Utilisation of Non-Edible Source (Pongamia pinnata Seeds Shells) for Producing Methyl Esters as Cleaner Fuel in the Presence of a Novel Heterogeneous Catalyst Synthesized from Waste Eggshells
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Catalyst Synthesis
2.3. Catalyst Characterisation
2.4. Biodiesel Production and Its Quality Determination
3. Results and Discussion
3.1. Catalyst Characterisation
3.1.1. X-ray Diffraction Analysis
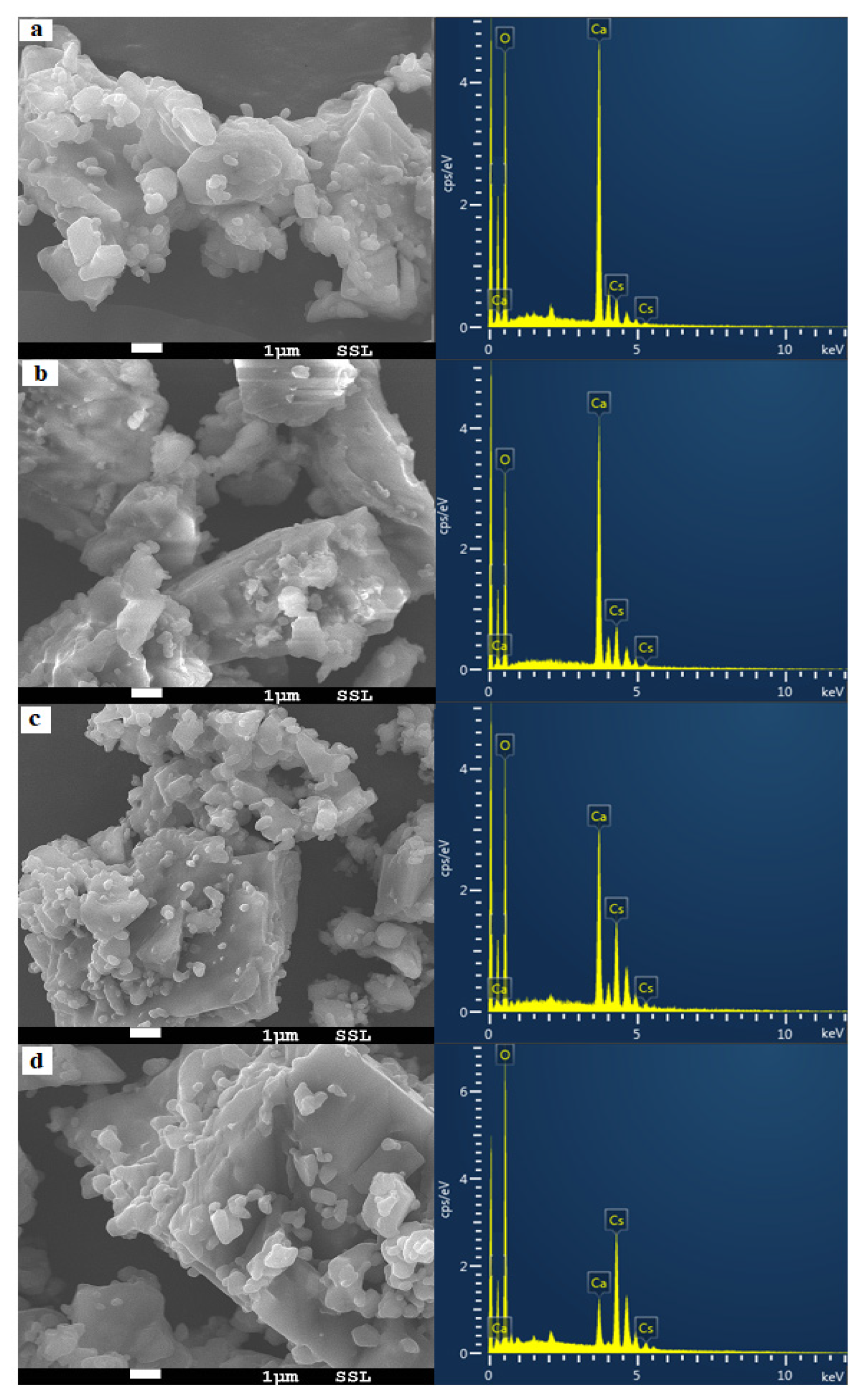
3.1.2. Scanning Electron Microscopy and Energy Dispersive Spectroscopy Analysis
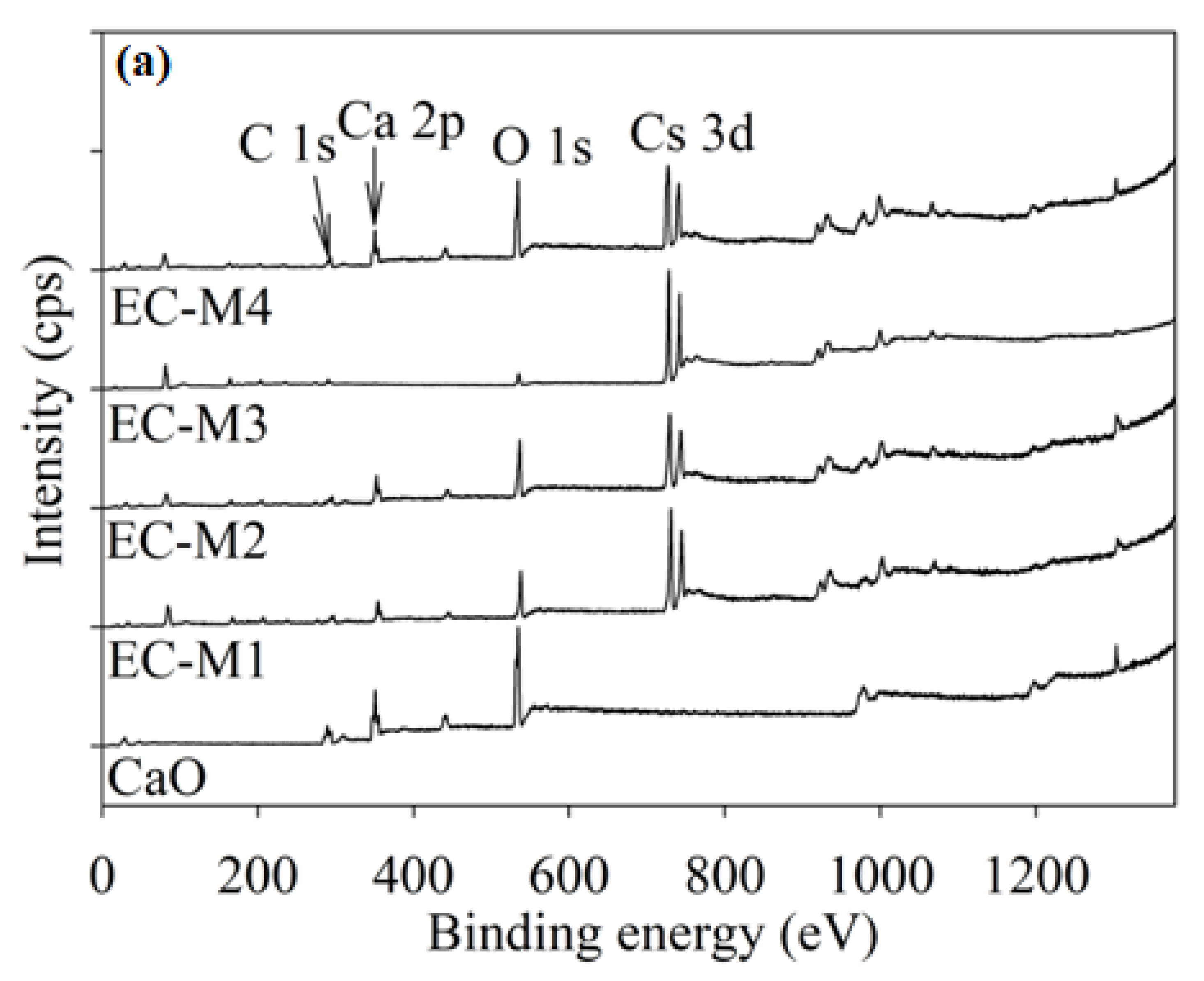
3.1.3. Surface Characterisation: X-ray Photoelectron Spectroscopy
3.1.4. CO2-Temperature Programmed Desorption Analysis
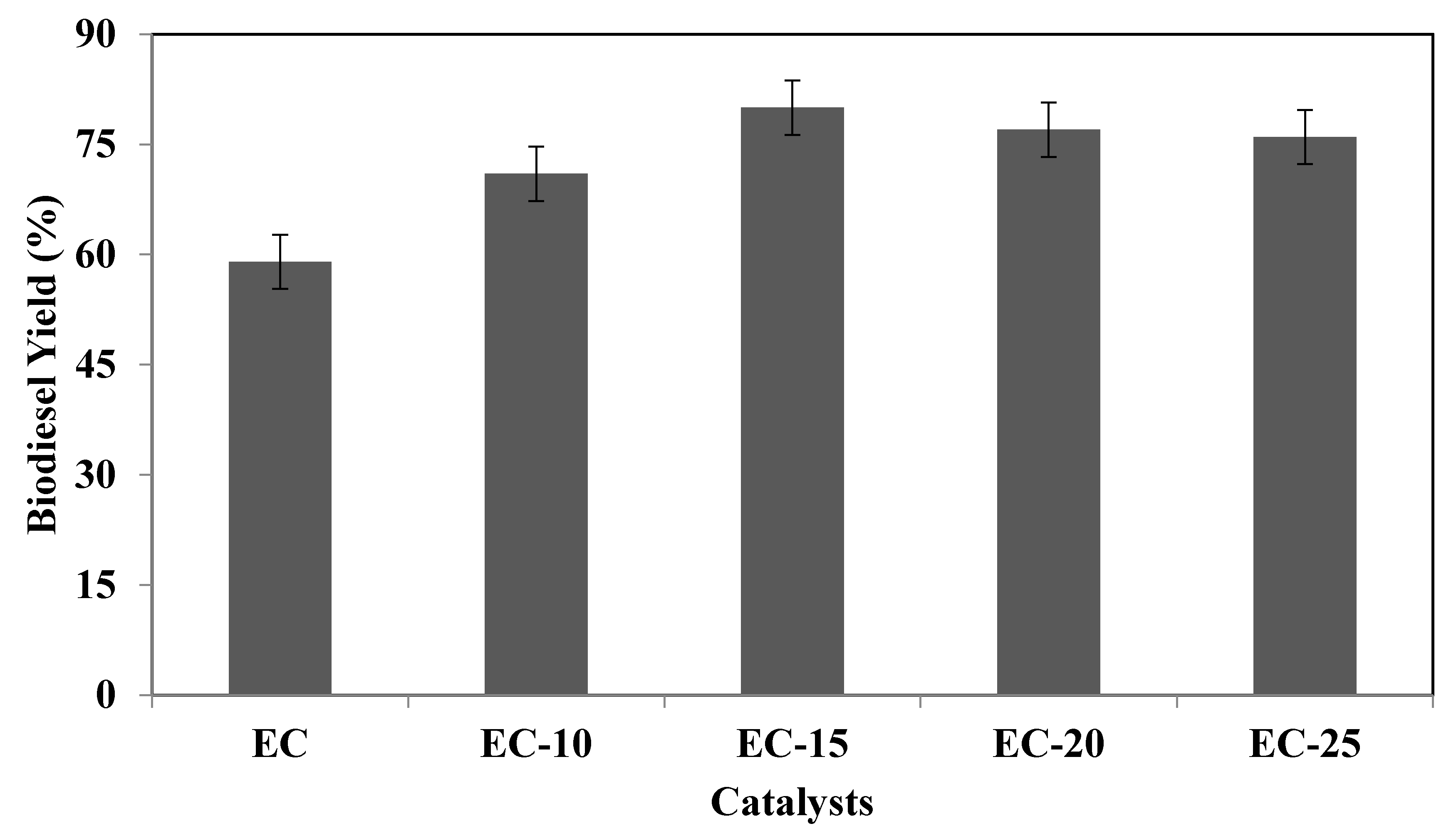
3.2. Catalyst Evaluation
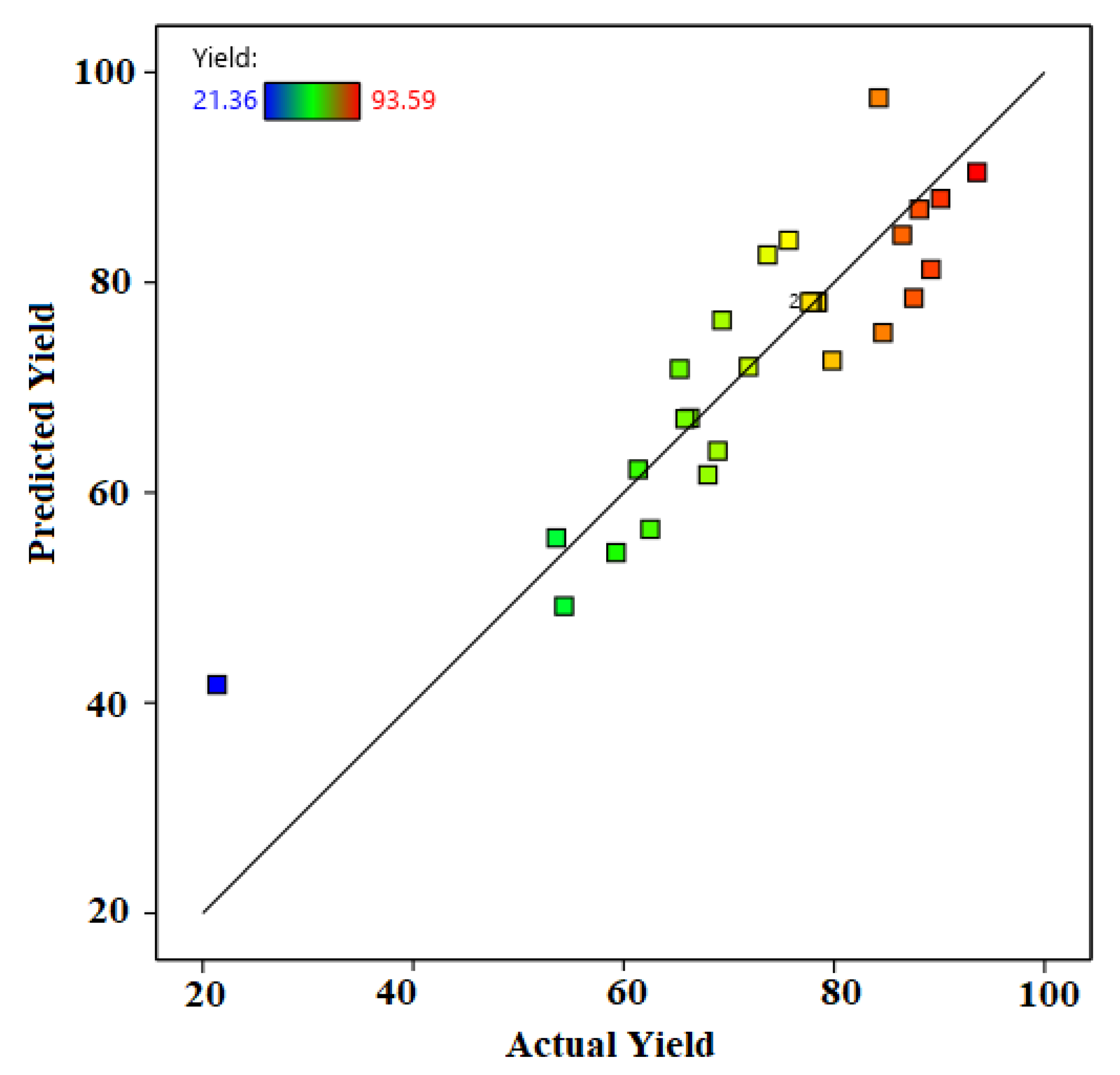
3.3. Model Prediction and Statistical Analysis
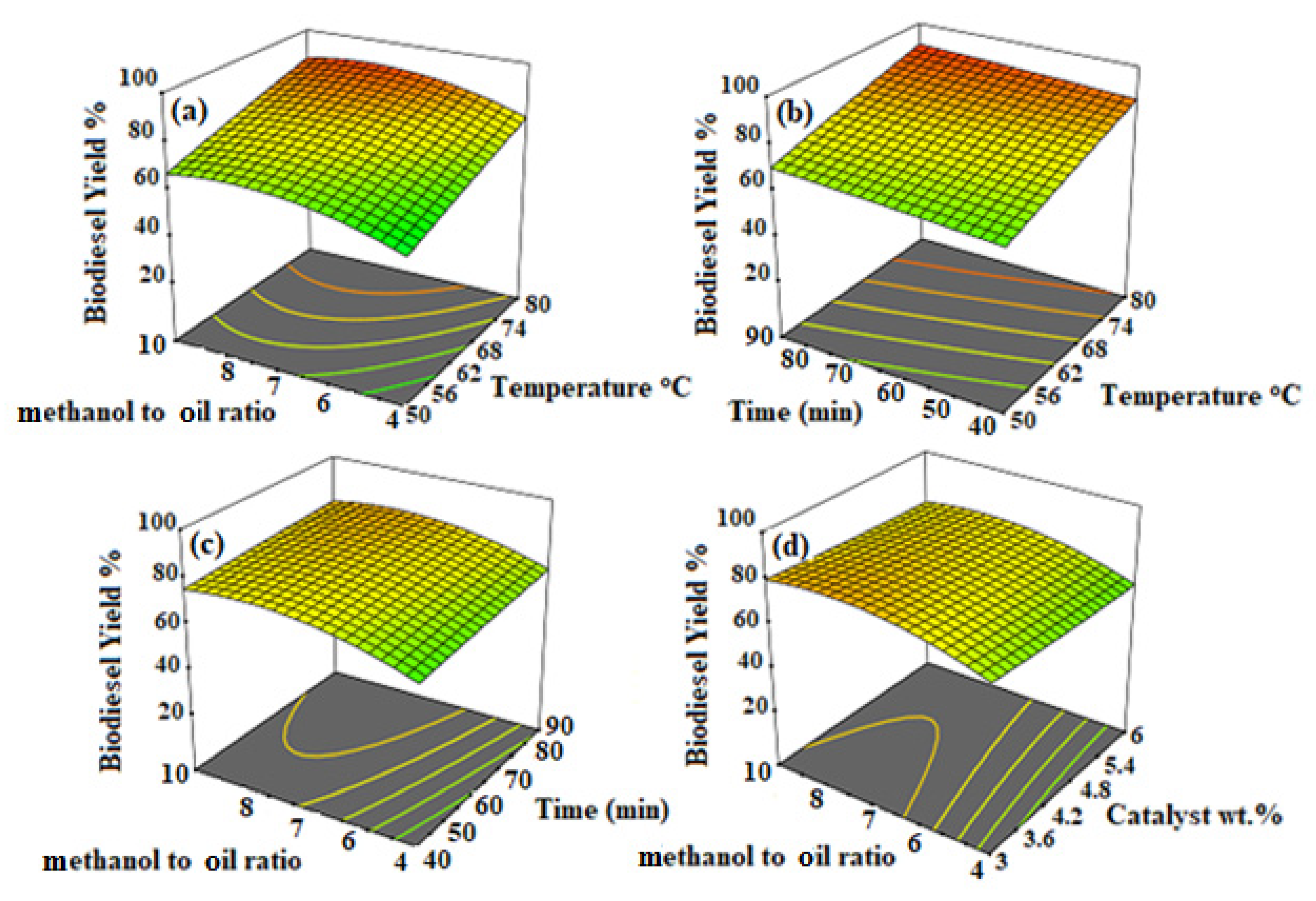
3.4. Parametric Study
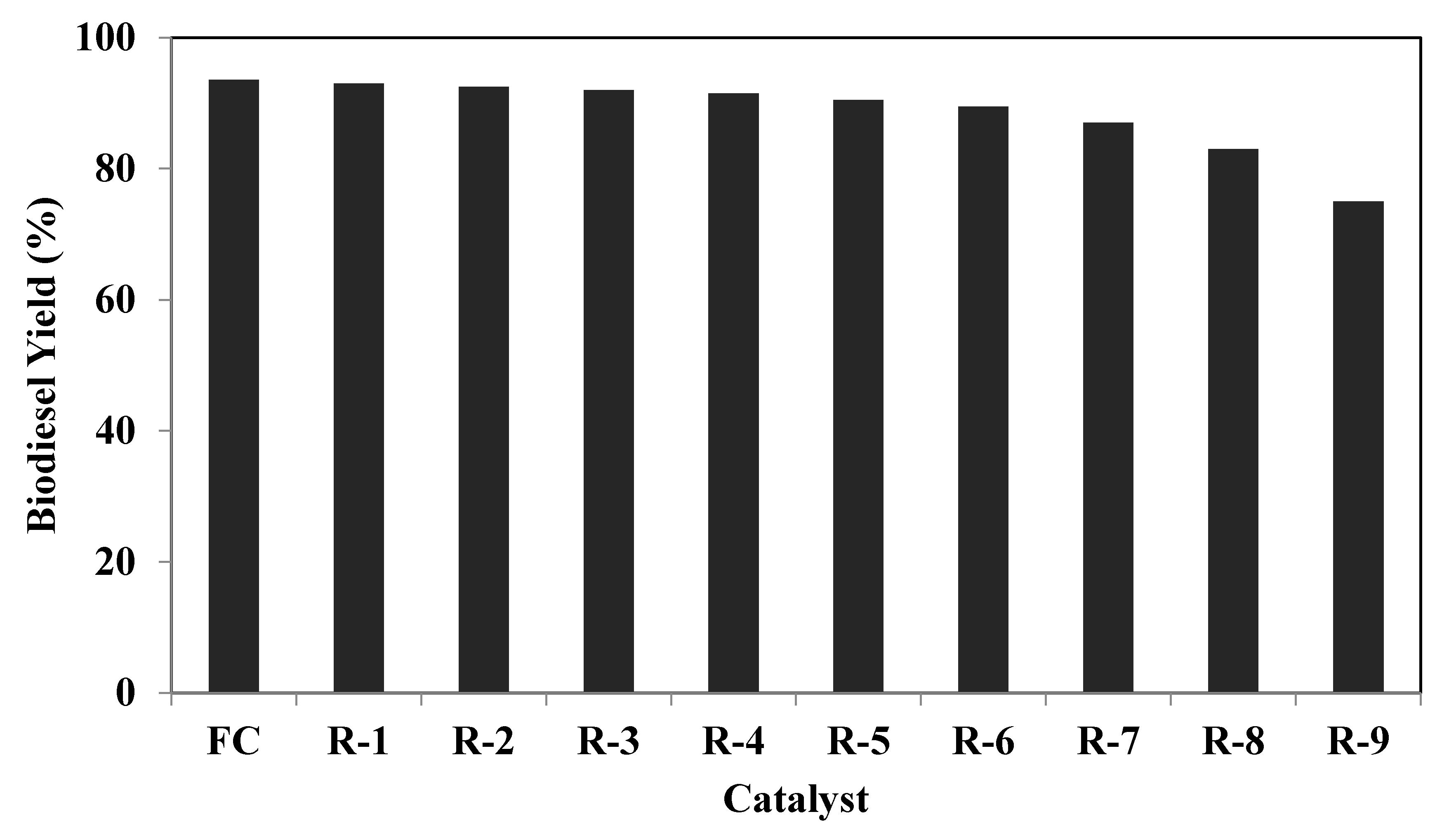
3.5. Reusability
3.6. Properties of Biodiesel
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hamza, M.; Ayoub, M.; Shamsuddin, R.B.; Mukhtar, A.; Saqib, S.; Zahid, I.; Ameen, M.; Ullah, S.; Al-Sehemi, A.G.; Ibrahim, M. A review on the waste biomass derived catalysts for biodiesel production. Environ. Technol. Innov. 2020, 21, 101200. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523. [Google Scholar] [CrossRef]
- Jamil, F.; Murphin Kumar, P.S.; Al-Haj, L.; Tay Zar Myint, M.; Al-Muhtaseb, A.a.H. Heterogeneous carbon-based catalyst modified by alkaline earth metal oxides for biodiesel production: Parametric and kinetic study. Energy Convers. Manag. X 2020, 10, 100047. [Google Scholar]
- Habib, M.S.; Tayyab, M.; Zahoor, S.; Sarkar, B. Management of animal fat-based biodiesel supply chain under the paradigm of sustainability. Energy Convers. Manag. 2020, 225, 113345. [Google Scholar] [CrossRef]
- Jamil, F.; Ahmad, M.M.; Yusup, S.; Abdullah, B. Upgrading of bio-oil from palm kernel shell by catalytic cracking in the presence of HZSM-5. Int. J. Green Energy 2016, 13, 424–429. [Google Scholar] [CrossRef]
- Hellier, P.; Jamil, F.; Zaglis-Tyraskis, E.; Al-Muhtaseb, A.a.H.; Al Haj, L.; Ladommatos, N. Combustion and emissions characteristics of date pit methyl ester in a single cylinder direct injection diesel engine. Fuel 2019, 243, 162–171. [Google Scholar] [CrossRef]
- Jume, B.H.; Gabris, M.A.; Rashidi Nodeh, H.; Rezania, S.; Cho, J. Biodiesel production from waste cooking oil using a novel heterogeneous catalyst based on graphene oxide doped metal oxide nanoparticles. Renew. Energy 2020, 162, 2182–2189. [Google Scholar] [CrossRef]
- Esan, A.O.; Adeyemi, A.D.; Ganesan, S. A review on the recent application of dimethyl carbonate in sustainable biodiesel production. J. Clean. Prod. 2020, 257, 120561. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Hassan, A.O.; Abu-jrai, A.; Al-Muhatseb, A.a.H.; Jamil, F. Impact of EGR and engine speed on HCCI engine performance and tail pipe emissions. Energy Procedia 2017, 136, 208–212. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, scharacterisation and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Pang, Y.L. Investigation of carbon-based solid acid catalyst from Jatropha curcas biomass in biodiesel production. Energy Convers. Manag. 2017, 144, 10–17. [Google Scholar] [CrossRef]
- Chua, S.Y.; Periasamy, L.A.P.; Goh, C.M.H.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Walvekar, R.; Abdullah, E.C. Biodiesel synthesis using natural solid catalyst derived from biomass waste—A review. J. Ind. Eng. Chem. 2020, 81, 41–60. [Google Scholar] [CrossRef]
- Jamil, U.; Husain Khoja, A.; Liaquat, R.; Raza Naqvi, S.; Nor Nadyaini Wan Omar, W.; Aishah Saidina Amin, N. Copper and calcium-based metal organic framework (MOF) catalyst for biodiesel production from waste cooking oil: A process soptimisation study. Energy Convers. Manag. 2020, 215, 112934. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Chong, C.T.; Ge, Y.; Ong, H.C.; Ng, J.-H.; Tian, B.; Ashokkumar, V.; Lim, S.; Seljak, T.; Józsa, V. Progress in utilisation of waste cooking oil for sustainable biodiesel and biojet fuel production. Energy Convers. Manag. 2020, 223, 113296. [Google Scholar] [CrossRef]
- Qu, S.; Chen, C.; Guo, M.; Lu, J.; Yi, W.; Ding, J.; Miao, Z. Synthesis of MgO/ZSM-5 catalyst and soptimisation of process parameters for clean production of biodiesel from Spirulina platensis. J. Clean. Prod. 2020, 276, 123382. [Google Scholar] [CrossRef]
- Du, L.; Li, Z.; Ding, S.; Chen, C.; Qu, S.; Yi, W.; Lu, J.; Ding, J. Synthesis and scharacterisation of carbon-based MgO catalysts for biodiesel production from castor oil. Fuel 2019, 258, 116122. [Google Scholar] [CrossRef]
- Sahani, S.; Roy, T.; Sharma, Y.C. Studies on fast and green biodiesel production from an indigenous non-edible Indian feedstock using single phase strontium titanate catalyst. Energy Convers. Manag. 2020, 203, 112180. [Google Scholar] [CrossRef]
- Bastos, R.R.C.; da Luz Corrêa, A.P.; da Luz, P.T.S.; da Rocha Filho, G.N.; Zamian, J.R.; da Conceição, L.R.V. Optimization of biodiesel production using sulfonated carbon-based catalyst from an amazon agro-industrial waste. Energy Convers. Manag. 2020, 205, 112457. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. Optimisation of biodiesel production from mixture of edible and non-edible vegetable oils. Biocatal. Agric. Biotechnol. 2016, 8, 112–120. [Google Scholar] [CrossRef]
- Kumar, D.; Park, C.H.; Kim, C.S. Solvent-free K–CaO/Ca(OH)2 mixed-phase nanocatalytic single-step methanolysis, ethanolysis and aminolysis of Pongamia pinnata triglycerides. Sustain. Chem. Pharm. 2020, 18, 100317. [Google Scholar] [CrossRef]
- Siroha, A.K.; Punia, S.; Kaur, M.; Sandhu, K.S. A novel starch from Pongamia pinnata seeds: Comparison of its thermal, morphological and rheological behaviour with starches from other botanical sources. Int. J. Biol. Macromol. 2020, 143, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Ajala, E.O.; Ajala, M.A.; Ajao, A.O.; Saka, H.B.; Oladipo, A.C. Calcium-carbide residue: A precursor for the synthesis of CaO–Al2O3–SiO2–CaSO4 solid acid catalyst for biodiesel production using waste lard. Chem. Eng. J. Adv. 2020, 4, 100033. [Google Scholar] [CrossRef]
- Yang, X.-X.; Wang, Y.-T.; Yang, Y.-T.; Feng, E.-Z.; Luo, J.; Zhang, F.; Yang, W.-J.; Bao, G.-R. Catalytic transesterification to biodiesel at room temperature over several solid bases. Energy Convers. Manag. 2018, 164, 112–121. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Haj, L.; Al-Muhtaseb Ala’a, H.; Al-Hinai Mohab, A.; Baawain, M.; Rashid, U.; Ahmad Mohammad, N.M. Current scenario of catalysts for biodiesel production: A critical review. In Reviews in Chemical Engineering; De Gruyter: Berlin, German, 2017. [Google Scholar] [CrossRef]
- Rahman, W.U.; Fatima, A.; Anwer, A.H.; Athar, M.; Khan, M.Z.; Khan, N.A.; Halder, G. Biodiesel synthesis from eucalyptus oil by sutilising waste egg shell derived calcium based metal oxide catalyst. Process. Saf. Environ. Prot. 2019, 122, 313–319. [Google Scholar] [CrossRef]
- Risso, R.; Ferraz, P.; Meireles, S.; Fonseca, I.; Vital, J. Highly active Cao catalysts from waste shells of egg, oyster and clam for biodiesel production. Appl. Catal. A Gen. 2018, 567, 56–64. [Google Scholar] [CrossRef]
- Torres-Rodríguez, D.A.; Romero-Ibarra, I.C.; Ibarra, I.A.; Pfeiffer, H. Biodiesel production from soybean and Jatropha oils using cesium impregnated sodium zirconate as a heterogeneous base catalyst. Renew. Energy 2016, 93, 323–331. [Google Scholar] [CrossRef]
- Amani, H.; Asif, M.; Hameed, B.H. Transesterification of waste cooking palm oil and palm oil to fatty acid methyl ester using cesium-modified silica catalyst. J. Taiwan Inst. Chem. Eng. 2016, 58, 226–234. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rashid, U.; Saiman, M.I.; Tan, Y.P.; Alsultan, G.A.; Taufiq-Yap, Y.H. Modified waste egg shell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 2018, 82, 3645–3655. [Google Scholar] [CrossRef]
- Piker, A.; Tabah, B.; Perkas, N.; Gedanken, A. A green and low-cost room temperature biodiesel production method from waste oil using egg shells as catalyst. Fuel 2016, 182, 34–41. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Naeem, A.; Mahmood, T.; Ahmad, S.; Humayun, M.; Islam, M.G.U. Biodiesel production from date seed oil (Phoenix dactylifera L.) via egg shell derived heterogeneous catalyst. Chem. Eng. Res. Des. 2018, 132, 644–651. [Google Scholar] [CrossRef]
- Rabie, A.M.; Shaban, M.; Abukhadra, M.R.; Hosny, R.; Ahmed, S.A.; Negm, N.A. Diatomite supported by CaO/MgO nanocomposite as heterogeneous catalyst for biodiesel production from waste cooking oil. J. Mol. Liq. 2019, 279, 224–231. [Google Scholar] [CrossRef]
- Roy, T.; Sahani, S.; Madhu, D.; Chandra Sharma, Y. A clean approach of biodiesel production from waste cooking oil by using single phase BaSnO3 as solid base catalyst: Mechanism, kinetics & E-study. J. Clean. Prod. 2020, 265, 121440. [Google Scholar]
- Bokhari, A.; Chuah, L.F.; Yusup, S.; Klemeš, J.J.; Kamil, R.N.M. Optimisation on pretreatment of rubber seed (Hevea brasiliensis) oil via esterification reaction in a hydrodynamic cavitation reactor. Bioresour. Technol. 2016, 199, 414–422. [Google Scholar] [CrossRef]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M.; Chong, Z.K. Kinetic studies on waste cooking oil into biodiesel via hydrodynamic cavitation. J. Clean. Prod. 2017, 146, 47–56. [Google Scholar] [CrossRef]
- Ahmad, J.; Yusup, S.; Bokhari, A.; Kamil, R.N.M. Study of fuel properties of rubber seed oil based biodiesel. Energy Convers. Manag. 2014, 78, 266–275. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Subbarao, D. Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Clean. Prod. 2013, 59, 131–140. [Google Scholar] [CrossRef]
- Ijaz, M.; Bahtti, K.H.; Anwar, Z.; Dogar, U.F.; Irshad, M. Production, soptimisation and quality assessment of biodiesel from Ricinus communis L. oil. J. Radiat. Res. Appl. Sci. 2016, 9, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Jamil, F.; Al-Muhtaseb, A.a.H.; Al-Haj, L.; Al-Hinai, M.A.; Hellier, P.; Rashid, U. Optimisation of oil extraction from waste “Date pits” for biodiesel production. Energy Convers. Manag. 2016, 117, 264–272. [Google Scholar] [CrossRef]


| Range | Temperature (°C) | Methanol-to-Oil Molar Ratio | Time (min) | Catalyst Loading (wt.%) |
|---|---|---|---|---|
| Axial point | 35 | 2 | 15 | 1.5 |
| Low | 50 | 4 | 40 | 3 |
| Mid | 65 | 6 | 65 | 4.5 |
| High | 80 | 8 | 90 | 6 |
| Axial point | 95 | 10 | 115 | 7.5 |
| Exp. Run | Temperature (°C) | Time (Minutes) | Catalyst (wt.%) | Methanol:Oil Ratio | Biodiesel Yield (%) |
|---|---|---|---|---|---|
| 1 | 80 | 90 | 6 | 8 | 90.12 |
| 2 | 50 | 40 | 6 | 4 | 54.35 |
| 3 | 65 | 65 | 4.5 | 6 | 77.89 |
| 4 | 50 | 90 | 6 | 4 | 62.52 |
| 5 | 50 | 90 | 3 | 8 | 71.87 |
| 6 | 80 | 40 | 6 | 8 | 86.47 |
| 7 | 80 | 90 | 3 | 8 | 93.59 |
| 8 | 35 | 65 | 4.5 | 6 | 53.61 |
| 9 | 65 | 65 | 4.5 | 6 | 78.12 |
| 10 | 80 | 90 | 6 | 4 | 87.58 |
| 11 | 80 | 40 | 3 | 4 | 84.65 |
| 12 | 80 | 90 | 3 | 4 | 89.21 |
| 13 | 65 | 65 | 4.5 | 6 | 78.37 |
| 14 | 80 | 40 | 6 | 4 | 79.82 |
| 15 | 65 | 65 | 7.5 | 6 | 69.34 |
| 16 | 65 | 65 | 1.5 | 6 | 75.69 |
| 17 | 50 | 40 | 3 | 4 | 59.29 |
| 18 | 65 | 65 | 4.5 | 6 | 78.39 |
| 19 | 80 | 40 | 3 | 8 | 88.11 |
| 20 | 50 | 90 | 3 | 4 | 68.01 |
| 21 | 65 | 15 | 4.5 | 6 | 65.32 |
| 22 | 65 | 115 | 4.5 | 6 | 73.68 |
| 23 | 65 | 65 | 4.5 | 6 | 77.69 |
| 24 | 50 | 40 | 3 | 8 | 66.29 |
| 25 | 65 | 65 | 4.5 | 2 | 21.36 |
| 26 | 65 | 65 | 4.5 | 6 | 78.39 |
| 27 | 50 | 90 | 6 | 8 | 65.84 |
| 28 | 95 | 65 | 4.5 | 6 | 84.25 |
| 29 | 65 | 65 | 4.5 | 10 | 68.95 |
| 30 | 50 | 40 | 6 | 8 | 61.38 |
| Catalyst | Basicity (mmol/g) |
|---|---|
| EC | 3.15 ± 0.14 |
| EC-10 | 5.96 ± 0.26 |
| EC-15 | 6.46 ± 0.11 |
| EC-20 | 4.86 ± 0.12 |
| EC-25 | 5.17 ± 0.10 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Mean vs. Total | 1.599 × 105 | 1 | 1.599 × 105 | ||
| Linear vs. Mean | 3635.98 | 4 | 909.00 | 9.18 | 0.0001 |
| 2FI vs. Linear | 15.32 | 6 | 2.55 | 0.02 | 1.000 |
| Quadratic vs. 2FI | 1162.27 | 4 | 290.57 | 3.36 | 0.037 |
| Cubic vs. Quadratic | 586.27 | 8 | 73.28 | 0.72 | 0.674 |
| Residual | 712.40 | 7 | 101.77 | ||
| Total | 1.660 × 105 | 30 | 5533.47 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 4813.58 | 14 | 343.83 | 3.97 | 0.006 |
| A—Temperature | 2630.90 | 1 | 2630.90 | 30.39 | <0.0001 |
| B—Time | 176.58 | 1 | 176.58 | 2.04 | 0.173 |
| C—Catalyst | 86.79 | 1 | 86.79 | 1.00 | 0.332 |
| D—Me:Oil | 741.70 | 1 | 741.70 | 8.57 | 0.010 |
| AB | 1.88 | 1 | 1.88 | 0.021 | 0.884 |
| AC | 6.00 | 1 | 6.00 | 0.069 | 0.795 |
| AD | 1.09 | 1 | 1.09 | 0.012 | 0.912 |
| BC | 0.005 | 1 | 0.005 | 0.0001 | 0.993 |
| BD | 6.30 | 1 | 6.30 | 0.072 | 0.791 |
| CD | 0.044 | 1 | 0.044 | 0.0005 | 0.982 |
| A2 | 3.94 | 1 | 3.94 | 0.045 | 0.834 |
| B2 | 1.53 | 1 | 1.53 | 0.017 | 0.895 |
| C2 | 7.34 | 1 | 7.34 | 0.084 | 0.774 |
| D2 | 1096.50 | 1 | 1096.50 | 12.66 | 0.002 |
| Run | Catalyst | Calcium (%) | Caesium (%) |
|---|---|---|---|
| 1 | FC | 71.89 | 14.89 |
| 2 | R-1 | 71.88 | 14.84 |
| 3 | R-2 | 71.81 | 14.77 |
| 4 | R-3 | 71.78 | 14.71 |
| 5 | R-4 | 71.60 | 14.64 |
| 6 | R-5 | 71.55 | 14.51 |
| 7 | R-6 | 71.40 | 14.06 |
| 8 | R-7 | 70.96 | 13.38 |
| 9 | R-8 | 70.10 | 12.31 |
| 10 | R-9 | 69.03 | 11.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Muhtaseb, A.H. Utilisation of Non-Edible Source (Pongamia pinnata Seeds Shells) for Producing Methyl Esters as Cleaner Fuel in the Presence of a Novel Heterogeneous Catalyst Synthesized from Waste Eggshells. Molecules 2021, 26, 5772. https://doi.org/10.3390/molecules26195772
Al-Muhtaseb AH. Utilisation of Non-Edible Source (Pongamia pinnata Seeds Shells) for Producing Methyl Esters as Cleaner Fuel in the Presence of a Novel Heterogeneous Catalyst Synthesized from Waste Eggshells. Molecules. 2021; 26(19):5772. https://doi.org/10.3390/molecules26195772
Chicago/Turabian StyleAl-Muhtaseb, Ala’a H. 2021. "Utilisation of Non-Edible Source (Pongamia pinnata Seeds Shells) for Producing Methyl Esters as Cleaner Fuel in the Presence of a Novel Heterogeneous Catalyst Synthesized from Waste Eggshells" Molecules 26, no. 19: 5772. https://doi.org/10.3390/molecules26195772
APA StyleAl-Muhtaseb, A. H. (2021). Utilisation of Non-Edible Source (Pongamia pinnata Seeds Shells) for Producing Methyl Esters as Cleaner Fuel in the Presence of a Novel Heterogeneous Catalyst Synthesized from Waste Eggshells. Molecules, 26(19), 5772. https://doi.org/10.3390/molecules26195772






