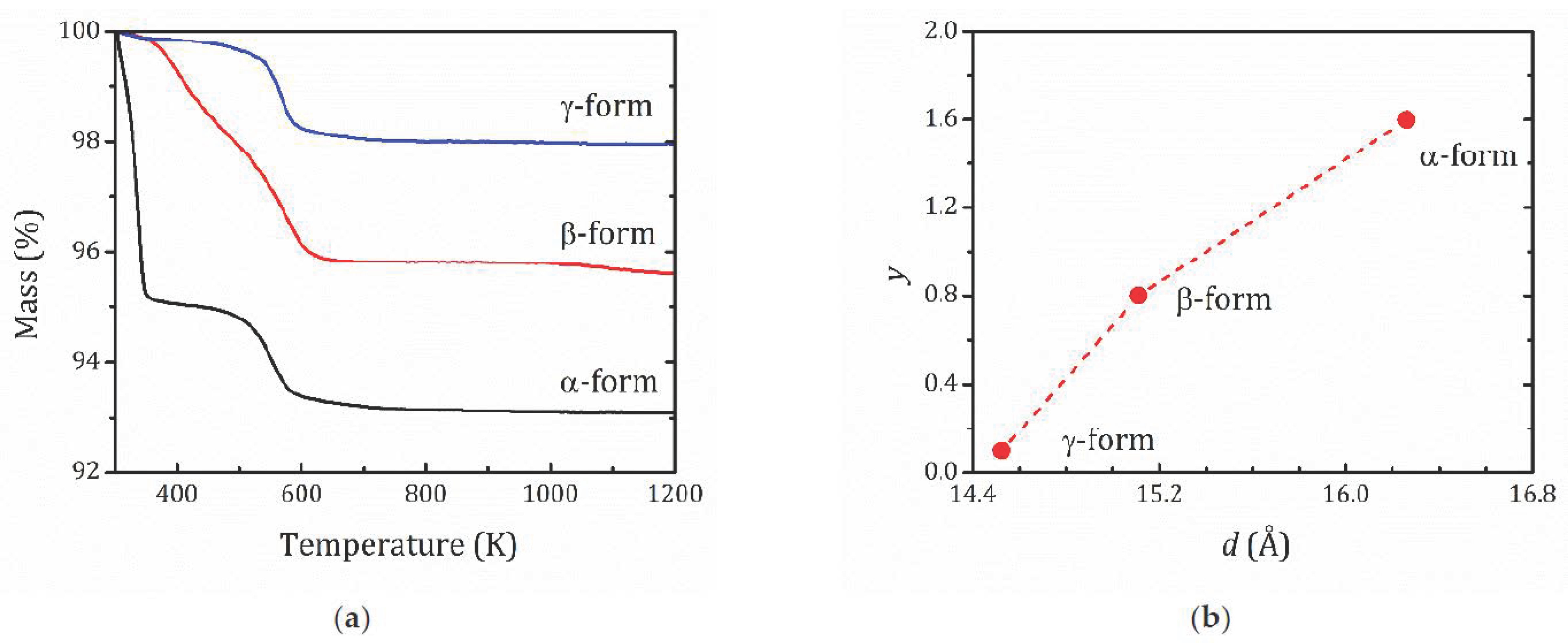
2.1. X-ray and TG Analysis
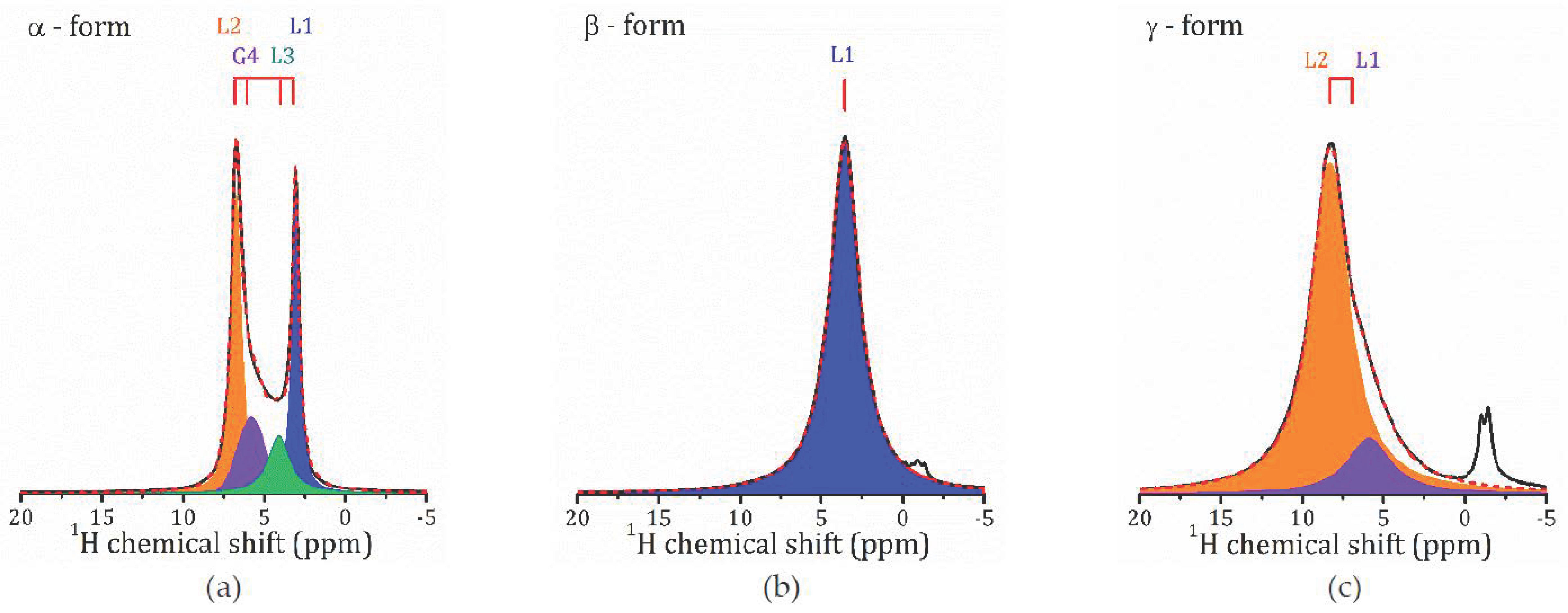
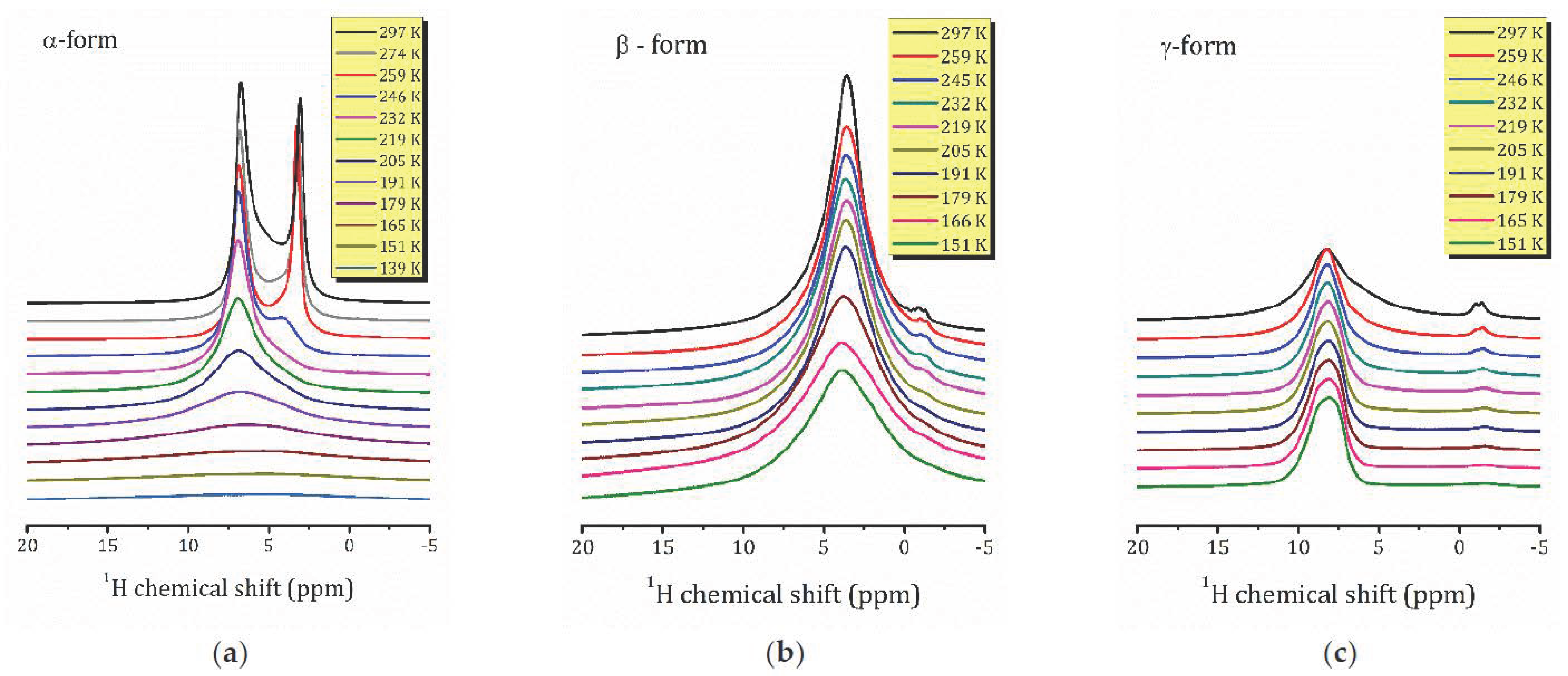
2.2. 1H MAS NMR Study
2.3. 1H T1ρ Study
3. Materials and Methods
4. Conclusions
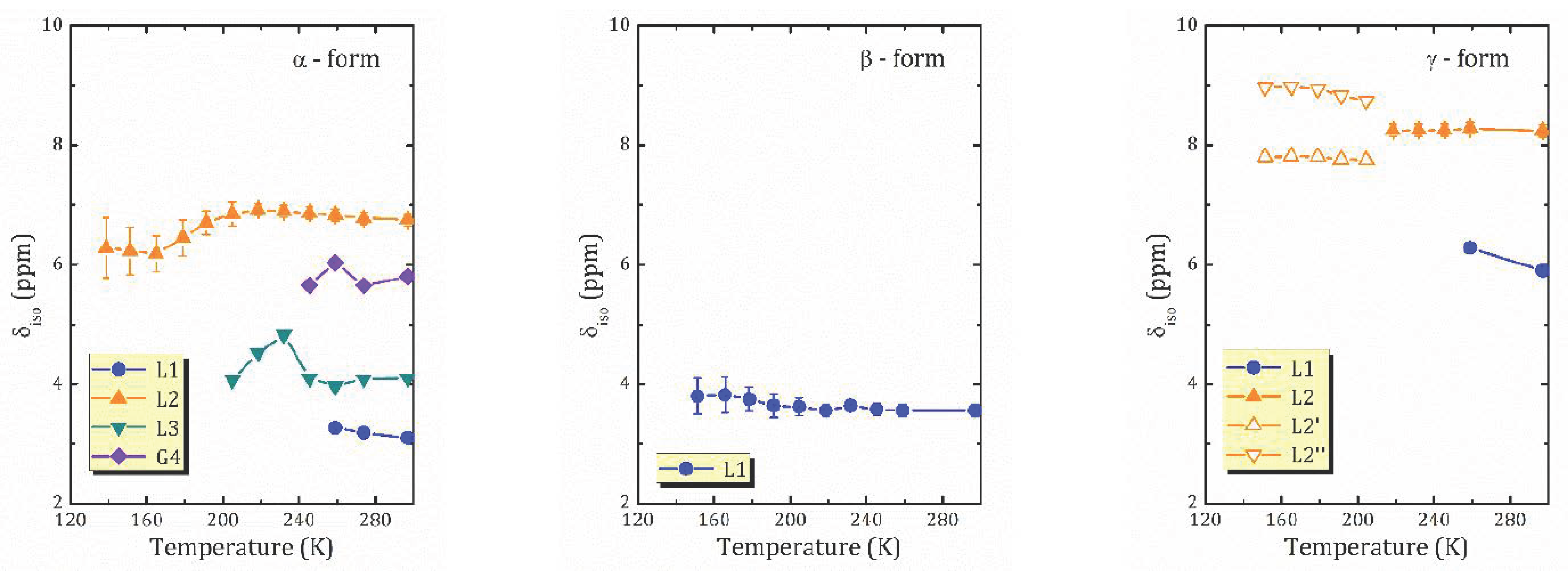
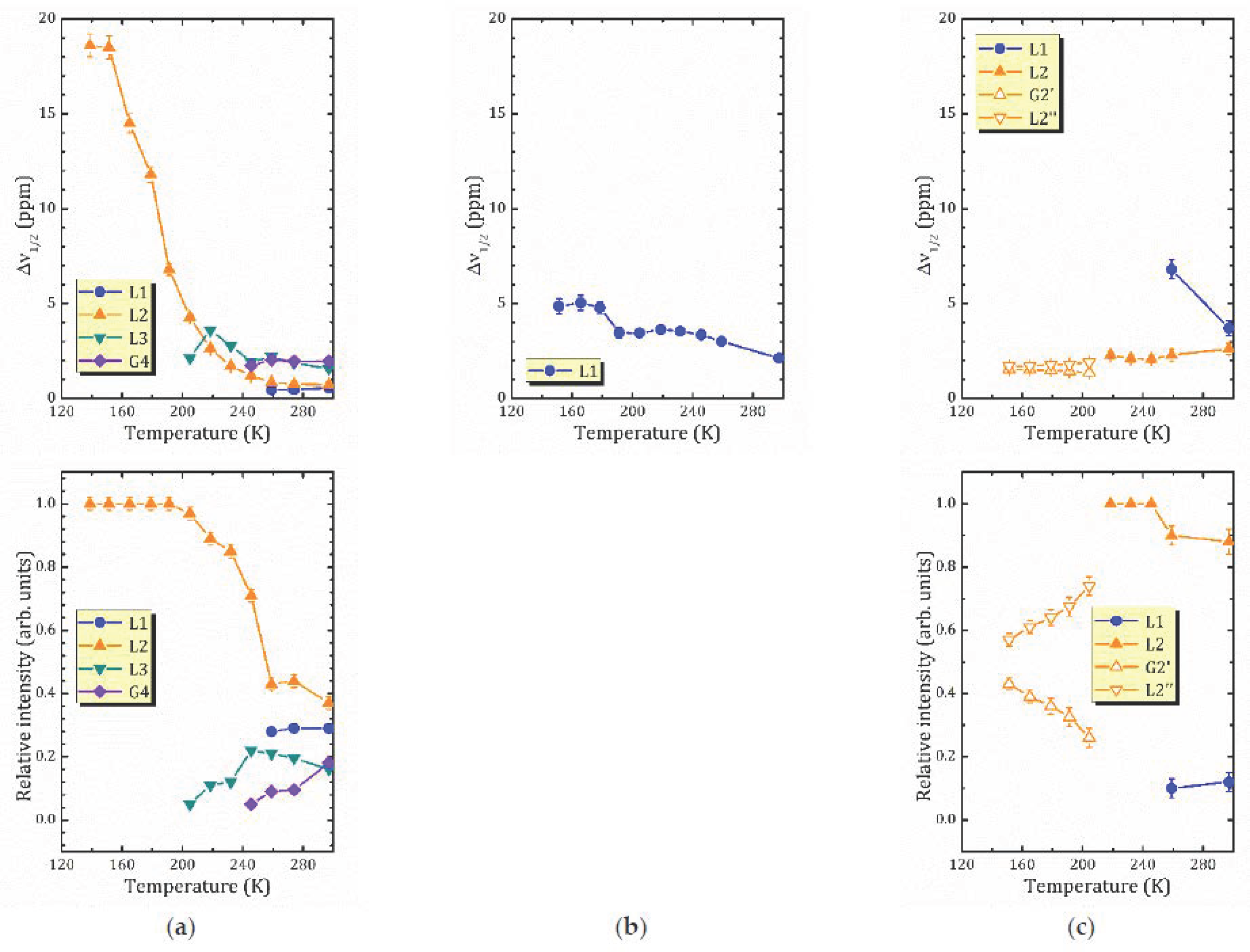
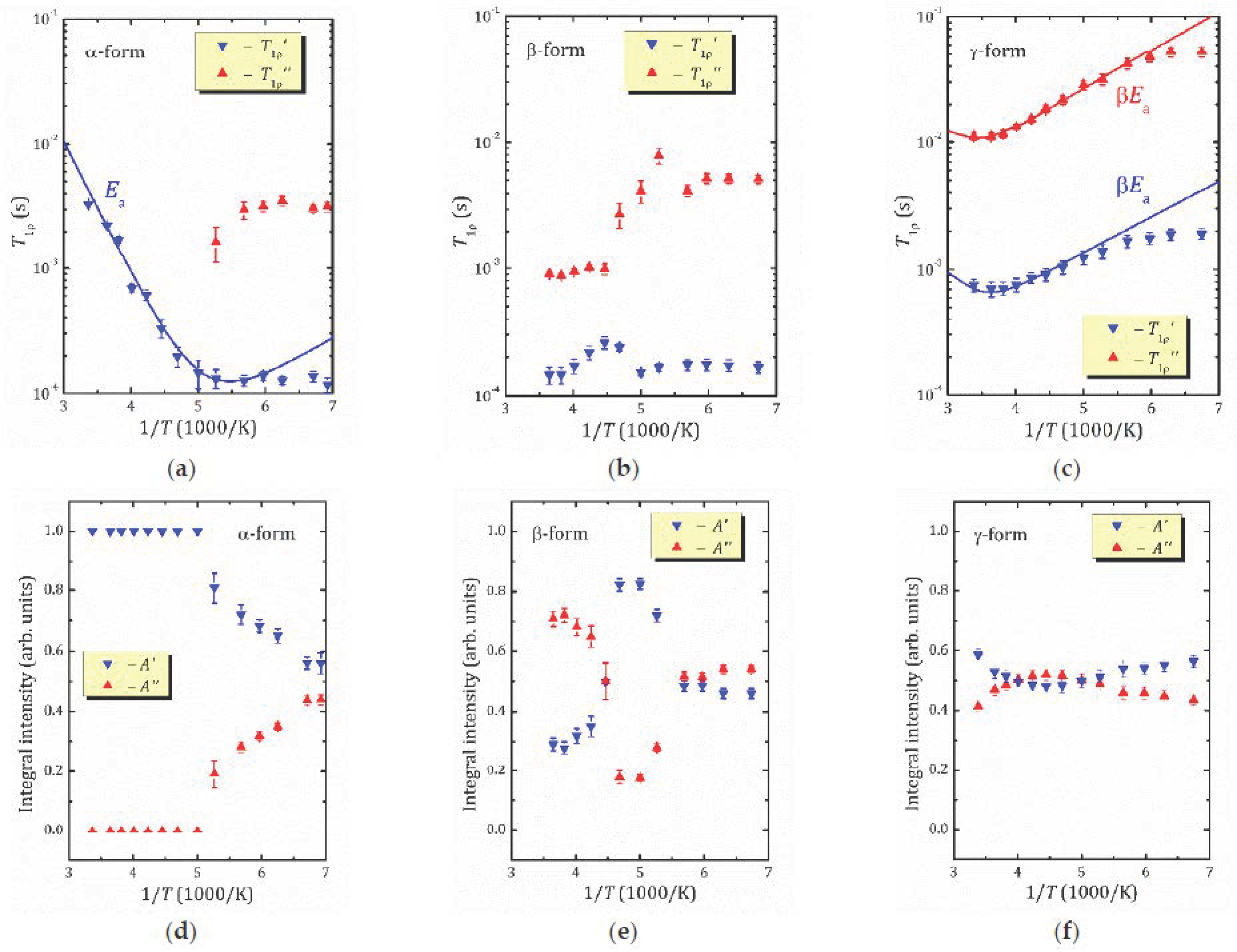
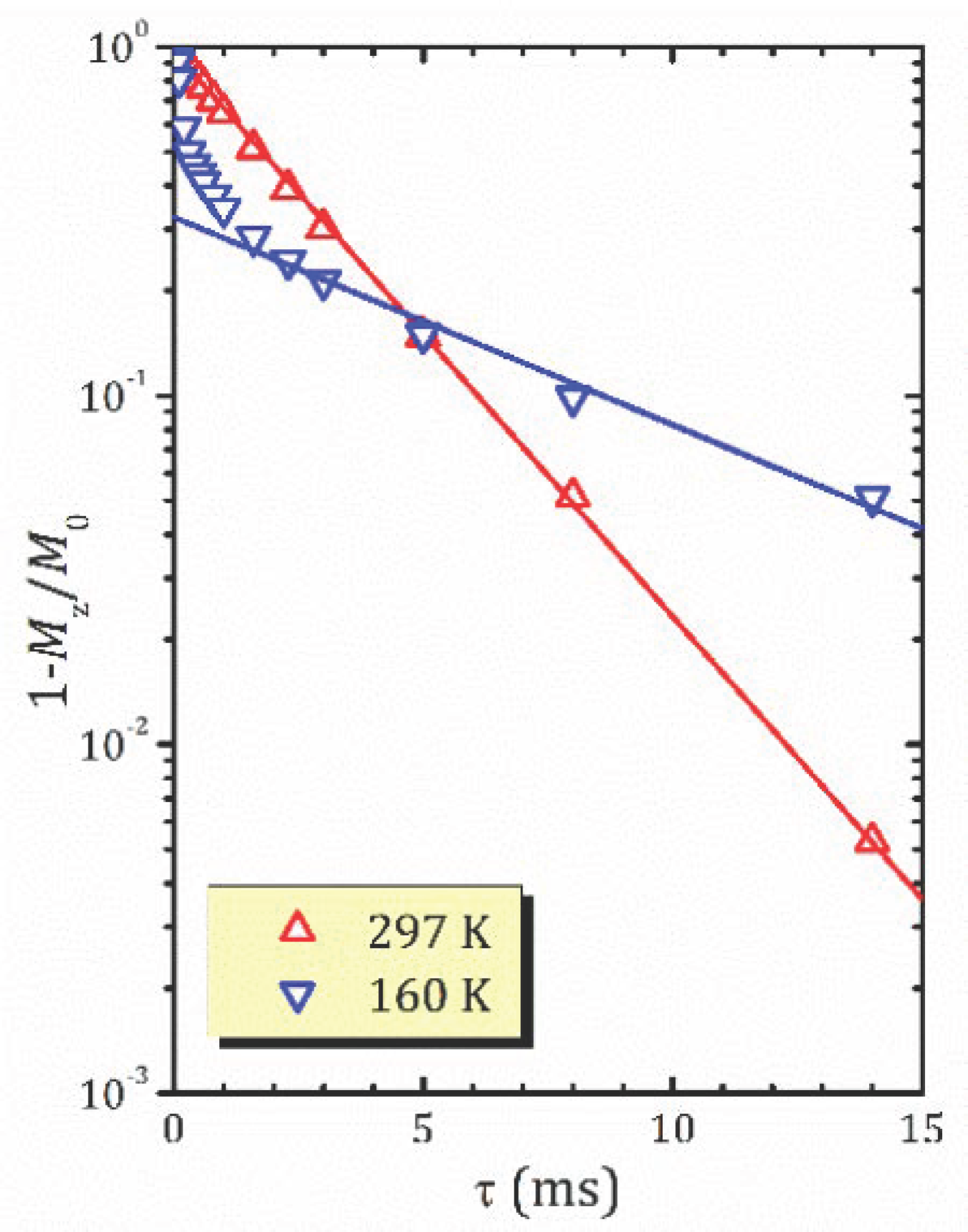
- For the most hydrated α-form, HCa2Nb3O10·1.6H2O, 1H MAS NMR spectra reveal the presence of different proton-containing species: H3O+, which comprises all the lattice protons (there is no signal that can be associated with H+ in regular sites), and water molecules that are localized in different sites of the interlayer slab and supposedly participate in the formation of charged complexes like H+…2H2O. With the temperature decreasing, the signal from the water proton is so broad that it is undetectable; the only detectable signal is from H3O+. The activation energy determined from the onset temperature of motional narrowing of 0.24(2) eV is in fair agreement with the relaxation data. Application of the KWW model with the stretching exponent β = 0.28 to the T1ρ(1/T) experimental dependence results in the following molecular motion parameters, which can be associated with the translational diffusion of H3O+: eV, s. Below 200 K, with the slowing down of the translational motion, other types of motion, such as reorientation, became more important.
- 1H MAS NMR spectrum of the β-form, HCa2Nb3O10·0.8H2O, within the studied temperature range consists of one line centered at about 3.6 ppm, which is the result of an exchange between lattice protons and the non-hydrogen-bounded water protons. The temperature dependence of the relaxation time evidences the presence of two characteristic points (224 and 176 K), at which proton dynamics changes.
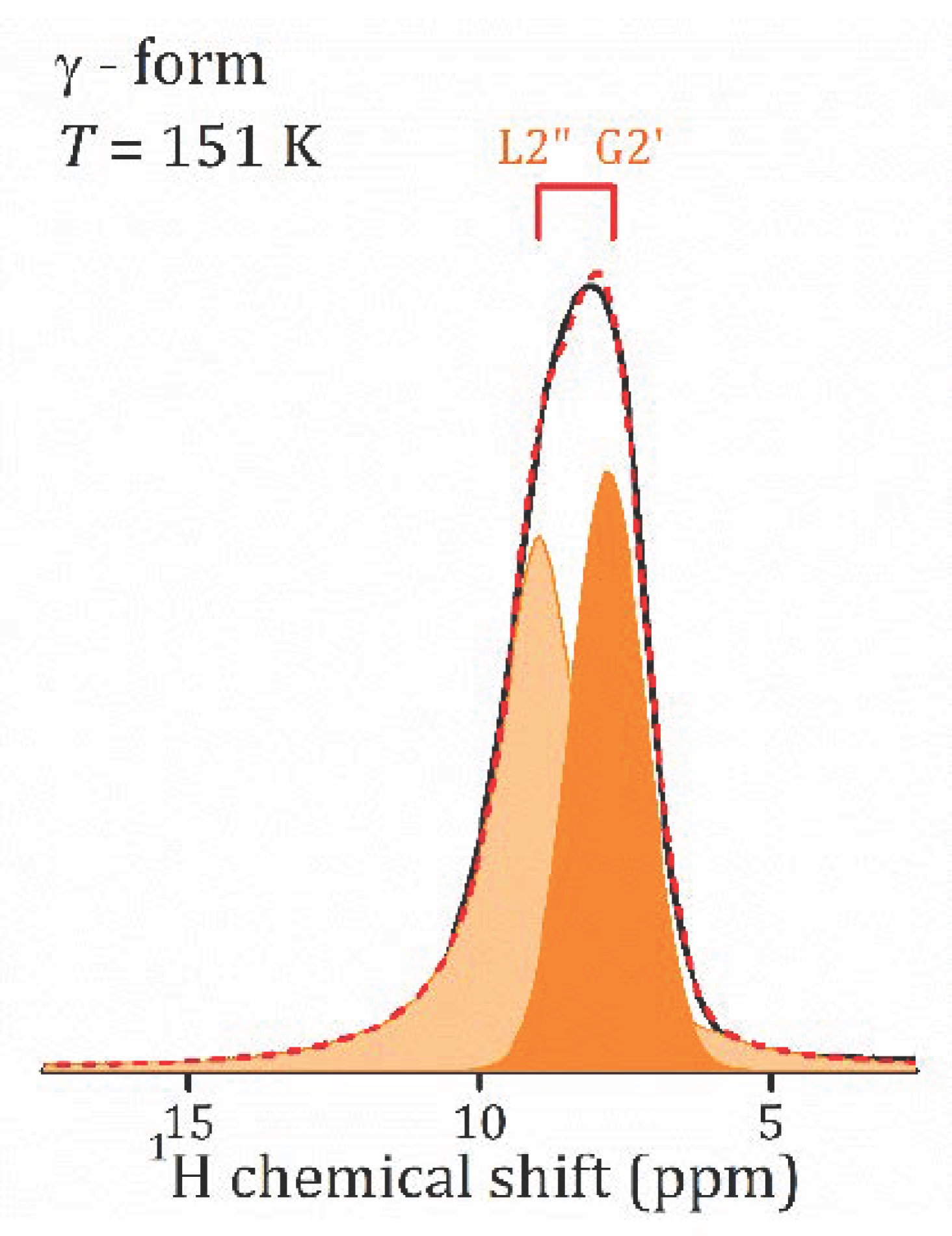
- The proton NMR spectroscopy study of the γ-form, HCa2Nb3O10·0.1H2O, which is characterized by the lowest water content, reveals the presence of bulk water and interlayer H+ in two inequivalent positions. The temperature dependencies of the spin-lattice relaxation time in the rotating frame treated withing the KWW model with β = 0.28 results in the following parameters of the proton motion: eV, s.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, Y.; Mao, Z.-Q. Unconventional superconductivity in Sr2RuO4. Phys. C Supercond. Its Appl. 2015, 514, 339–353. [Google Scholar] [CrossRef]
- Ogino, H.; Sato, S.; Kishio, K.; Shimoyama, J. Relationship between crystal structures and physical properties in iron arsenides with perovskite-type layers. Phys. Procedia 2012, 36, 722–726. [Google Scholar] [CrossRef]
- Moritomo, Y.; Asamitsu, A.; Kuwahara, H.; Tokura, Y. Giant magnetoresistance of manganese oxides with a layered perovskite structure. Nature 1996, 380, 141–144. [Google Scholar] [CrossRef]
- Domen, K.; Kondo, J.N.; Hara, M.; Takata, T. Photo- and mechano-catalytic overall water splitting reactions to form hydrogen and oxygen on heterogeneous catalysts. Bull. Chem. Soc. Jpn. 2000, 73, 1307–1331. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Toda, K.; Kameo, Y.; Kurita, S.; Sato, M. Crystal structure determination and ionic conductivity of layered perovskite compounds NaLnTiO4 (Ln = rare earth). J. Alloys Compd. 1996, 234, 19–25. [Google Scholar] [CrossRef]
- Petrov, A.A.; Melnikova, N.A.; Petrov, A.V.; Silyukov, O.I.; Murin, I.V.; Zvereva, I.A. Experimental investigation and modelling of the Na+ mobility in NaLnTiO4 (Ln = La, Nd) ceramics. Ceram. Int. 2017, 43, 10861–10865. [Google Scholar] [CrossRef]
- Thangadurai, V.; Shukla, A.; Gopalakrishnan, J. Proton conduction in layered perovskite oxides. Solid State Ion. 1994, 73, 9–14. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Zvereva, I.A. Photocatalytic activity of layered perovskite-like oxides in practically valuable chemical reactions. Russ. Chem. Rev. 2016, 85, 248–279. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Kulish, L.D.; Zvereva, I.A. Study on thermolysis process of a new hydrated and protonated perovskite-like oxides H2K0.5Bi2.5Ti4O13·yH2O. Ceram. Int. 2019, 45, 2704–2709. [Google Scholar] [CrossRef]
- Silyukov, O.I.; Minich, I.A.; Zvereva, I.A. Synthesis of protonated derivatives of layered perovskite-like bismuth titanates. Glas. Phys. Chem. 2018, 44, 115–119. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Nefedov, D.Y.; Kostromin, A.V.; Silyukov, O.I.; Zvereva, I.A. Proton mobility in Ruddlesden–Popper phase H2La2Ti3O10 studied by 1H NMR. Ceram. Int. 2019, 45, 5788–5795. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Ma, H.; Ma, S.; Huang, G.; Makita, Y.; Bai, X.; Yang, X. Structure and dehydration of layered perovskite niobate with bilayer hydrates prepared by exfoliation/self-assembly process. J. Solid State Chem. 2008, 181, 1684–1694. [Google Scholar] [CrossRef]
- Guo, T.; Wang, L.; Evans, D.G.; Yang, W. Synthesis and photocatalytic properties of a pPolyaniline-intercalated layered protonic titanate nanocomposite with a p−n heterojunction structure. J. Phys. Chem. C 2010, 114, 4765–4772. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Silyukov, O.I.; Lushpinskaia, I.P.; Kurnosenko, S.A.; Mazur, A.S.; Shenderovich, I.G.; Zvereva, I.A. NMR study of intercalates and grafted organic derivatives of H2La2Ti3O10. Molecules 2020, 25, 5229. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, L.; Hao, Q.; Zhu, X.; Chen, X.; Tang, K. Preparation of interlayer surface tailored protonated double-layered perovskite H2CaTa2O7 with n-alcohols, and their photocatalytic activity. RSC Adv. 2014, 4, 4047–4054. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Lushpinskaya, I.P.; Kurnosenko, S.A.; Silyukov, O.I.; Zvereva, I.A. Identification of intercalates and grafted organic derivatives of H2La2Ti3O10 by multinuclear NMR. Russ. J. Gen. Chem. 2020, 90, 760–761. [Google Scholar] [CrossRef]
- Voytovich, V.; Kurnosenko, S.; Silyukov, O.; Rodionov, I.; Bugrov, A.; Minich, I.; Malygina, E.; Zvereva, I. Synthesis of n-alkoxy derivatives of layered perovskite-like niobate HCa2Nb3O10 and study of their photocatalytic activity for hydrogen production from an aqueous solution of methanol. Catalysts 2021, 11, 897. [Google Scholar] [CrossRef]
- Asai, Y.; Ariake, Y.; Saito, H.; Idota, N.; Matsukawa, K.; Nishino, T.; Sugahara, Y. Layered perovskite nanosheets bearing fluoroalkoxy groups: Their preparation and application in epoxy-based hybrids. RSC Adv. 2014, 4, 26932–26939. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Bekheet, M.F.; Zahedi, E.; Wagata, H.; Kamei, Y.; Yubuta, K.; Gurlo, A.; Matsushita, N.; Domen, K.; Teshima, K. New Dion-Jacobson phase three-layer perovskite CsBa2Ta3O10 and its conversion to nitrided Ba2Ta3O10 nanosheets via a nitridation-protonation-intercalation-exfoliation route for water splitt. Cryst. Growth Des. 2016, 16, 2302–2308. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Lewandowski, J.T.; Johnson, J.W. Ion exchange of the layered perovskite KCa2Nb3O10 by protons. J. Less Common Met. 1986, 116, 137–146. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J.T. Intercalation of the layered solid acid HCa2Nb3O10 by organic amines. Mater. Res. Bull. 1987, 22, 45–51. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J.T. Interlayer chemistry between thick transition-metal oxide layers: Synthesis and intercalation reactions of K[Ca2Nan−3NbnO3n+1] (3.ltoreq. n.ltoreq. 7). Inorg. Chem. 1985, 24, 3727–3729. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Prying apart Ruddlesden-Popper phases: Exfoliation into sheets and nanotubes for assembly of perovskite thin films for assembly of perovskite thin films. Chem. Mater. 2000, 3427–3434. [Google Scholar] [CrossRef]
- Domen, K.; Yoshimura, J.; Sekine, T.; Kondo, J.; Tanaka, A.; Maruya, K.; Onishi, T. A novel series of photocatalysts with an ion-exchangeable layered structure of niobate. Catal. Lett. 1993, 75, 2159–2162. [Google Scholar] [CrossRef]
- Oshima, T.; Ishitani, O.; Maeda, K. Non-sacrificial water photo-oxidation activity of lamellar calcium niobate induced by exfoliation. Adv. Mater. Interfaces 2014, 1, 2–5. [Google Scholar] [CrossRef]
- Sabio, E.M.; Chamousis, R.L.; Browning, N.D.; Osterloh, F.E. Photocatalytic water splitting with suspended calcium niobium oxides: Why nanoscale is better than bulk—A kinetic analysis. J. Phys. Chem. C 2012, 116, 3161–3170. [Google Scholar] [CrossRef]
- Takata, T.; Furumi, Y.; Shinohara, K.; Tanaka, A.; Hara, M.; Kondo, J.N.; Domen, K. Photocatalytic decomposition of water on spontaneously hydrated layered perovskites. Chem. Mater. 1997, 9, 1063–1064. [Google Scholar] [CrossRef]
- Wang, T.H.; Henderson, C.N.; Draskovic, T.I.; Mallouk, T.E. Synthesis, exfoliation, and electronic/protonic conductivity of the Dion-Jacobson phase layer perovsikite HLa2TiTa2O10. Chem. Mater. 2014, 26, 898–906. [Google Scholar] [CrossRef]
- Oshima, T.; Yokoi, T.; Eguchi, M.; Maeda, K. Synthesis and photocatalytic activity of K2CaNaNb3O10, a new Ruddlesden-Popper phase layered perovskite. Dalt. Trans. 2017, 46, 10594–10601. [Google Scholar] [CrossRef]
- Essayem, N.; Tong, Y.Y.; Jobic, H.; Vedrine, J.C. Characterization of protonic sites in H3PW12O40 and Cs1.9H1.1PW12O40: A solid-state 1H, 2H, 31P MAS-NMR and inelastic neutron scattering study on. Appl. Catal. A Gen. 2000, 194, 109–122. [Google Scholar] [CrossRef]
- Raciulete, M.; Papa, F.; Negrila, C.; Bratan, V.; Munteanu, C.; Pandele-Cusu, J.; Culita, D.C.; Atkinson, I.; Balint, I. Strategy for modifying layered perovskites toward efficient solar light-driven photocatalysts for removal of chlorinated pollutants. Catalysts 2020, 10, 637. [Google Scholar] [CrossRef]
- Arribart, H.; Piffard, Y. Indication from NMR of Grotthuss mechanism for proton conduction in H2Sb4O11·nH2O. Solid State Commun. 1983, 45, 571–575. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Yocupicio-Gaxiola, R.I.; Zhelezniak, I.V.; Chislov, M.V.; Antúnez-García, J.; Murrieta-Rico, F.N.; Galván, D.H.; Petranovskii, V.; Fuentes-Moyado, S. Local structures of two-dimensional zeolites—Mordenite and ZSM-5—Probed by multinuclear NMR. Molecules 2020, 25, 4678. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakai, T.; Deguchi, K.; Yamada, K.; Yue, B.; Ye, J. A visible-light-responsive photocatalyst of nitrogen-doped solid-acid HNb3O8-N studied by ultrahigh-field 1 H MAS NMR and 1H93Nb/1H15N HETCOR NMR in solids. Chem. Lett. 2014, 43, 80–82. [Google Scholar] [CrossRef]
- Cattaneo, A.S.A.S.; Ferrara, C.; Marculescu, A.M.A.M.; Giannici, F.; Martorana, A.; Mustarelli, P.; Tealdi, C. Solid-state NMR characterization of the structure and thermal stability of hybrid organic-inorganic compounds based on a HLaNb2O7 Dion-Jacobson layered perovskite. Phys. Chem. Chem. Phys. 2016, 18, 21903–21912. [Google Scholar] [CrossRef][Green Version]
- Krylova, E.A.; Shelyapina, M.G.; Nowak, P.; Harańczyk, H.; Chislov, M.; Zvereva, I.A.; Privalov, A.F.; Becker, M.; Vogel, M.; Petranovskii, V. Mobility of water molecules in sodium- and copper-exchanged mordenites: Thermal analysis and 1H NMR. Microporous Mesoporous Mater. 2018, 265, 132–142. [Google Scholar] [CrossRef]
- Tani, S.; Komori, Y.; Hayashi, S.; Sugahara, Y. Local environments and dynamics of hydrogen atoms in protonated forms of ion-exchangeable layered perovskites estimated by solid-state 1H NMR. J. Solid State Chem. 2006, 179, 3357–3364. [Google Scholar] [CrossRef]
- Silyukov, O.; Chislov, M.; Burovikhina, A.; Utkina, T.; Zvereva, I. Thermogravimetry study of ion exchange and hydration in layered oxide materials. J. Therm. Anal. Calorim. 2012, 110, 187–192. [Google Scholar] [CrossRef]
- Utkina, T.; Chislov, M.; Silyukov, O.; Burovikhina, A.; Zvereva, I.A. TG and DSC investigation of water intercalation and protonation processes in perovskite-like layered structure of titanate K2Nd2Ti3O10. J. Therm. Anal. Calorim. 2016, 125, 281–287. [Google Scholar] [CrossRef]
- Zvereva, I.A.; Silyukov, O.I.; Chislov, M.V. Ion-exchange reactions in the structure of perovskite-like layered oxides: I. Protonation of NaNdTiO4 complex oxide. Russ. J. Gen. Chem. 2011, 81, 1434–1441. [Google Scholar] [CrossRef]
- Mäemets, V.; Koppel, I. 17O and 1H NMR chemical shifts of hydroxide and hydronium ion in aqueous solutions of strong electrolytes. J. Chem. Soc. Faraday Trans. 1997, 93, 1539–1542. [Google Scholar] [CrossRef]
- Takagaki, A.; Sugisawa, M.; Lu, D.; Kondo, J.N.; Hara, M.; Domen, K.; Hayashi, S. Exfoliated nanosheets as a new strong solid acid catalyst. J. Am. Chem. Soc. 2003, 125, 5479–5485. [Google Scholar] [CrossRef] [PubMed]
- Jeanneau, E.; Le Floch, M.; Bureau, B.; Audebrand, N.; Louër, D. X-ray diffraction, 133Cs and 1H NMR and thermal studies of CdZrCs1.5(H3O)0.5(C2O4)4·xH2O displaying Cs and water dynamic behavior. J. Phys. Chem. Solids 2004, 65, 1213–1221. [Google Scholar] [CrossRef]
- Grube, E.; Lipton, A.S.; Nielsen, U.G. Identification of hydrogen species in alunite-type minerals by multi-nuclear solid-state NMR spectroscopy. Phys. Chem. Miner. 2019, 46, 299–309. [Google Scholar] [CrossRef]
- Skripov, A.V.; Shelyapina, M.G. Nuclear magnetic resonance. In Neutron Scattering and Other Nuclear Techniques for Hydrogen in Materials; Fritzsche, H., Huot, J., Fruchart, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 337–376. ISBN 978-3-319-22792-4. [Google Scholar]
- Shelyapina, M.G.; Skryabina, N.E.; Surova, L.S.; Dost, A.; Ievlev, A.V.; Privalov, A.F.; Fruchart, D. Proton NMR study of hydrogen mobility in (TiCr1.8)1-xVx hydrides. J. Alloys Compd. 2019, 778, 962–971. [Google Scholar] [CrossRef]
- Waugh, J.S.; Fedin, E.I. Determination of hindered-rotation barriers in solids. Sov. Phys. Solid State 1963, 4, 1633–1636. [Google Scholar]
- Chizhik, V.I.; Chernyshev, Y.S.; Donets, A.V.; Frolov, V.V.; Komolkin, A.V.; Shelyapina, M.G. Magnetic Resonance and Its Applications; Springer International Publishing: Cham, Switzerland, 2014; ISBN 9783319052984. [Google Scholar]
- Ailion, D.; Slichter, C.P. Study of ultraslow atomic motions by magnetic resonance. Phys. Rev. Lett. 1964, 12, 168–171. [Google Scholar] [CrossRef]
- Slichter, C.P.; Ailion, D. Low-field relaxation and the study of ultraslow atomic motions by magnetic resonance. Phys. Rev. 1964, 135, A1099–A1110. [Google Scholar] [CrossRef]
- Look, D.C.; Lowe, I.J. Nuclear magnetic dipole-dipole relaxation along the static and rotating magnetic fields: Application to gypsum. J. Chem. Phys. 1966, 44, 2995–3000. [Google Scholar] [CrossRef]
- Wolf, D. Effect of correlated self-diffusion on the low-field nuclear-spin relaxation in the rotating reference frame. Phys. Rev. B 1974, 10, 2724–2732. [Google Scholar] [CrossRef]
- Kuhn, A.; Kunze, M.; Sreeraj, P.; Wiemhöfer, H.D.; Thangadurai, V.; Wilkening, M.; Heitjans, P. NMR relaxometry as a versatile tool to study Li ion dynamics in potential battery materials. Solid State Nucl. Magn. Reson. 2012, 42, 2–8. [Google Scholar] [CrossRef]
- Kohlrausch, R. Theorie des elektrischen Rückstandes in der Leidener Flasche. Ann. Phys. 1854, 167, 179–214. [Google Scholar] [CrossRef]
- Williams, G.; Watts, D. Non-symmetrical dielectric relaxation behaviour arising from a simple empirical decay function. Trans. Faraday Soc. 1970, 66, 80–85. [Google Scholar] [CrossRef]
- Williams, G.; Watts, D.C.; Dev, S.B.; North, A. Further considerations of non symmetrical dielectric relaxation behaviour arising from a simple empirical decay function. Trans. Faraday Soc. 1971, 67, 1323–1335. [Google Scholar] [CrossRef]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation effects in nuclear magnetic resonance absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Kasperovich, V.S.; Shelyapina, M.G.; Khar’Kov, B.; Rykov, I.; Osipov, V.; Kurenkova, E.; Ievlev, A.V.; Skryabina, N.E.; Fruchart, D.; Miraglia, S.; et al. NMR study of metal-hydrogen systems for hydrogen storage. J. Alloys Compd. 2011, 509, S804–S808. [Google Scholar] [CrossRef]
- Chizhik, V.I.; Kasperovich, V.S.; Shelyapina, M.G.; Chernyshev, Y.S. Exchange model for proton relaxation in disordered metallic hydrides. Int. J. Hydrogen Energy 2011, 36, 1601–1605. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Vyvodtceva, A.V.; Klyukin, K.A.; Bavrina, O.O.; Chernyshev, Y.S.; Privalov, A.F.; Fruchart, D. Hydrogen diffusion in metal-hydrogen systems via NMR and DFT. Int. J. Hydrogen Energy 2015, 40, 17038–17050. [Google Scholar] [CrossRef]
- Chizhik, V.I.; Rykov, I.A.; Shelyapina, M.G.; Fruchart, D. Proton relaxation and hydrogen mobility in Ti-V-Cr alloys: Improved exchange model. Int. J. Hydrogen Energy 2014, 39, 17416–17421. [Google Scholar] [CrossRef]
- Cardona, M.; Chamberlin, R.V.; Marx, W. The history of the stretched exponential function. Ann. Phys. 2007, 16, 842–845. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism I. A gauge-invariant LCAO method for N.M.R. Chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
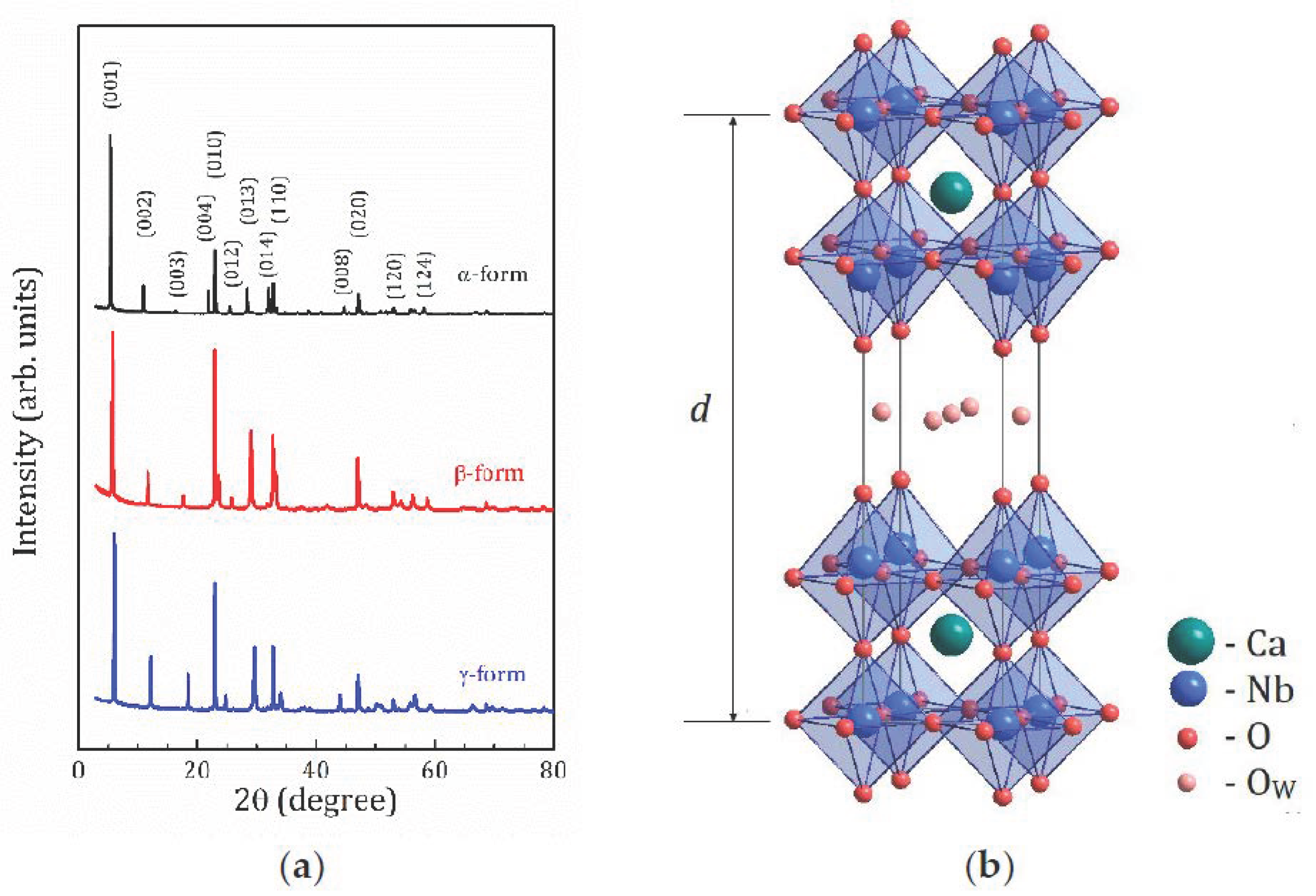
| Parameters | α-Form | β-Form | γ-Form |
|---|---|---|---|
| a (Å) | 3.86517(3) | 3.86550(9) | 3.89267(8) |
| c (Å) | 16.2627(2) | 15.1125(7) | 14.5254(4) |
| V (Å3) | 242.957(6) | 225.812(18) | 220.102(14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelyapina, M.G.; Silyukov, O.I.; Andronova, E.A.; Nefedov, D.Y.; Antonenko, A.O.; Missyul, A.; Kurnosenko, S.A.; Zvereva, I.A. 1H NMR Study of the HCa2Nb3O10 Photocatalyst with Different Hydration Levels. Molecules 2021, 26, 5943. https://doi.org/10.3390/molecules26195943
Shelyapina MG, Silyukov OI, Andronova EA, Nefedov DY, Antonenko AO, Missyul A, Kurnosenko SA, Zvereva IA. 1H NMR Study of the HCa2Nb3O10 Photocatalyst with Different Hydration Levels. Molecules. 2021; 26(19):5943. https://doi.org/10.3390/molecules26195943
Chicago/Turabian StyleShelyapina, Marina G., Oleg I. Silyukov, Elizaveta A. Andronova, Denis Y. Nefedov, Anastasiia O. Antonenko, Alexander Missyul, Sergei A. Kurnosenko, and Irina A. Zvereva. 2021. "1H NMR Study of the HCa2Nb3O10 Photocatalyst with Different Hydration Levels" Molecules 26, no. 19: 5943. https://doi.org/10.3390/molecules26195943
APA StyleShelyapina, M. G., Silyukov, O. I., Andronova, E. A., Nefedov, D. Y., Antonenko, A. O., Missyul, A., Kurnosenko, S. A., & Zvereva, I. A. (2021). 1H NMR Study of the HCa2Nb3O10 Photocatalyst with Different Hydration Levels. Molecules, 26(19), 5943. https://doi.org/10.3390/molecules26195943








