Well-designed mathematical models of in-vivo processes may be used not only as surrogates of in-vivo experiments but they provide a significantly larger piece of information and deeper knowledge than mere in-vivo experiments. This allegation follows from the generality and flexibility of mathematical modelling. This is the reason that, in this study, the philosophy of compartmental pharmacokinetic modelling was adopted.
3.1. Structure of the Model
The specific structure of any compartment model follows from the preliminary analysis of in-vivo measurements. To synthetize a model which would possess sufficient predictive accuracy of the drug amounts in particular parts of the body is a rather difficult task. Practical experience shows that the model with too many tuneable parameters may be rather fragile in the sense that it may accurately predict the system behaviour for one set of the input data but does a poor job for other inputs. Such a model may be too sensitive to even small variations of its structure and/or parameters.
A passable way to overcome some of these problems may be to design a sufficiently parsimonious model structure, which would support the maximal likelihood of the physiologically acceptable hypothesis about the behaviour of the original. According to the principle of parsimony, the model should be maximally descriptive and simple. The model should be designed in accord with the Rule I. of Newton’s Principia, which says: We are to admit no more causes of natural things than those which are both true and sufficient to explain their appearances [
6].
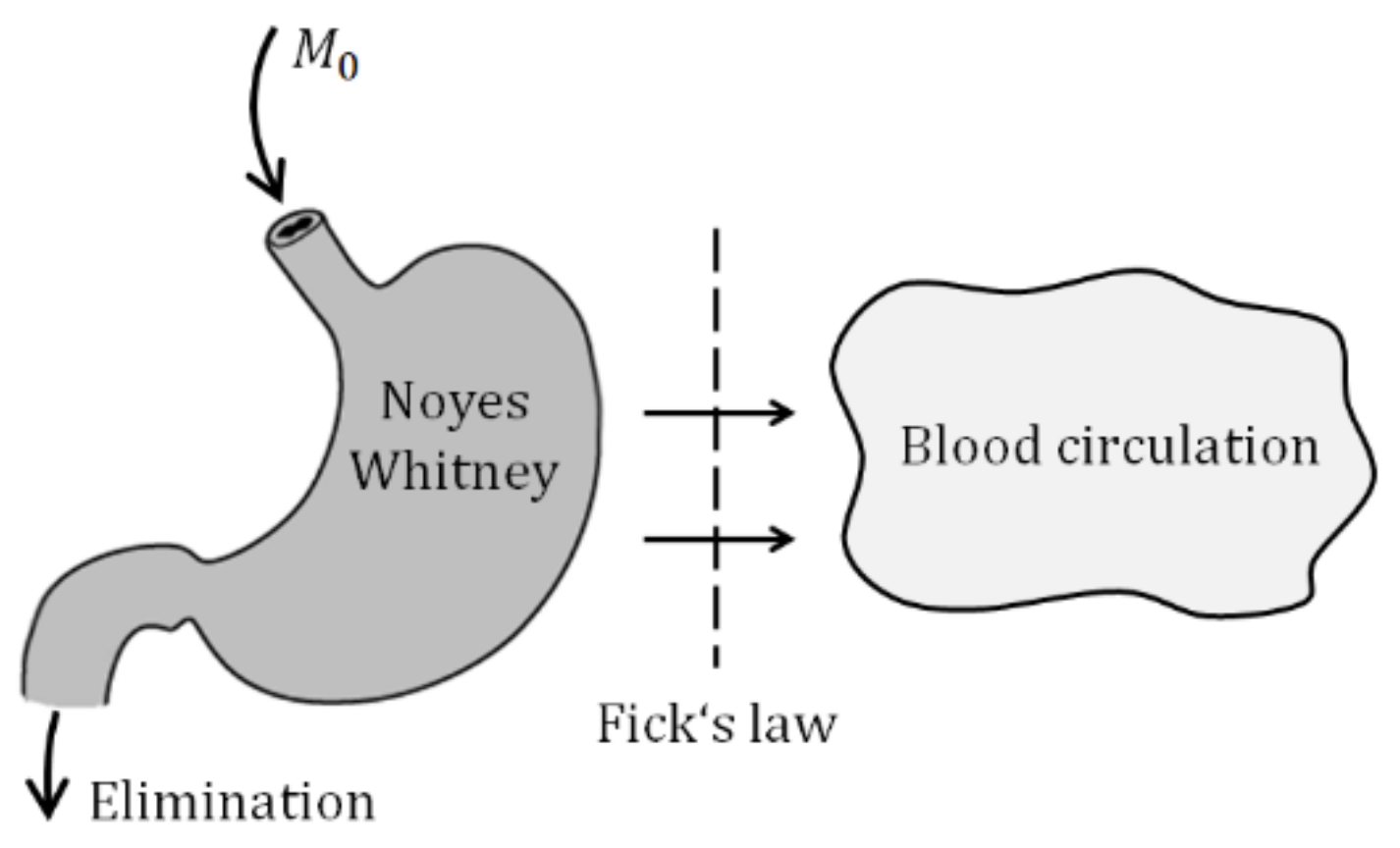
A stepping stone of the model derivation is illustrated by two figures and two basic laws describing the drug release and absorption. The solid particles contained in the suspension administrated into the gastrointestinal tract (GIT) must first dissolve and only then they can be absorbed. In parallel with the drug liberation, it is gradually absorbed into the blood circulation and partially eliminated from the body (
Figure 3).
The liberation of solid particles in solutions is generally approximated by Noyes–Whitney equation [
1].
The M means the mass of particles, dM/dt is a rate of liberation, CS is a saturated concentration at the close vicinity around the particle, C is the concentration of the bulk solution, h is a thickness of the saturated layer around the particle, A is a surface area of the particle, D is a diffusion coefficient. As the mass is equal to the product of the volume and concentration, Equation (1) is a first-order differential equation indicating that the liberation obeys the first-order dynamics.
In parallel with the liberation of particles, the drug diffuses through the biological membrane into the blood circulation. The process is commonly described by the Fick law.
The rate of the diffusion
dM(t)/dt is proportional to the concentration gradient
dC/dx, which is commonly approximated by the difference of concentrations between the two sides of the biological membrane. Therefore, one can write (2) in the form (3), which says that the diffusion obeys the first-order dynamics as well.
Symbols
m1 and
m2 are drug amounts and
V1,
V2 are volumes of distribution. The constant parameters in (3) are commonly lumped into the membrane permeability
G = DA/d, which is experimentally determinable. The Equation (3) is illustrated in
Figure 4.
Equations (2) and (3) describe two essential sub-processes, namely the drug liberation which is followed by the diffusion through a biologic membrane. These sub-processes drive the drug into motion throughout the body. From the perspective of modelling, Equations (11) and (3) decide about the structure of the future compartment model.
3.3. State-Space (Internal) Model
The paper uses a linear compartment model with a single input and single-output (SISO) model. It is generally described by the set of linear differential Equations (4). By convention, the matrices are typed by the ordinary upper-case fonts while the lower-case bolt fronts are reserved for vectors. The state vector
x has
n components―the number of compartments, and components of
x are concentrations or amounts of drug in particular compartments.
Meanings of denotations:
x(t) is a vector of the state variables, e.g., drug amounts or concentrations at the time t
x(t0) is an initial state of the system, i.e., a vector of initial values of the state variables
u(t) is an input to the system, e.g., the time profile of the drug amount delivered into the body
y(t) is an output from the system, e.g., a drug amount or concentration in the output compartment
A is a system matrix―a relation between the state and its time derivative
b and c are control and observation vectors, respectively
cT is the transposition of c, hence cT is a row vector
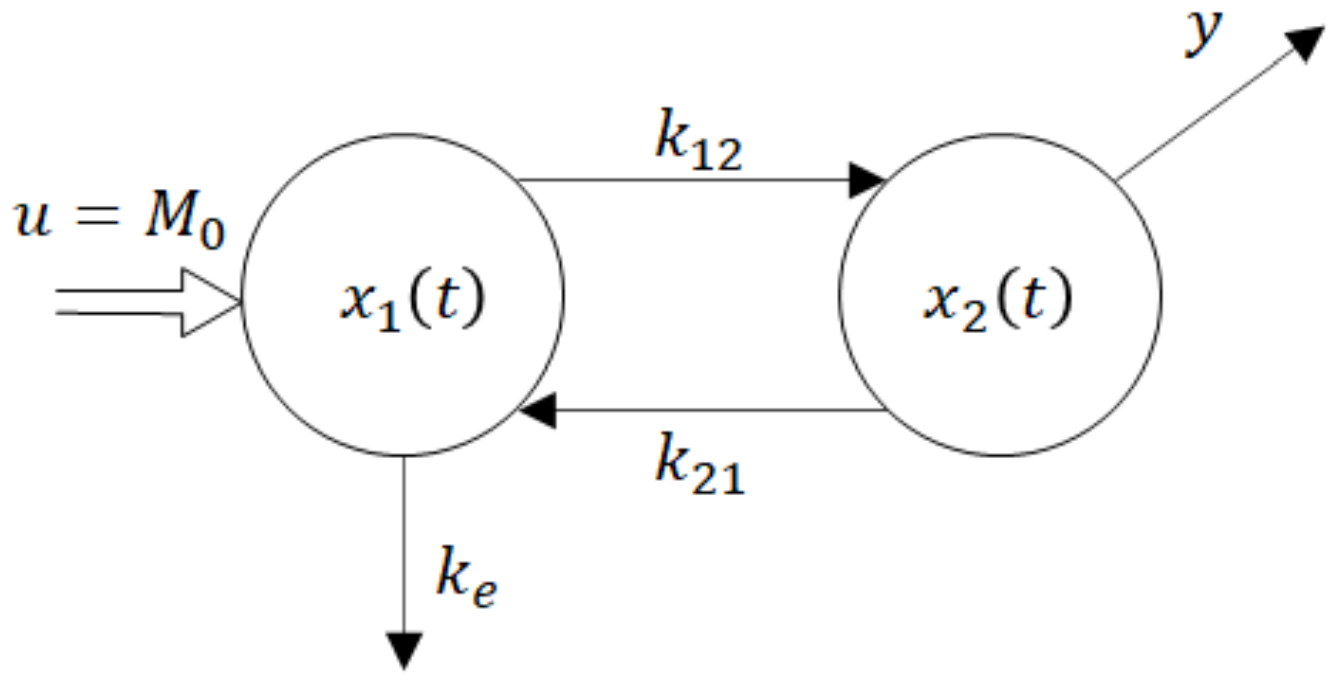
As was said earlier, every compartment model should be physiologically substantiated. The compartment models belong to the category of so-called positive systems, i.e., the systems whose output
y and all components of the vector
x, as responses to any positive (nonnegative) input
u (e.g., the dose administrated) must be positive (nonnegative). That is a natural requirement as to the drug amounts/concentrations, as well as the rates of the drug flows between compartments are always nonnegative. As a consequence, the state trajectory
x(t) starting at the time
t0 from a nonnegative initial state vector
x(t0) must remain consistently nonnegative. It can be shown that this happens if and only if the vectors
b and
c are component-wise nonnegative and the matrix A is the so-called Metzler matrix, i.e., a non-zero matrix with nonnegative off-diagonal entries [
13,
14,
15]. Let us note that the requirement of the system positivity significantly hardens the synthesis of possible control algorithms, e.g., the dosing regimes.
So as the state-space model (4) to be a true representative of a compartment model should match the theory of positive and compartment systems [
15,
16]. In the realm of drug design, this means that the fulfilment of conditions (5) must be checked during the design of the model’s structure.
Remark 1. Clearly, the drug concentration in an extravascular site of administration is not accessible for measurements. This is one of the reasons why the researchers sometimes evaluate the absorption from the in-vitro 3. Strictly speaking, the (in-vivo) rate of absorption cannot be directly evaluated either from the in-vitro or in-vivo experiment. The only passable way of evaluating the rate of absorption leads through its prediction by a mathematical model.
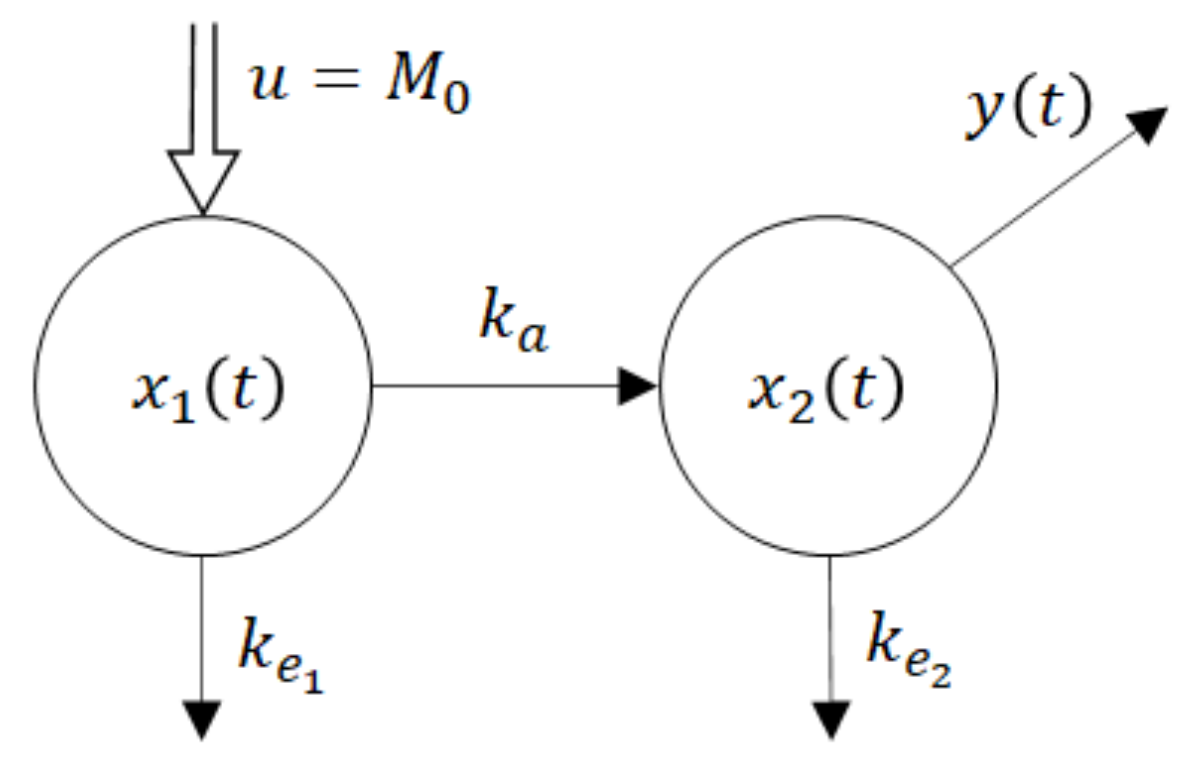
One of the parsimonious models solving this problem is shown in Figure 5. Obviously, its structure is physiologically acceptable as the drug flows from the site of administration―the GIT into the central compartment―the blood. However, at the same time, this fact is the model’s beauty bug as to the model does not quite correspond to the Ficks law, according to which the rate of the drug flow through the membrane is proportional to the difference between concentrations (amounts) at the opposite sides of the membrane. Namely, in accordance with the law of donor control, the rate of absorption from the GIT equals the product (
x1(t) ×
ka).
However, in the case of the model shown in Figure 5, it means that the rate of absorption does not depend on the drug amount x2(t) in the blood compartment. In the cybernetic parlance, one would say that the rate of absorption “lacks the feedback information” about the current drug amount in the acceptor compartment. As a consequence, the rate of absorption is not influenced by the drug amount x2(t).
Clearly, in reality, such a feedback always exists. No wonder that in some cases the authors approximate the rate of absorption by the exponential K ×
exp (−kat), which in general is not quite correct, especially for the special drug forms like the transdermal patches, chewing gums, etc. To put things in the right place, in the Appendix A, another compartment model is briefly mentioned, which perfectly matches the law of donor control. However, contrary to the previous case, its physiological correctness may be disputable for a simple reason: Under normal (healthy) conditions the drug does not flow from the blood circulation into the content of the stomach and intestine. Nevertheless, for the purposes of this study, both models are quite good as they generate exactly the same values of the absorption rate constant. The reasons are rather deep, and we omit here the related discussion. End of the remark 1.
u = M0 is a system input―an instantaneously administered dose
ke1, ke2 are rate constants of the drug elimination
x1(t) is a time course of the drug amount in the peripheral compartment―the GIT
x2(t) is a time course of the drug amount in the central compartment—the blood
y(t) is a system output―the observed time course of the drug amount
The dynamic behaviour of the compartment model in
Figure 5 is described by the state-space model (6). For the sake of notational simplicity, the time dependence of variables is not explicitly expressed.
As indicated earlier, an important issue that ought to be examined is identifiability of the matrix A, i.e., the determination of numerical values of its entries from in-vivo samples. However, the crux of the problem lies in the fact that in-vivo samples bear only information about the output
y as a response to an input
u, and nothing more. Information about the state variables
x1,
x2 is not included in these samples. The reason is simple: The state variables
x1,
x2 are in general artificial quantities, which may not have a realistic interpretation. Their meanings are defined by the model’s structure which was chosen by the designer. Hence, to resolve the problem of identification of the matrix A one should make a record of an input–output model (I/O model). We decided to use the I/O model in the form of the transfer function
G(s), which is the ubiquitous concept in the realm of system analysis. The
G(s) is defined by the ratio of the image
y(s) of the output
y(t) to the image
u(s) of the input
u(t). Omitting the detailed explanations, we only declare that the symbol “
s” is a certain complex variable. The transfer function
G(s) of the system (4) can be computed in accordance with Expression (7).
Application of Expression (7) on Equations (6) gives (8).
After doing requested inversion and multiplications one obtains (9).
Finally, after doing the product in the denominator of (9) one will obtain (10).
The reason for using different denotations for parameters in (10), namely (
b0,
a0,
a1) and (
ka,
ke1,
ke2) is that while the former were identified (!) from the in-vivo samples, the latter were calculated (!) from already known values of the coefficients
b0,
a0, and
a1). Comparing the homothetic coefficients in the numerator and denominator in (10) the following relations will be obtained:
Solutions to the Equation (11) for unknown rate constant
k12,
k21 and
ke take the form (12).
If it would be possible to find unique values of rate constants ke1, ke2, ka, for already known values of the parameters b0, a0, a1, the compartment model (6) would be parametrically identifiable. However, looking at the second expression in (12), it is clear that there exist two possible solutions for ke2. Hence, the compartment model (6) is not uniquely identifiable.
Remark 2. The dose u = M0 in Figure 5 corresponds to the instantaneous administration of 50 mg of sulfathiazole. Let us recall that the computer implementation of the dose u may be either in the form of the function u(t) = 50 δ(t) mg, where δ(t) means the Dirac unit function [15], or in the form of the initial condition x1(0) = 50 mg though the latter alternative may seem to be more natural (because the suspension Though the latter alternative may seem to be more natural (because the suspension was instantly inserted into the stomach), this paper prefers the first one. A reason is that the drug must first transit from the stomach into the intestine where it is dissolved, and only then it can be absorbed. In other words, the drug amount in the GIT―x1(t) in (6) cannot instantly jump to the initial value X1(0) = 50 mg. Using the initial condition in the role of the input can be fully accepted in the case of an instantaneous intravenous administration but not in the case discussed here. As to the Dirac function δ(t) is defined as an infinitely short and infinitely high impulse with unit surface area, the input u(t) = 50 δ(t) was approximated by a very thin and very high rectangle of the surface area equal to 50 units. In particular, the following dimensions of this rectangle were used: width × height = 0.083333 × 600 m = 50 h·mg. End of remark 2.
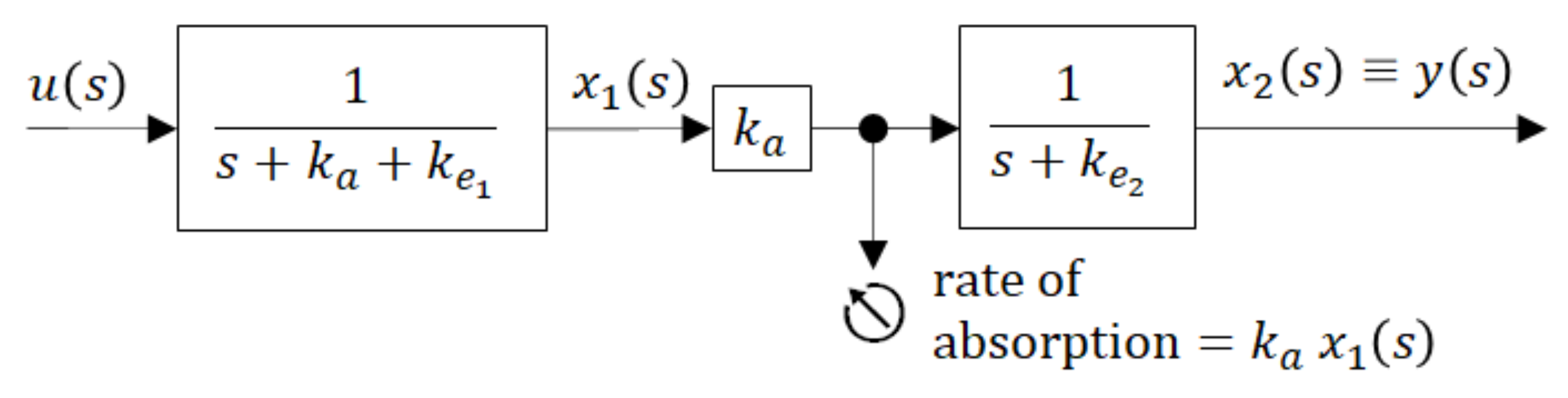
To gain better insight into the inner dynamics of the compartment model shown in
Figure 5 it may be illustrative to present it by the blocking scheme with partial transfer functions (in rectangles) as shown in
Figure 6.
The block scheme shown in
Figure 6 is an elegant representation of the compartment model. As a serial connection of partial transfer functions, it distinguishes between the dynamics of the individual compartments and shows the directions of causality. The global transfer function
G(s) is given by the product of partial transfer functions. It is obviously the same as it is in (9), but it explicitly indicates that the sum (
ka +
ke1) exclusively influences the dynamic behaviour of the 1st compartment, while the dynamics of the 2nd compartment is exclusively influenced by
ke2. The lager is the sum (
ka +
ke1) the more quickly the 1st compartment responds to the input
u(t).
Parameters
b0,
a0,
a1 were identified from in-vivo measurements and the following results were obtained:
b0 = 0.030272,
a0 = 0.230137,
a1 = 1.018397. With parameters
b0,
a0,
a1 identified, the rate constants
ka,
ke1,
ke2 were calculated in accordance with (12) and two different sets of solutions were obtained:
Though values of ka are the same in both sets, values of ke1 and ke2 are different. The existence of two different sets of solutions means that model (6) is parametrically un-identifiable. Nevertheless, this fact is not relevant for the purposes of this paper, as information about the rate of absorption is exclusively carried by the absorption rate constant ka, which was uniquely identified. Keeping in mind that the major route of the drug elimination is renal excretion, in case of need a passable way to determine which of these two sets of solutions is correct can lead through evaluation of the drug amount excreted into the urine. In that case, it would be enough to evaluate the half-time (t1/2) and then use the known relation ke1 = 0.693/t1/2.
3.4. Prediction of the Tenside’s Influence on the Rate of Absorption
In
Section 2, based on the in-vivo samples without added tensides, the compartment model was designed and parametrically identified allowing determination of the rate of absorption from the intestine into the blood circulation. In this section, the parameters of the same model will be identified, but for the drug with the added tenside.
The in-vivo samples of the drug with the tenside are given in
Table 1 (or by the squares in
Figure 1). Based on them, the transfer function (10) was identified and the following values were obtained:
As before, using (12) were computed the following sets of model parameters:
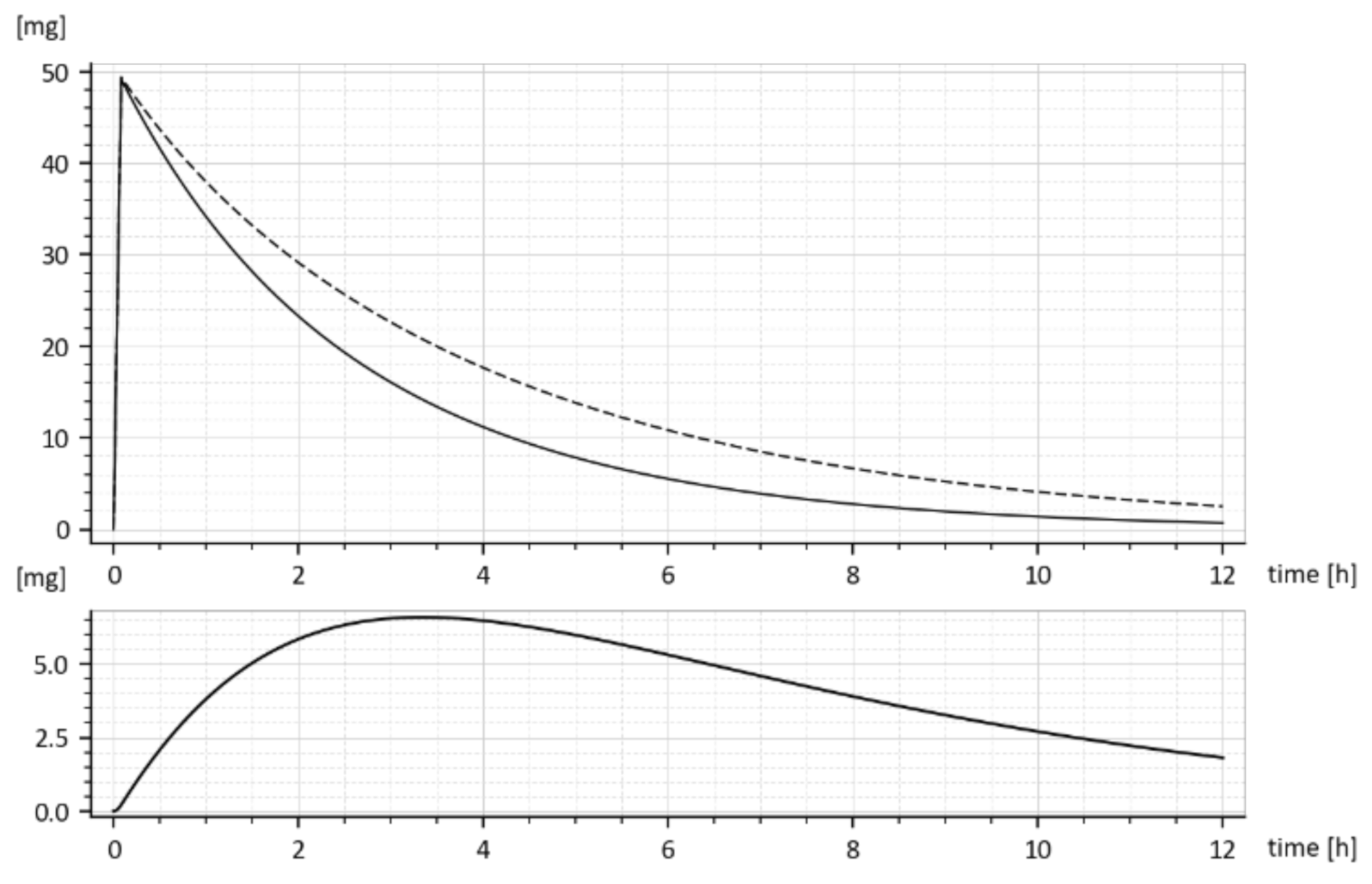
The dashed curve in
Figure 7 represents the time course of the drug amount in the blood that was predicted by the model with parameters (15). Obviously, the time course is the same as the upper curve in
Figure 1, though these curves were obtained in different ways. Namely, the upper (dashed) curve in
Figure 7 was predicted by the model while the upper (dashed) curve in
Figure 1 was obtained by the in-vivo measurements. Their sameness proves that both sets of parameters (15) are correct and validates the suggested compartment model.
Let us look at
Figure 1. While
Figure 1 shows the results of in-vivo measurements, the dotted curve in
Figure 7 represents the predicted amounts of the drug with the tenside. The difference between corresponding absorption rate constants
ka is a measure of the strength of the tenside’s influence. As follows from (13) and (15), the 0.001% solution of tenside increased absorption rate constant
ka from 0.030272 h
−1 to 0.045815 h
−1, that is by 51.34%.
The
model predicted drug amounts it the GIT, i.e., the
x1(t), without and with the tenside are shown in
Figure 8. The dashed curve corresponds to parameters (
ka = 0.030272 h
−1 ke1 = 0.649656 h
−1 ke2 = 0.338477 h
−1) while the full one corresponds to the increased absorption rate constant
ka, namely (
ka = 0.045815 h
−1,
ke1 = 0.959041 h
−1,
ke2 = 0.242913 h
−1).
One can read, from
Figure 8, the free drug amount
x1(t) (dashed curve) at a particular time instant, say
t = 2 h was
cca 28 mg, but after the addition of the tenside (full curve), it decreased to
cca 25 mg. The decrease is due to faster outflow from the 1st compartment caused by the increased
ka.
Let us note that
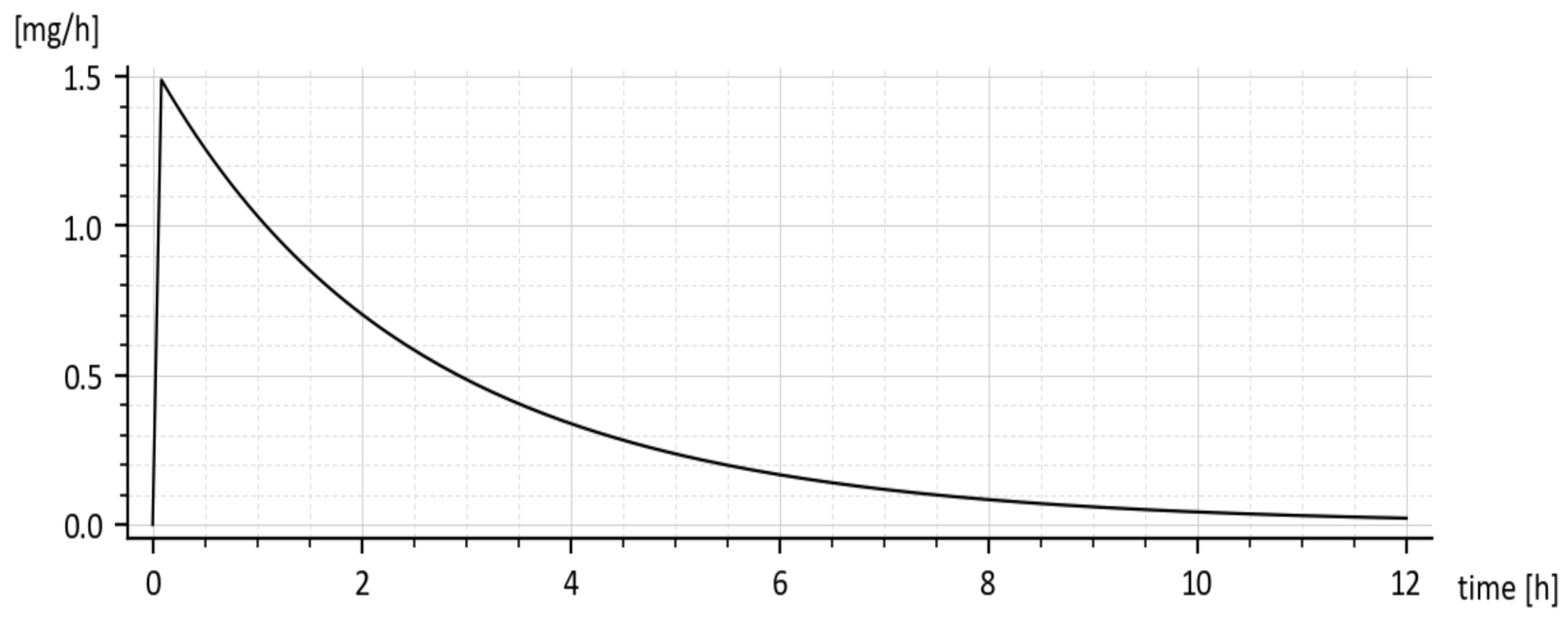
x1(t) represents the drug amount in the peripheral compartment (GIT) and for that reason, it cannot be measured in-vivo. It can be only predicted by the compartment model (6). The same goes for the predicted rate of absorption given by Expression (16).
The predicted rate of absorption with added tenside is shown in
Figure 9. It copies the global shape of
x1(
t) without tenside.
The reader is invited to look that the initial onset of the curves in
Figure 8 and
Figure 9. The curves emanate from the origin (0, 0) and slightly deviate from the vertical axis. It is caused by using the input
u(t) = 50 δ(
t)mg rather than the initial condition
x1(0) = 50 mg. The deviation is natural and indicates that the drug amount cannot jump instantly to its initial value.
In the end, it would be worth comparing the predicted results with those obtained in-vivo. As follows from
Table 1 and
Figure 1, the maximal increase in the drug amount in the blood was caused by the added tenside, which appears at the time
t = 2 h. The drug in the blood increased from 1.093 to 1.403 mg that is by 28.4%. On other hand, in accordance with
Figure 2, (which shows results of the quite independent experiment), the added tenside caused the maximal increase from 1.1 mg to 1.4 mg that is by 27.2%. Taking into account the measurement inaccuracy, the difference between the increase of 28.4% and the increase of 27.2% is virtually insignificant. These findings are another proof of the model’s validity.
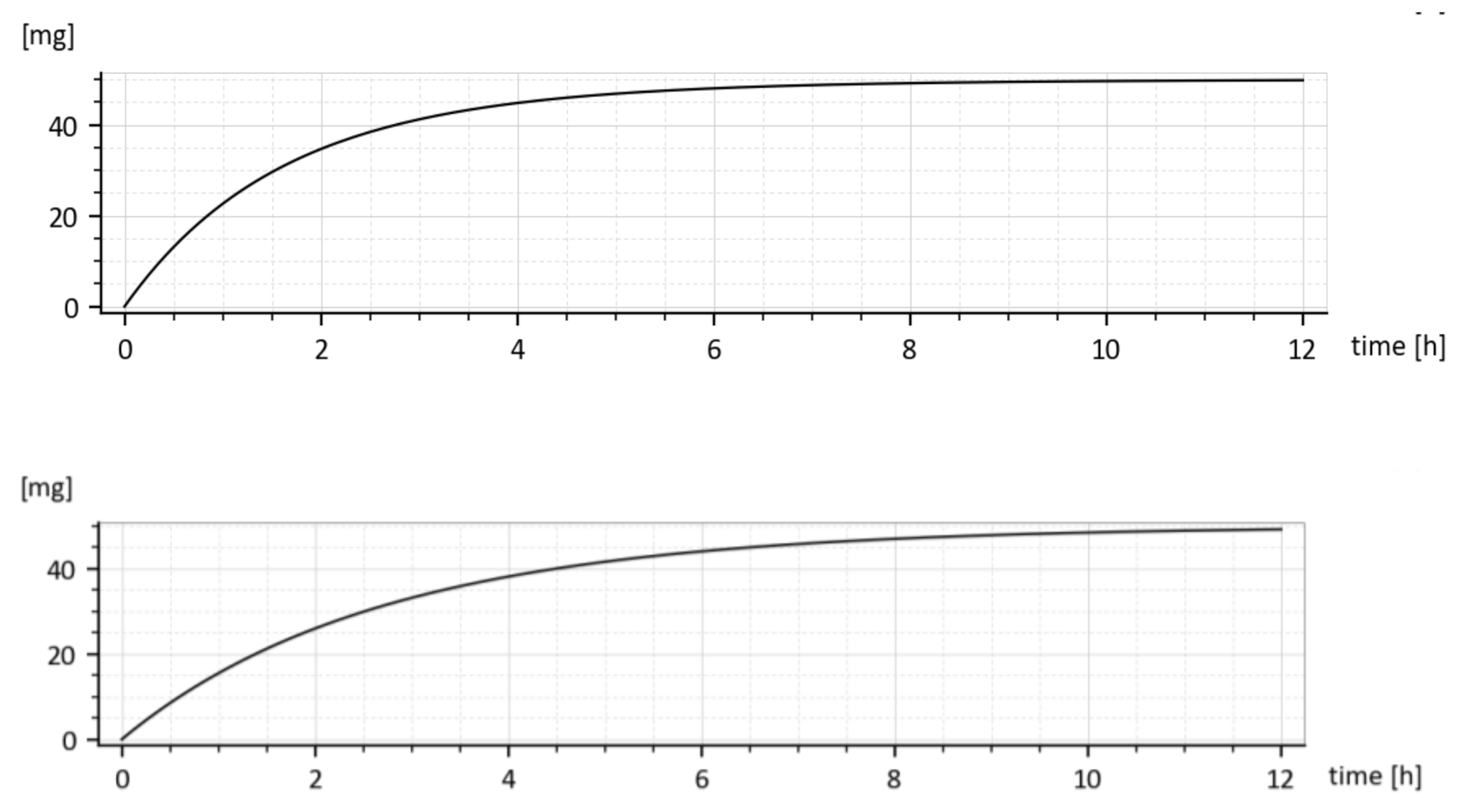
3.4.1. Cumulative Eliminated Amounts in Time
As it was already said, the fact that two different values for every elimination constant
ke1 and
ke2 were identified is irrelevant w.r.t. purposes of the paper. This is demonstrated in
Figure 10 showing cumulative drug amounts which left the 1st compartment in the time
t, (given by the integral
for two different parameter sets. Let us consider the parameter sets (15). As shown in
Figure 10, both cumulative amounts converge to the administrated dose
M0 = 50 mg, though their shapes are slightly different.
Clearly, the drug leaves the 1st compartment more quickly for the upper triplet of (15) because the sum (ka + ke1) is larger than in the lower triplet.
3.4.2. Total Amount of Absorbed Drug and Absolute Bioavailability
The total amount of the absorbed drug is given is by the time integral (17).
The time dependences
x1(t) for the drug with and without added tenside are shown in
Figure 8. Due to the added tenside, the absorption rate constant
ka increased from 0.030272 h
−1 to 0.045815 h
−1, i.e., by 51.34447% and the total absorbed amount (18) increased virtually two-fold, namely from 4.384808 mg to 8.919179 mg.
Proportionally to that, the absorbed fraction F of the administered dose M0, which is nothing else than the absolute bio-availability defined by (19), increased virtually two-fold, namely from 8.76964% to 17.83835%. Hence, the presence of surfactant significantly increases the bioavailability of sulfathiazole from the suspension. Similarly, it was found that the addition of 0.001% solution of the tenside increased the rate constant ka by cca 51.34%