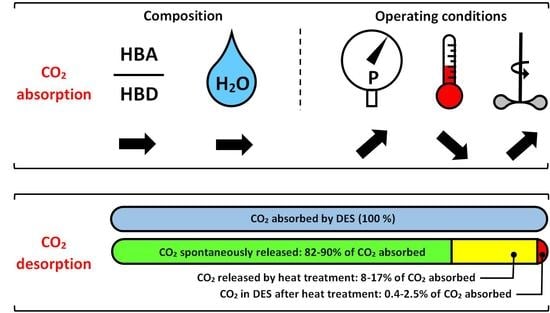
A Comprehensive Study of CO2 Absorption and Desorption by Choline-Chloride/Levulinic-Acid-Based Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the DESs
2.2. CO2 Absorption Measurements
2.3. Thermodynamic Analysis of CO2 Absorption
2.4. Sorbent Regeneration Measurements
2.5. FTIR Spectra Measurements
2.6. Recyclability of the DESs
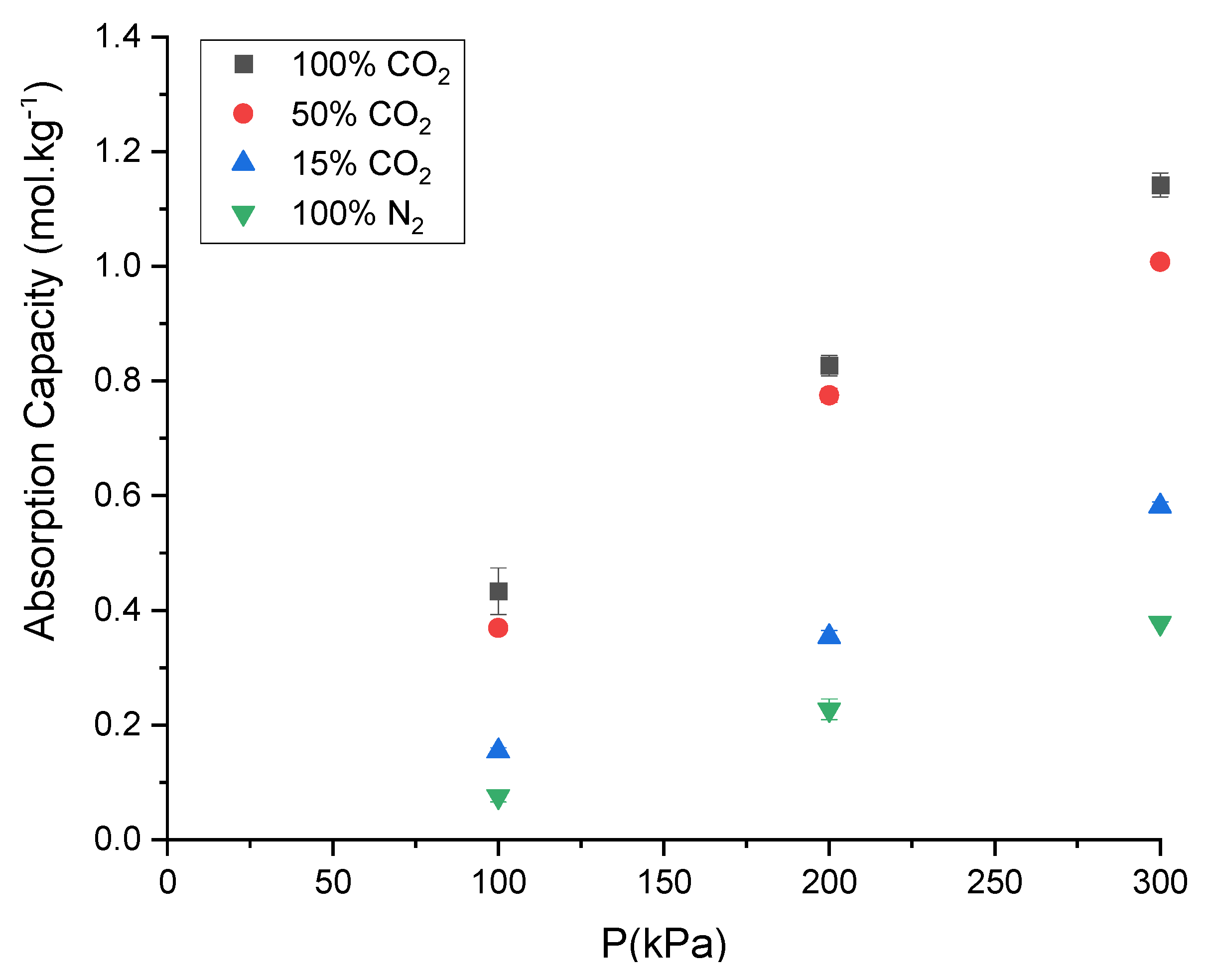
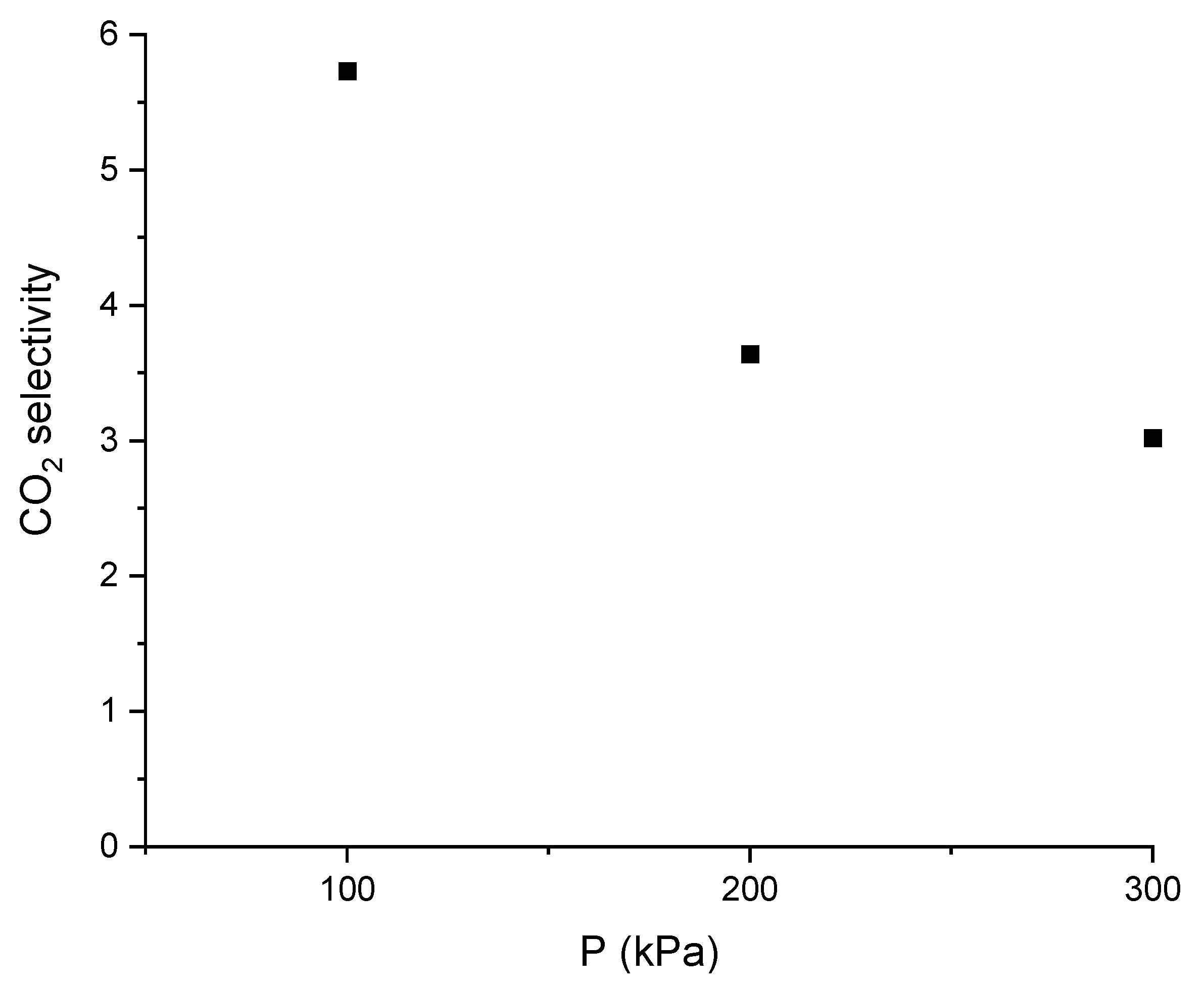
2.7. Selectivity of the DESs towards CO2
3. Results and Discussions
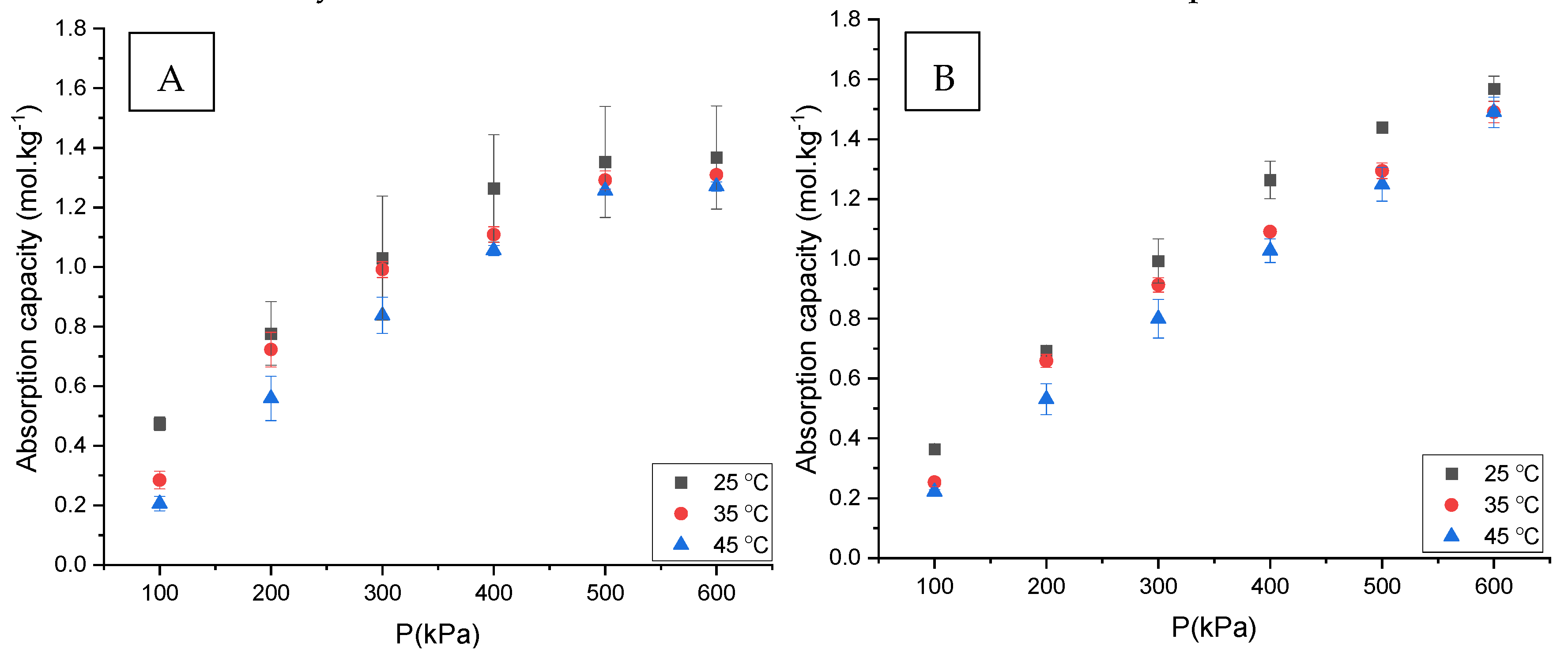
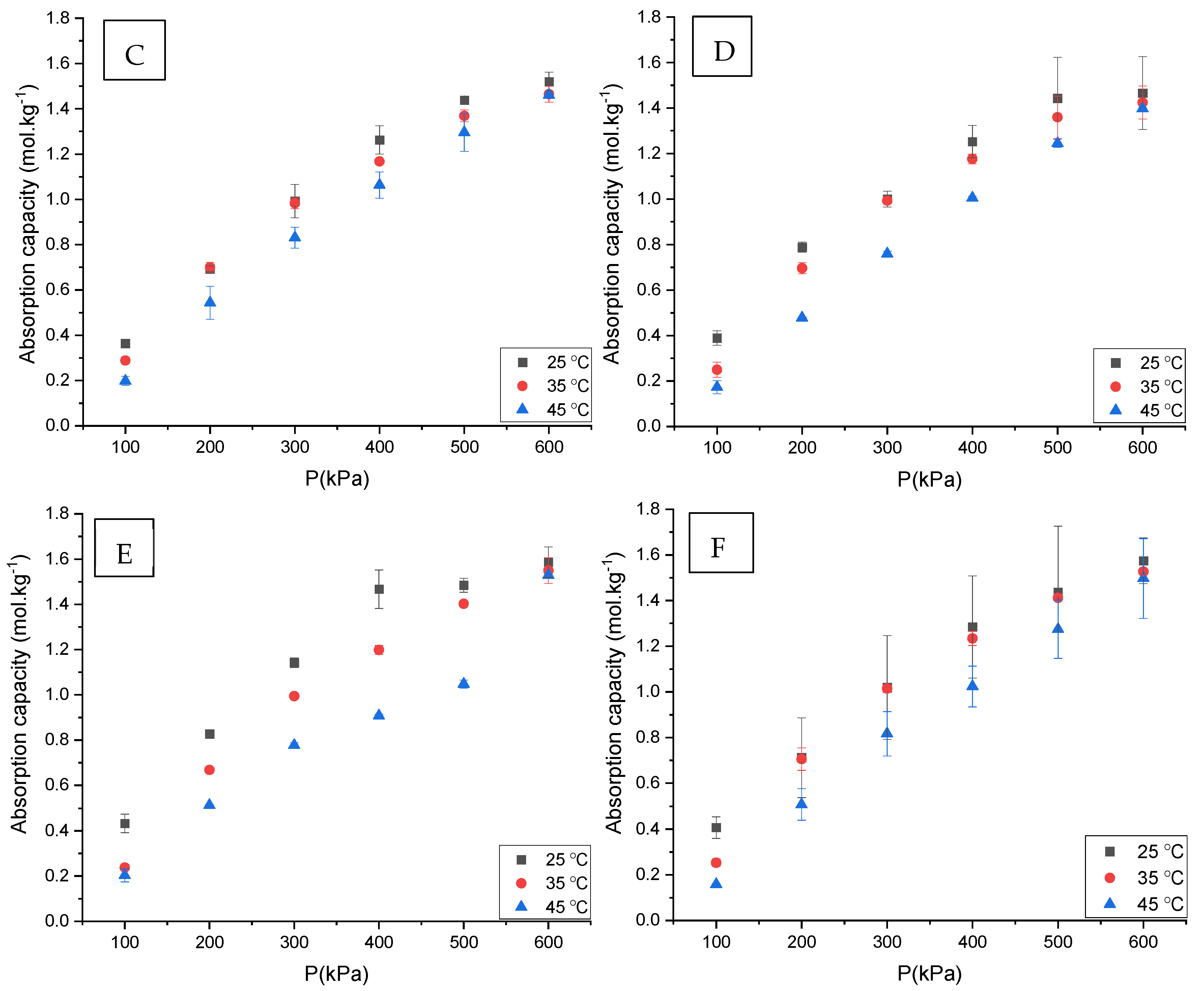
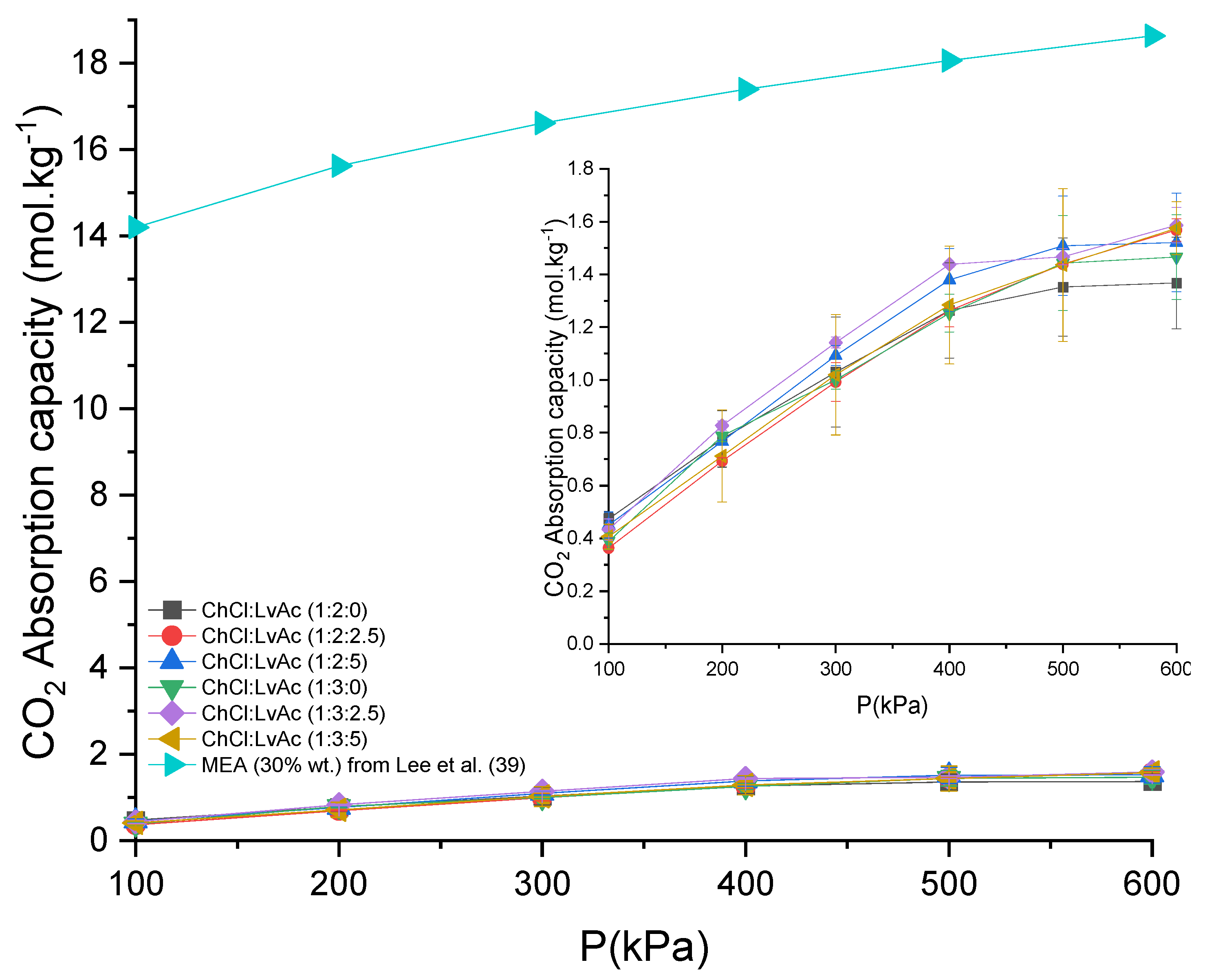
3.1. CO2 Absorption
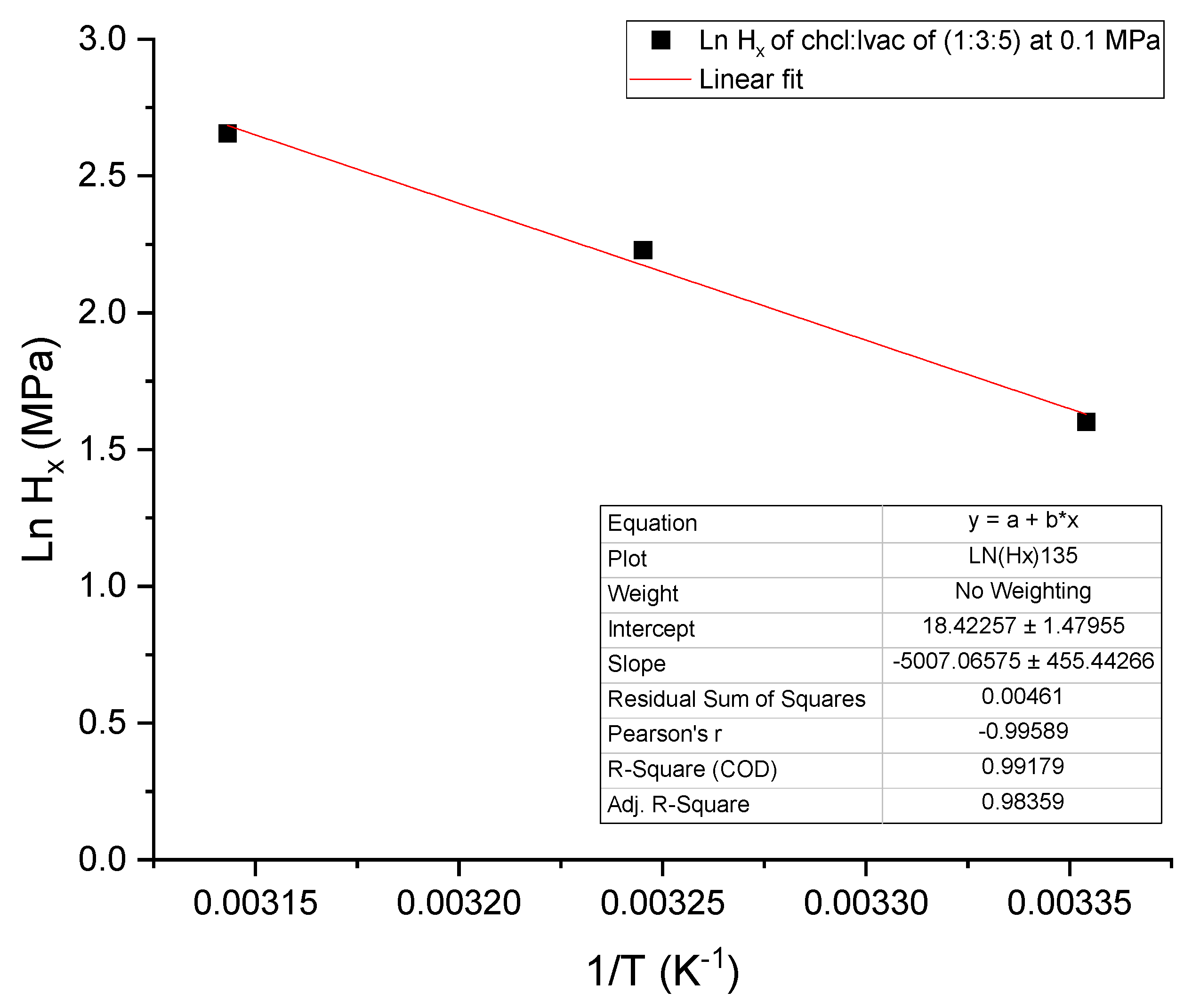
3.2. Thermodynamic Analysis of CO2 Absorption
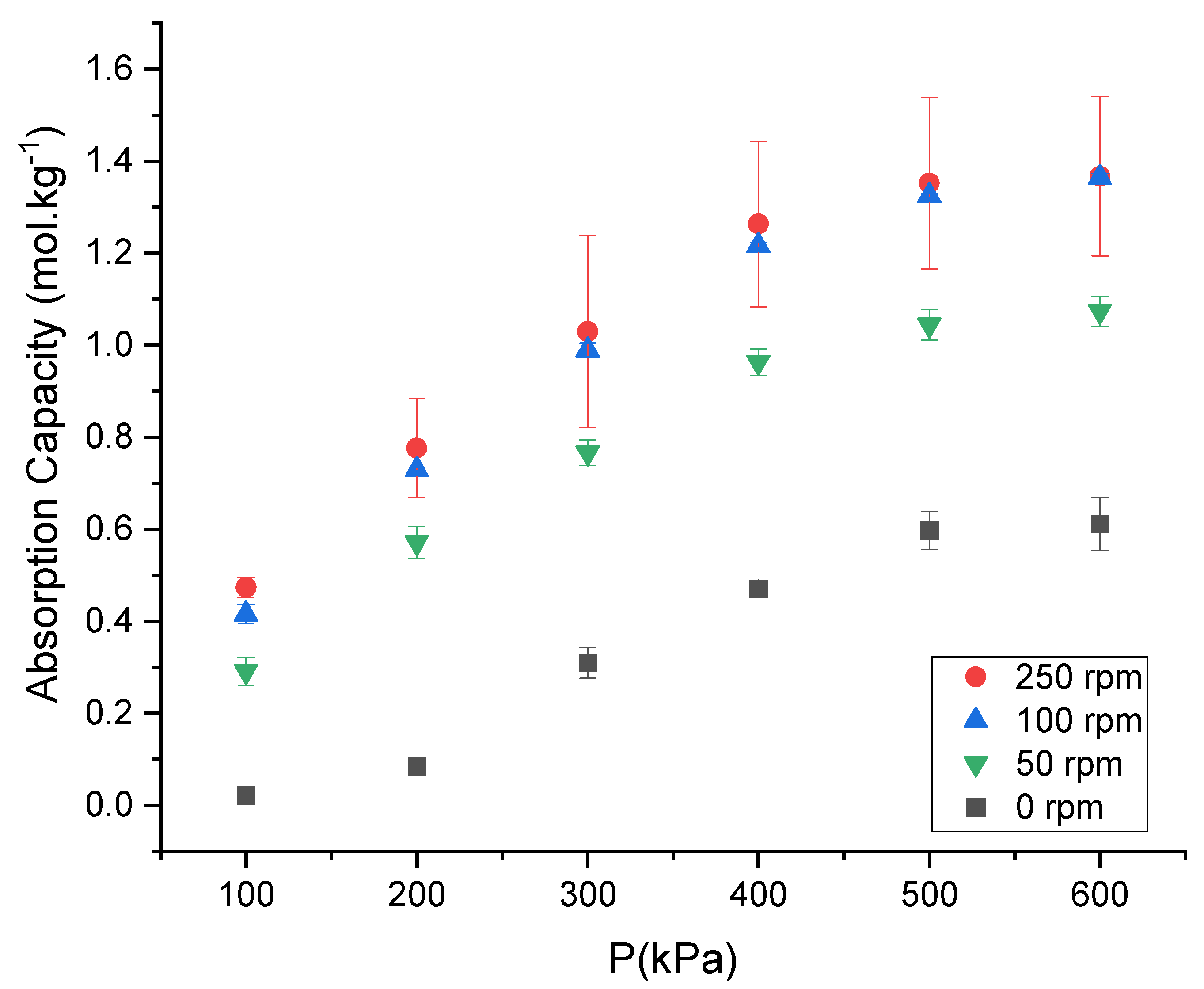
3.3. The Effect of Stirring Speed on the CO2 Absorption Capacity of the DES
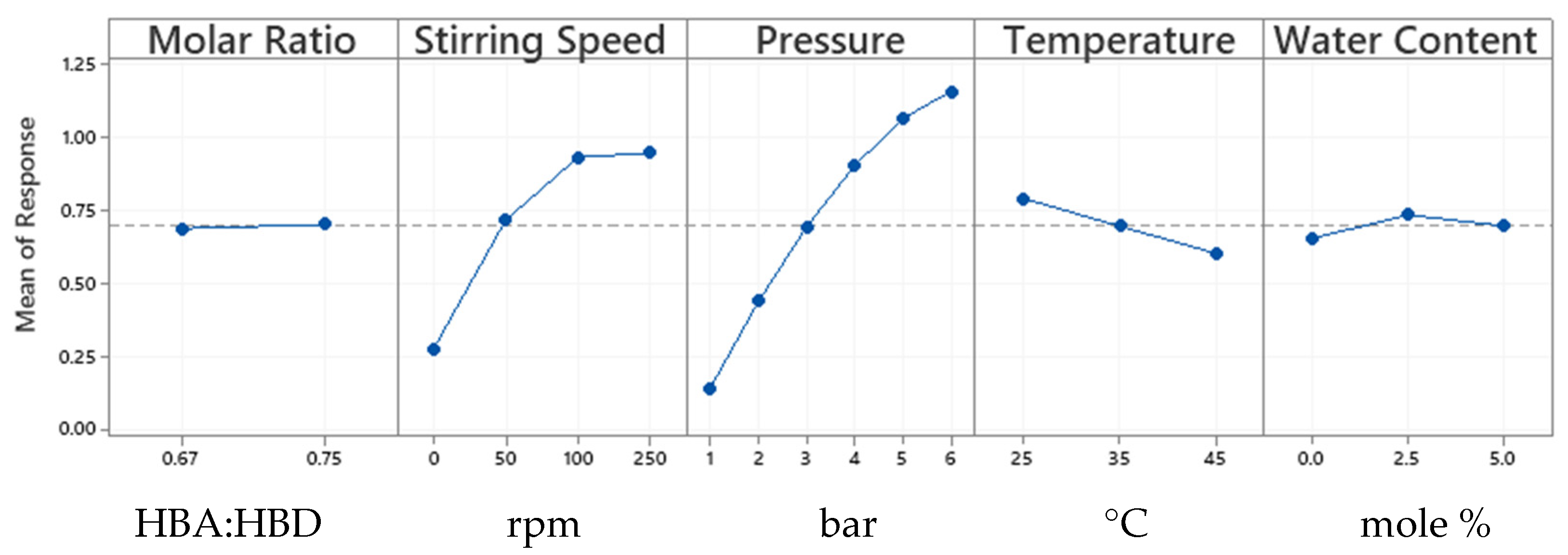
3.4. Statistical Analysis of CO2 Absorption Results
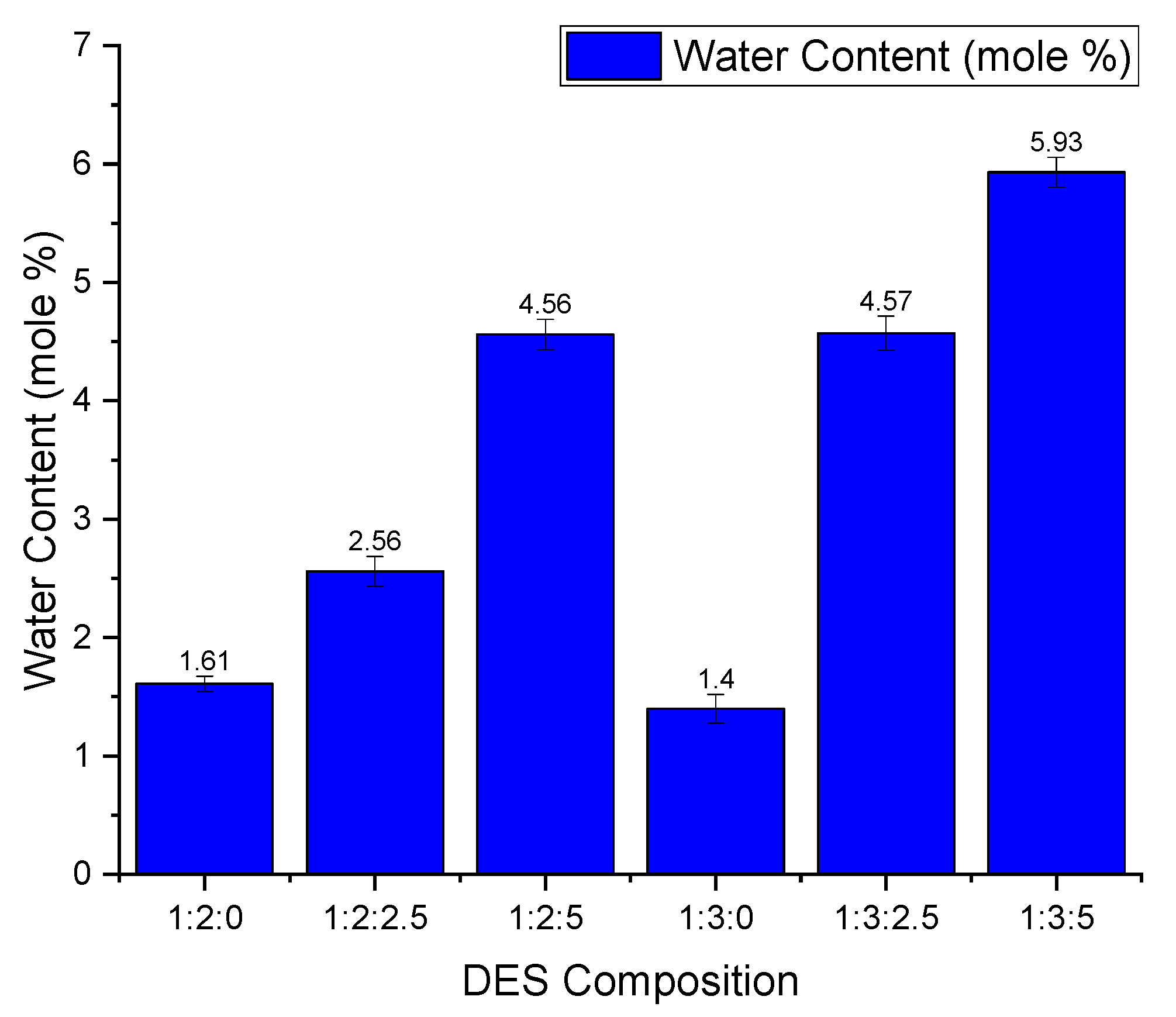
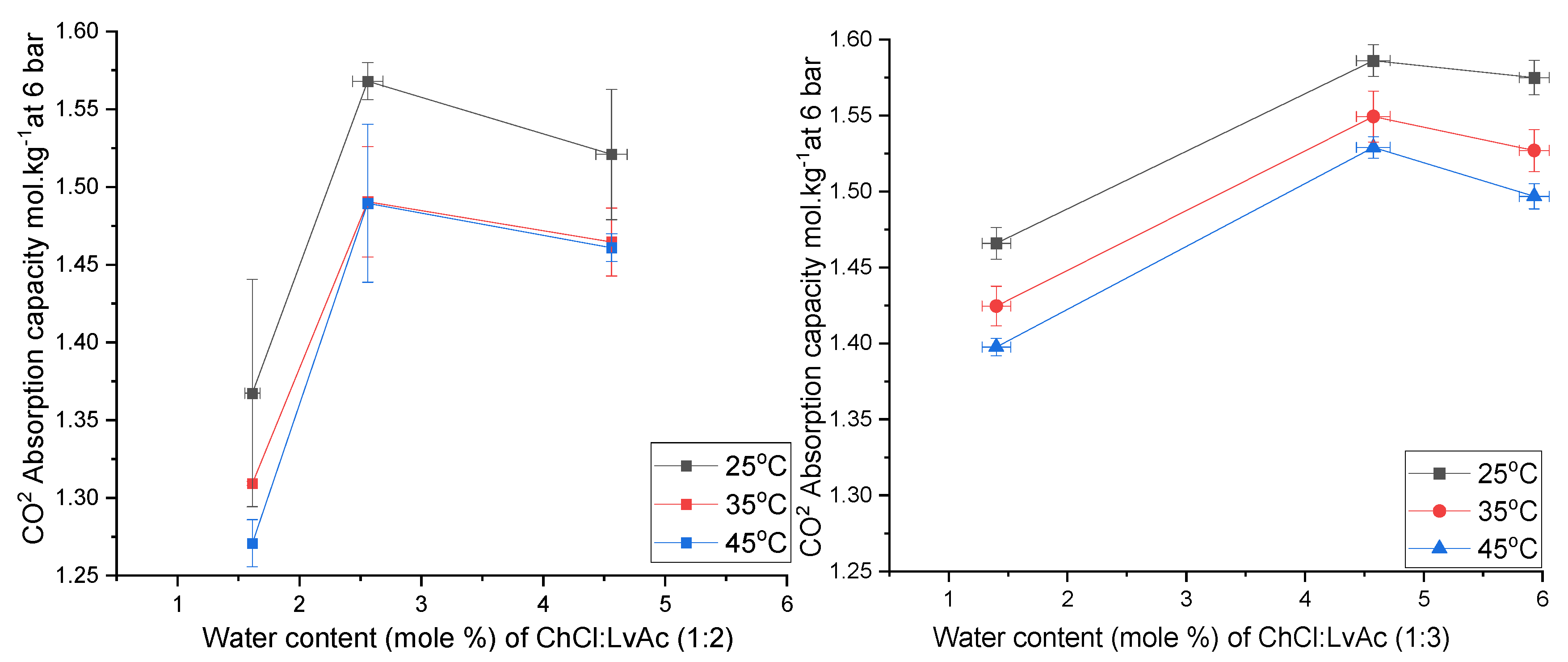
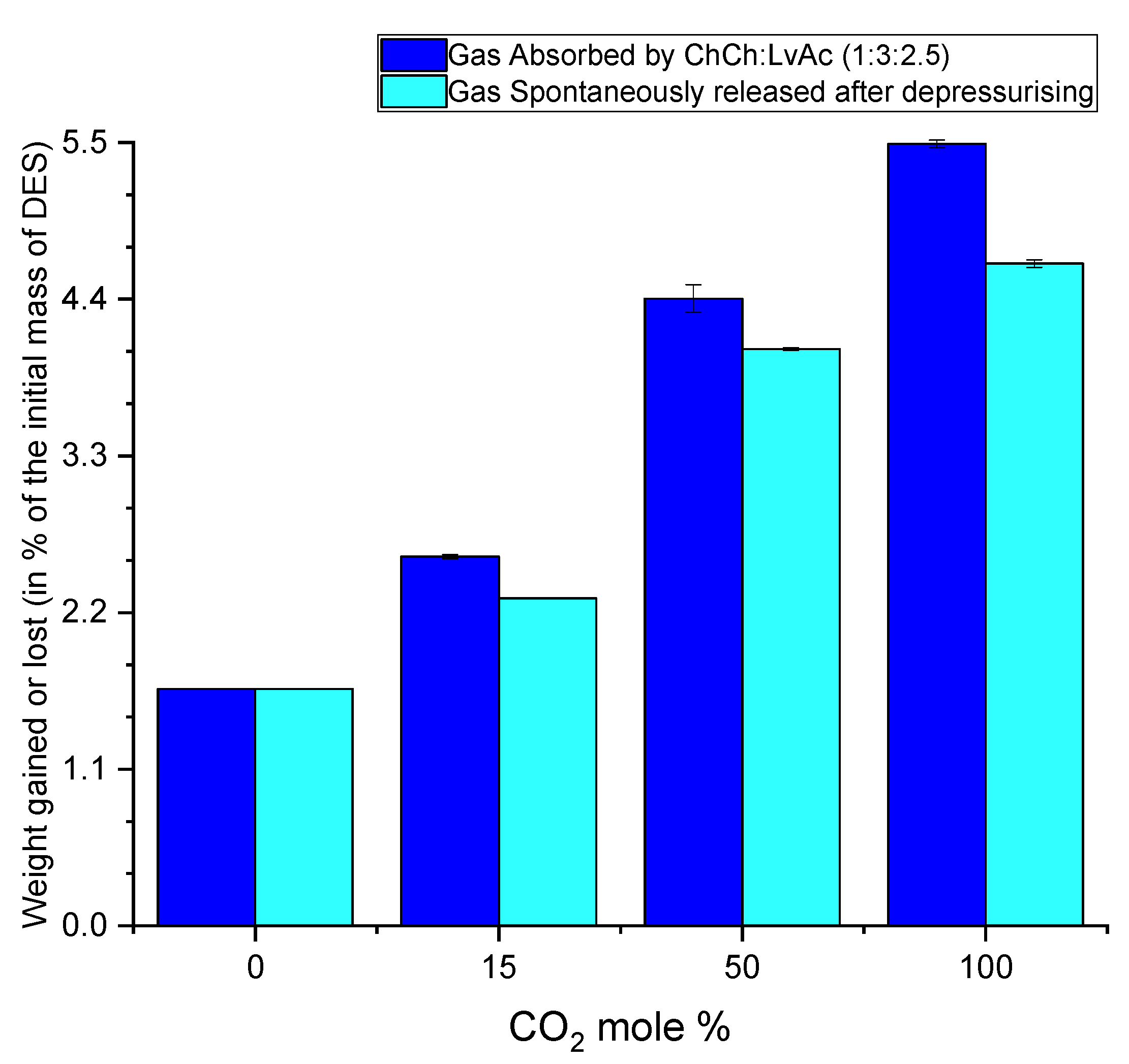
3.5. The Effect of Water Content on CO2 Absorption
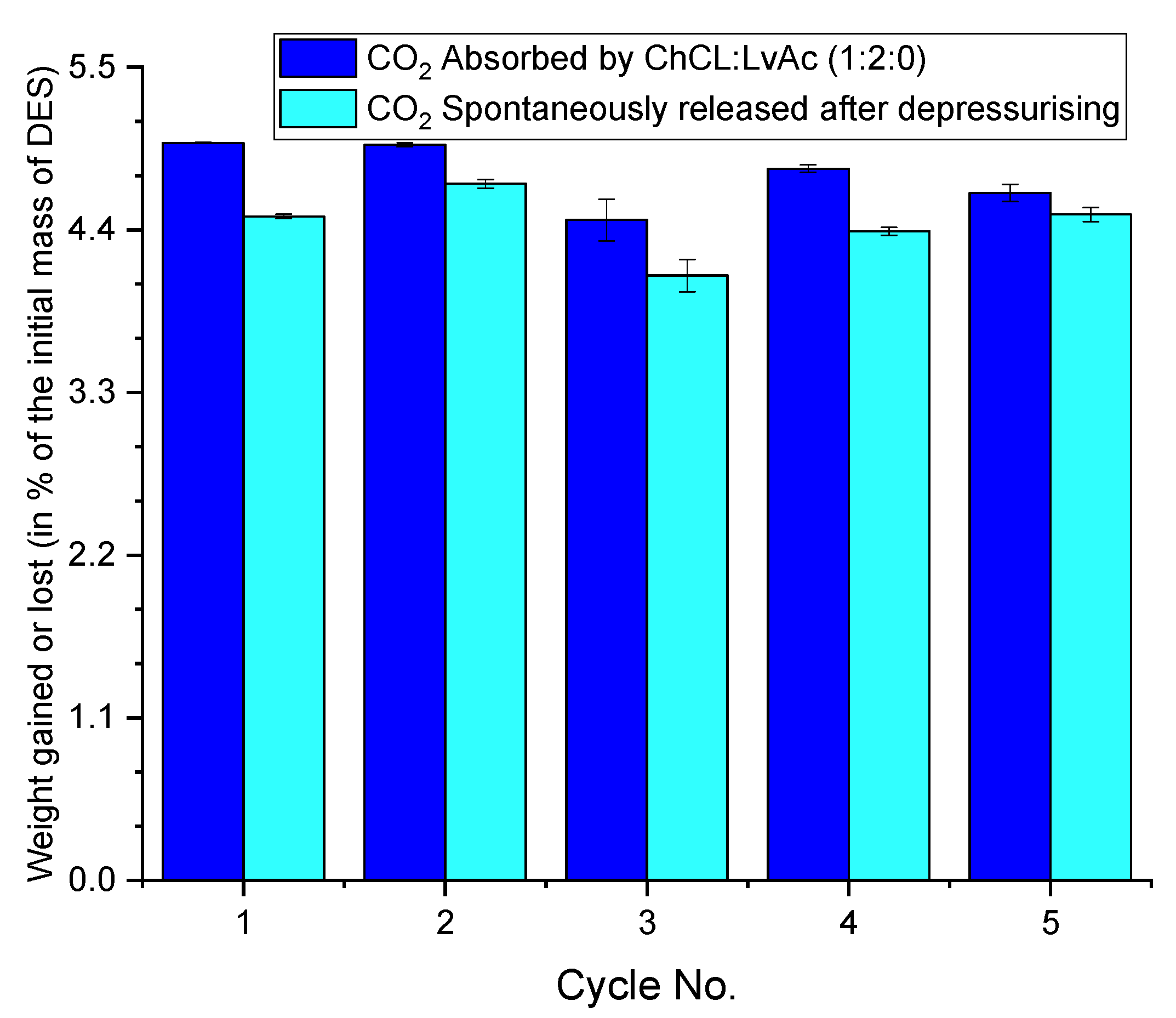
3.6. CO2 Desorption Results
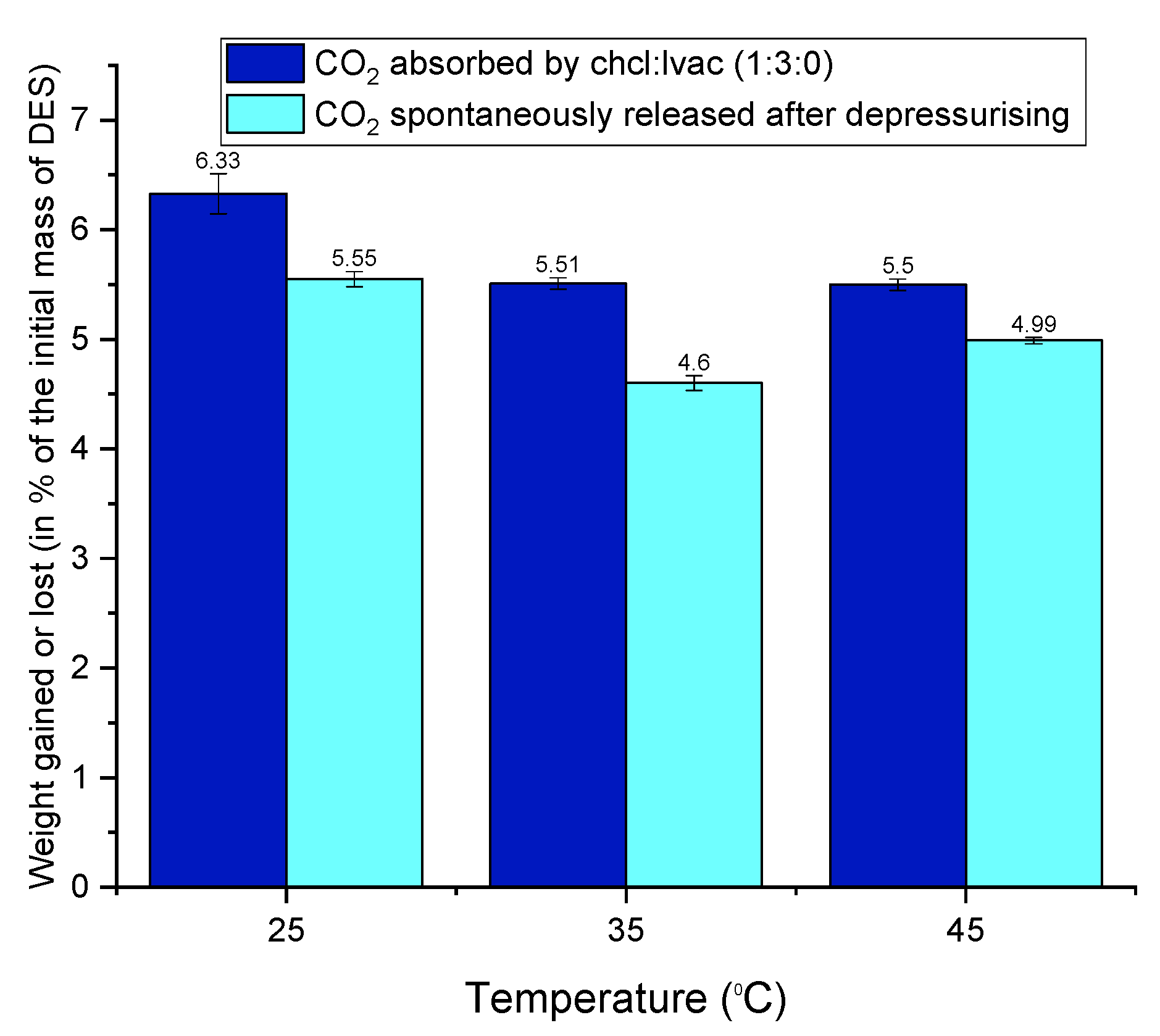
3.6.1. Indication of Nonspontaneous Released (Residual) CO2 by Weight Gain Measurements
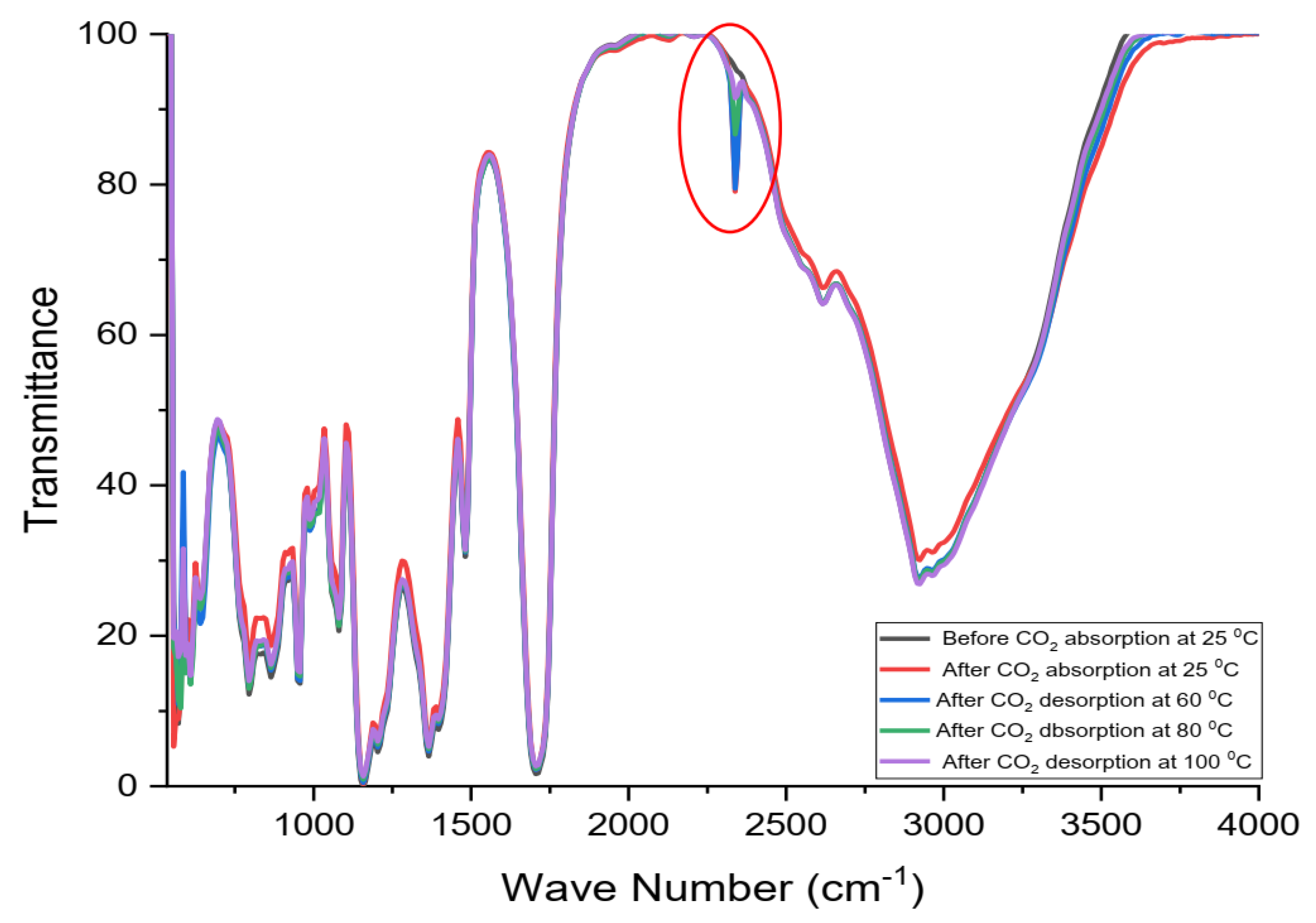
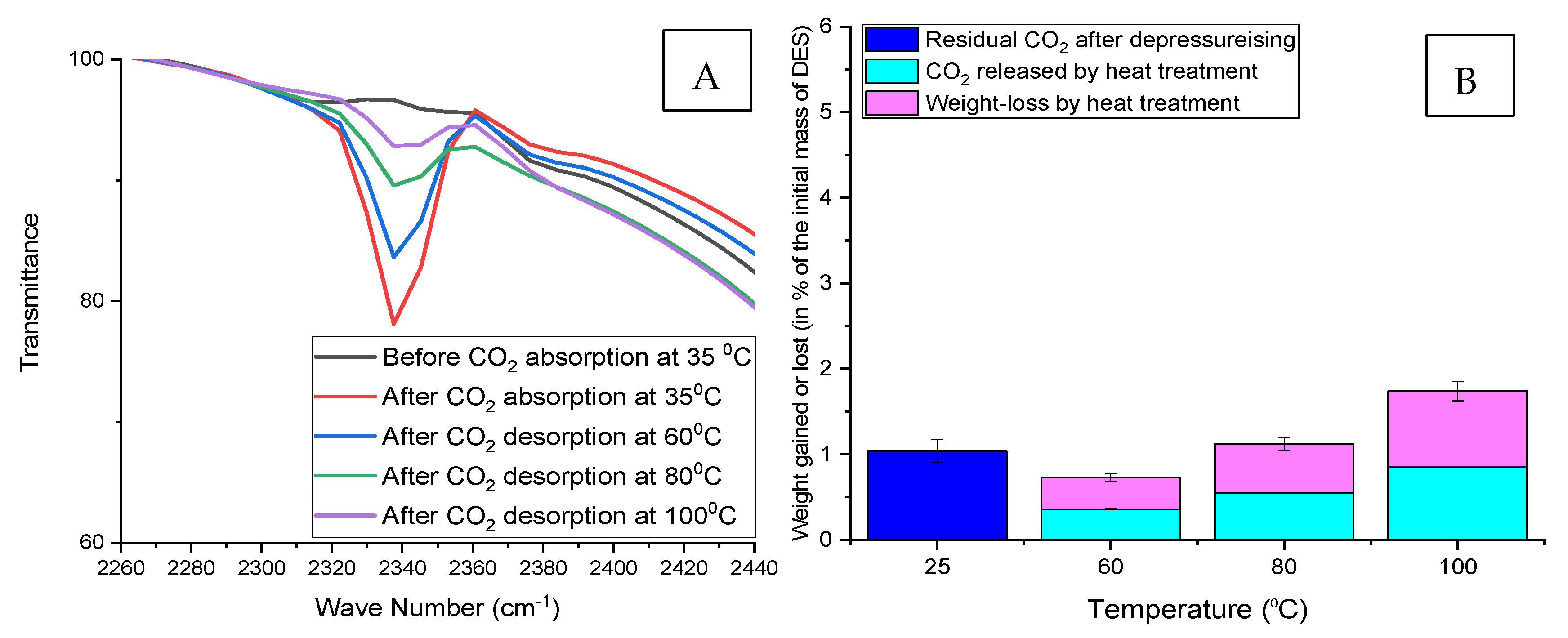
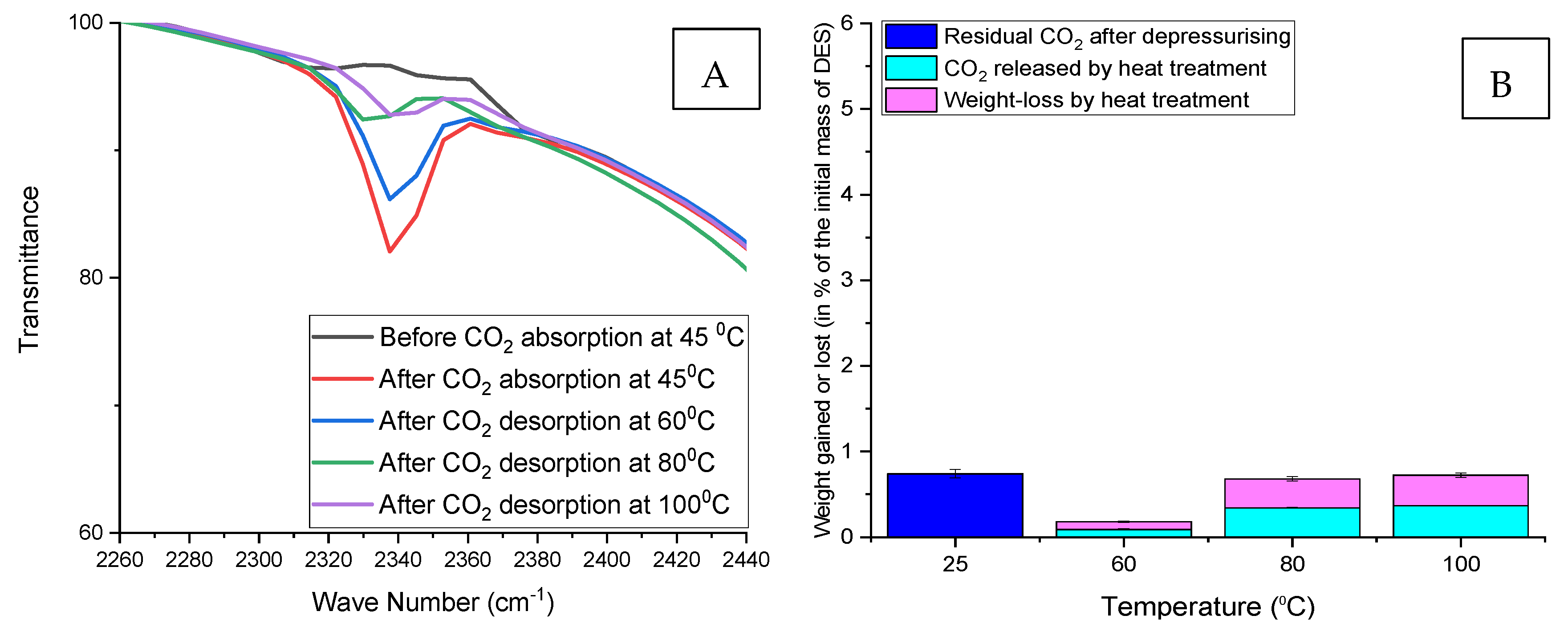
3.6.2. Quantitative Use of FTIR to Follow the Desorption of Nonspontaneous Released (Residual) CO2
3.6.3. Indication of CO2 Desorption by Weight Loss Measurements
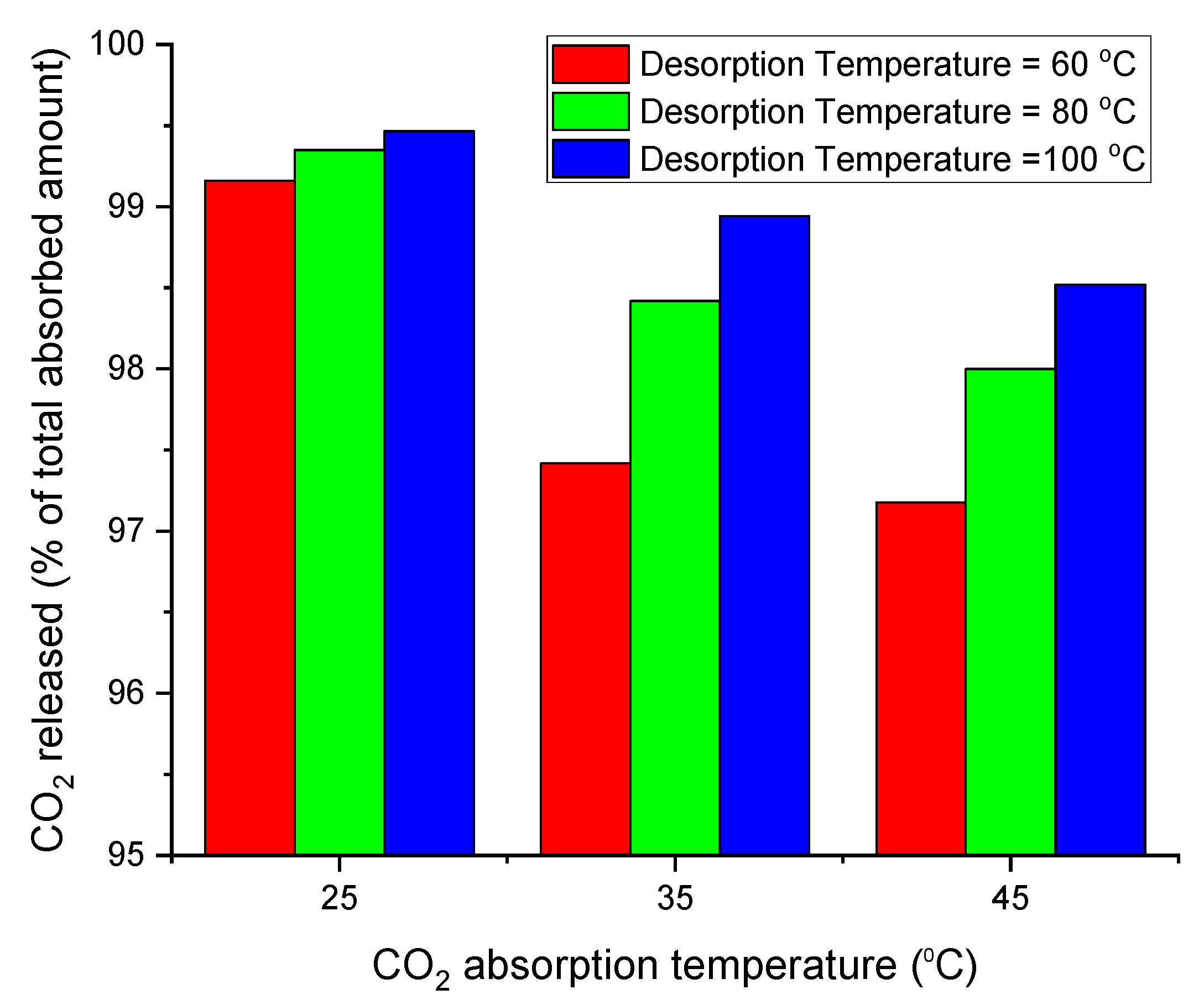
3.7. Total CO2 Released
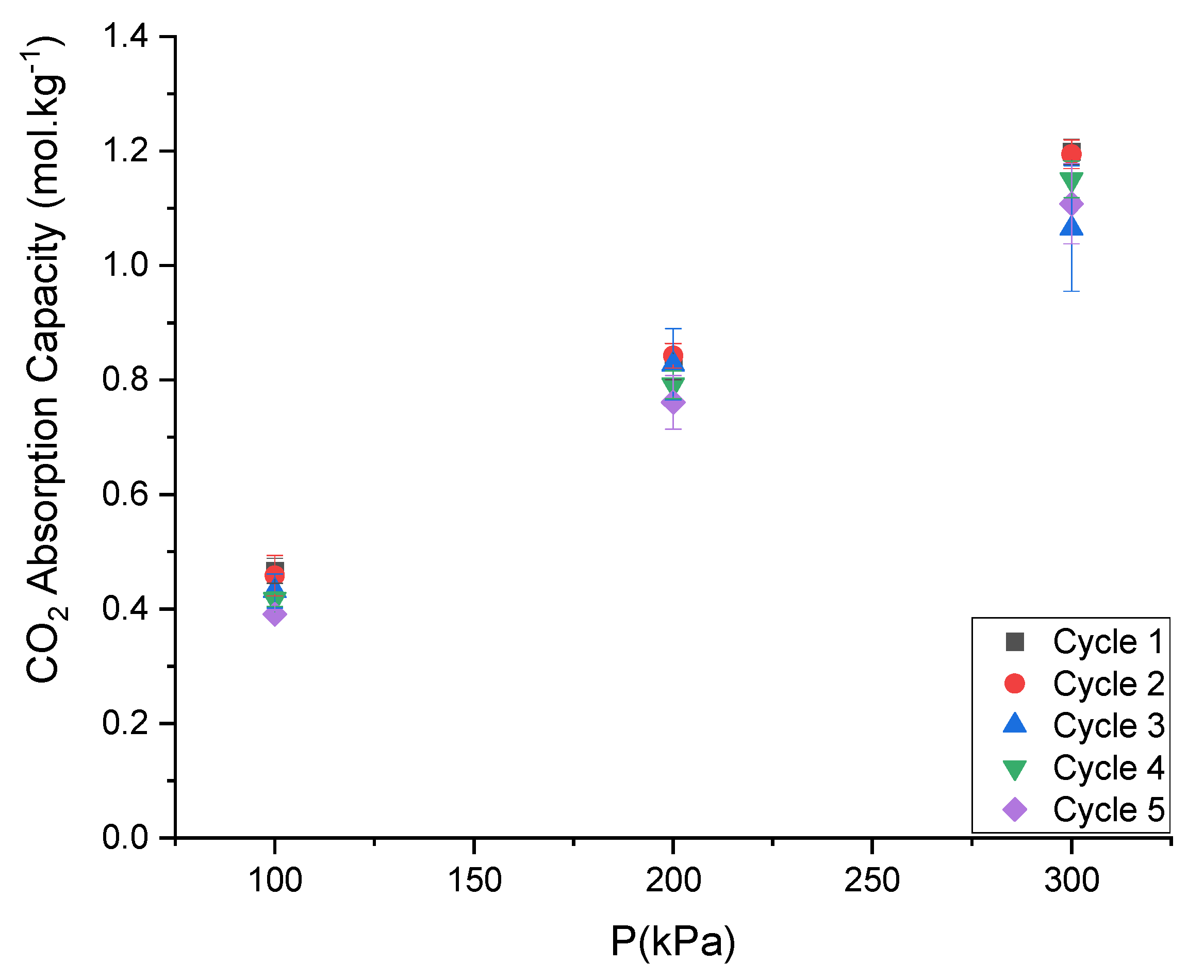
3.8. Recyclability of the DESs
3.9. Selectivity of the DESs towards CO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Scripps Institution of Oceanography. The Keeling Curve. Available online: https://scripps.ucsd.edu/programs/keelingcurve/ (accessed on 27 June 2021).
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Cao, L.; Huang, J.; Zhang, X.; Zhang, S.; Gao, J.; Zeng, S. Imidazole tailored deep eutectic solvents for CO2 capture enhanced by hydrogen bonds. Phys. Chem. Chem. Phys. 2015, 17, 27306–27316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, X.; Lu, X. Choline-based deep eutectic solvents for CO2 separation: Review and thermodynamic analysis. Renew. Sustain. Energy Rev. 2018, 97, 436–455. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Evaluation of toxicity and biodegradability for cholinium-based deep eutectic solvents. RSC Adv. 2015, 5, 83636–83647. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A. Natural deep eutectic solvents from choline chloride and betaine-Physicochemical properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Leron, R.B.; Li, M.-H. Solubility of carbon dioxide in a choline chloride-ethylene glycol based deep eutectic solvent. Thermochim. Acta 2013, 551, 14–19. [Google Scholar] [CrossRef]
- Haider, M.; Jha, D.; Sivagnanam, B.M.; Kumar, R. Modelling and simulation of CO2 removal from shale gas using deep eutectic solvents. J. Environ. Chem. Eng. 2019, 7, 102747. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, S. Densities and viscosities of (choline chloride + urea) deep eutectic solvent and its aqueous mixtures in the temperature range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Amhamed, A. Review on carbon dioxide absorption by choline chloride/urea deep eutectic solvents. Adv. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Lin, C.-M.; Leron, R.B.; Caparanga, A.R.; Li, M.-H. Henry’s constant of carbon dioxide-aqueous deep eutectic solvent (choline chloride/ethylene glycol, choline chloride/glycerol, choline chloride/malonic acid) systems. J. Chem. Thermodyn. 2014, 68, 216–220. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, H.; Zhang, S.; Lu, X.; Ji, X. Effect of water on the density, viscosity, and CO2 solubility in choline chloride/urea. J. Chem. Eng. Data 2014, 59, 3344–3352. [Google Scholar] [CrossRef]
- Ren, H.; Lian, S.; Wang, X.; Zhang, Y.; Duan, E. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Maugeri, Z.; De María, P.D. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Ullah, R.; Atilhan, M.; Anaya, B.; Khraisheh, M.; García, G.; Elkhatat, A.; Tariq, M.; Aparicio, S. A detailed study of cholinium chloride and levulinic acid deep eutectic solvent system for CO2 capture via experimental and molecular simulation approaches. Phys. Chem. Chem. Phys. 2015, 17, 20941–20960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Han, G.; Jiang, Y.; Zhang, X.; Deng, D.; Ai, N. Solubilities of carbon dioxide in the eutectic mixture of levulinic acid ( or furfuryl alcohol ) and choline chloride. J. Chem. Thermodyn. 2015, 88, 72–77. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Larriba, M.; Navarro, P.; Rigual, V.; Ayuso, M.; García, J.; Rodríguez, F. Thermal stability of choline chloride deep eutectic solvents by TGA/FTIR-ATR analysis. J. Mol. Liq. 2018, 260, 37–43. [Google Scholar] [CrossRef]
- Pashaei, H.; Ghaemi, A.; Nasiri, M. Modeling and experimental study on the solubility and mass transfer of CO2 into aqueous DEA solution using a stirrer bubble column. RSC Adv. 2016, 6, 108075–108092. [Google Scholar] [CrossRef]
- Kim, Y.E.; Lim, J.A.; Jeong, S.K.; Yoon, Y.I.; Bae, S.T.; Nam, S.C. Comparison of carbon dioxide absorption in aqueous MEA, DEA, TEA, and AMP solutions. Bull. Korean Chem. Soc. 2013, 34, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Pashaei, H.; Zarandi, M.N.; Ghaemi, A. Experimental study and modelling of CO2 absorption into diethanolamine solutions using stirrer bubble column. Chem. Eng. Res. Des. 2017, 121, 32–43. [Google Scholar] [CrossRef]
- Chen, Y.; Han, X.; Liu, Z.; Yu, D.; Guo, W.; Mu, T. Capture of toxic gases by deep eutectic solvents. ACS Sustain. Chem. Eng. 2020, 8, 5410–5430. [Google Scholar] [CrossRef]
- Liu, F.; Chen, W.; Mi, J.; Zhang, J.; Kan, X.; Zhong, F.; Huang, K.; Zheng, A.; Jiang, L. Thermodynamic and molecular insights into the absorption of H2S, CO2, and CH4 in choline chloride plus urea mixtures. AIChE J. 2019, 65, e16574. [Google Scholar] [CrossRef]
- Deng, D.; Deng, X.; Duan, X.; Gong, L. Protic guanidine isothiocyanate plus acetamide deep eutectic solvents with low viscosity for efficient NH3 capture and NH3/CO2 separation. J. Mol. Liq. 2021, 324, 114719. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Mjalli, F.S.; AlNashef, I. A new processing route for cleaner production of biodiesel fuel using a choline chloride-based deep eutectic solvent. J. Clean. Prod. 2014, 65, 246–251. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.; Fernandes, A.M.; Marrucho, I. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Sze, L.L.; Pandey, S.; Ravula, S.; Pandey, S.; Zhao, H.; Baker, G.A.; Baker, S.N. Ternary deep eutectic solvents tasked for carbon dioxide capture. ACS Sustain. Chem. Eng. 2014, 2, 2117–2123. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Murshid, G.; Farrukh, S.; Shariff, A.M. Investigation of various process parameters on the solubility of carbon dioxide in phosphonium-based deep eutectic solvents and their aqueous mixtures: Experimental and modelling. Int. J. Greenh. Gas Control 2017, 66, 147–158. [Google Scholar] [CrossRef]
- Wu, S.-H.; Caparanga, A.; Leron, R.B.; Li, M.-H. Vapor pressure of aqueous choline chloride-based deep eutectic solvents (ethaline, glyceline, maline and reline) at 30–70 °C. Thermochim. Acta 2012, 544, 1–5. [Google Scholar] [CrossRef]
- Mirza, N.R.; Nicholas, N.J.; Wu, Y.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Experiments and thermodynamic modeling of the solubility of carbon dioxide in three different deep eutectic solvents (DESs). J. Chem. Eng. Data 2015, 60, 3246–3252. [Google Scholar] [CrossRef]
- Hasib-Ur-Rahman, M.; Siaj, M.; Larachi, F. Ionic liquids for CO2 capture—Development and progress. Chem. Eng. Process. Process. Intensif. 2010, 49, 313–322. [Google Scholar] [CrossRef]
- Li, G.; Deng, D.; Chen, Y.; Shan, H.; Ai, N. Solubilities and thermodynamic properties of CO2 in choline-chloride based deep eutectic solvents. J. Chem. Thermodyn. 2014, 75, 58–62. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert law. J. Chem. Educ. 1962, 39, 333–335. [Google Scholar] [CrossRef]
- Lee, J.I.; Otto, F.D.; Mather, A.E. Equilibrium between carbon dioxide and aqueous monoethanolamine solutions. J. Appl. Chem. Biotechnol. 2007, 26, 541–549. [Google Scholar] [CrossRef]
- Akachuku, A.; Osei, P.A.; Decardi-Nelson, B.; Srisang, W.; Pouryousefi, F.; Ibrahim, H.; Idem, R. Experimental and kinetic study of the catalytic desorption of CO2 from CO2-loaded monoethanolamine (MEA) and blended monoethanolamine-Methyl-diethanolamine (MEA-MDEA) solutions. Energy 2019, 179, 475–489. [Google Scholar] [CrossRef]
- Huertas, J.I.; Gomez, M.D.; Giraldo, N.; Garzon, J.P. CO2 absorbing capacity of MEA. J. Chem. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ai, N.; Li, G.; Shan, H.; Cui, Y.; Deng, D. Solubilities of carbon dioxide in eutectic mixtures of choline chloride and dihydric alcohols. J. Chem. Eng. Data 2014, 59, 1247–1253. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.M.; van Ness, H.C.; Abbott, M.M.; Swihart, M.T. Introduction To Chemical Engineering Thermodynamics, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Moioli, S.; Pellegrini, L.A. Regeneration section of CO2 capture plant by MEA scrubbing with a rate-based model. Chem. Eng. Trans. 2013, 32, 1849–1854. [Google Scholar] [CrossRef]
- Fan, W.; Liu, Y.; Wang, K. Detailed experimental study on the performance of Monoethanolamine, Diethanolamine, and Diethylenetriamine at absorption/regeneration conditions. J. Clean. Prod. 2016, 125, 296–308. [Google Scholar] [CrossRef]
- Blauwhoff, P.M.M.; van Swaaij, W.P.M. Selective absorption of hydrogen sulfide from natural gas. I2-Procestechnology 1986, 7–8, 13–18. [Google Scholar]
- Jacquemin, J.; Gomes, M.F.C.; Husson, P.; Majer, V. Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon, and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K and 343 K and at pressures close to atmospheric. J. Chem. Thermodyn. 2006, 38, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Jacquemin, J.; Husson, P.; Majer, V.; Gomes, M.F.C. Low-pressure solubilities and thermodynamics of solvation of eight gases in 1-butyl-3-methylimidazolium hexafluorophosphate. Fluid Phase Equilibria 2006, 240, 87–95. [Google Scholar] [CrossRef] [Green Version]
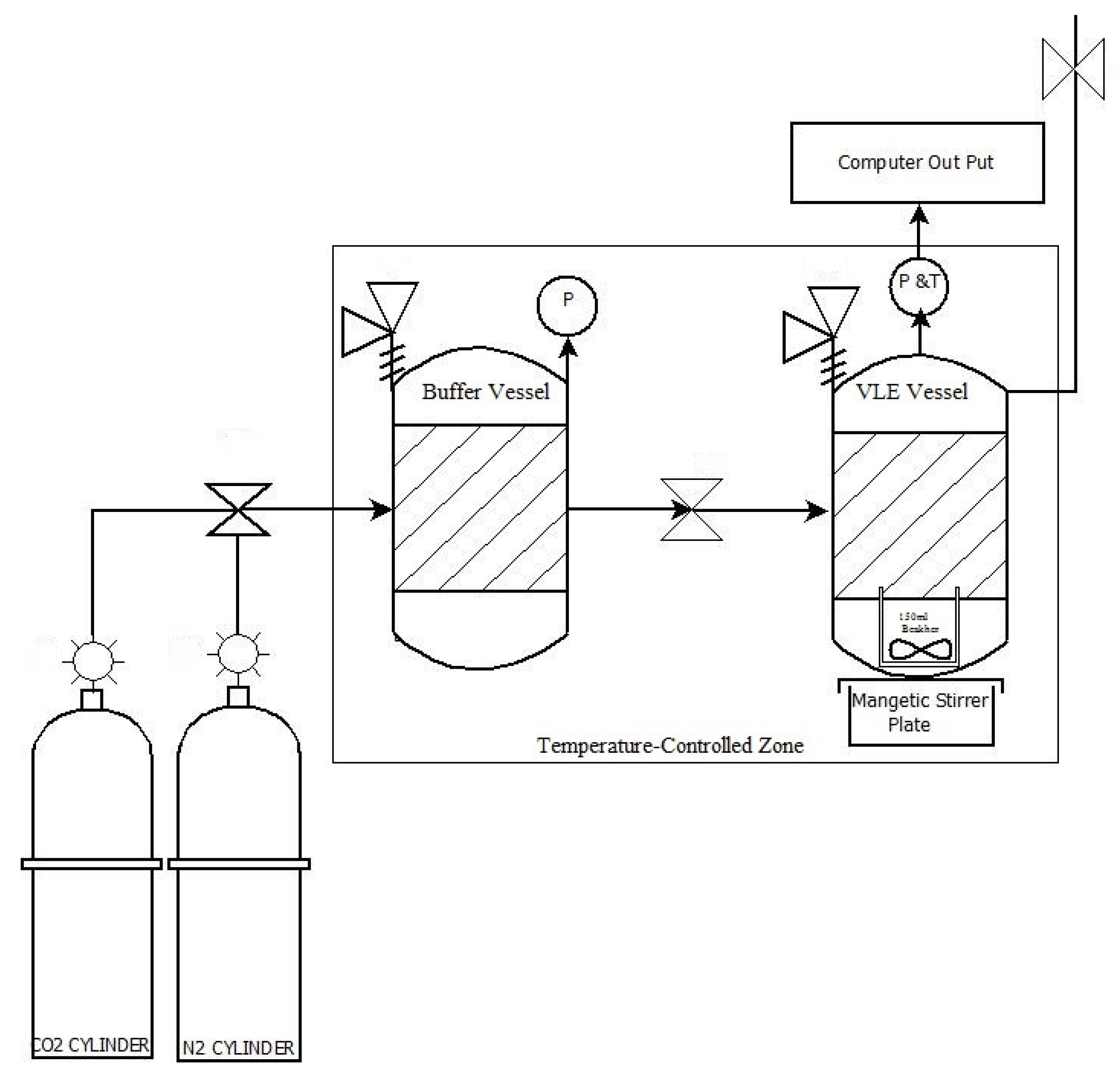

| Run | Before Measurements: Pressure in Buffer Tank Only (During Temperature Equilibration) | At the Start of the Measurements: Pressure in the Buffer and Measuring Tanks | ||
|---|---|---|---|---|
| pCO2 (bar) | pN2 (bar) | pCO2 (bar) | pN2 (bar) | |
| Pure CO2 | 6 | 0 | 3 | 0 |
| 50% CO2/50% N2 | 3 | 3 | 1.5 | 1.5 |
| 15% CO2/85% N2 | 0.9 | 5.1 | 0.45 | 2.55 |
| Pure N2 | 0 | 6 | 0 | 3 |
| CO2−DESs | T (K) | Hx (MPa) | ∆H (kJ·mole−1) | ∆S (J·mole−1·K−1) | ∆G (kJ·mole−1) |
|---|---|---|---|---|---|
| ChCl:LvAc (1:2:0) | 298.15 | 5.34 | −41.5 | −172.4 | 9.86 |
| 308.15 | 6.92 | −49.6 | −196.1 | 10.9 | |
| 318.15 | 12.1 | −65.9 | −246.8 | 12.7 | |
| ChCl:LvAc (1:2:2.5) | 298.15 | 6.14 | −45.0 | −185.2 | 10.2 |
| 308.15 | 8.88 | −56.0 | −218.9 | 11.5 | |
| 318.15 | 10.5 | −62.2 | −234.4 | 12.3 | |
| ChCl:LvAc (1:2:5) | 298.15 | 5.47 | −42.1 | −174.6 | 9.92 |
| 308.15 | 8.17 | −53.8 | −211.3 | 11.3 | |
| 318.15 | 10.3 | −61.8 | −232.8 | 12.3 | |
| ChCl:LvAc (1:3:0) | 298.15 | 5.60 | −42.7 | −176.8 | 9.97 |
| 308.15 | 10.1 | −59.3 | −230.7 | 11.8 | |
| 318.15 | 10.8 | −63.0 | −237.1 | 12.4 | |
| ChCl:LvAc (1:3:2.5) | 298.15 | 4.61 | −37.9 | −159.0 | 9.49 |
| 308.15 | 9.25 | −57.0 | −222.6 | 11.6 | |
| 318.15 | 10.8 | −63.0 | −237.1 | 12.4 | |
| ChCl:LvAc (1:3:5) | 298.15 | 4.96 | −39.7 | −165.7 | 9.67 |
| 308.15 | 9.29 | −57.1 | −223.0 | 11.6 | |
| 318.15 | 14.2 | −70.3 | −262.0 | 13.1 |
| Material | Description | Molar % of Gases (CO2/N2) | Selectivity (S) | Reference |
|---|---|---|---|---|
| DES | ChCl:LvAc (1:3:2.5) | 50/50 | 5.63 | This work |
| 15/85 | 4.94 | |||
| IL | bmim (BF4) | 28.7 * | [43] | |
| IL | bmim (BF6) | 22.6 * | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboshatta, M.; Magueijo, V. A Comprehensive Study of CO2 Absorption and Desorption by Choline-Chloride/Levulinic-Acid-Based Deep Eutectic Solvents. Molecules 2021, 26, 5595. https://doi.org/10.3390/molecules26185595
Aboshatta M, Magueijo V. A Comprehensive Study of CO2 Absorption and Desorption by Choline-Chloride/Levulinic-Acid-Based Deep Eutectic Solvents. Molecules. 2021; 26(18):5595. https://doi.org/10.3390/molecules26185595
Chicago/Turabian StyleAboshatta, Mohaned, and Vitor Magueijo. 2021. "A Comprehensive Study of CO2 Absorption and Desorption by Choline-Chloride/Levulinic-Acid-Based Deep Eutectic Solvents" Molecules 26, no. 18: 5595. https://doi.org/10.3390/molecules26185595
APA StyleAboshatta, M., & Magueijo, V. (2021). A Comprehensive Study of CO2 Absorption and Desorption by Choline-Chloride/Levulinic-Acid-Based Deep Eutectic Solvents. Molecules, 26(18), 5595. https://doi.org/10.3390/molecules26185595