2.1. Preliminary Contact Angle Measurement Results
Indiana limestone samples were cleaned using toluene and heated for three days in the oven at 50 °C to remove the remaining toluene. Samples were then saturated with crude oil and oil-aged in the oven at 90 °C for more than 15 days. All of the oil-aged samples were then treated with surfactants solutions. Finally, CA measurements were conducted on the samples (
Figure 1).
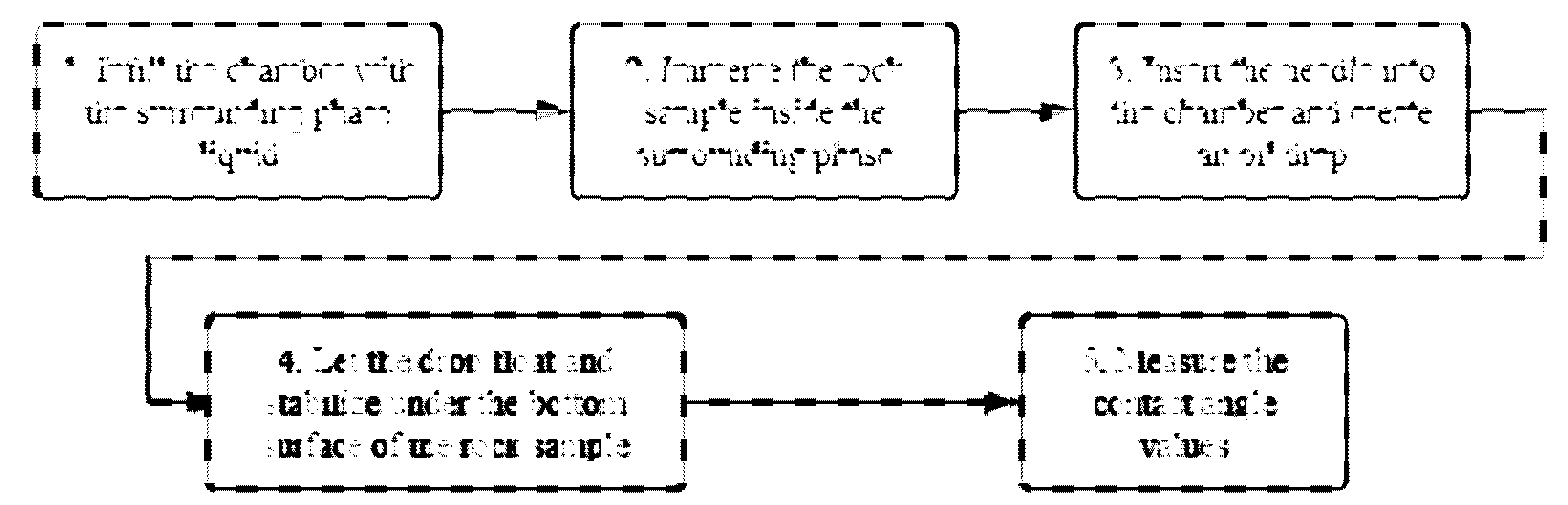
In the preliminary measurements, the process for CA measurement is arranged in this way:
Infill the liquid chamber with the surrounding liquid phase.
Immerse the rock sample inside the surrounding phase.
Insert the needle into the chamber and generate an oil drop.
Let the drop float and stabilize under the bottom surface of the rock sample.
Measure the contact angle values.
A batch of 331 samples was used. At least two measurements were conducted for each sample. After that, the average CA value and the corresponding standard deviation were calculated for each sample. Standard deviation (SD) was calculated to represent the measurement error. A dataset of 331 points, the preliminary dataset, was obtained.
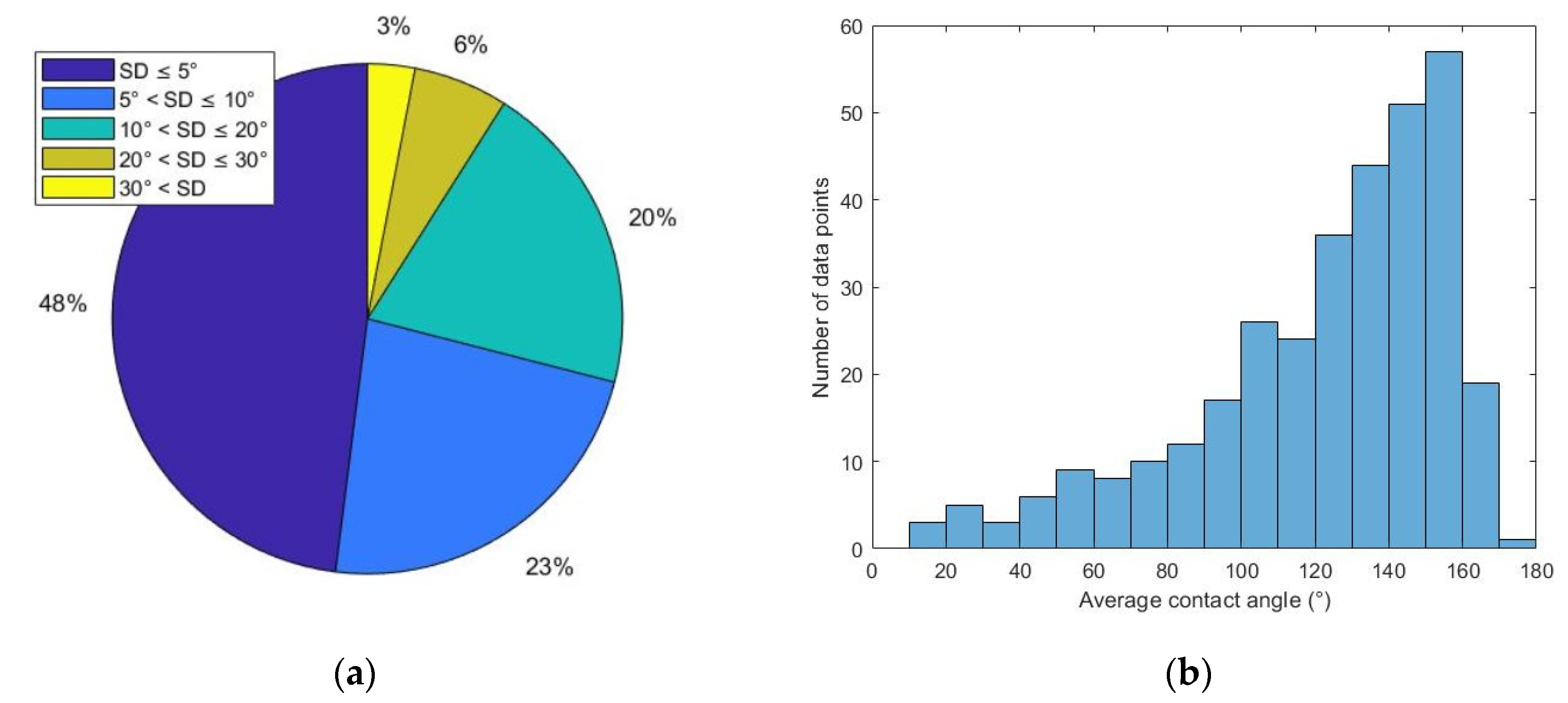
Figure 2a is a pie chart showing the percentage of data with different SD ranges. A total of 161 points (48% of total) have SD less than 5°. A total of 75 points (23% of total) have SD values in the range of 5°~10°. A total of 66 points (20% of total) have SD values in the range of 10°~20°. A total of 20 points (6% of total) have SD values in the range of 20°~30°. Nine points (3% of total) have SD values in the range of 30°~60°.
Figure 2b shows that most of the CA values are in the range of 90°~180°. There are 53 points in the range of 0°~90° and 278 points in the range of 90°~180°. This unbalanced distribution can be explained by the experimental condition. Samples were oil-aged to oil-wet or strongly oil-wet conditions. Then, they were treated with surfactant solutions that have poor to moderate wettability alteration (WA) performance. Most of the surfactant-treated samples had intermediate-wet to oil-wet wettability after treatment. It is reasonable that the resulting contact angle values are more concentrated in the range of 90°~180°.
52% of the collected data has a standard deviation larger than 5°, indicating that the preliminary dataset has moderate to large error. Since the oil drop spreading phenomenon was frequently observed during measurements, a study on the connection between measurement error and the oil drop spreading phenomenon was conducted.
2.2. A Study on The Oil Drop Spreading Phenomenon
During the measurements, it was frequently observed that the oil drop spread on the rock surface. This phenomenon can indicate oil-wetness [
35,
36]. However, in our study, it was observed on samples that showed water-wetness. There seemed no or weak connection between the phenomenon and surface wettability. Besides, the oil drop could spread out till it became a thin oil film. It causes measurement difficulties or even failure due to the lack of an ellipsoidal droplet.
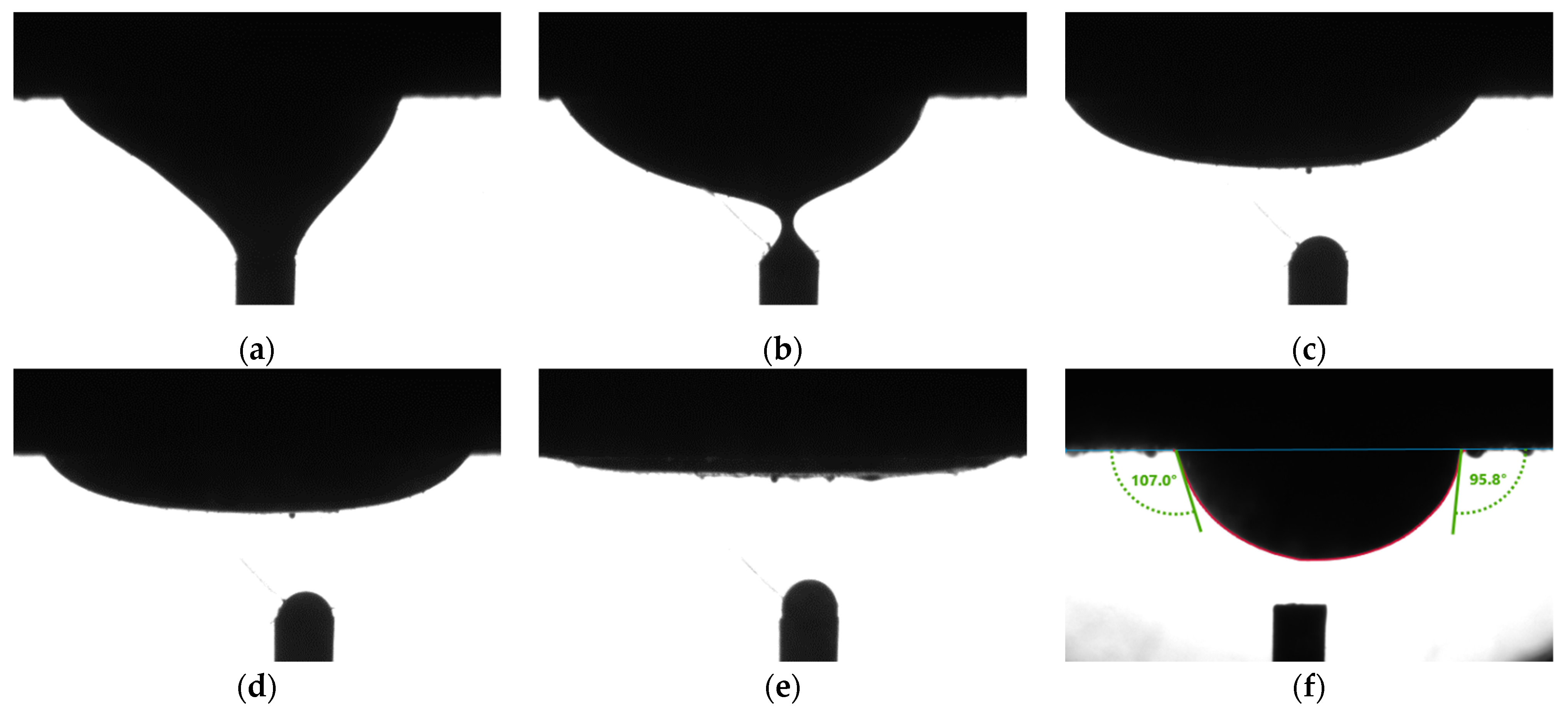
Figure 3a to
Figure 3c shows the spreading of an oil drop. The drop gradually diminished to a thin film in 10 min. On the same rock surface, another oil drop at a different spot didn’t spread. The CA values showed the intermediate-wet condition, as shown in
Figure 3f.
Figure 4 shows curvatures of oil drops in normal cases. Comparing
Figure 3 and
Figure 4, it is obvious that the curvatures of oil drops in
Figure 3a–e are flatter.
The drop spreading phenomenon can be qualitatively defined with three levels. When the oil drop becomes slightly flattered without significant change in CA value, it is referred to as “slight spreading”. When the oil drop continues spreading to the extent that capturing CA values on both sides simultaneously became impossible, it is referred to as “severe spreading”. The level between “slight spreading” and “severe spreading”, in which CA values on both sides could still be captured simultaneously, is referred to as “moderate spreading” in the following context.
Another feature related to the oil drop spreading phenomenon is the irregular contour of the drop. When moderate or severe spreading happened, the contact contour of oil drop became irregular. An artifact of the irregular contour is shown in
Figure 5. On an ideal surface, the contact contour should be a circle. The irregular contour of oil drop indicates the rock surface heterogeneity, nonuniformity of the surrounding phase, or both.
Factors that influence CA measurements include sample heterogeneity [
37,
38], surface roughness [
39,
40], phase properties [
41,
42], temperature [
43,
44], pressure [
45,
46], humidity [
47], electrical field [
48], and others. Rock has some degree of heterogeneity by nature. However, Indiana limestone is usually regarded as relatively homogeneous [
49]. XRD result shows that the samples have a mineral composition of 100% calcite. Besides, sample surfaces were smoothened in the same way, with sandpaper of 500cw. The same crude oil was used in all measurements. All of the measurements were carried out in the lab with a maintained room temperature of around 21 °C and stable humidity. The only factor that changed significantly among measurements was the surrounding phase properties, and the treatment time (which refers to the time of sample soakage inside surfactant solutions). Additionally, the measurement process could also have an impact.
One possible reason for oil drop spreading could be the presence of surfactants. The definition of spreading coefficient, as given by Equation (1), emphasizes the impact of surface tension and interfacial tension (IFT) on the spreading phenomenon. In the equation,
is the spreading coefficient.
are the IFT between gas and the surface, nonaqueous liquid and the surface, gas and the nonaqueous liquid, respectively [
50]. In our case, the surface is rock surface. Water and crude oil are the liquid phases on the surface. Surfactants reduce the IFT between phases and cause changes in the spreading coefficient. The oil drop spreading phenomenon is reported in studies regarding low IFT systems. Reed and Healy suggested that if spreading happens, the IFT value must be lower than
[
51]. Njobuenwu et al. proposed an equation to describe the relationship between the radius of the spreading oil drop and IFT, in which lower IFT links with a higher oil drop radius [
52]. Hirasaki and Zhang analyzed how the interplay of IFT and buoyancy affected the equatorial radius [
53]. Their method of analysis can also be applied to the cases in our study. IFT maintained the ellipsoid contour. When surfactants were added, IFT was significantly reduced. As a result, the oil drop spread on the horizontal plane.
Another possible cause relates to the remaining oil in the surrounding phase. The surfactant solutions were used as the WA treatment fluid and then used as the surrounding phase. During WA treatment, some oil phase dispersed into the surfactant solution. Consequently, the surfactant solutions contained some oil as an oil layer on top of the surfactant solution, as oil droplets inside the solution, or both. When there was an oil phase dispersed in the surfactant solution, the solution appeared brownish or “dirty”. The oil phase inside surfactant solutions may contaminate the rock surface and affect the spreading of oil drop.
The remaining oil on the rock surface could also affect the oil drop spreading phenomenon. The rock samples usually had some remaining oil phase distributed on the surface after WA treatment. When the generated oil drop contacted the rock surface, the remaining oil on the rock surface could contact the generated oil drop and affect the spreading of the oil drop.
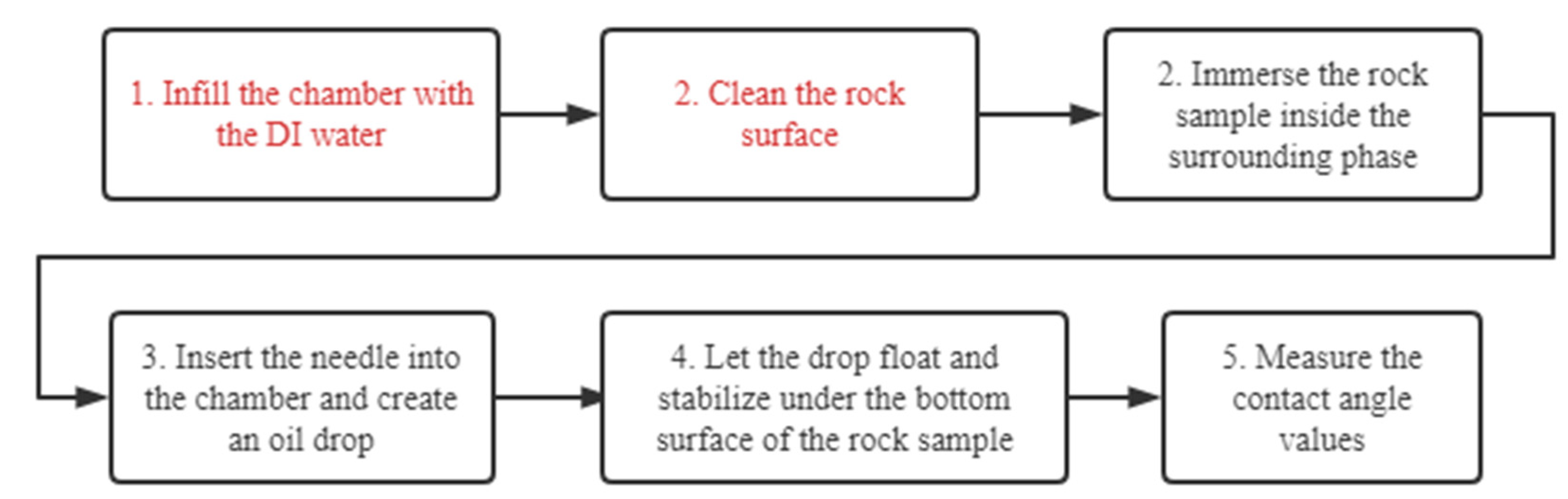
Tests were conducted to study the influence of the remaining oil on rock samples and the surrounding phase. A type of surfactant with moderate WA performance was selected to prepare surfactant solutions. Measurements were conducted on 32 rock samples. Three different fluids, DI water, newly prepared surfactant solution (labeled as “Clean”), and surfactant solution used in the WA treatment (labeled as “Dirty”), were used as the surrounding phases. In some cases, rock samples were used for CA measurement right after WA treatment (labeled as “Dirty”). In other cases, rock samples were cleaned (labeled as “Clean”) by softly rubbing on paper to remove the remaining oil on rock surface before CA measurements. Results are listed in
Table A1. Rows carrying the same sample number contain the measurement results from the same core sample. CA1, CA2, CA3, and CA4 represent values from repeated measurements on the same sample, after the same treatment, and at the same measurement condition. Apart from CA values, the qualitative extent of the spreading phenomenon was also recorded.
To analyze the impact of oil drop spreading on CA values,
Table 1 was made to compare the data obtained under different oil drop spreading conditions. On the same sample, the angles generally tended to be larger when moderate or severe spreading happened. For example, Sample 11, Sample 17, and Sample 19 showed an intermediate-wet state when slight or no spreading happened. However, when severe or moderate spreading happened, those samples showed an oil-wet state. In a word, the oil drop spreading phenomenon leads to deviated CA values that generally indicate stronger oil-wetness.
Comparing the number of times when spreading happened,
Table 2 is obtained. According to
Table 2, when DI water was used as the surrounding phase, oil drop spreading happened more often on dirty rock surface than on clean rock surface, indicating that surface cleaning reduces the chance of oil drop spreading. When the rock surface was clean, oil drop spreading happened more often in clean surfactant solutions than in DI water, indicating that surfactants add to the chance of oil drop spreading. When surfactant solution containing oil was used as the surrounding phase, severe oil drop spreading phenomenon was more frequently observed than when clean surfactant solution was used, indicating that oil phase inside surfactant solution also adds to the chance of oil drop spreading.
In summary, a few observations can be drawn about the oil drop spreading phenomenon:
The oil drop spreading phenomenon has a significant impact on static contact angle measurement by resulting in measurement failures or deviated CA values higher than the values measured without oil drop spreading.
The existence of surfactants in the surrounding phase largely increases the chance of oil drop spreading.
The existence of the oil phase inside the surfactant solution or on the rock surface adds to the chance of oil drop spreading.
2.5. Comparison between Preliminary and Secondary Measurement Results
Table 3 compares the percentage of each SD range of both datasets. The modified process exhibited significant improvement in precision which came mainly from data of moderate error level (23% vs. 33%).
Both preliminary and secondary datasets are collections of the CA values obtained in different conditions. The varying conditions include surfactant type, surfactant concentration, solution volume, treatment time, and brine composition. With so many varying conditions, the significant improvement may not be solely attributed to the process modification. To further confirm that the modification helps reduce error, several detailed comparison analyses were conducted.
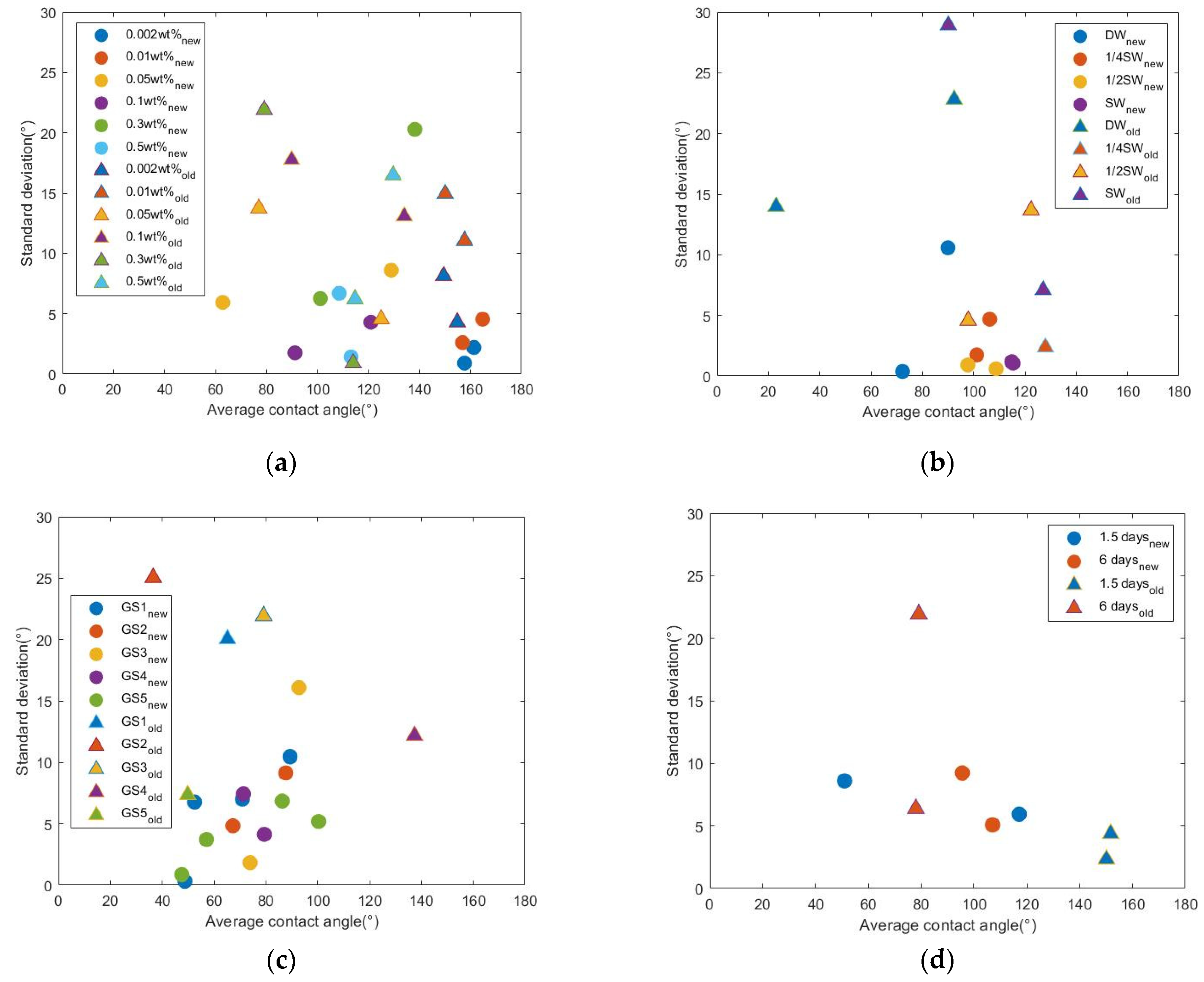
Four data subsets are selected from the preliminary and secondary datasets. Surfactant solutions of the same surfactant GS3, but different concentrations, were prepared and used for the wettability alteration on oil-aged limestone samples. Two batches of rock samples were used. Both batches were divided into six groups. Each group had two samples. In the same batch, different groups of samples were treated with surfactant solutions with different concentrations. After the treatment, two CA measurements were conducted on each sample.
Figure 8a shows the distribution of SD over average CA of all samples. The measurements of Batch 1 followed the modified process (labeled ‘new’). The measurement of Batch 2 followed the old process (labeled ‘old’). Data points of samples treated with surfactant solutions of the same concentration have the same color but different shapes. For example, the first groups in Batch 1 and Batch 2 were treated with GS3 solution of the same concentration (0.002 wt %). The data points of the first group in Batch 1 are shown by blue dots. The data points of the first group in Batch 2 are shown by blue triangles. In the figure, each dot or triangle represents one sample. Regardless of the surfactant concentration, most dots are lower than triangles of the same color, which indicates that measurements followed the modified process has a lower error level. For example, the red dots lower than the red triangles, suggesting that the error level of the red dots is lower than that of the red triangles. This means when all of the conditions are kept the same, measurements following the modified process have a lower error level.
The other three figures in
Figure 8 are plotted in the same way as
Figure 8a.
Figure 8b compares the results from measurements where the changing conditions were the salinity of the surfactant solutions and the measurement process. The surfactant solutions were prepared by dissolving surfactants in diluted synthetic seawater. In
Figure 8c the changing conditions were the surfactant type and the measurement process. In
Figure 8d the changing conditions were the surfactant treatment time and the measurement process. In these figures, data of the same salinity, or surfactant type, or treatment time, show the improved precision brought by the modified process, such as what is concluded from
Figure 8a.
When all of the other conditions are kept the same between two batches, a lower error level is repeatedly observed in the measurements from Batch 1 that followed the modified process. These analyses confirmed that the suggested process modification can improve measurement precision.
Another important observation from these figures indicates the limitation of the modified process. When all of the other conditions are kept the same, the average CA values from both batches appear to be close in many cases. For example, in
Figure 8a, the two average CA values of red dots are close to 160°. The two average values of red triangles are close to 152°. This observation suggests consistency between the measurements following two different processes. However, this consistency does not apply to all data in the figures. For example,
Figure 8d shows a large difference in average CA values between two batches. The possible difference brought by the modified process is discussed in the following section.
2.6. Limitation of The Modified Process
Using DI water as the surrounding phase has several advantages: (1) it reduces the chance of oil drop spreading; (2) it keeps IFT constant. When IFT is reduced, the volume of oil drop generated will reduce. Researchers have observed that changes in oil drop volume will lead to changes in CA value [
16,
54]. Some studies highlight the influence of the oil drop volume. Korhonen et al. suggested a “surprisingly large” initial drop size to be necessary to achieve reliable receding angle results [
26]. If CA values are measured inside surfactant solutions, the interpretation from CA results to wettability will have to consider differences in IFT and drop size. Using DI water as the surrounding phase ensures a relatively constant oil drop volume. The CA value difference before and after WA treatment can be regarded as the “net wettability change” that minimizes the influences of IFT change or drop size change.
On the other hand, the change of the surrounding phase may affect rock wettability. For cationic surfactants, the main WA mechanism is reported to be taking away the negatively charged organic materials adsorbed on the rock surface by ion-pairing [
55]. In that case, the WA is irreversible. Using DI water as the surrounding phase will not change the rock wettability. When surfactants of reversible WA effect are used, such as anionic surfactants that change wettability mainly by adsorbing onto a rock surface, using DI water as the surrounding phase can wash away part of the surfactant molecules on rock surface, resulting in a changed wettability of rock.
Although the modified process showed improved precision, the change of surrounding phase from surfactant solution to DI water makes a different three-phase system. In the study or simulation of oil displacement, contact angle is involved in the calculation of important parameters such as work of adhesion, capillary pressure, the bond number, the trapping number and so on. If the contact angle value changes with the surrounding phase, it will be necessary to use surfactant solution as the surrounding phase when the in-situ CA values are needed.
A test was conducted to study how changing the surrounding phase affects the CA values. Measurements on 43 samples were conducted inside clean surfactant solutions. After that, measurements were repeated inside DI water.
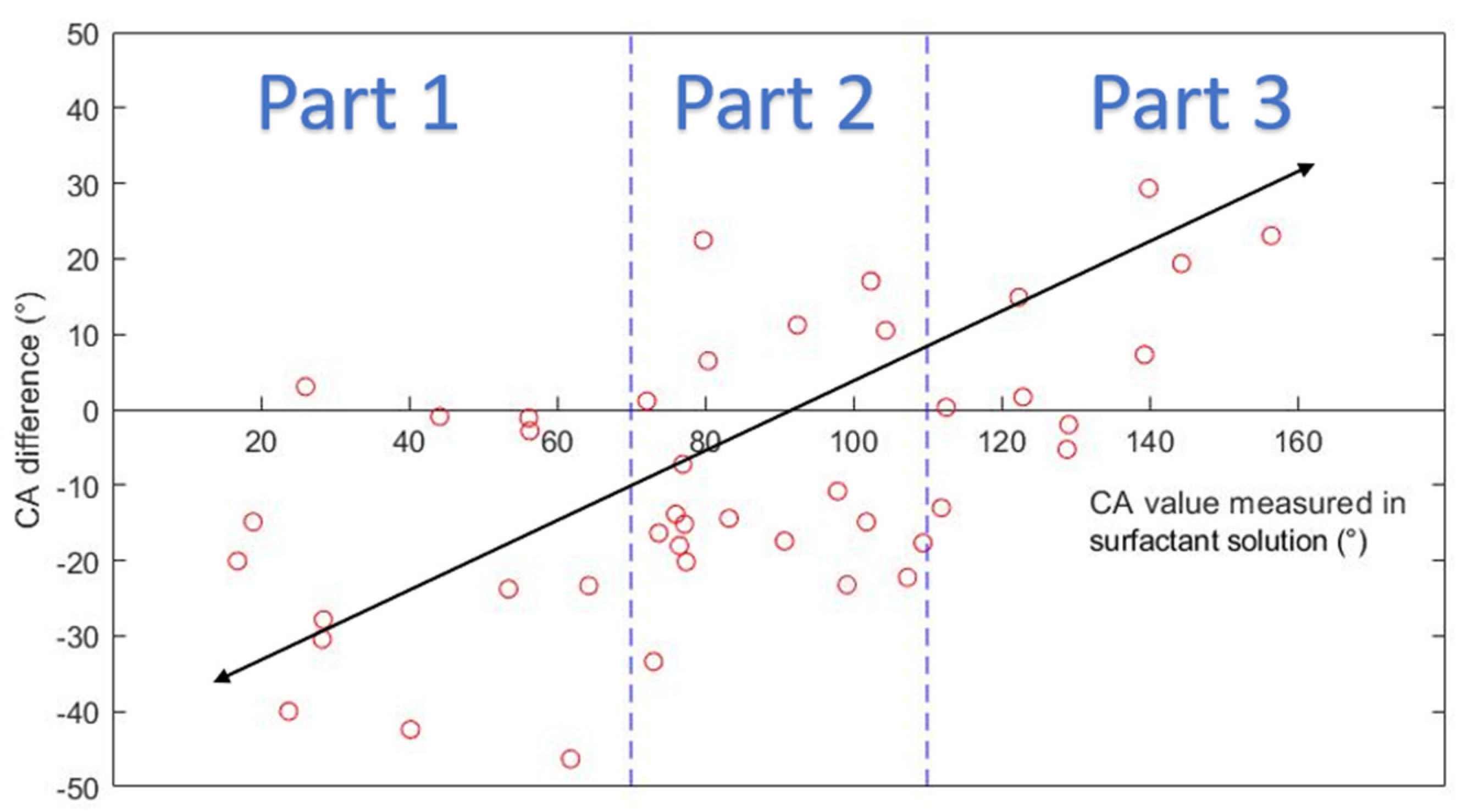
Table A2 compiles the averaged CA values obtained inside DI water and inside clean surfactant solutions. The CA difference is calculated by deducting the angle measured in DI water from the angle measured in surfactant solutions. The CA difference ranges from 0.3° to 46.3°. The average difference between CA values is about −6.2°.
Figure 9 plots the CA difference along the CA. The figure can be divided into three parts:
Part 1: in the range of 0°~70°, most of the CA values measured in DI water were larger than those measured in surfactant solutions. When CA values in surfactant solutions indicate water-wet condition, CA values in DI water indicate less water-wet or intermediate-wet condition.
Part 2: in the range of 70°~110°, CA values measured in DI water could be larger or smaller than those measured in surfactant solutions.
Part 3: in the range 110°~180°, most of the CA values measured in DI water were smaller than those measured in surfactant solutions. When CA values in surfactant solutions indicate oil-wet condition, CA values in DI water indicate less oil-wet or intermediate-wet condition.
Results show that changing the surrounding phase from surfactant solutions to DI water can cause a significant difference in the measured CA values, especially when the surface is strongly water-wet or strongly oil-wet. The significance of the difference is related to the wetting state of the surface. Based on the result, a conclusion can be drawn that DI water should not be used as the surrounding phase when in-situ CA values are needed.
A similar comparison study is conducted regarding the advancing and receding contact angle. In this study, the advancing and receding contact angle was measured by expanding and decreasing the oil droplet size. Similar measurement difficulty induced by the oil drop spreading was observed.
The comparison study was conducted on two rock samples. Advancing and receding contact angle values were measured in surfactant solution and in DI water. Results are listed in
Table 4. The rock sample saturated with DI water had an initial wettability condition of strongly water-wet. The surrounding phase seemed to have a negligible impact on the advancing and receding angles. The other rock sample was oil aged and then treated with a surfactant solution. There was a difference of 35.2° between the advancing angle, indicating that changing the surrounding phase can lead to significant changes in the advancing angle value.
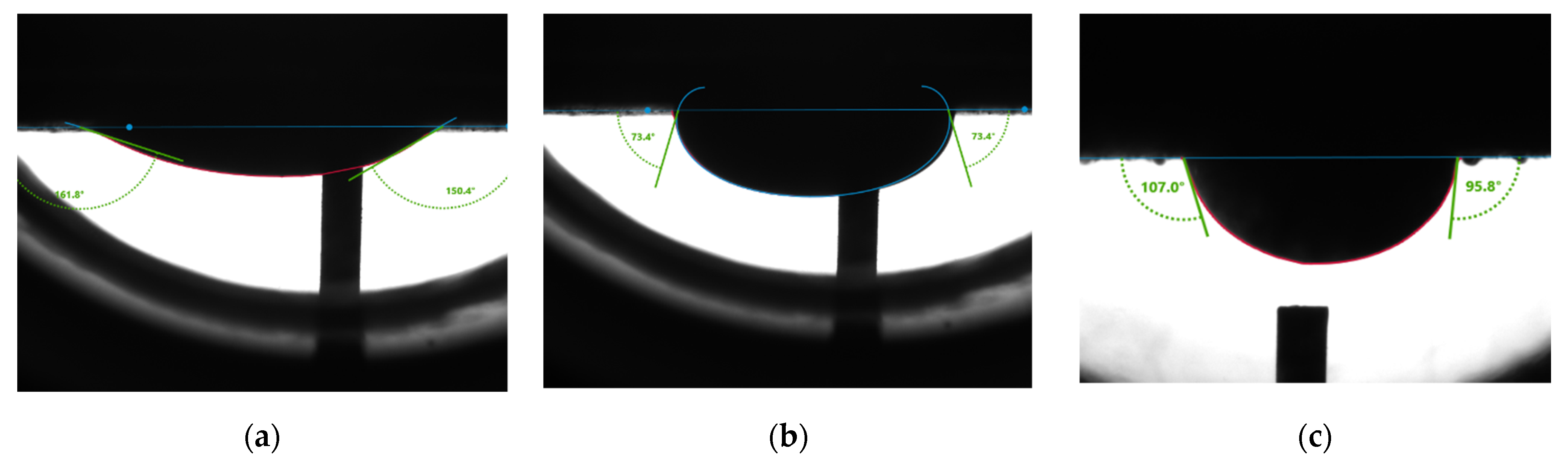
Figure 10 shows measurement results from the second rock sample. The average receding angle (
Figure 10a) is 156.1°. The average advancing angle (
Figure 10b) is 73.4°.
Figure 10c showed an average water contact angle of 101.4°, indicating intermediate wetness of the rock surface.
In a word, the modified process may not apply to all conditions. There are two prerequisites for applying the modified process:
If either of the prerequisites is not satisfied, this modified process is not applicable. In that case, either different contact angle measurement techniques or other wettability assessment methods should be applied. One alternative method is to relate the spreading phenomenon with surface wettability and other interfacial properties. For example, Chen related the drop spreading process with dynamic contact angle when the capillary and viscous forces are the only dominant factors [
56]. Lavi and Marmur proposed an empirically derived equation that matched the drop spreading phenomenon very well [
57]. Chen and Bonaccurso showed that the power-law developed for the description of the dynamic wetting process has an exponent that is dependent only on the surface wettability [
58].
Another limitation of this modified process is that it is based on static contact angle measurement which cannot ensure reaching the lowest Gibbs energy condition. As a result, contact angle hysteresis still introduces error to the measurement results.