Fluorescein Hydrazide-Appended Metal–Organic Framework as a Chromogenic and Fluorogenic Chemosensor for Mercury Ions
Abstract
:1. Introduction
2. Results and Discussions
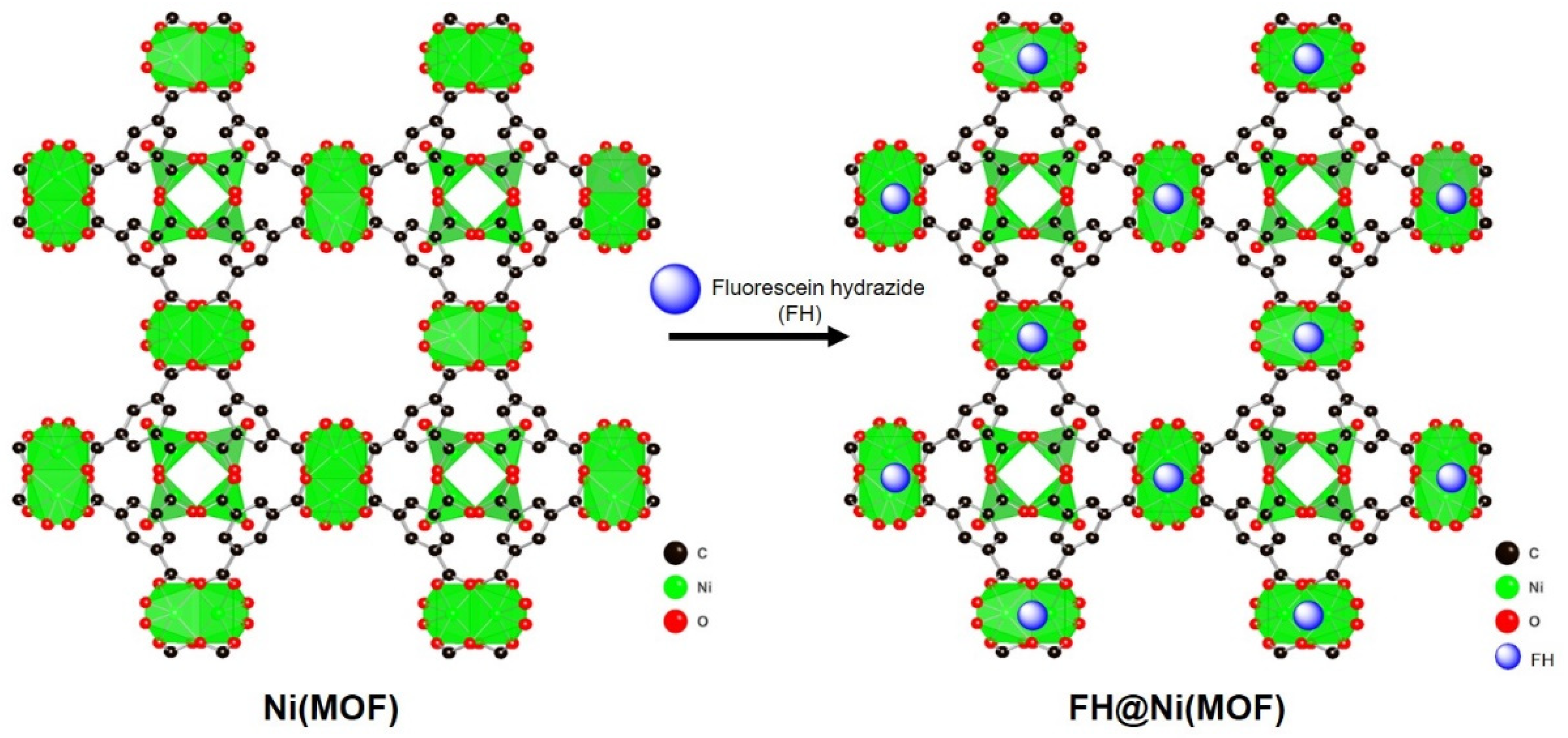
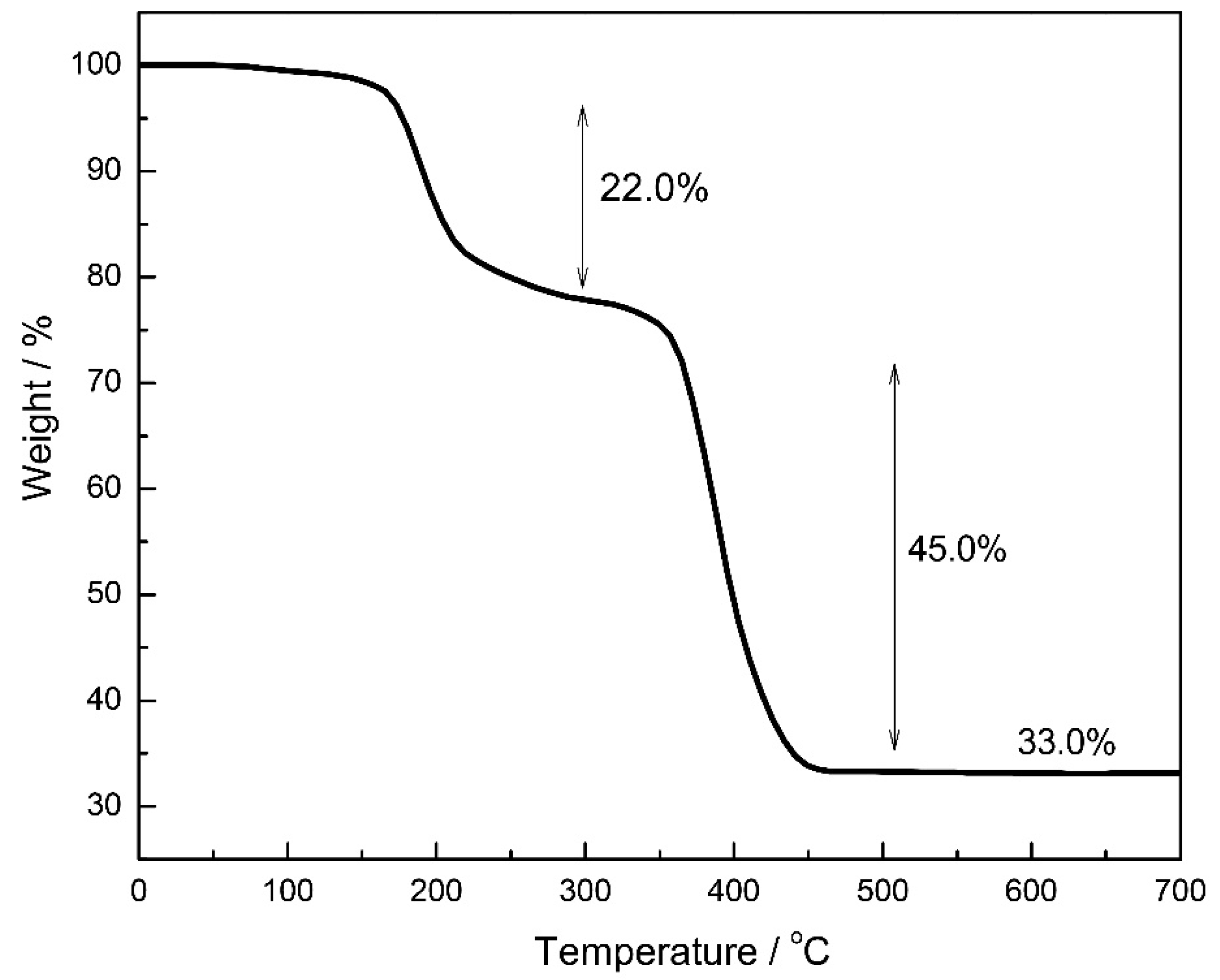
2.1. Synthesis and Characterization of FH@Ni(MOF)
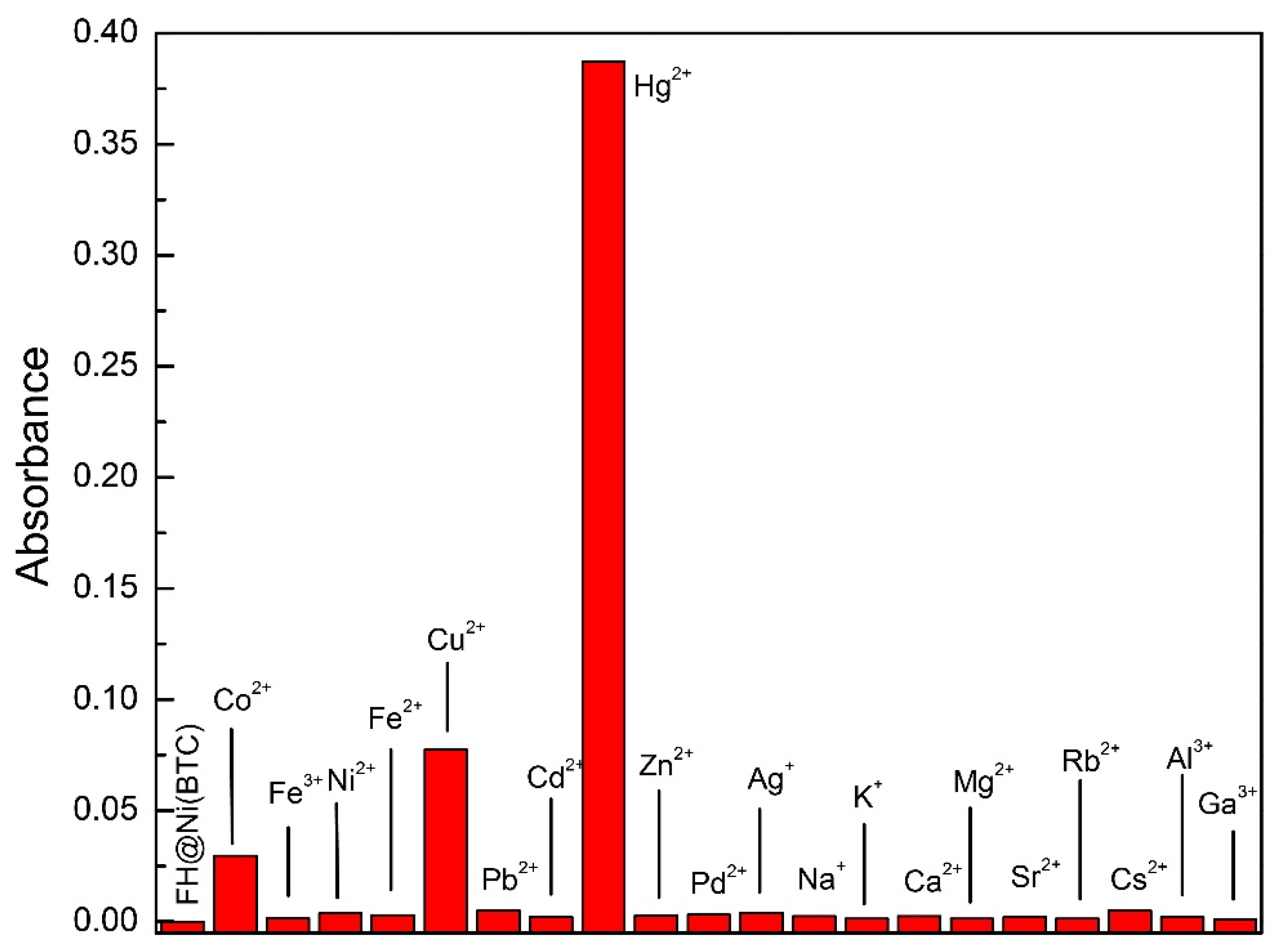
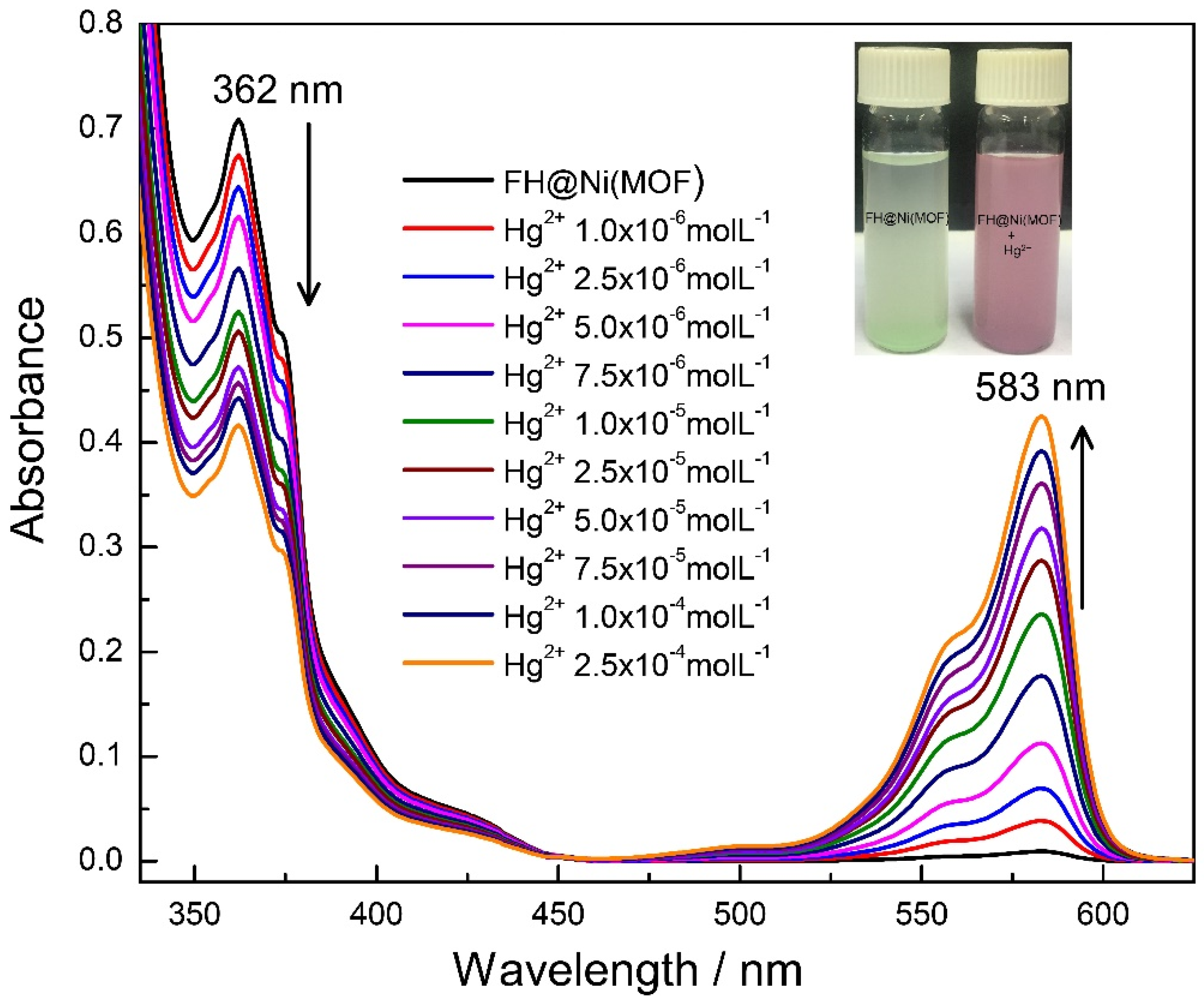
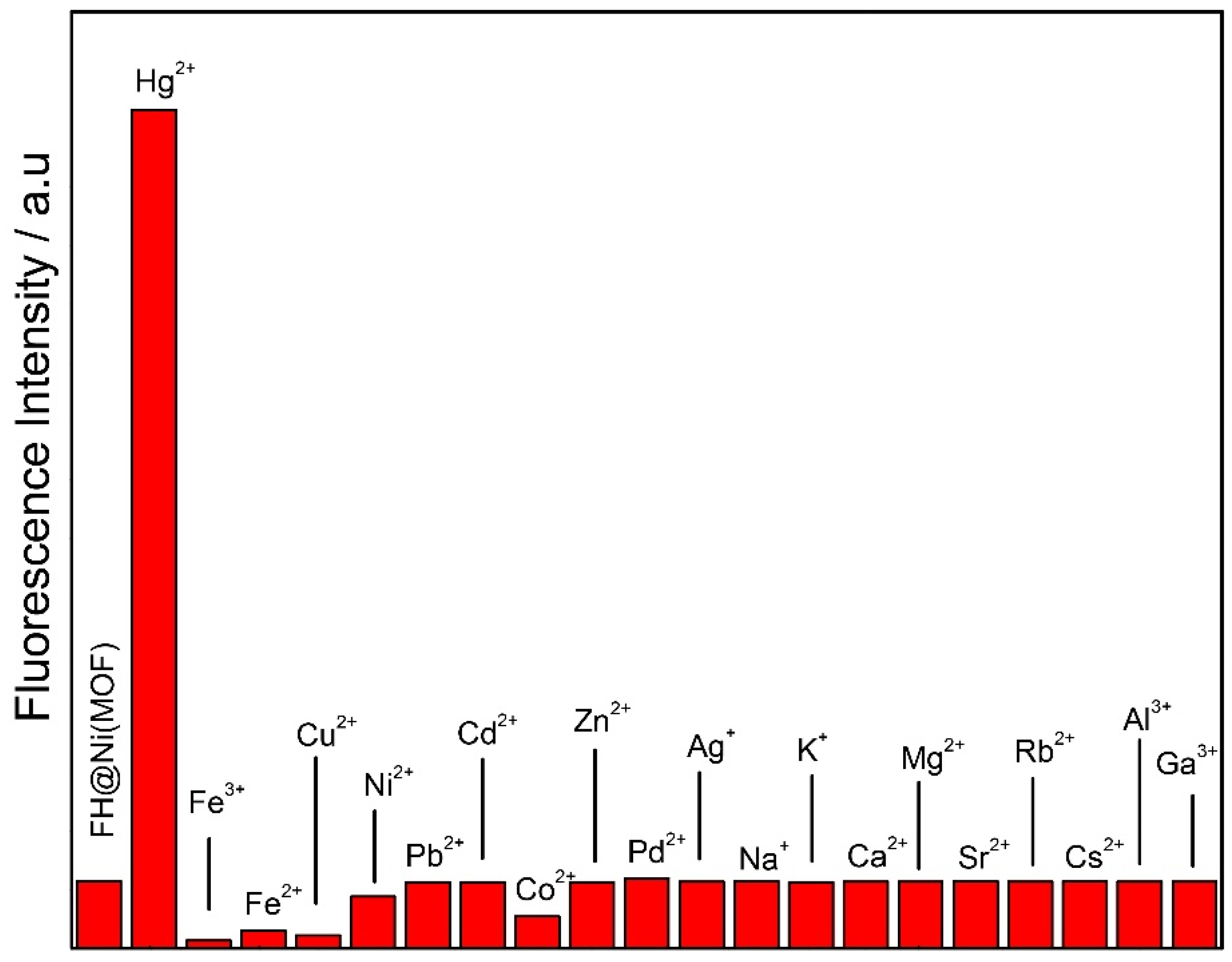
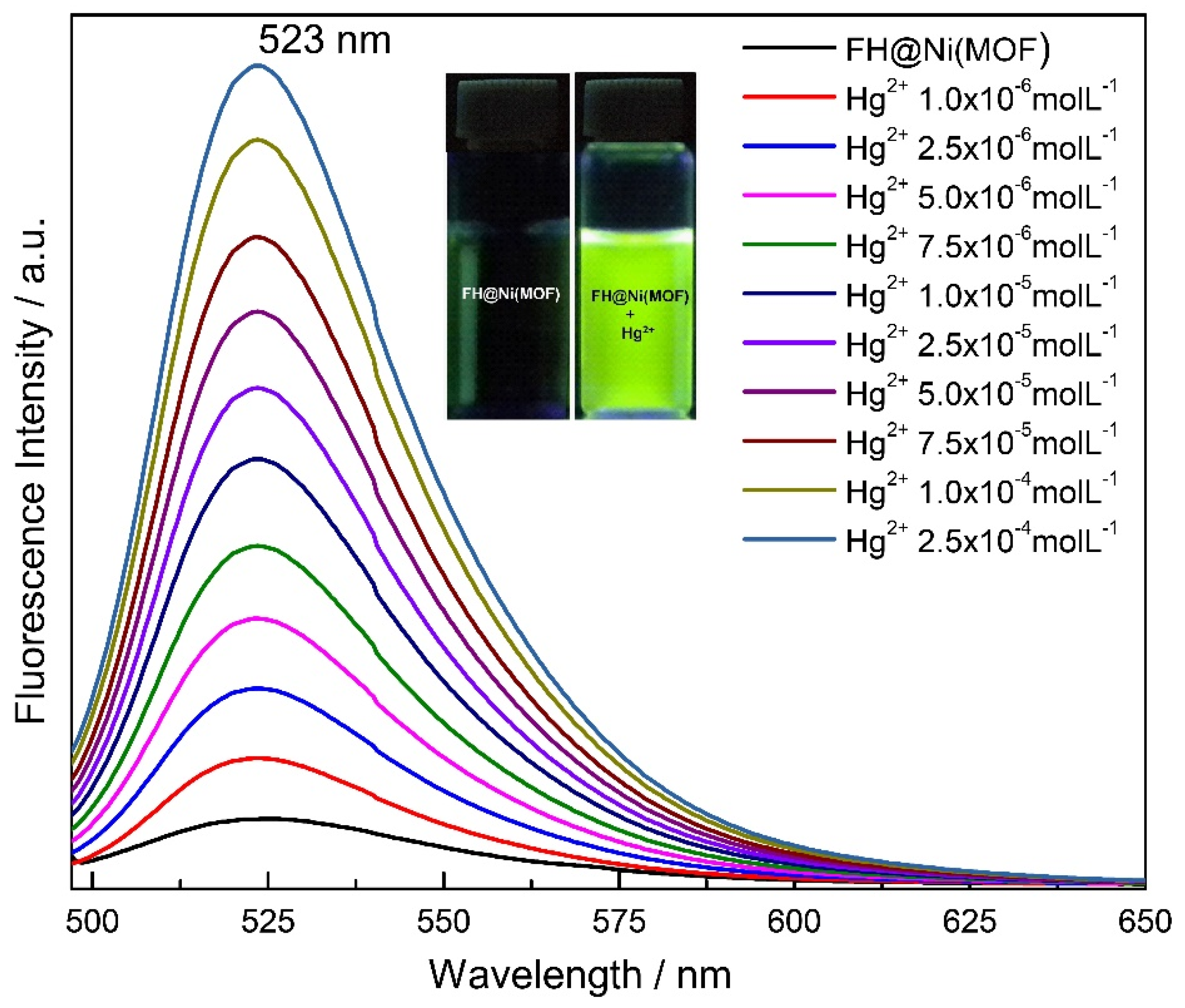
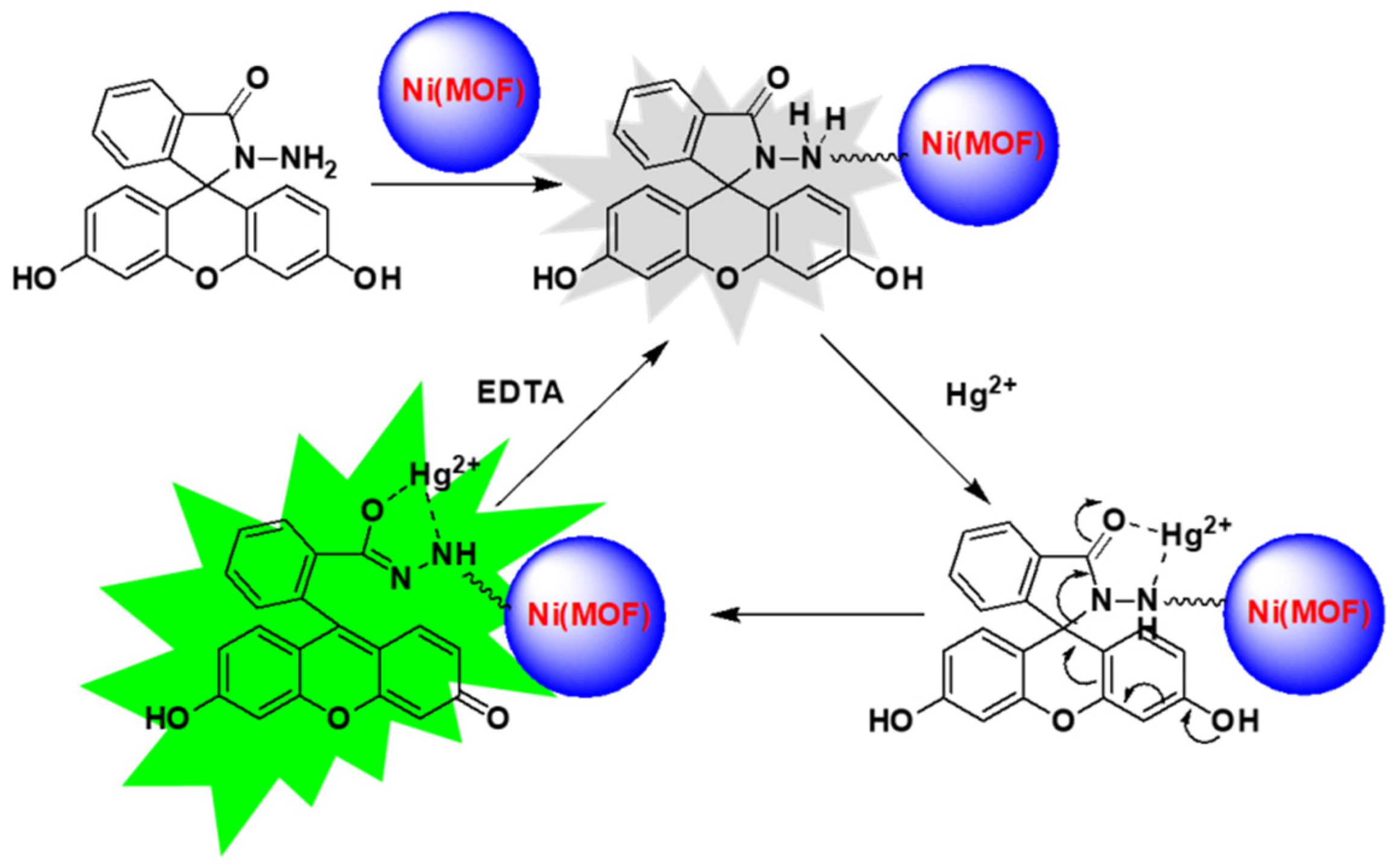
2.2. Cation Sensing Properties of FH@Ni(MOF)
3. Experimental
3.1. Materials and General Methods
3.1.1. Chemicals Used in This Work
3.1.2. Instrumentation
3.1.3. Sample Preparation for Photophysical Studies
3.1.4. Practical Application in Water Samples
3.2. Synthesis
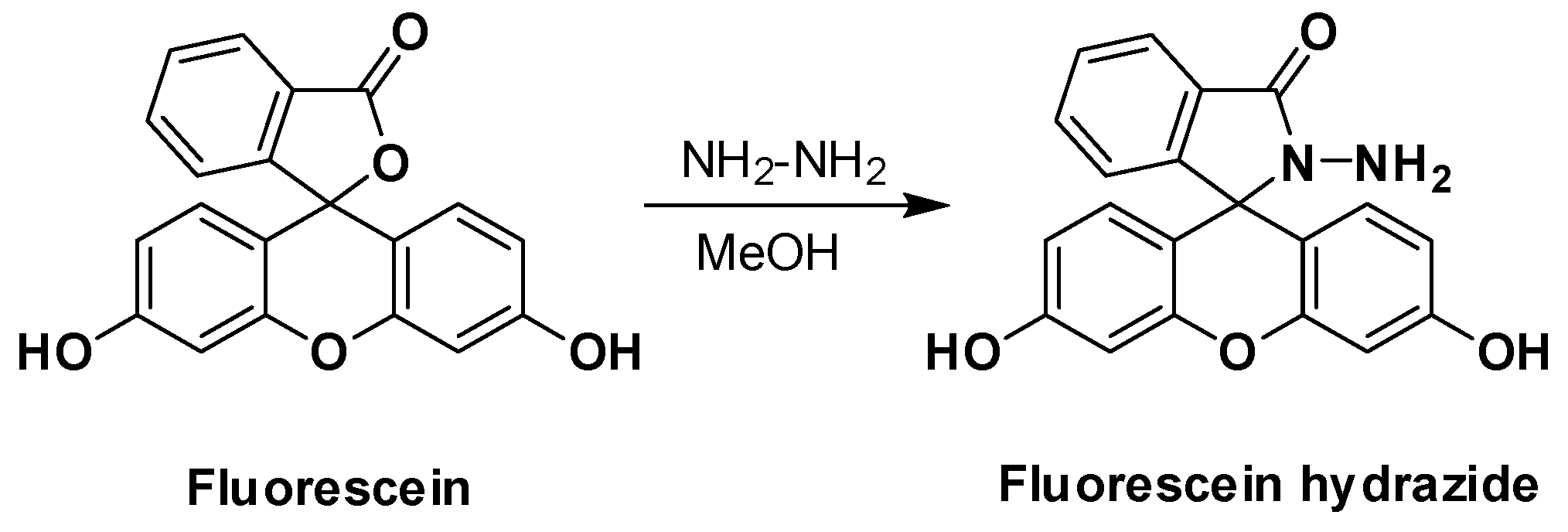
3.2.1. Synthesis of Fluorescein Hydrazide (FH)
3.2.2. Synthesis of Ni(MOF)
3.2.3. Synthesis of FH@Ni(MOF)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Budnik, L.T.; Casteleyn, L. Mercury pollution in modern times and its socio-medical consequences. Sci. Total Environ. 2019, 654, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Denmark, I.S.; Begu, E.; Arslan, Z.; Han, F.X.; Seiter-Moser, J.M.; Pierce, E.M. Removal of inorganic mercury by selective extraction and coprecipitation for determination of methylmercury in mercury-contaminated soils by chemical vapor generation inductively coupled plasma mass spectrometry (CVG-ICP-MS). Anal. Chim. Acta 2018, 1041, 68–77. [Google Scholar] [CrossRef]
- Harris, H.H.; Pickering, I.J.; George, G.N. The chemical form of mercury in fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Review: Environmental exposure to mercury and its toxico pathologic implications for public health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef]
- Fu, L.; Xie, K.; Wang, A.; Lyu, F.; Ge, J.; Zhang, L.; Zhang, H.; Su, W.; Hou, Y.L.; Zhou, C.; et al. High selective detection of mercury (II) ions by thioether side groups on metal-organic frameworks. Anal. Chim. Acta. 2019, 1081, 51–58. [Google Scholar] [CrossRef]
- Letelier, M.E.; Lepe, A.M.; Faundez, M.; Salazar, J.; Marín, R.; Aracena, P.; Speisky, H. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem. Interact. 2005, 151, 71–82. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Silva, I.A.; Nyland, J.F. Mercury and autoimmunity: Implications for occupational and environmental health. Toxicol. Appl. Pharm. 2005, 207, 282–292. [Google Scholar] [CrossRef]
- Hoyle, I.; Handy, R. Dose-dependent inorganic mercury absorption by isolated perfused intestine of rainbow trout, Oncorhynchus mykiss, involves both amiloride-sensitive and energy-dependent pathways. Aquat. Toxicol. 2005, 72, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Fang, S.; Qin, D.; Zhou, J.; Yang, G.; Jin, S.; Duan, H. Two novel fluorescent probes based on phenothiazine: Synthesis and “naked-eye” colorimetric recognition of Hg2+. Res. Chem. Intermed. 2019, 45, 2045–2063. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, C.; Meng, F.-L.; Li, H.-H.; Wang, L.; Liu, J.-H.; Huang, X.-J. SnO2/Reduced Graphene Oxide Nanocomposite for the Simultaneous Electrochemical Detection of Cadmium(II), Lead(II), Copper(II), and Mercury(II): An Interesting Favorable Mutual Interference. J. Phys. Chem. C 2012, 116, 1034–1041. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Jin, X. Determination of ultra-trace amount methyl-, phenyl- and inorganic mercury in environmental and biological samples by liquid chromatography with inductively coupled plasma mass spectrometry after cloud point extraction preconcentration. Talanta 2009, 77, 1381–1387. [Google Scholar] [CrossRef]
- de Wuilloud, J.C.; Wuilloud, R.; Silva, M.F.; A Olsina, R.; Martinez, L.D. Sensitive determination of mercury in tap water by cloud point extraction pre-concentration and flow injection-cold vapor-inductively coupled plasma optical emission spectrometry. Spectrochim. Acta Part. B At. Spectrosc. 2002, 57, 365–374. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Z.; Zeng, G.; Tang, L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst 2015, 140, 5400. [Google Scholar] [CrossRef]
- Bhowmick, R.; Alam, R.; Mistri, T.; Bhattacharya, D.; Karmakar, P.; Ali, M. Morphology-Directing Synthesis of Rhodamine-Based Fluorophore Microstructures and Application toward Extra- and Intracellular Detection of Hg2+. ACS Appl. Mater. Interfaces 2015, 7, 7476–7485. [Google Scholar] [CrossRef] [PubMed]
- Ratner, N.; Mandler, D. Electrochemical Detection of Low Concentrations of Mercury in Water Using Gold Nanoparticles. Anal. Chem. 2015, 87, 5148. [Google Scholar] [CrossRef]
- Qi, G.; Fu, C.; Chen, G.; Xu, S.; Xu, W. Highly sensitive SERS sensor for mercury ions based on the catalytic reaction of mercury ion decorated Ag nanoparticles. RSC Adv. 2015, 5, 49759–49764. [Google Scholar] [CrossRef]
- Ma, C.; Zeng, F.; Huang, L.; Wu, S. FRET-Based Ratiometric Detection System for Mercury Ions in Water with Polymeric Particles as Scaffolds. J. Phys. Chem. B 2011, 115, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Z.; Yang, L.; Tian, S.; Hou, C.; Lu, Y. Lysozyme-stabilized gold fluorescent cluster: Synthesis and application as Hg2+ sensor. Analyst 2010, 135, 1406–1410. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, H.; Yoshimura, K.; Hamaguchi, K.; Arakawa, R. Trypsin-Stabilized Fluorescent Gold Nanocluster for Sensitive and Selective Hg2+ Detection. Anal. Sci. 2011, 27, 591. [Google Scholar] [CrossRef] [Green Version]
- Dave, N.; Chan, M.Y.; Huang, P.-J.J.; Smith, B.D.; Liu, J. Regenerable DNA-Functionalized Hydrogels for Ultrasensitive, Instrument-Free Mercury(II) Detection and Removal in Water. J. Am. Chem. Soc. 2010, 132, 12668–12673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, D.; He, X.; Wang, K.; Zhao, Y.; Zou, Z. Regenerable Multifunctional Mesoporous Silica Nanocomposites for Simultaneous Detection and Removal of Mercury(II). Langmuir 2013, 29, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Wu, L.; Hu, G.; Cui, Y. SERS Detection and Removal of Mercury(II)/Silver(I) using Oligonucleotide-Functionalized Core/Shell Magnetic Silica Sphere@Au Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 7371. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; García, H.; Llabrés, F.; Xamen, X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Ouyang, S.; Ye, J. Metal-organic frameworks for photocatalysis. Phys. Chem. Chem. Phys. 2016, 18, 7563–7572. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Férey, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal–Organic Frameworks for Biological and Medical Applications. Angew. Chem. Int. Ed. 2010, 49, 6260–6266. [Google Scholar] [CrossRef]
- Morozan, A.; Jaouen, F. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.G.; Lu, X.M.; Yang, C.D.; Fan, N.N.; Yang, Z.T.; Wang, L.Y.; Ma, L.F. {Zn6} Cluster Based Metal–Organic Framework with Enhanced Room-Temperature Phosphorescence and Optoelectronic Performances. Inorg. Chem. 2019, 58, 6215–6221. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Fan, N.-N.; Han, M.-L.; Yang, G.-P.; Ma, L. Porous Zn(II)-Based Metal–Organic Frameworks Decorated with Carboxylate Groups Exhibiting High Gas Adsorption and Separation of Organic Dyes. Cryst. Growth Des. 2018, 18, 7114–7121. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.-Y.; Cheng, C.; Shao, Z.-S.; Wang, H.-S. Strategies to fabricate metal–organic framework (MOF)-based luminescent sensing platforms. J. Mater. Chem. C 2019, 7, 10743–10763. [Google Scholar] [CrossRef]
- Au, V.K.M. Recent Advances in the Use of Metal-Organic Frameworks for Dye Adsorption. Front. Chem. 2020, 8, 708. [Google Scholar] [CrossRef]
- Yang, L.-Z.; Wang, J.; Kirillov, A.; Dou, W.; Xu, C.; Fang, R.; Xu, C.-L.; Liu, W.-S. 2D lanthanide MOFs driven by a rigid 3,5-bis(3-carboxy-phenyl)pyridine building block: Solvothermal syntheses, structural features, and photoluminescence and sensing properties. CrystEngComm 2016, 18, 6425–6436. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, J.; Kirillov, A.M.; Zhang, Y.; Hu, M.; Liu, W.; Yang, L.; Fang, R.; Liu, W. Construction of a Two-Dimensional Composite Derived from TiO2 and SnS2 for Enhanced Photocatalytic Reduction of CO2 into CH4. ACS Sustain. Chem. Eng. 2019, 7, 3203–3212. [Google Scholar] [CrossRef]
- Helal, A.; Qamaruddin, M.; Aziz, M.A.; Shaikh, M.N.; Yamani, Z.H. MB-UiO-66-NH2 Metal-Organic Framework as Chromogenic and Fluorogenic Sensor for Hydrazine Hydrate in Aqueous Solution. ChemistrySelect 2017, 2, 7630–7636. [Google Scholar] [CrossRef]
- Mondal, T.; Bose, S.; Husain, A.; Ghorai, U.K.; Saha, S.K. White light emission from single dye incorporated metal organic framework. Opt. Mater. 2020, 100, 109706. [Google Scholar] [CrossRef]
- Wang, C.; Tian, L.; Zhu, W.; Wang, S.; Wang, P.; Liang, Y.; Zhang, W.; Zhao, H.; Li, G. Dye@bio-MOF-1 Composite as a Dual-Emitting Platform for Enhanced Detection of a Wide Range of Explosive Molecules. ACS Appl. Mater. Interfaces 2017, 9, 20076–20085. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, D.; Zhao, J.; Xia, Z. Fabrication of a dual-emitting dye-encapsulated metal–organic framework as a sSection fluorescent sensor for metal ion detection. Dalton Trans. 2019, 48, 6794–6799. [Google Scholar] [CrossRef]
- Diet, D.; Hintz, H.; Rühle, B.; Günne, J.S.; Langhals, H.; Wuttke., S. Switch-On Fluorescence of a Perylene-Dye-Functionalized Metal–Organic Framework through Postsynthetic Modification. Chem. Eur. J. 2015, 21, 10714–10720. [Google Scholar]
- Zhang, T.; Guo, X.; Shi, Y.; He, C.; Duan, C. Dye-incorporated coordination polymers for direct photocatalytic trifluoromethylation of aromatics at metabolically susceptible positions. Nat. Commun. 2018, 9, 4024. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Cui, Y.; Xu, Y.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Confinement of pyridinium hemicyanine dye within an anionic metal-organic framework for two-photon-pumped lasing. Nat. Commun. 2013, 4, 2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chueh, C.-C.; Chen, C.-I.; Su, Y.-A.; Konnerth, H.; Gu, Y.-J.; Kung, C.-W.; Wu, K.C.-W. Harnessing MOF materials in photovoltaic devices: Recent advances, challenges, and perspectives. J. Mater. Chem. A 2019, 7, 17079–17095. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, M.; Sun, W.; Wang, M.; Liu, S.; Li, Q. Recent Progress on Synthesis of Fluorescein Probes. Mini-Rev. Org. Chem. 2009, 6, 35–43. [Google Scholar] [CrossRef]
- Oliveira, E.; Bértolo, E.; Núñez, C.; Pilla, V.; Santos, H.M.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Djafari, J.; Capelo, J.L.; Lodeiro, C. Green and Red Fluorescent Dyes for Translational Applications in Imaging and Sensing Analytes: A Dual-Color Flag. ChemistryOpen 2018, 7, 9–52. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Fettouhi, M.; Arafat, E.; Khan, M.Y.; Sanhoob, M.A. Nickel based metal-organic framework as catalyst for chemical fixation of CO2 in oxazolidinone synthesis. J. CO2 Util. 2021, 50, 101603. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Le Bris, M.-T.; Guett, J.-P.; Valeur, B. Ion-responsive fluorescent compounds. 1. Effect of cation binding on photophysical properties of benzoxazinone derivative linked to monoaza-15-crown-5. J. Phys. Chem. 1988, 92, 6233–6237. [Google Scholar] [CrossRef]
- Lv, R.; Wang, J.; Zhang, Y.; Li, H.; Yang, L.; Liao, S.; Gu, W.; Liu, X. An amino-decorated dual-functional metal–organic framework for highly selective sensing of Cr (III) and Cr (VI) ions and detection of nitroaromatic explosives. J. Mater. Chem. A 2016, 4, 15494–15500. [Google Scholar] [CrossRef]
- Roditi, E.; Tsetsoni, M.; Kokkinos, C.; Economou, A. Integrated on-chip sensor with sputtered Ag-Au-Au electrodes for the voltammetric determination of trace Hg(II). Sens. Actuators B Chem. 2019, 286, 125–130. [Google Scholar] [CrossRef]
- Lin, W.C.; Hu, J.W.; Chen, K.Y. A ratiometric chemodosimeter for highly selective naked-eye and fluorogenic detection of cyanide. Anal. Chim. Acta 2015, 893, 91–100. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, Y.; Duan, H. Four xanthene–fluorene based probes for the detection of Hg2+ ions and their application in strip tests and biological cells. N. J. Chem. 2021, 45, 685–695. [Google Scholar] [CrossRef]
- Saha, S.; Das, S.; Das, S.; Samanta, A.; Maitra, S.; Sahoo, P. Prompt detection of endogenous hypochlorite (ClO−) in murine macrophages and zebrafish embryos facilitated by a distinctive chemodosimetric mode. Org. Biomol. Chem. 2020, 18, 6716–6723. [Google Scholar] [CrossRef] [PubMed]
- Christus, A.A.B.; Panneerselvam, P.; Ravikumar, A.; Marieeswaran, M.; Sivanesan, S. MoS2 nanosheet mediated ZnO–g-C3N4 nanocomposite as a peroxidase mimic: Catalytic activity and application in the colorimetric determination of Hg(II). RSC Adv. 2019, 9, 4268. [Google Scholar] [CrossRef] [Green Version]


| Sample | Hg2+ Ion (M) Spiked | Hg2+ Ion (M) Detected | Recovery (%) |
|---|---|---|---|
| Tap Water | 10 | 10.09 | 100.9 |
| 15 | 14.69 | 97.93 | |
| Drinking Water | 10 | 10.1 | 101.0 |
| 15 | 14.58 | 97.2 | |
| Groundwater | 10 | 9.71 | 97.1 |
| 15 | 14.71 | 98.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helal, A.; Naeem, M.; Fettouhi, M.; Zahir, M.H. Fluorescein Hydrazide-Appended Metal–Organic Framework as a Chromogenic and Fluorogenic Chemosensor for Mercury Ions. Molecules 2021, 26, 5773. https://doi.org/10.3390/molecules26195773
Helal A, Naeem M, Fettouhi M, Zahir MH. Fluorescein Hydrazide-Appended Metal–Organic Framework as a Chromogenic and Fluorogenic Chemosensor for Mercury Ions. Molecules. 2021; 26(19):5773. https://doi.org/10.3390/molecules26195773
Chicago/Turabian StyleHelal, Aasif, Muhammed Naeem, Mohammed Fettouhi, and Md. Hasan Zahir. 2021. "Fluorescein Hydrazide-Appended Metal–Organic Framework as a Chromogenic and Fluorogenic Chemosensor for Mercury Ions" Molecules 26, no. 19: 5773. https://doi.org/10.3390/molecules26195773
APA StyleHelal, A., Naeem, M., Fettouhi, M., & Zahir, M. H. (2021). Fluorescein Hydrazide-Appended Metal–Organic Framework as a Chromogenic and Fluorogenic Chemosensor for Mercury Ions. Molecules, 26(19), 5773. https://doi.org/10.3390/molecules26195773







