Gram-Scale Synthesis of an Ultrastable Microporous Metal-Organic Framework for Efficient Adsorptive Separation of C2H2/CO2 and C2H2/CH4
Abstract
:1. Introduction
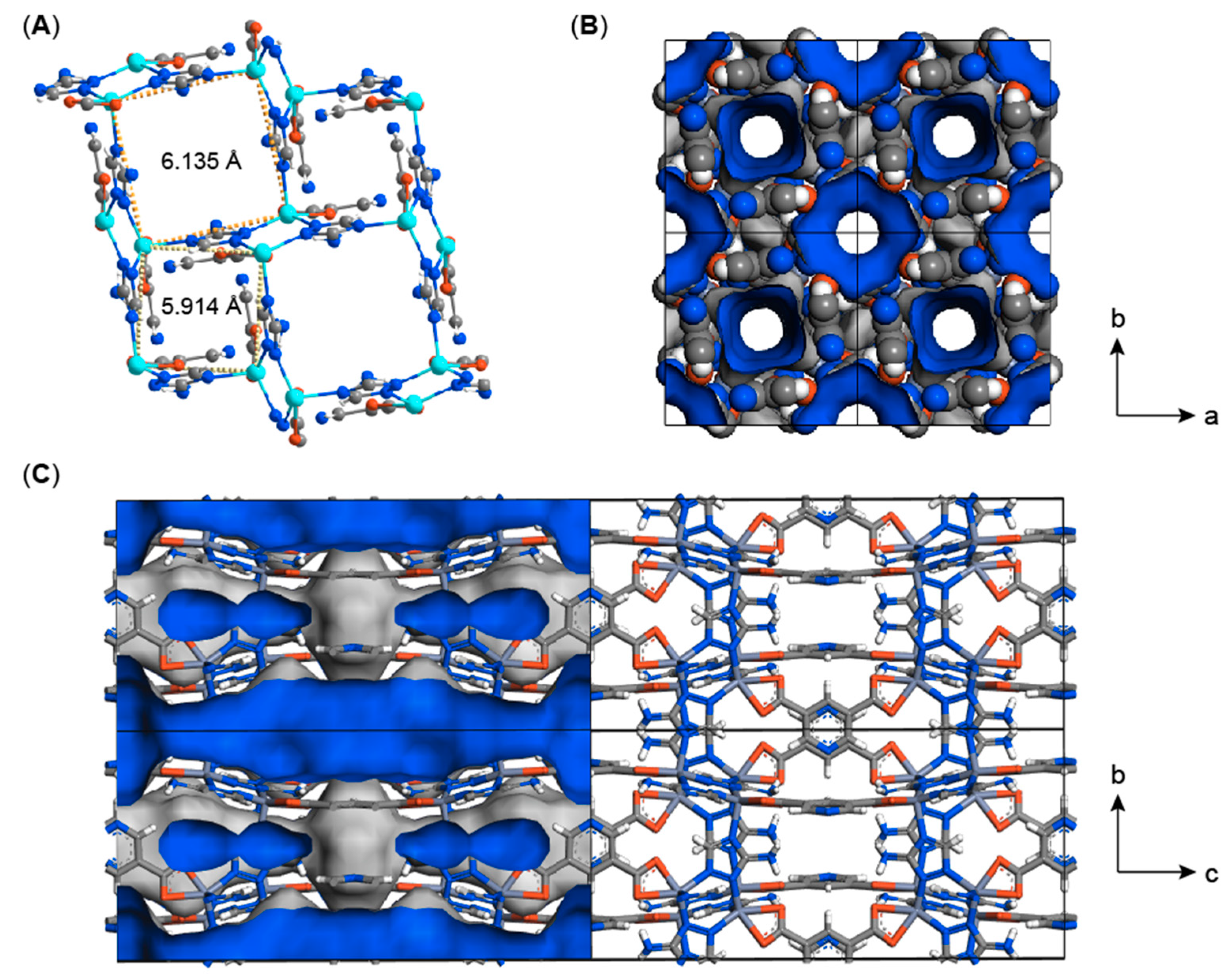
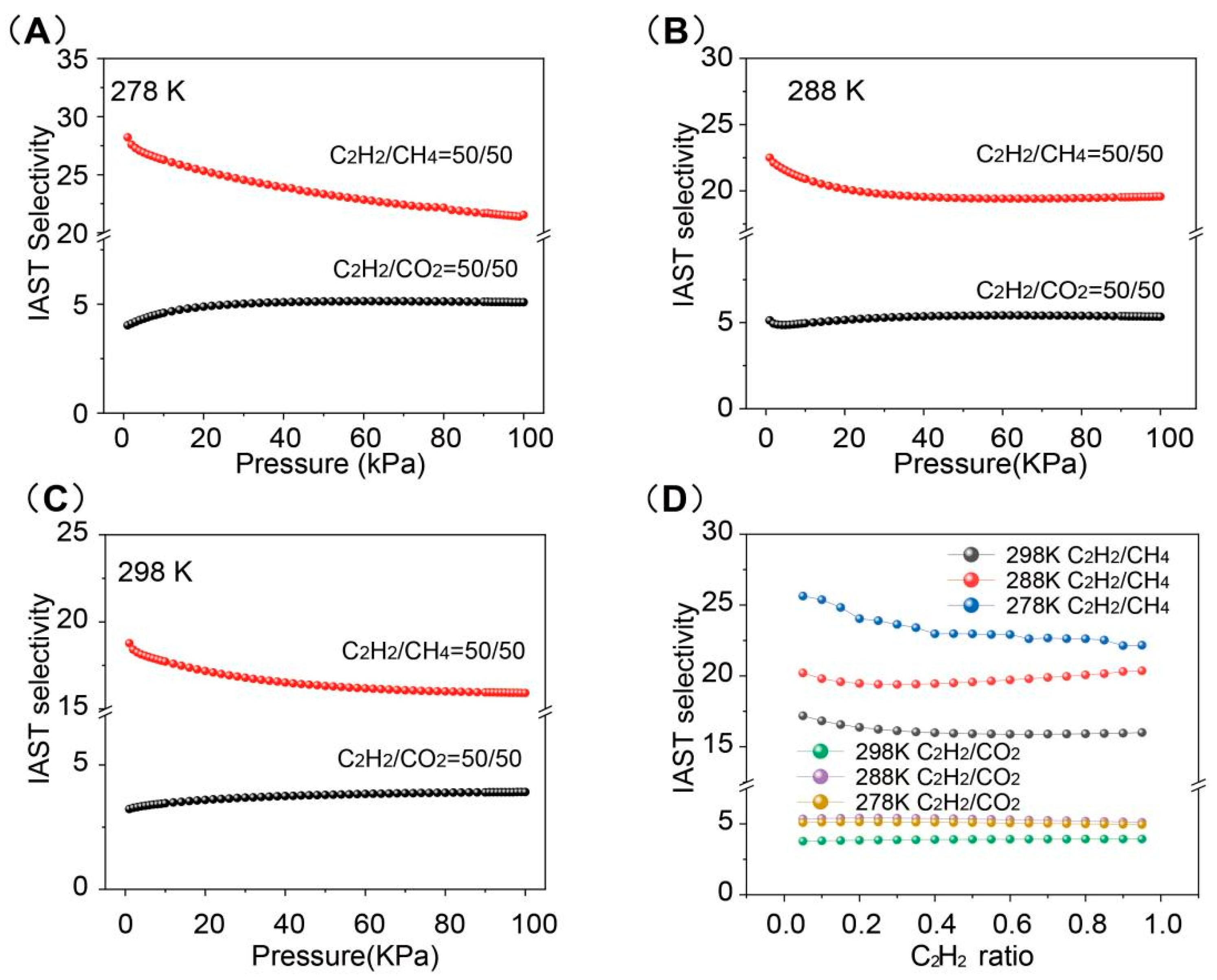
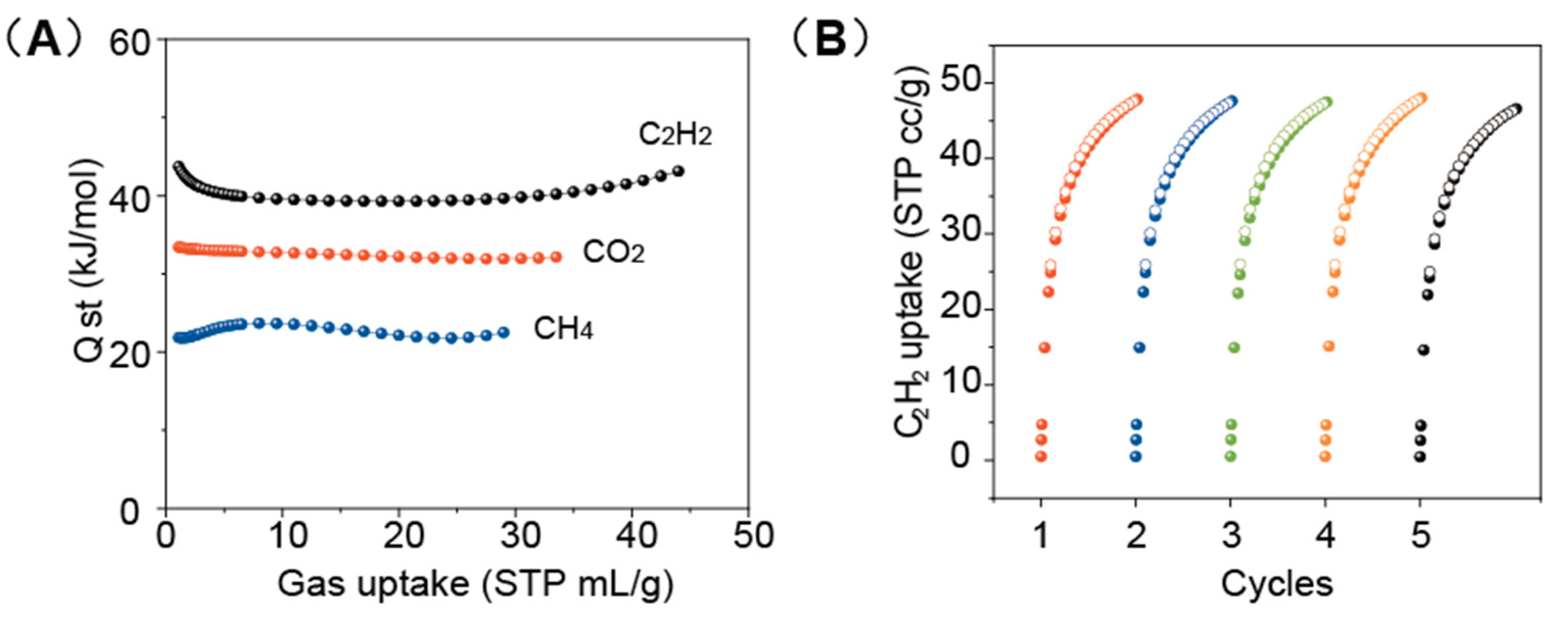
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Zn2(Pydc)(Ata)2 1
3.3. Characterization
3.4. Adsorption Measurements
3.5. Calculation for Adsorption Selectivity and Isosteric Heat of Adsorption
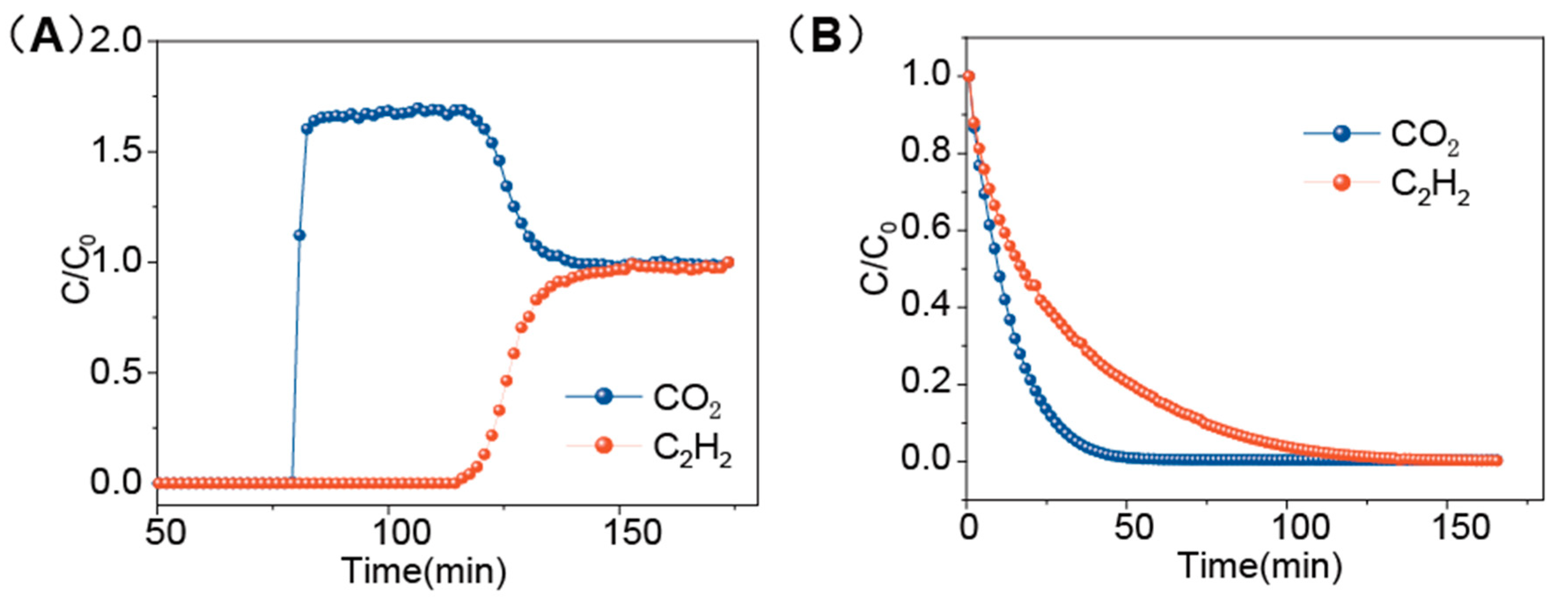
3.6. Breakthrough Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stang, P.J.; Diederich, F. Modern Acetylene Chemistry; WileyVCH: Weinheim, Germany, 2008. [Google Scholar]
- Alduhaish, O.; Wang, H.; Li, B.; Hu, T.-L.; Arman, H.D.; Alfooty, K.; Chen, B. A Twofold Interpenetrated Metal–Organic Framework with High Performance in Selective Separation of C2H2/CH4. ChemPlusChem 2016, 81, 770–774. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, K.; Li, Y.; Zhao, D.; Yang, Y.; Cui, Y.; Chen, B.; Qian, G. Microporous Metal−Organic Framework with Exposed Amino Functional Group for High Acetylene Storage and Excellent C2H2/CO2 and C2H2/CH4 Separations. Cryst. Growth Des. 2017, 17, 2319–2322. [Google Scholar] [CrossRef]
- Hou, X.-J.; He, P.; Li, H.; Wang, X. Understanding the Adsorption Mechanism of C2H2, CO2, and CH4 in Isostructural Metal−Organic Frameworks with Coordinatively Unsaturated Metal Sites. J. Phys. Chem. C 2013, 117, 2824–2834. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–438. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Krishna, R.; Wang, L.; Yang, L.; Cui, X.; Duttwyler, S.; Xing, H. Rational Design of Microporous MOFs with Anionic Boron Cluster Functionality and Cooperative Dihydrogen Binding Sites for Highly Selective Capture of Acetylene. Angew. Chem. Int. Ed. 2020, 59, 17664–17669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Li, D.S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Chang, G.; Xing, H.; Krishna, R.; Ren, Q.; Chen, B. Potential of microporous metal–organic frameworks for separation of hydrocarbon mixtures. Energy Environ. Sci. 2016, 9, 3612–3641. [Google Scholar] [CrossRef]
- Liao, P.-Q.; Huang, N.-Y.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Controlling guest conformation for efficient purification of butadiene. Science 2017, 356, 1193–1196. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-P.; Wang, Y.; Xue, Y.-Y.; Li, H.-P.; Zhai, Q.-G.; Li, S.-N.; Jiang, Y.-C.; Hu, M.-C.; Bu, X. Ultramicroporous Building Units as a Path to Bi-microporous Metal-Organic Frameworks with High Acetylene Storage and Separation Performance. Angew. Chem. Int. Ed. 2019, 58, 13590–13595. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, P.; Xu, T.; Fan, L.; Wang, X.; Yue, L.; Jiang, Z.; Zhang, Y.; Zhang, Z.; He, Y. Modulation of Topological Structures and Adsorption Properties of Copper-Tricarboxylate Frameworks Enabled by the Effect of the Functional Group and Its Position. Inorg. Chem. 2021, 60, 8111–8122. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, X.; Liu, X.; Zhang, L.; Liu, Y. Two urea-functionalized pcu metal-organic frameworks based on a pillared-layer strategy for gas adsorption and separation. Inorg. Chem. Front. 2020, 7, 3500–3508. [Google Scholar] [CrossRef]
- Hua, G.-F.; Xie, X.-J.; Lu, W.-G.; Li, D. Optimizing supramolecular interactions in metal-organic frameworks for C2 separation. Dalton Trans. 2020, 49, 15548–15559. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, S.; Hu, Z.; Kundu, T.; Zhang, J.; Peh, S.B.; Cheng, Y.; Dong, J.; Yuan, D.; Zhou, H.-C.; et al. Pore Size Reduction in Zirconium Metal−Organic Frameworks for Ethylene/Ethane Separation. ACS Sustain. Chem. Eng. 2019, 7, 7118–7126. [Google Scholar] [CrossRef]
- Peng, Y.-L.; Pham, T.; Li, P.; Wang, T.; Chen, Y.; Chen, K.-J.; Forrest, K.A.; Space, B.; Cheng, P.; Zaworotko, M.J.; et al. Robust Ultramicroporous Metal-Organic Frameworks with Benchmark Affinity for Acetylene. Angew. Chem. Int. Ed. 2018, 57, 10971–10975. [Google Scholar] [CrossRef]
- Jiang, Z.; Zou, Y.; Xu, T.; Fan, L.; Zhou, P.; He, Y. A Hydrostable Cage-Based MOF with Open Metal Sites and Lewis Basic Sites Immobilized in Pore Surface for Efficient Separation and Purification of Natural Gas and C2H2. Dalton Trans. 2020, 49, 3553–3561. [Google Scholar] [CrossRef]
- Xu, T.; Fan, L.; Jiang, Z.; Zhou, P.; Li, Z.; Lu, H.; He, Y. Immobilization of N-Oxide Functionality into Nbo-Type Mofs for Significantly Enhanced C2H2/CH4 and CO2/CH4 Separations. Dalton Trans. 2020, 49, 7174–7181. [Google Scholar] [CrossRef]
- Li, N.; Chang, Z.; Huang, H.; Feng, R.; He, W.-W.; Zhong, M.; Madden, D.G.; Zaworotko, M.-J.; Bu, X.-H. Specific K+ Binding Sites as CO2 Traps in a Porous MOF for Enhanced CO2 Selective Sorption. Small 2019, 15, 1900426. [Google Scholar] [CrossRef]
- Kumar, A.; Madden, D.G.; Lusi, M.; Chen, K.-J.; Daniels, E.A.; Curtin, T.; Perry, J.J., IV; Zaworotko, M.J. Direct Air Capture of CO2 by Physisorbent Materials. Angew. Chem. Int. Ed. 2015, 54, 14372–14377. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Duan, J.; Bai, J. Constructing and finely tuning the CO2 traps of stable and various-pore-containing MOFs towards highly selective CO2 capture. Chem. Commun. 2019, 55, 3477–3480. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Zhang, Y.-Z.; Yan, H.; Zhang, W.-J.; Li, Y.-W.; Wang, S.-N.; Li, D.-C.; Dou, J.-M.; Li, J.-R. Two microporous CoII-MOFs with dual active sites for highly selective adsorption of CO2/CH4 and CO2/N2. Dalton Trans. 2019, 48, 13541–13545. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Xiao, Q.; Lv, D.; Li, F.; Li, Z.; Xia, Q. Rapid room temperature conversion of hydroxy double salt to MOF-505 for CO2 capture. Cryst. Eng. Comm. 2019, 21, 165–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Xing, H. Recent advances in the capture and abatement of toxic gases and vapors by metal-organic frameworks. Mater. Chem. Front. 2021, 5, 5970–6013. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Liu, Y.; Hupp, J.T.; Farha, O.K. Instantaneous Hydrolysis of Nerve-Agent Simulants with a Six-Connected Zirconium-Based Metal-Organic Framework. Angew. Chem. Int. Ed. 2015, 54, 6795–6799. [Google Scholar] [CrossRef] [PubMed]
- Sabyrov, K.; Jiang, J.; Yaghi, O.M.; Somorjai, G.A. Hydroisomerization of n-Hexane Using Acidified Metal−Organic Framework and Platinum Nanoparticles. J. Am. Chem. Soc. 2017, 139, 12382–12385. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhang, W.; Lan, P.C.; Aguila, B.; Ma, S. Promoting Frustrated Lewis Pairs for Heterogeneous Chemoselective Hydrogenation via the Tailored Pore Environment within Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 7420–7424. [Google Scholar] [CrossRef]
- Yue, D.; Zhao, D.; Zhang, J.; Zhang, L.; Jiang, K.; Zhang, X.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. A luminescent cerium metal-organic framework for the turn-on sensing of ascorbic acid. Chem. Commun. 2017, 53, 11221–11224. [Google Scholar] [CrossRef]
- Han, X.; Gu, C.; Ding, Y.; Yu, J.; Li, K.; Zhao, D.; Chen, B. Stable Eu3+/Cu2+-Functionalized Supramolecular Zinc(II) Complexes as Fluorescent Probes for Turn-On and Ratiometric Detection of Hydrogen Sulfide. ACS Appl. Mater. Interfaces 2021, 13, 20371–20379. [Google Scholar] [CrossRef]
- Jiang, J.; Furukawa, H.; Zhang, Y.-B.; Yaghi, O.M. High Methane Storage Working Capacity in Metal−Organic Frameworks with Acrylate Links. J. Am. Chem. Soc. 2016, 138, 10244–10251. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Ye, W.Q.; Yang, Y.; Zhong, Y.; Hu, Y. Zn-ion hybrid supercapacitors: Achievements, challenges and future perspectives. Nano Energy 2021, 85, 105942. [Google Scholar] [CrossRef]
- Hanikel, N.; Prevot, M.S.; Yaghi, O.M. MOF water harvesters. Nat. Nanotechnol. 2020, 15, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Shao, K.; Wang, J.-X.; Wen, H.-M.; Yang, Y.; Cui, Y.; Krishna, R.; Li, B.; Qian, G. A Chemically Stable Hofmann-Type Metal−Organic Framework with Sandwich-Like Binding Sites for Benchmark Acetylene Capture. Adv. Mater. 2020, 32, 1908275. [Google Scholar] [CrossRef]
- Niu, Z.; Cui, X.; Pham, T.; Verma, G.; Lan, P.C.; Shan, C.; Xing, H.; Forrest, K.A.; Suepaul, S.; Space, B.; et al. A MOF-based Ultra-Strong Acetylene Nano-trap for Highly Efficient C2H2/CO2 Separation. Angew. Chem. Int. Ed. 2021, 60, 5283–5288. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, T.; Duttwyler, S.; Zhang, Y. Supramolecular Cu(II)-dipyridyl frameworks featuring weakly coordinating dodecaborate dianions for selective gas separation. Cryst. Eng. Comm. 2021, 23, 282–291. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Duttwyler, S.; Zhang, Y. Efficient adsorption separation of methane from CO2 and C2–C3 hydrocarbons in a microporous closo-dodecaborate [B12H12]2− pillared metal-organic framework. J. Solid State Chem. 2021, 299, 122167. [Google Scholar] [CrossRef]
- Yang, L.; Cui, X.; Zhang, Y.; Wang, Q.; Zhang, Z.; Suo, X.; Xing, H. Anion Pillared Metal−Organic Framework Embedded with Molecular Rotors for Size-Selective Capture of CO2 from CH4 and N2. ACS Sustain. Chem. Eng. 2019, 7, 3138–3144. [Google Scholar] [CrossRef]
- Lin, R.-B.; Li, L.; Zhou, H.-L.; Wu, H.; He, C.; Li, S.; Krishna, R.; Li, J.; Zhou, W.; Chen, B. Molecular sieving of ethylene from ethane using a rigid metal-organic framework. Nat. Mater. 2018, 17, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Qu, Y.; Zhang, X.; Ma, H.; Xu, P.; Sun, J. A novel water-stable MOF Zn(Py)(Atz) as heterogeneous catalyst for chemical conversion of CO2 with various epoxides under mild conditions. J. CO2 Util. 2020, 35, 216–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Wang, L.; Duttwyler, S.; Xing, H. A Microporous Metal-Organic Framework Supramolecularly Assembled from a Cu(II) Dodecaborate Cluster Complex for Selective Gas Separation. Angew. Chem. Int. Ed. 2019, 58, 8145–8150. [Google Scholar] [CrossRef]
- Xu, T.; He, M.; Fan, L.; Zhou, P.; Jiang, Z.; He, Y. Engineering ligand conformation by substituent manipulation towards diverse copper-tricarboxylate frameworks with tuned gas adsorption properties. Dalton Trans. 2021, 50, 638–646. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Wang, L.; Cui, X.; Xing, H. Pillar iodination in functional boron cage hybrid supramolecular frameworks for high performance separation of light hydrocarbons. J. Mater. Chem. A 2019, 7, 27560–27566. [Google Scholar] [CrossRef]
- Chang, G.; Li, B.; Wang, H.; Hu, T.; Bao, Z.; Chen, B. Control of interpenetration in a microporous metal–organic framework for significantly enhanced C2H2/CO2 separation at room temperature. Chem. Commun. 2016, 52, 3494–3496. [Google Scholar] [CrossRef]
- Li, L.; Yin, Q.; Li, H.-F.; Liu, T.-F.; Cao, R. Rational design of phosphonocarboxylate metal–organic frameworks for light hydrocarbon separations. Mater. Chem. Front. 2018, 2, 1436–1440. [Google Scholar] [CrossRef]
- Fan, L.; Lin, S.; Wang, X.; Yue, L.; Xu, T.; Jiang, Z.; He, Y. A Series of Metal−Organic Framework Isomers Based on Pyridinedicarboxylate Ligands: Diversified Selective Gas Adsorption and the Positional Effect of Methyl Functionality. Inorg Chem. 2021, 60, 2704–2715. [Google Scholar] [CrossRef]
- Das, M.C.; Xu, H.; Xiang, S.; Zhang, Z.; Arman, H.D.; Qian, G.; Chen, B. A New Approach to Construct a Doubly Interpenetrated Microporous Metal–Organic Framework of Primitive Cubic Net for Highly Selective Sorption of Small Hydrocarbon Molecules. Chem. Eur. J. 2011, 17, 7817–7822. [Google Scholar] [CrossRef]
- Ding, T.; Zhang, S.; Zhang, W.; Zhang, G.; Gao, Z.W. Highly selective C2H2 and CO2 capture and magnetic properties of robust Co-chain based metal–organic framework. Dalton Trans. 2019, 48, 7938–7945. [Google Scholar] [CrossRef]
- Lin, R.-G.; Lin, R.-B.; Chen, B. A microporous metal–organic framework for selective C2H2 and CO2 Separation. J. Solid State Chem. 2017, 252, 138–141. [Google Scholar] [CrossRef]
- Huang, P.; Chen, C.; Wu, M.; Jiang, F.; Hong, M. An indium-organic framework for efficient storage of light hydrocarbons and selective removal of organic dyes. Dalton Trans. 2019, 48, 5527–5533. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, M.; Huang, Y.L.; Zhao, Y.; Xie, X.J.; Bai, J.P.; Wan, M.Y.; Krishna, R.; Lu, W.; Li, D. Induced Fit of C2H2 in a Flexible MOF Through Cooperative Action of Open Metal Sites. Angew. Chem. Int. Ed. 2019, 58, 8515–8519. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Q.Y.; Krishna, R.; Wang, W.; He, C.T.; Wang, Y.L. Water-Stable Europium 1,3,6,8-Tetrakis(4-carboxylphenyl)pyrene Framework for Efficient C2H2/CO2 Separation. Inorg. Chem. 2019, 58, 5089–5095. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Z.; Ye, Y.; Chen, L.; Lin, Q.; Yang, Y.; Zhang, Z.; Xiang, S. Robustness, Selective Gas Separation, and Nitrobenzene Sensing on Two Isomers of Cadmium Metal–Organic Frameworks Containing Various Metal–O–Metal Chains. Inorg. Chem. 2018, 57, 12961–12968. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Zhang, Y.; Xu, N.; Krishna, R.; Hu, J.; Jiang, Y.; He, Y.; Xing, H. Interpenetration symmetry control within ultramicroporous robust boron cluster hybrid MOFs for benchmark purification of acetylene from carbon dioxide. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhang, J.W.; Lv, H.J.; Hu, M.C.; Li, S.N.; Jiang, Y.C.; Zhai, Q.G. Tailoring the Pore Environment of a Robust Ga-MOF by Deformed [Ga3O(COO)6] Cluster for Boosting C2H2 Uptake and Separation. Inorg. Chem. 2020, 59, 10368–10373. [Google Scholar] [CrossRef] [PubMed]


| Uptake (cm3 g−1) (298 K, 100 kPa) | IAST Selectivity (298K, 100 kPa) | Ref | ||||
|---|---|---|---|---|---|---|
| C2H2 | CO2 | CH4 | C2H2/CO2 | C2H2/CH4 | ||
| FJI-C3 | 43.6 | -- | 11.6 | -- | 14.6 | [43] |
| ZJNU-27 | 93.7 | 79.3 | 26.0 | -- | 16.6 | [44] |
| USTA-36 | 58.6 a | -- | 13.2 a | -- | 13.8 a | [45] |
| [Co3(L)(OH)2(H2O)]·2DMF·2H2O | 107.3 | 62.0 | 17.7 | -- | 13 | [46] |
| QMOF-1 | 41.5 | 24.6 | 3.9 | -- | 13.5 | [47] |
| FJI-H21 | 92.4 | -- | 7.10 | -- | 16.3 | [48] |
| JNU-1 | 63 | 51 | -- | 3 | -- | [49] |
| JXNU-5a | 55.9 | 34.8 | -- | 5 | -- | [50] |
| UTSA-68 | 70.1 a | 39.6 a | -- | 4 a | -- | [42] |
| FJU-36a | 52.2 a | 35.5 a | 10.5 a | 2.8 a | 17.7 a | [51] |
| BSF-1 | 52.5 | 39.7 | 10.5 | 3.3 | 46.9 | [39] |
| BSF-2 | 41.5 | 29.7 | 5.4 | 5.1 | 324 | [41] |
| BSF-3 | 81.8 | 47.3 | 13.4 | 16.3 | 205 | [6] |
| ZNU-1 (BSF-9) | 76.3 | 38.1 | -- | 56.6 | -- | [52] |
| SNNU-63 | 91.1 | 43.7 | 10.3 | 3.3 | 12.9 | [53] |
| ZJNU-109 | 104.6 | 60.0 | 14.3 | 3.8 | 21.6 | [40] |
| Zn2(Pydc)(Ata)2 | 52.6 | 35.3 | 23.6 | 3.9 | 15.9 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Jiang, Y.; Sun, W.; Li, J.; Wang, L.; Jin, Y.; Zhang, Y.; Wang, D.; Duttwyler, S. Gram-Scale Synthesis of an Ultrastable Microporous Metal-Organic Framework for Efficient Adsorptive Separation of C2H2/CO2 and C2H2/CH4. Molecules 2021, 26, 5121. https://doi.org/10.3390/molecules26175121
Xu N, Jiang Y, Sun W, Li J, Wang L, Jin Y, Zhang Y, Wang D, Duttwyler S. Gram-Scale Synthesis of an Ultrastable Microporous Metal-Organic Framework for Efficient Adsorptive Separation of C2H2/CO2 and C2H2/CH4. Molecules. 2021; 26(17):5121. https://doi.org/10.3390/molecules26175121
Chicago/Turabian StyleXu, Nuo, Yunjia Jiang, Wanqi Sun, Jiahao Li, Lingyao Wang, Yujie Jin, Yuanbin Zhang, Dongmei Wang, and Simon Duttwyler. 2021. "Gram-Scale Synthesis of an Ultrastable Microporous Metal-Organic Framework for Efficient Adsorptive Separation of C2H2/CO2 and C2H2/CH4" Molecules 26, no. 17: 5121. https://doi.org/10.3390/molecules26175121
APA StyleXu, N., Jiang, Y., Sun, W., Li, J., Wang, L., Jin, Y., Zhang, Y., Wang, D., & Duttwyler, S. (2021). Gram-Scale Synthesis of an Ultrastable Microporous Metal-Organic Framework for Efficient Adsorptive Separation of C2H2/CO2 and C2H2/CH4. Molecules, 26(17), 5121. https://doi.org/10.3390/molecules26175121








