Lead Assays with Smartphone Detection Using a Monolithic Rod with 4-(2-Pyridylazo) Resorcinol
Abstract
:1. Introduction
2. Results
2.1. The Monolithic PUF–PAR Rod
2.2. The Proposed Water Monitoring Procedure
3. Discussion
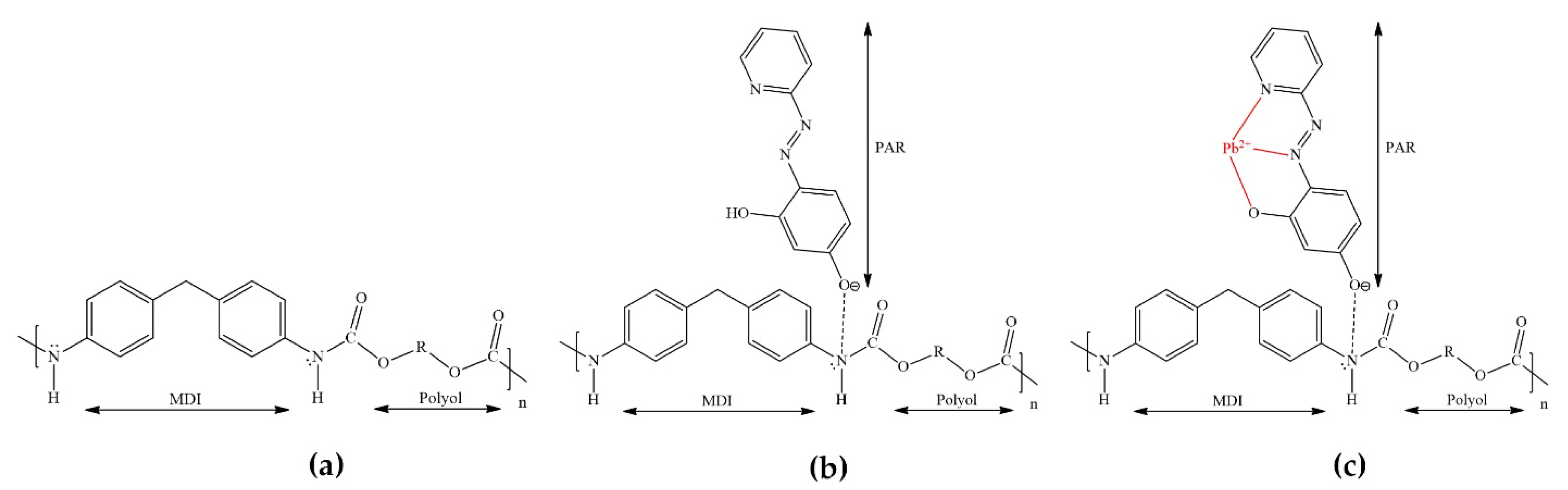
3.1. Properties of the Monolithic PUF–PAR Rod
3.2. Parameters Affecting the Color Development: PAR Concentration and pH
3.2.1. PAR Concentrations
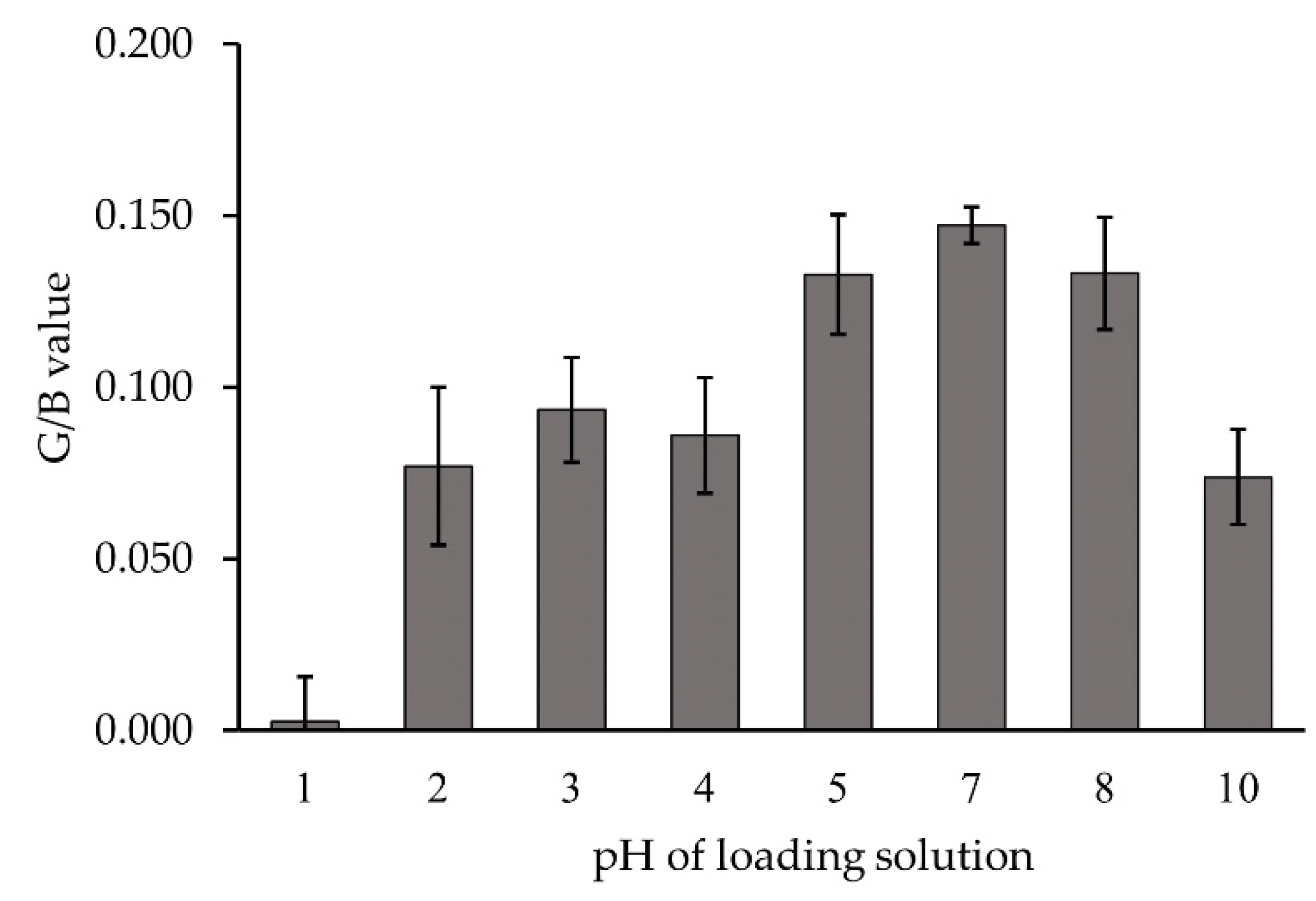
3.2.2. Effect of pH
3.3. Single Standard Calibration
3.4. Interference Study
4. Materials and Methods
4.1. Apparatus
4.2. Reagents and Materials
4.3. Preparation of Monolithic PUF–PAR Rod
4.4. General Procedure for Lead Determination Using Monolithic PUF–PAR Rod
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollution Control Department of Thailand. Available online: http://pcd.go.th/info_serv/reg_std_water01.html (accessed on 15 August 2021).
- Gottler, R.A. Part 3000 metals. In Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; pp. 3-15–3-83. [Google Scholar]
- Asgharinezhad, A.A.; Ebrahimzadeh, H.; Rezvani, M.; Shekari, N.; Loni, M.A. Novel 4-(2-pyridylazo) resorcinol functionalised magnetic nanosorbent for selective extraction of Cu(II) and Pb(II) ions from food and water samples. Food Addit. Contam. 2014, 31, 1196–1204. [Google Scholar] [CrossRef]
- Cai, Y.; Ren, B.; Peng, C.; Zhang, C.; Wei, X. Highly sensitive and selective fluorescence turn-on detection of Pb (II) based on Fe3O4@Au-FITC nanocomposite. Molecules 2021, 26, 3180. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on water quality detection by UV-vis spectroscopy. Appl. Sci. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Asghari, A.; Mohammadi, B. Nano-alumina coated with sodium dodecyl sulfate and modified with 4-(2-Pyridylazo) resorcinol for extraction of heavy metals in different matrixes. J. Ind. Eng. Chem. 2014, 20, 824–829. [Google Scholar] [CrossRef]
- Lu, W.; Lin, C.; Yang, J.; Wang, X.; Yao, B.; Wang, M. A DNAzyme assay coupled with effective magnetic separation and rolling circle amplification for detection of lead cations with a smartphone camera. Anal. Bioanal. Chem. 2019, 411, 5383–5391. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Chu, S.; Liu, B.; Zhang, Q.; Zou, L.; Yu, S.; Jiang, C. Semiquantitative Visual Detection of Lead Ions with a Smartphone via a Colorimetric Paper-Based Analytical Device. Anal. Chem. 2019, 91, 9292–9299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, C.; Liu, F.; Zou, X.; Xu, Y.; Xu, X. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Kurrey, R.; Deb, M.K.; Shrivas, K.; Karbhal, I.; Khalkho, B.R. A simple and cost-effective paper-based and colorimetric dual-mode detection of arsenic(III) and lead(II) based on glucose-functionalized gold nanoparticles. RSC Adv. 2021, 11, 20769–20780. [Google Scholar] [CrossRef]
- Satarpai, T.; Shiowatana, J.; Siripinyanond, A. Paper-based analytical device for sampling, on-site preconcentration and detection of ppb lead in water. Talanta 2016, 154, 504–510. [Google Scholar] [CrossRef]
- Kang, W.; Pei, X.; Rusinek, C.A.; Bange, A.; Haynes, E.N.; Heineman, W.R.; Papautsky, I. Determination of lead with a copper-based electrochemical sensor. Anal. Chem. 2017, 89, 3345–3352. [Google Scholar] [CrossRef] [Green Version]
- Alam, A.U.; Howlader, M.M.R.; Hu, N.-X.; Deen, M.J. Electrochemical sensing of lead in drinking water using β-cyclodextrin-modified MWCNTs. Sens. Actuators B 2019, 296, 126632. [Google Scholar] [CrossRef] [Green Version]
- Frutos-Puerto, S.; Miró, C.; Pinilla-Gil, E. Nafion-protected sputtered-bismuth screen-printed electrode for on-site voltammetric measurements of Cd(II) and Pb(II) in natural water samples. Sensors 2019, 19, 279. [Google Scholar] [CrossRef] [Green Version]
- Dagnal, R.M.; West, T.S.; Young, P. Determination of Lead with 4-(2-pyridylazo)-resorcinol-I: Spectrophotometry and solvent extraction. Talanta 1965, 12, 583–588. [Google Scholar] [CrossRef]
- Rahman, I.; Furusho, Y.; Begum, Z.; Sato, R.; Okumura, H.; Honda, H.; Hasegawa, H. Determination of lead in solution by solid phase extraction, elution, and spectrophotometric detection using 4-(2-pyridylazo)-resorcinol. Cent. Eur. J. Chem. 2013, 11, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Shvoeva, O.P.; Dedkova, V.P.; Savvin, S.B. Determination of lead with 4-(2-pyridylazo)resorcinol after the sorption of lead as its thiosulfate complex on a fibrous sorbent filled with AV-17. J. Anal. Chem. 2001, 56, 1080–1083. [Google Scholar] [CrossRef]
- Dedkova, V.P.; Shvoeva, O.P.; Savvin, S.B. Effect of anions on the development of color reactions of cadmium, lead, and mercury with dithizone and 4-(2-pyridylazo)resorcinol on a fibrous material filled with the anion exchanger AV-17. J. Anal. Chem. 2003, 58, 230–235. [Google Scholar] [CrossRef]
- Dedkova, V.; Shvoeva, O.; Savvin, S. Test method for the separate determination of mercury(II), cadmium, and lead in one sample on the fibrous sorbent PANV-AV-17. J. Anal. Chem. 2006, 61, 813–818. [Google Scholar] [CrossRef]
- Santos, W.L.D.; Santos, C.M.M.D.; Costa, J.L.O.; Andrade, H.M.C.; Ferreira, S.L.C. Multivariate optimization and validation studies in on-line pre-concentration system for lead determination in drinking water and saline waste from oil refinery. Microchem. J. 2004, 77, 123–129. [Google Scholar] [CrossRef]
- Tokalioglu, S.; Kartal, S. Preconcentration of iron(III), lead(II), cobalt(II) and chromium(III) on amberlite XAD-1180 resin loaded with 4-(2-pyridylazo)-resorcinol (PAR) and their determination by FAAS. Bull. Korean Chem. Soc. 2006, 27, 1293–1296. [Google Scholar]
- Klamtet, J.; Sanguthai, S.; Sriprang, S. Determination of lead in aqueous samples using a flow injection analysis system with on-line preconcentration and spectrophotometric detection. NU. Int. J. Sci. 2007, 4, 122–131. [Google Scholar]
- Yanovskaya, E.S.; Nazarenko, E.V. Formation of the mixed-ligand complexes of lead(II), cadmium(II), and zinc(II) sorbed on the surface of silicas with chemically immobilized N-propyl-N′-allylthiourea and mercaptopropyl groups. Russ. J. Inorg. Chem. 2007, 52, 505–509. [Google Scholar] [CrossRef]
- Bojdi, M.K.; Mashhadizadeh, M.H.; Behbahani, M.; Farahani, A.; Davarani, S. Synthesis, characterization and application of novel lead imprinted polymer nanoparticles as a high selective electrochemical sensor for ultra-trace determination of lead ions in complex matrixes. Electrochim. Acta 2014, 136, 59–65. [Google Scholar] [CrossRef]
- Mattio, E.; Robert-Peillard, F.; Branger, C.; Puzio, K.; Margaillan, A.; Brach-Papa, C.; Knoery, J.; Boudenne, J.L.; Coulomb, B. 3D-printed flow system for determination of lead in natural waters. Talanta 2017, 168, 298–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, G.; Sardar, M.R.; Lin, B.; Lin, J.-M. Analysis of trace metals in water samples using NOBIAS chelate resins by HPLC and ICP-MS. Talanta 2019, 204, 50–56. [Google Scholar] [CrossRef]
- Duangthong, S.; Kamhang, R.; Wararatananuruk, P.; Chooto, P.; Tapachai, W.A. Simple on-line preconcentration spectrophotometry for detecting lead contamination from drinking water coolers and glazed bowl samples. Anal. Bioanal. Chem. Res. 2020, 7, 473–482. [Google Scholar]
- Rodríguez-Maese, R.; Ferrer, L.; Cerdà, V.; Leal, L.O. Fully automatic system for lead monitoring in water. Microchem. J. 2020, 154, 104550. [Google Scholar] [CrossRef]
- Smirnova, S.V.; Ilin, D.V.; Pletnev, I.V. Extraction and ICP-OES determination of heavy metals using tetrabutylammonium bromide aqueous biphasic system and oleophilic collector. Talanta 2021, 221, 121485. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.; Ferreira, S. On-line preconcentration system for lead determination in seafood samples by flame atomic absorption spectrometry using polyurethane foam loaded with 2-(2-benzothiazolylazo)-2-p-cresol. Anal. Chim. Acta 2001, 441, 281–289. [Google Scholar] [CrossRef]
- Gama, E.M.; Lima, A.D.S.; Lemos, V.A. Preconcentration system for cadmium and lead determination in environmental samples using polyurethane foam/Me-BTANC. J. Hazard. Mater. 2006, 136, 757–762. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Zachariadis, G.A.; Stratis, J.A. On-line preconcentration and determination of copper, lead and chromium(VI) using unloaded polyurethane foam packed column by flame atomic absorption spectrometry in natural waters and biological samples. Talanta 2002, 58, 831–840. [Google Scholar] [CrossRef]
- Azeem, S.A.; Arafa, W.A.A.; El-shahat, M. Synthesis and application of alizarin complexone functionalized polyurethane foam: Preconcentration/separation of metal ions from tap water and human urine. J. Hazard. Mater. 2010, 182, 286–294. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Arruda, M.A.Z. OnLine coupling of a flow injection system to TS-FF-AAS for preconcentration and determination of lead in water and vegetables. Anal. Lett. 2005, 38, 1427–1443. [Google Scholar] [CrossRef]
- Burham, N. Separation and preconcentration system for lead and cadmium determination in natural samples using 2-aminoacetylthiophenol modified polyurethane foam. Desalination 2009, 249, 1199–1205. [Google Scholar] [CrossRef]
- Lemos, V.A.; Lima, A.D.S.; Santos, J.S.; Castro, J.T.; Ferreira, S.L.C. Determination of lead in water samples after its separation and preconcentration by 4,5-dihydroxy-1,3-benzenedisulfonic acid functionalised polyurethane foam. Int. J. Environ. Anal. Chem. 2012, 92, 1121–1134. [Google Scholar] [CrossRef]
- Moawed, E.; Elhagrasy, M.; Kamal, M. Detection and removing of lead from wastewater using chemical treatment of polyurethane foam waste: Batch and column experiments. Desalin. Water Treat. 2019, 159, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Santos, W.N.L.D.; Santos, J.V.S.; Silva, L.O.B.; Araújo, A.S.; Lemos, V.A.; Miró, M.; Ferreira, S.L.C. On-line simultaneous pre-concentration procedure for the determination of cadmium and lead in drinking water employing sequential multi-element flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2011, 91, 1425–1435. [Google Scholar] [CrossRef]
- Burham, N.; Abdel-Azeem, S.M.; El-Shahat, F. Determination of lead and cadmium in tap water and apple leaves after preconcentration on a new acetylacetone bonded polyurethane foam sorbent. Int. J. Environ. Anal. Chem. 2008, 88, 775–789. [Google Scholar] [CrossRef]
- Sant’Ana, O.D.; Jesuino, L.S.; Cassella, R.J.; Carvalho, M.S.; Santelli, R.E. Determination of lead by electrothermal atomic absorption spectrometry employing a novel sampling strategy of polyurethane foam impregnated with thiazolylazo-p-cresol (TAC). J. Braz. Chem. Soc. 2004, 15, 96–102. [Google Scholar] [CrossRef]
- Ferreira, S.; Santos, W.; Bezerra, M.; Lemos, V.A.; Bosque-Sendra, J. Use of factorial design and doehlert matrix for multivariate optimisation of an on-line preconcentration system for lead determination by flame atomic absorption spectrometry. Anal. Bioanal. Chem. 2003, 375, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.A.; Guardia, M.D.L.; Ferreira, S.L.C. An on-line system for preconcentration and determination of lead in wine samples by FAAS. Talanta 2002, 58, 475–480. [Google Scholar] [CrossRef]
- Quináia, S.P.; Silva, J.B.B.D.; Rollemberg, M.D.C.E.; Curtius, A.J. Preconcentration of lead complexed with O,O-diethyl-dithiophosphate by column solid-phase extraction using different sorbents in a flow injection system coupled to a flame atomic absorption spectrometer. Talanta 2001, 54, 687–696. [Google Scholar] [CrossRef]
- Silva, J.B.D.; Quináia, S.P.; Rollemberg, M.C. On-line preconcentration with different solid adsorbents for lead determination. Fresenius J. Anal. Chem. 2001, 369, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, L.; Zhang, Z.; Sun, L.; Shi, Y.; Xie, L.; Xu, D.; Jin, J.; Xue, Z.; Ma, X. Synthesis of polyethyleneimine modified polyurethane foam for removal of Pb(II) ion from aqueous solution. Desalin. Water Treat. 2019, 160, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Muginova, S.V.; Veselova, I.A.; Parova, L.M.; Shekhovtsova, T.N. Enzymatic determination of cadmium, zinc, and lead in plant materials. J. Anal. Chem. 2008, 63, 1005–1014. [Google Scholar] [CrossRef]
- Burham, N.; Abdel-Azeem, S.M.; El-Shahat, M.F. Separation and determination of trace amounts of zinc, lead, cadmium and mercury in tap and Qaroun lake water using polyurethane foam functionalized with 4-hydroxytoluene and 4-hydroxyacetophenone. Anal. Chim. Acta 2006, 579, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Yeerum, C.; Wongwilai, W.; Grudpan, K.; Vongboot, M. Green assay of anionic surfactant via ion-association with methylene blue sorbed on polyurethane foam monolithic rod and using a smartphone. Talanta 2018, 190, 85–88. [Google Scholar] [CrossRef]
- Ayutthaya, P.I.N.; Yeerum, C.; Kesonkan, K.; Kiwfo, K.; Grudpan, K.; Teshima, N.; Murakami, H.; Vongboot, M. Determination of lead employing simple flow injection AAS with monolithic alginate-polyurethane composite packed in-valve column. Molecules 2021, 26, 4397. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Marchisio, P.; Sales, A.; Cerutti, S.; Marchevsky, E.; Martinez, L. On-line preconcentration/determination of lead in Ilex paraguariensis samples (Mate tea) using polyurethane foam as filter and USN-ICP-OES. J. Hazard. Mater. 2005, 124, 113–118. [Google Scholar] [CrossRef]
- Bowen, H.J.M. Absorption by polyurethane foams; new method of separation. J. Chem. Soc. A 1970, 1082–1085. [Google Scholar] [CrossRef]
- Kocyła, A.; Pomorski, A.; Krężel, A. Molar absorption coefficients and stability constants of metal complexes of 4-(2-pyridylazo)resorcinol (PAR): Revisiting common chelating probe for the study of metalloproteins. J. Inorg. Biochem. 2015, 152, 82–92. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Amano, Y.; Machid, M. Enhanced lead(II) binding properties of heat-treated cattle-manure-compost-activated carbon. Desalin. Water Treat. 2014, 52, 6420–6429. [Google Scholar] [CrossRef]
- Shrivas, K.; Sahu, B.; Deb, M.K.; Thakur, S.S.; Sahu, S.; Kurrey, R.; Kant, T.; Patle, T.K.; Jangde, R. Colorimetric and paper-based detection of lead using PVA capped silver nanoparticles: Experimental and theoretical approach. Microchem. J. 2019, 150, 104156. [Google Scholar] [CrossRef]
- Nonova, D.; Evtimova, B. Complex formation of nickel(II) and cobalt(II) with 4-(2-pyridylazo)-resorcinol. Anal. Chim. Acta 1972, 62, 456–461. [Google Scholar] [CrossRef]
- Nonova, D.; Evtimova, B. Complexing of Iron(II) and Iron (III) by 4-(2-pyridylazo) resorcinol. J. Inorg. Nucl. Chem. 1973, 35, 3581–3586. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants Volume 2; Plenum Publishing Corporation: New York, NY, USA, 1975; pp. 178–179. [Google Scholar]
- Gomez, E.; Estela, J.M.; Cerda, V. Simultaneous spectrophotometric determination of calcium and magnesium in water. Anal. Chim. Acta 1991, 249, 513–518. [Google Scholar] [CrossRef]
- Saeidi, M.; Shamsipur, M. Complex formation between alkaline earth cations and 4-(2-pyridylazo)resorcinol in ethanol water mixtures. J. Coord. Chem. 1990, 22, 131–137. [Google Scholar] [CrossRef]
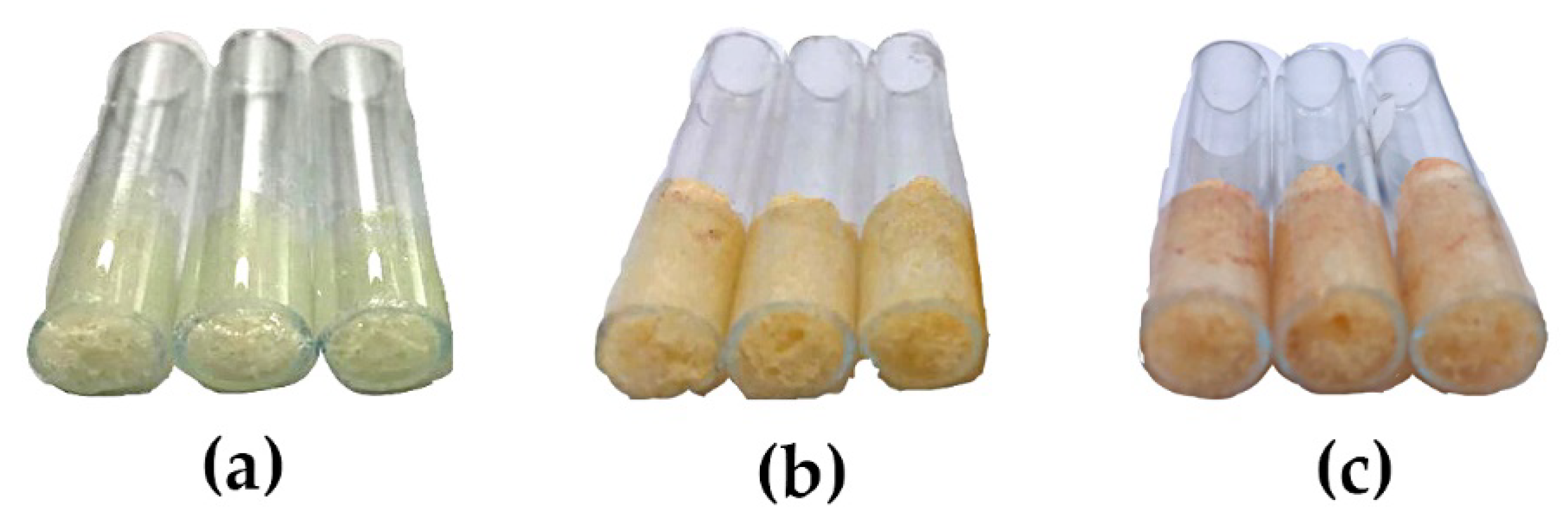
 ), B (
), B ( ), C (
), C ( ), D (
), D ( ), E (
), E ( ), F (
), F ( ).
).
 ), B (
), B ( ), C (
), C ( ), D (
), D ( ), E (
), E ( ), F (
), F ( ).
).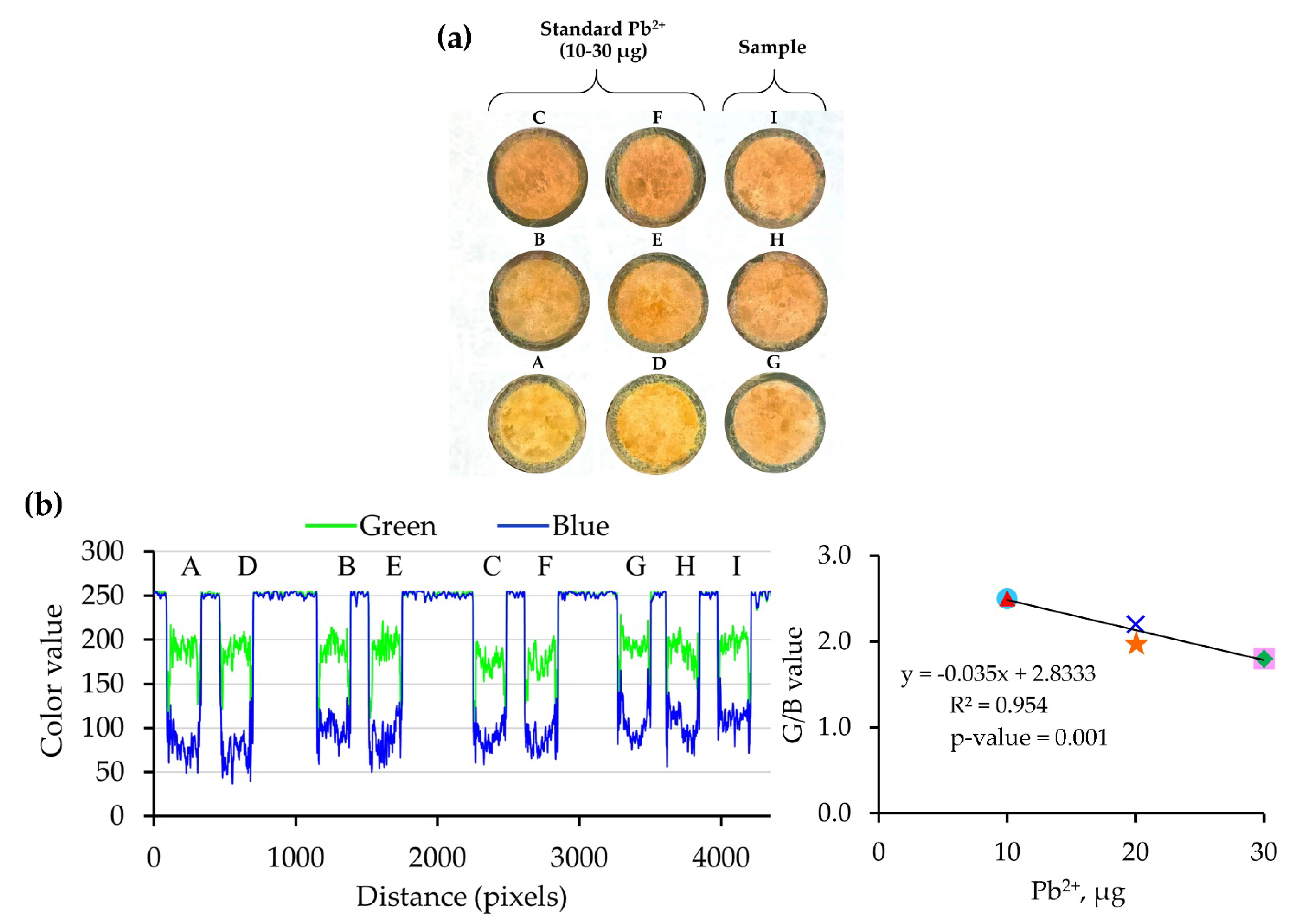

| Position (in Figure 2) | Loading Pb2+ | Intensity | G/B value c | Pb2+ found | ||||
|---|---|---|---|---|---|---|---|---|
| µg | µg mL−1 | Loading volume(mL) | G value | B value | µg a | Concentration (µg mL−1) | ||
| A | 10 | 0.2 | 50 | 200 | 80 | 2.5 | − | − |
| D | 10 | 0.5 | 20 | 200 | 80 | 2.5 | − | − |
| B | 20 | 0.1 | 200 | 200 | 100 | 2.0 | − | − |
| C | 20 | 0.1 | 200 | 200 | 90 | 2.2 | − | − |
| E | 30 | 0.3 | 100 | 170 | 95 | 1.8 | − | − |
| F | 30 | 0.5 | 60 | 170 | 95 | 1.8 | − | − |
| I | − | − | 40 | 200 | 90 | 2.2 | 18 | 0.4 b |
| H | − | − | 50 | 195 | 105 | 1.9 | 27 | 0.5 b |
| G | − | − | 70 | 200 | 115 | 1.7 | 32 | 0.4 b |
| Sample | Monitoring site a | Added Pb2+ std (µg L−1) | Found Pb2+ | tobserved d | |||
|---|---|---|---|---|---|---|---|
| Proposed method (n = 3) | ICP-OES (n = 3) | ||||||
| µg c | µg L−1 | % Recovery | µg L−1 | ||||
| S1 (Dindaeng) | 13° 46′ 12.80644″ N, 100° 33′ 31.986″ E | − | ND | − | − | ND | 2.70 |
| 50 b | 18 ± 2 | 60 ± 7 | 120 | 49 ± 0.4 | |||
| S2 (Bangkapi) | 13° 46′ 7.2408″ N, 100° 38′ 30.03″ E | − | ND | − | − | ND | 1.22 |
| 50 b | 18 ± 5 | 60 ± 17 | 120 | 48 ± 0.4 | |||
| S3 (Huai Khwang) | 13° 48′ 1.1916″ N, 100° 35′ 1.41″ E | − | ND | − | − | ND | 0.79 |
| 50 b | 15 ± 4 | 50 ± 13 | 100 | 44 ± 0.8 | |||
| S4 (Thungkru) | 13° 38′ 58.182″ N, 100° 29′ 46.9896″ E | − | ND | − | − | ND | 2.07 |
| 50 b | 18 ± 3 | 60 ± 10 | 120 | 48 ± 0.4 | |||
| Amount of Pb2+ (μg) | Concentration of Pb2+ (μg mL−1) | Loading Volume (mL) | G/B a ± SD b |
|---|---|---|---|
| 10 | 0.1 | 100 | 2.5 ± 0.3 |
| 10 | 0.2 | 50 | 2.5 ± 0.3 |
| 10 | 2.0 | 5 | 2.6 ± 0.2 |
| 20 | 0.2 | 100 | 2.1 ± 0.2 |
| 20 | 0.5 | 40 | 2.1 ± 0.3 |
| 20 | 2.0 | 10 | 2.0 ± 0.1 |
| 30 | 0.3 | 100 | 1.7 ± 0.1 |
| 30 | 0.5 | 60 | 1.8 ± 0.1 |
| 30 | 2.0 | 15 | 1.8 ± 0.1 |
| 40 | 0.5 | 80 | 1.6 ± 0.2 |
| 40 | 2.0 | 20 | 1.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issarangkura Na Ayutthaya, P.; Yeerum, C.; Kesonkan, K.; Kiwfo, K.; Grudpan, K.; Teshima, N.; Murakami, H.; Vongboot, M. Lead Assays with Smartphone Detection Using a Monolithic Rod with 4-(2-Pyridylazo) Resorcinol. Molecules 2021, 26, 5720. https://doi.org/10.3390/molecules26185720
Issarangkura Na Ayutthaya P, Yeerum C, Kesonkan K, Kiwfo K, Grudpan K, Teshima N, Murakami H, Vongboot M. Lead Assays with Smartphone Detection Using a Monolithic Rod with 4-(2-Pyridylazo) Resorcinol. Molecules. 2021; 26(18):5720. https://doi.org/10.3390/molecules26185720
Chicago/Turabian StyleIssarangkura Na Ayutthaya, Piyanat, Chonnipa Yeerum, Kullapon Kesonkan, Kanokwan Kiwfo, Kate Grudpan, Norio Teshima, Hiroya Murakami, and Monnapat Vongboot. 2021. "Lead Assays with Smartphone Detection Using a Monolithic Rod with 4-(2-Pyridylazo) Resorcinol" Molecules 26, no. 18: 5720. https://doi.org/10.3390/molecules26185720
APA StyleIssarangkura Na Ayutthaya, P., Yeerum, C., Kesonkan, K., Kiwfo, K., Grudpan, K., Teshima, N., Murakami, H., & Vongboot, M. (2021). Lead Assays with Smartphone Detection Using a Monolithic Rod with 4-(2-Pyridylazo) Resorcinol. Molecules, 26(18), 5720. https://doi.org/10.3390/molecules26185720








