A DFT Study on the Molecular Mechanism of Additions of Electrophilic and Nucleophilic Carbenes to Non-Enolizable Cycloaliphatic Thioketones †
Abstract
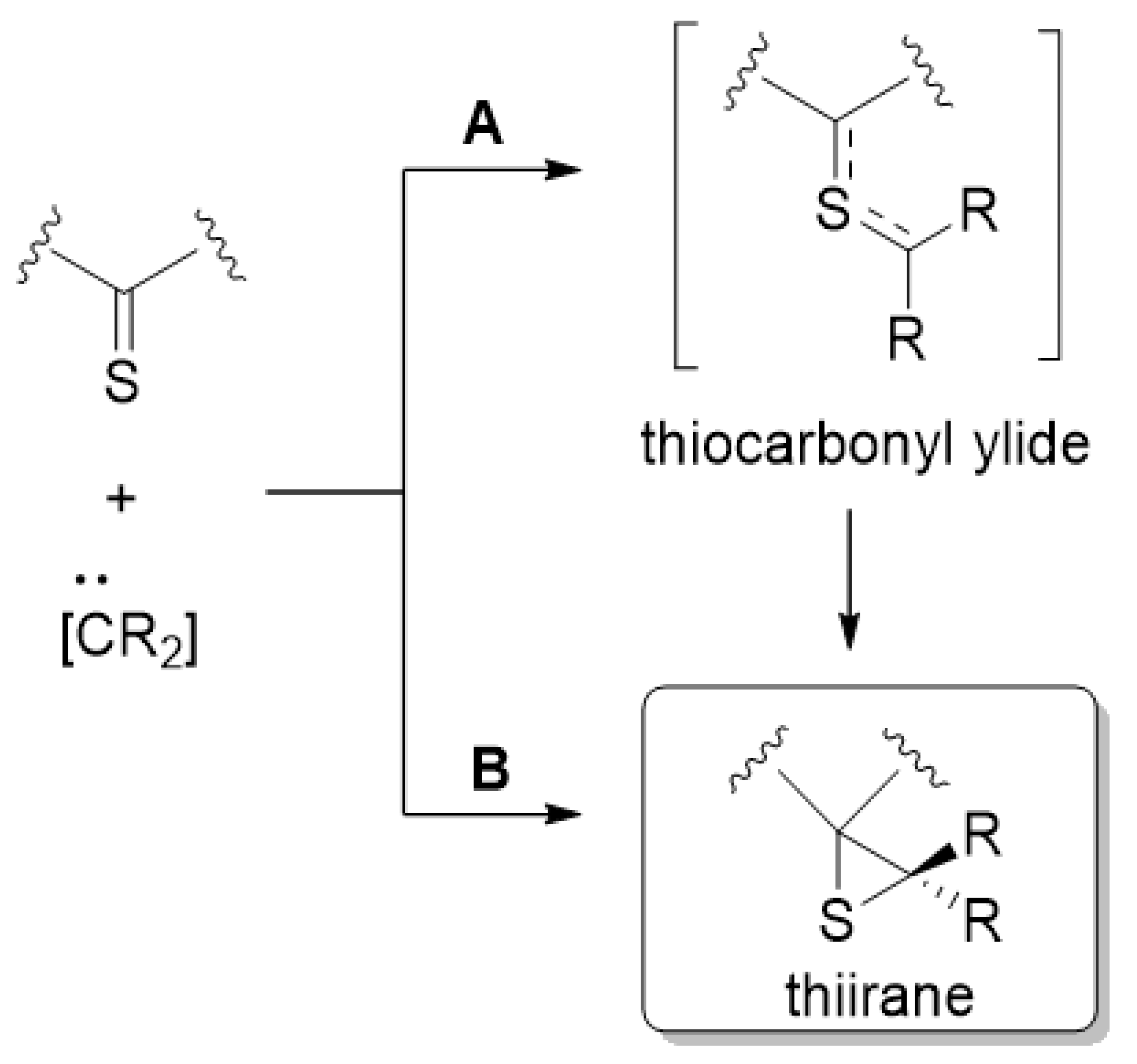
:1. Introduction
2. Results and Discussion
2.1. Nature of Intermolecular Interactions in the Light of a the Analysis of the CDFT Reactivity Indices of Reagents
2.1.1. Global Reactivity
2.1.2. Local Reactivity
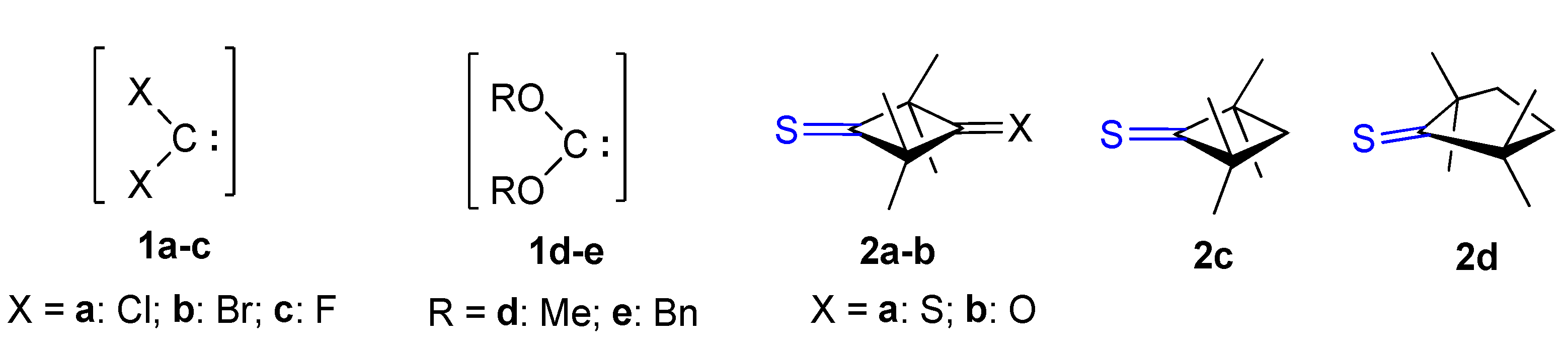
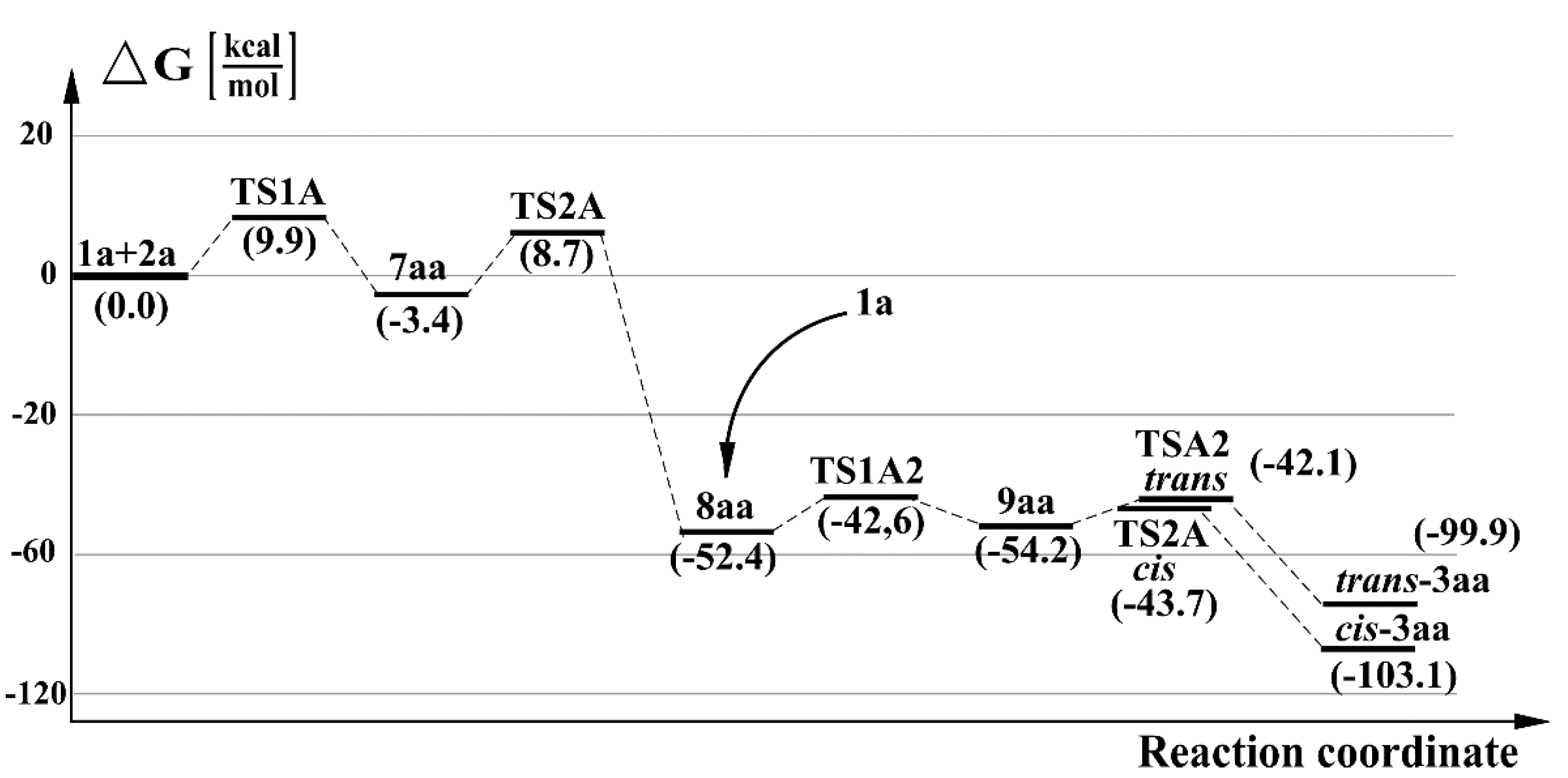
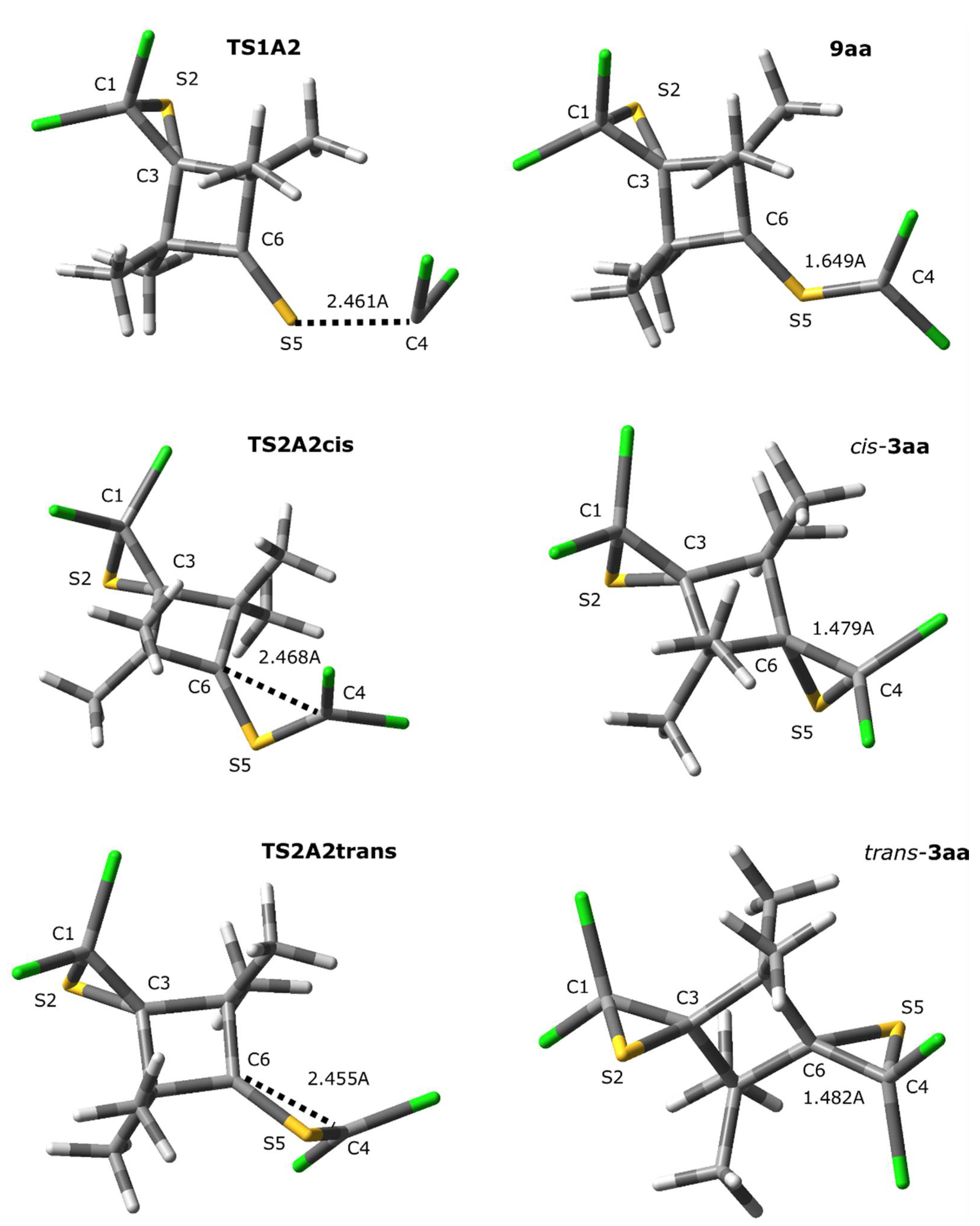
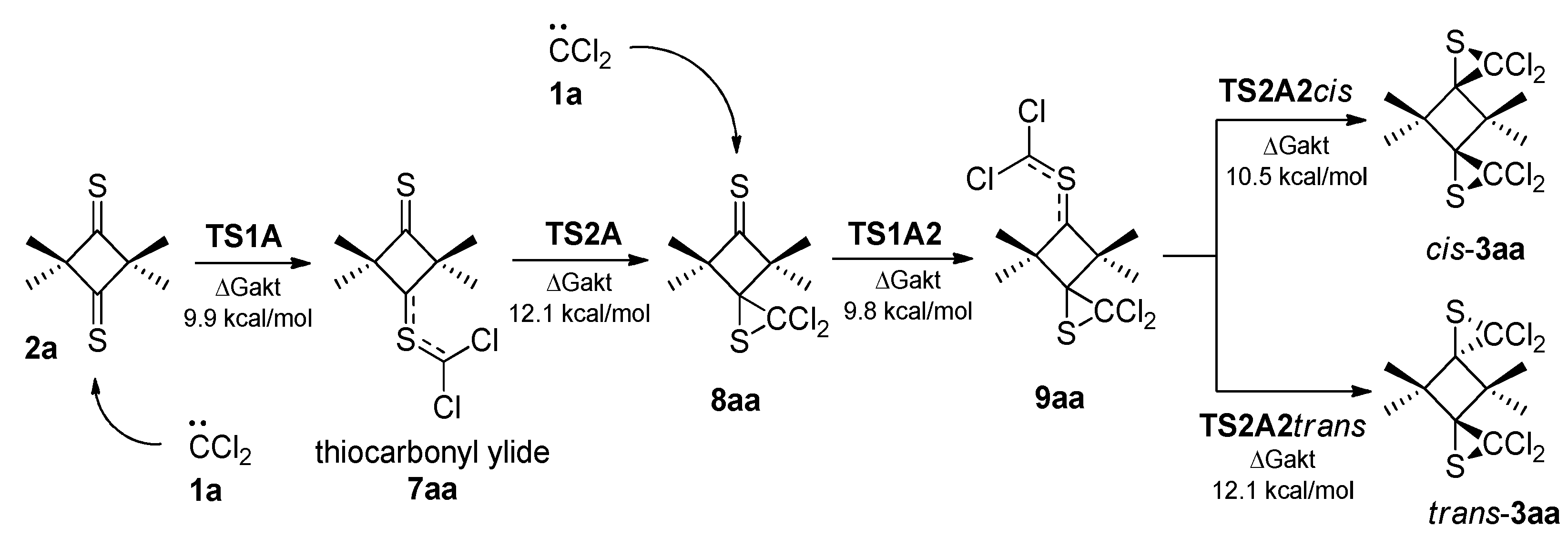
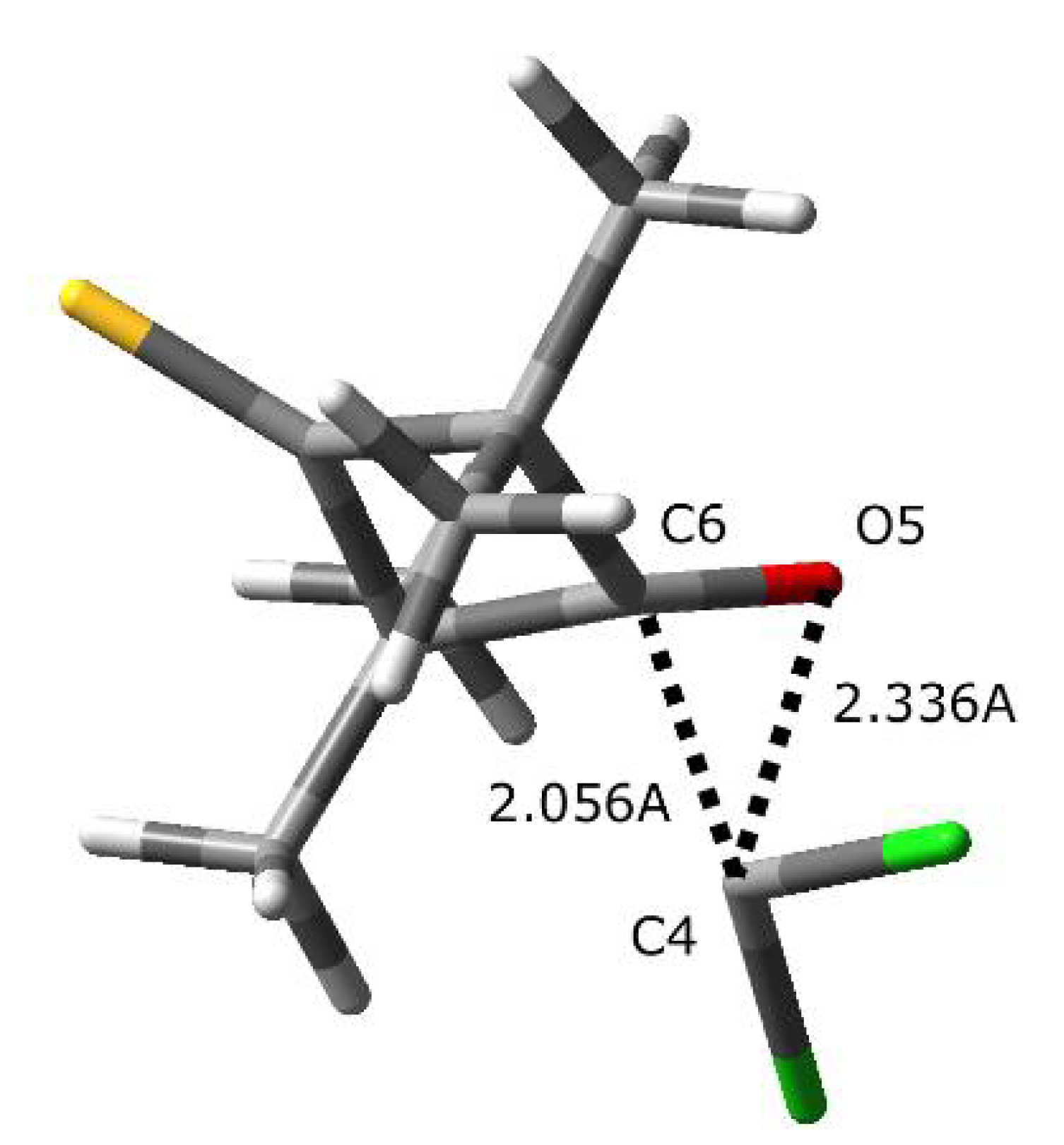
2.2. A Full Mechanistic Considerations Reaction of Dichlorocarbene (1a) with 2,2,4,4-Tetramethylcyclobutane-1,3-dithione (2a)
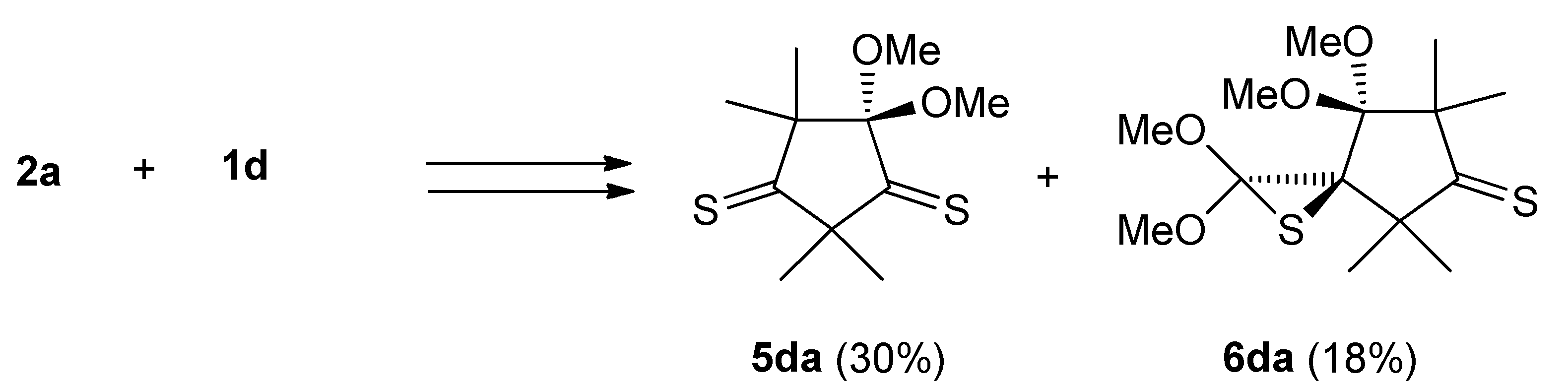
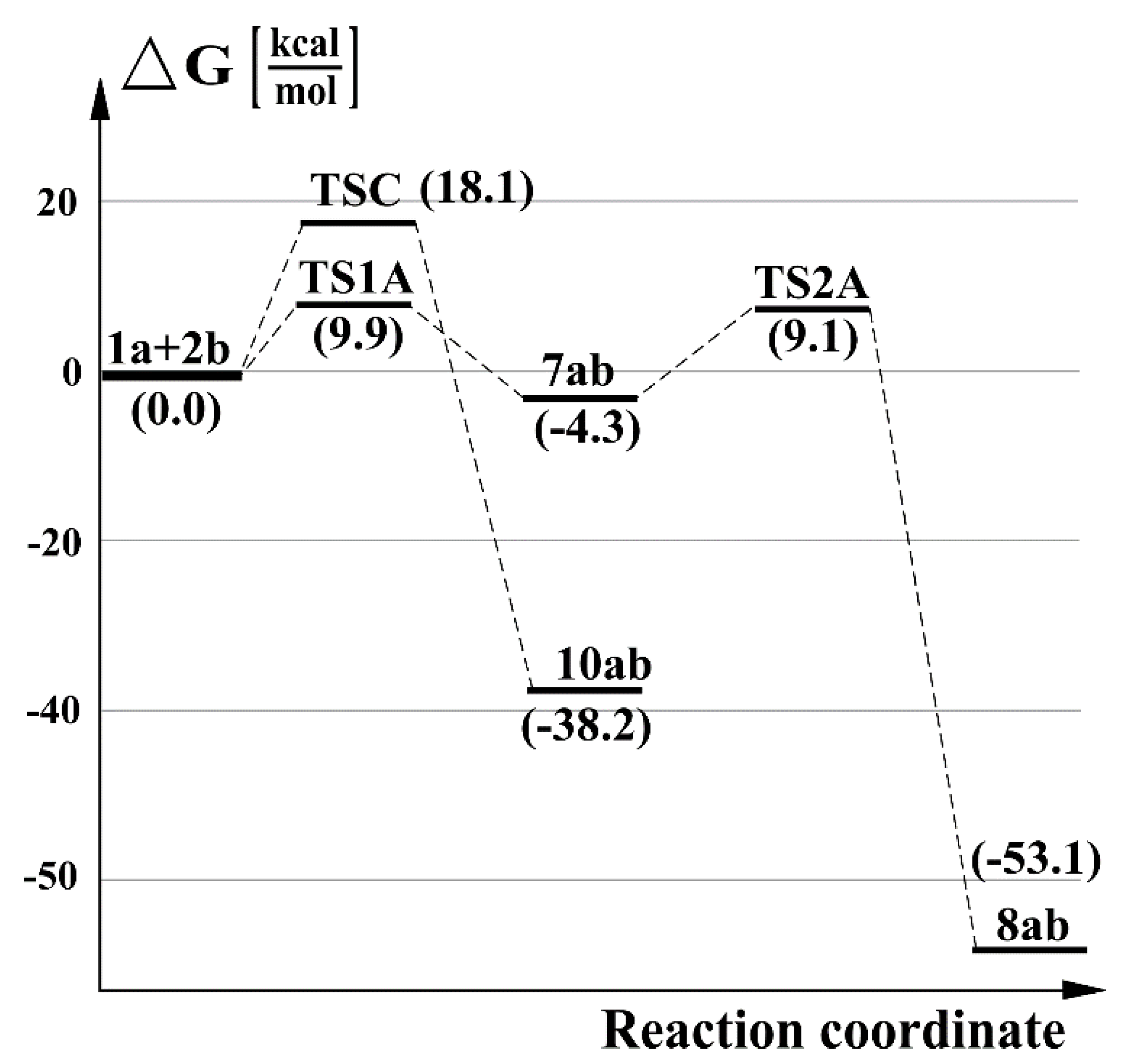
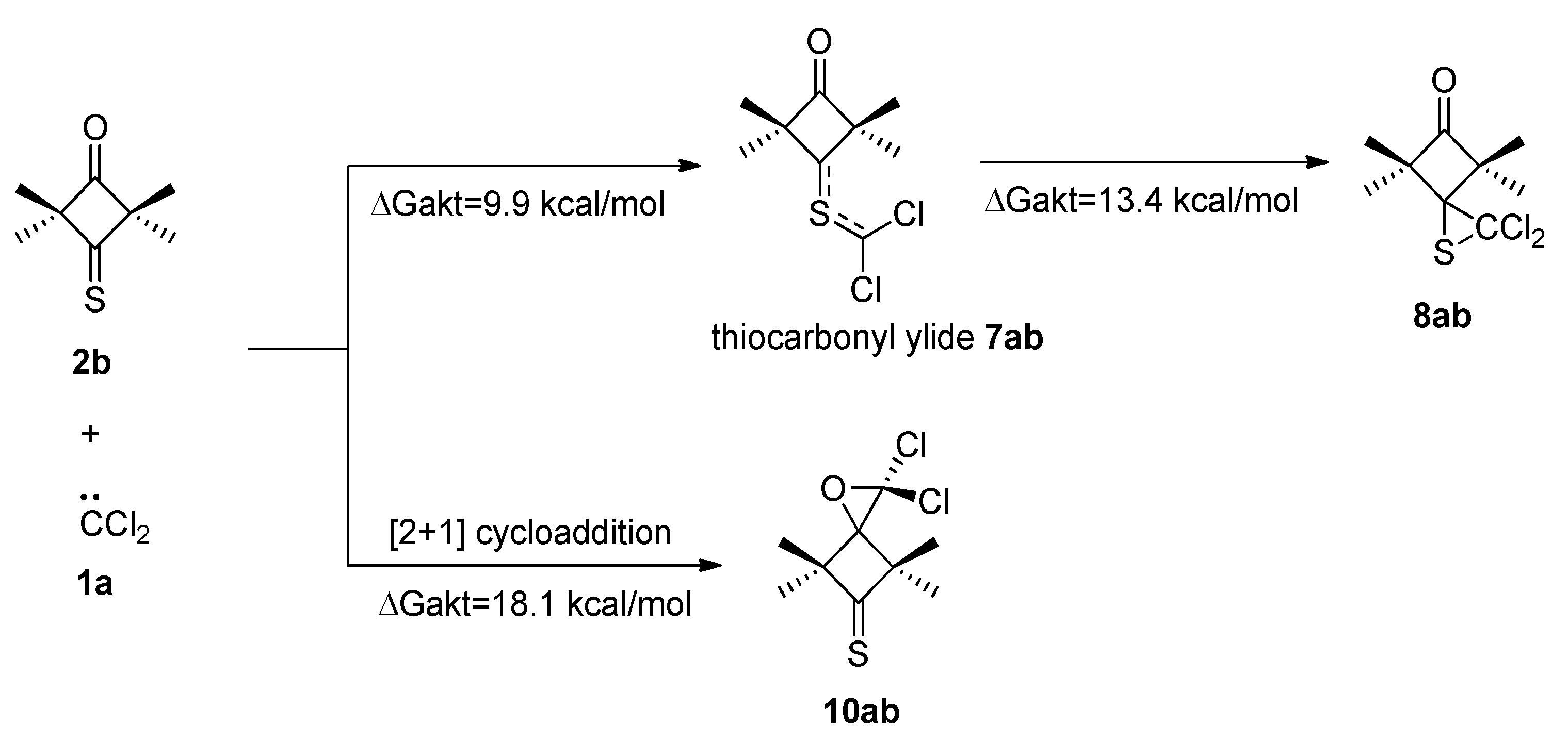
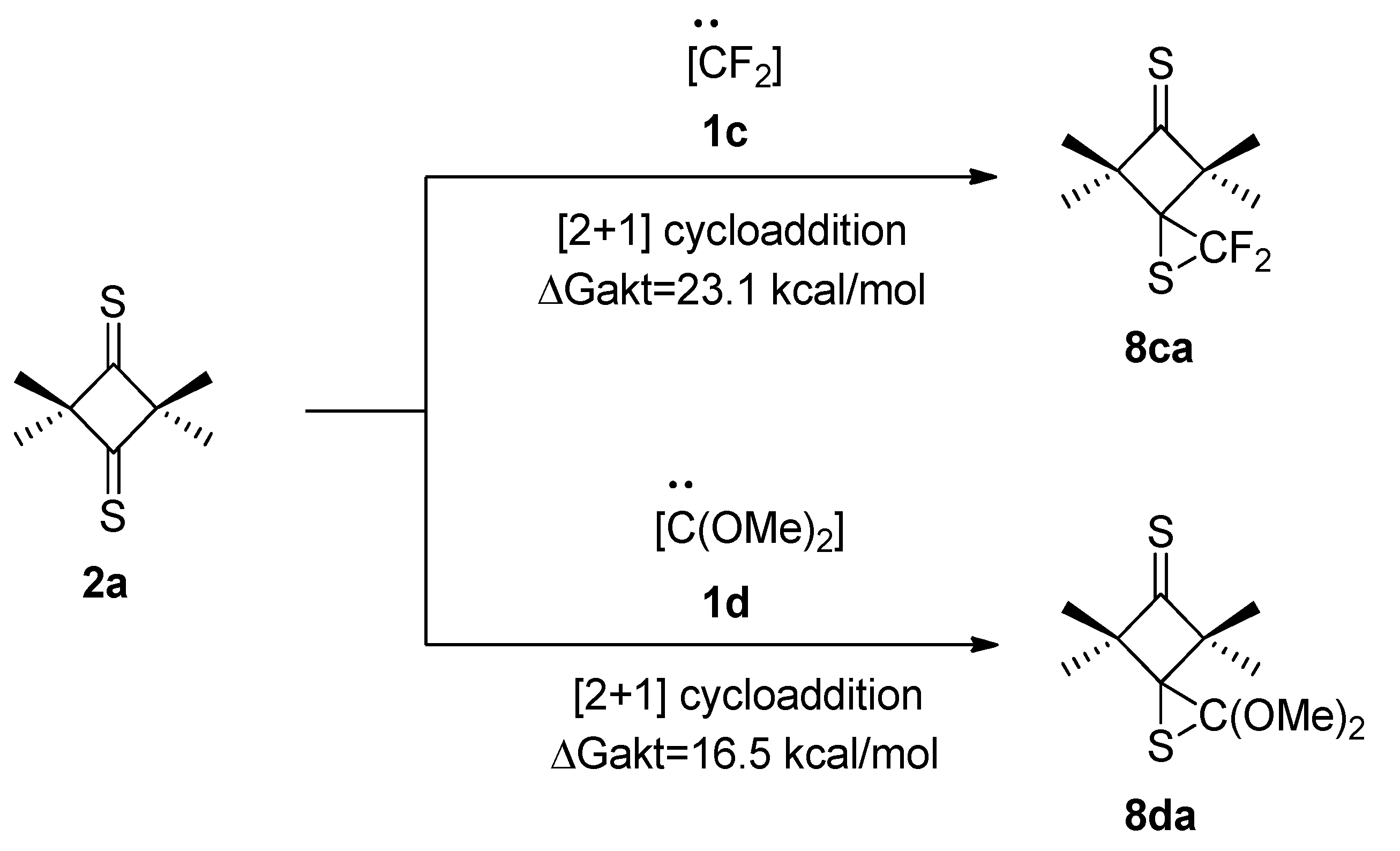
2.3. Further Aspects of Reactions between Carbenes 1a–d and Thioketones 2a–d
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Moss, R.A.; Doyle, M.P. (Eds.) Contemporary Carbene Chemistry, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hahn, F.E. Introduction: Carbene chemistry. Chem. Rev. 2018, 118, 9455–9456. [Google Scholar]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- de Fremont, P.; Nicolas, M.; Nolan, S.P. Carbenes: Synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, L.; Fan, H.; Yao, Q.; Zhu, S. Recent progress on donor and donor–donor carbenes. Chem. Soc. Rev. 2020, 49, 908–950. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, B.D.; Nickerson, L.A.; Shaw, J.T.; Souza, L.W. Transition metal catalyzed insertion reactions with donor/donor carbenes. Angew. Chem. Int. Ed. 2021, 60, 6864–6878. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.C.; Hota, P.K.; Mandal, S.K.; Soleilhavoup, M.; Bertrand, G. Stable abnormal N-heterocyclic carbenes and their applications. Chem. Soc. Rev. 2020, 49, 1233–1252. [Google Scholar] [CrossRef]
- Wentrup, C. Carbenes and nitrenes: Recent developments in fundamental chemistry. Angew. Chem. Int. Ed. 2018, 57, 11508–11521. [Google Scholar] [CrossRef]
- Soleilhavoup, M.; Bertrand, G. Stable carbenes, nitrenes, phosphinidenes, and borylenes: Past and future. Chem 2020, 6, 1275–1282. [Google Scholar] [CrossRef]
- Yang, Y.; Arnold, F.H. Unnatural reaction space: Directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 2021, 54, 1209–1225. [Google Scholar] [CrossRef]
- Jana, S.; Pei, C.; Empel, C.; Koenigs, R.M. Photochemical carbene transfer reactions of aryl/aryl diazoalkanes - Experiment and theory. Angew. Chem. Int. Ed. 2021, 60, 13271–13279. [Google Scholar] [CrossRef]
- Chen, R.; Ogunlana, A.A.; Fang, S.; Long, W.; Sun, H.; Bao, X.; Wan, X. In situ generation of nitrile oxides from copper carbene and tert-butyl nitrite: Synthesis of fully substituted isoxazoles. Org. Biomol. Chem. 2018, 16, 4683–4687. [Google Scholar] [CrossRef]
- Wróblewska, A.; Lauriol, G.; Mlostoń, G.; Bantreilc, X.; Lamaty, F. Expedient synthesis of N–Oxy-Heterocyclic Carbenes (NOHC) ligands and metal complexes using mechanochemistry. J. Organomet. Chem. 2021, 949, e121914. [Google Scholar] [CrossRef]
- Østby, R.B.; Didriksen, T.; Antonsen, S.G.; Nicolaisen, S.S.; Stenstrøm, Y. Two-phase dibromocyclopropanation of unsaturated alcohols using flow chemistry. Molecules 2020, 25, 2364. [Google Scholar] [CrossRef] [PubMed]
- Dehmlow, E.V. Houben-Weyl: Methoden der Organischen Chemie, 4th ed.; Regitz, M., Ed.; Thieme: Stuttgart, NY, USA, 1989; pp. 1568–1571. [Google Scholar]
- Mąkosza, M.; Wawrzynkiewicz, M. Reactions of organic anions. XXIV. Catalytic method for preparation of dichloro-cyclopropane derivatives in aqueous medium. Tetrahedron Lett. 1969, 10, 4659–4662. [Google Scholar] [CrossRef]
- Mąkosza, M.; Fedoryński, M. Reakcje anionów organicznych. XXXVIII. Reakcja włączania dwuchlorokarbenu wytwarzanego w środowisku wodnym do wiązania węgiel-wodór. Roczniki Chem. 1972, 46, 311–313. [Google Scholar]
- Mąkosza, M. Two-phase reactions in the chemistry of carbanions and halocarbenes. A useful tool in organic synthesis. Pure Appl. Chem. 1975, 43, 439–462. [Google Scholar] [CrossRef]
- Fedoryński, M. Syntheses of gem-dihalocyclopropanes and their use in organic synthesis. Chem. Rev. 2003, 103, 1099–1132. [Google Scholar] [CrossRef]
- Dilman, A.D.; Levin, V.V. Difluorocarbene as a building block for consecutive bond-forming reactions. Acc. Chem. Res. 2018, 51, 1272–1280. [Google Scholar] [CrossRef]
- Adekenova, K.S.; Wyatt, P.B.; Adekenov, S.M. The preparation and properties of 1,1-difluorocyclopropane derivatives. Beilstein J. Org. Chem. 2021, 17, 245–272. [Google Scholar] [CrossRef]
- Warkentin, J. 2,5-Dihydro-1,3,4-oxadiazoles and bis(heteroatom-substituted)carbenes. Acc. Chem. Res. 2009, 42, 205–213. [Google Scholar] [CrossRef]
- Mlostoń, G.; Romański, J.; Heimgartner, H. First synthesis of gem-difluorothiiranes from cycloaliphatic thioketones and difluorocarbene. Heterocycles 1999, 50, 403–410. [Google Scholar]
- Mlostoń, G.; Romański, J.; Świątek, A.; Heimgartner, H. Reactions of thioketones with dichlorocarbene. Helv. Chim. Acta 1999, 82, 946–956. [Google Scholar] [CrossRef]
- Dawid, M.; Warkentin, J.; Mloston, G. Relative reactivities of carbonyl and thiocarbonyl groups toward dimethoxycarbene and two new dimethoxythiiranes. Chem. Eur. J. 2002, 8, 2184–2187. [Google Scholar] [CrossRef]
- Moss, R.A. Carbenic reactivity revisited. Acc. Chem. Res. 1989, 22, 15–21. [Google Scholar] [CrossRef]
- Moss, R.A. “Carbon Dichloride”: Dihalocarbenes sixty years after Hine. J. Org. Chem. 2010, 75, 5773–5783. [Google Scholar] [CrossRef]
- Alnajjar, R.A.; Jasiński, R. Competition between [2 + 1]- and [4 + 1]-cycloaddition mechanisms in reactions of conjugated nitroalkenes with dichlorocarbene in the light of DFT computational study. J. Mol. Model. 2019, 25, e157. [Google Scholar] [CrossRef] [Green Version]
- Padwa, A.; Hornbuckle, S.F. Ylide Formation from the reaction of carbenes and carbenoids with heteroatom lone pairs. Chem. Rev. 1991, 91, 263–309. [Google Scholar] [CrossRef]
- Padwa, A. Catalytic decomposition of diazo compounds as a method for generating carbonyl-ylide dipoles. Helv. Chim. Acta. 2005, 88, 1357–1374. [Google Scholar] [CrossRef]
- Meyer, A.G.; Ryan, J.H. 1,3-Dipolar cycloaddition reactions of azomethine ylides with carbonyl dipolarophiles yielding oxazolidine derivatives. Molecules 2016, 21, 935. [Google Scholar] [CrossRef]
- Mlostoń, G.; Heimgartner, H. Generation and typical reactions of thiocarbonyl ylides. Pol. J. Chem. 2000, 74, 1503–1533. [Google Scholar]
- Mlostoń, G.; Heimgartner, H. Thiocarbonyl ylides. In 1,3-Dipolar Cycloaddition Chemistry towards Heterocycles and Natural Products; Padwa, A., Pearson, W.H., Eds.; Wiley: New York, NY, USA, 2002; pp. 315–360. [Google Scholar]
- Domingo, L.R. Molecular Electron Density Theory: A modern view of reactivity in organic chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules, 1st ed.; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Pérez, P.; Saez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Kula, K.; Zawadzinska, K. Local nucleophile-electrophile interactions in [3 + 2] cycloaddition reactions between benzonitrile N-oxide and selected conjugated nitroalkenes in the light of MEDT computational study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Mlostoń, G.; Heimgartner, H. Reactions of thiocarbonyl compounds with electrophilic and nucleophilic carbenes as well as with their metal complexes. J. Sulfur Chem. 2020, 41, 672–700. [Google Scholar] [CrossRef]
- Kącka-Zych, A. Understanding the uniqueness of the stepwise [4 + 1] cycloaddition reaction between conjugated nitroalkenes and electrophilic carbene systems with a molecular electron density theory perspective. Int. J. Quantum. Chem. 2021, 121, e26440. [Google Scholar] [CrossRef]
- Jasinski, R.; Dresler, E. On the question of zwitterionic intermediates in the [3 + 2] cycloaddition reactions: A critical review. Organics 2020, 49, 5. [Google Scholar] [CrossRef]
- Mlostoń, G.; Jasiński, R.; Kula, K.; Heimgartner, H. A DFT Study on the Barton-Kellogg Reaction—The molecular mechanism of the formation of thiiranes in the reaction between diphenyldiazomethane and diaryl thioketones. Eur. J. Org. Chem. 2020, 176–182. [Google Scholar] [CrossRef]
- Dawid, M.; Mlostoń, G.; Warkentin, J. The first 2,2-dialkoxythiirane. Org. Lett. 2002, 3, 2455–2456. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.R.; Duque-Noreña, M.; Chamorro, E. A condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions. J. Mol. Struct. 2009, 895, 86–91. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.; Domingo, L.R.; Aurell, M.J.; Contreras, R. Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1.3-dipolar cycloaddition reactions. Tetrahedron 2003, 59, 3117–3125. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Rev. A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, e084106. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Zawadzinska, K.; Kula, K. Application of ß-phosphorylated nitroethenes in [3 + 2] cycloaddition reactions involving benzonitrile N-oxide in the light of DFT computational study. Organics 2021, 2, 3. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Wzorek, Z.; Nowak, A.; Jasiński, R. Experimental and theoretical mechanistic study on the thermal decomposition of 3,3-diphenyl-4-(trichloromethyl)-5-nitropyrazoline. Molecules 2021, 26, 1364. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Analysis of the possibility and molecular mechanism of carbon dioxide consumption in the Diels-Alder processes. Pure Appl. Chem. 2021, 93, 427–446. [Google Scholar] [CrossRef]
- Jasiński, R. One-step versus two-step mechanism of Diels-Alder reaction of 1-chloro-1-nitroethene with cyclopentadiene and furan. J. Mol. Graph. Model. 2017, 75, 55–61. [Google Scholar] [CrossRef]
- Domingo, L.R.; Kula, K.; Ríos-Gutiérrez, M. Unveiling the reactivity of cyclic azomethine ylides in [3 + 2] cycloaddition reactions within the Molecular Electron Density Theory. Eur. J. Org. Chem. 2020, 5938–5948. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Chem. 1996, 225, 327–335. [Google Scholar] [CrossRef]
- Mlostoń, G.; Pipiak, P.; Heimgartner, H. Diradical reaction mechanisms in [3 + 2]-cycloadditions of hetaryl thioketones with alkyl- or trimethylsilyl-substituted diazomethanes. Beilstein J. Org. Chem. 2016, 12, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Huisgen, R.; Kalvinsch, I.; Li, X.; Mlostoń, G. 1,3-Dipolar cycloadditions, 116. The formation of 1,3-dithiolanes from aromatic thioketones and diazomethane - The mechanism of the Schonberg Reaction. Eur. J. Org. Chem. 2000, 2000, 1685–1694. [Google Scholar]
- Mlostoń, G.; Romański, J.; Schmidt, C.; Reisenauer, H.P.; Maier, G. Photochemische und thermische Erzeugung von Thiocarbonylyliden aus 2,5-Dihydro-1,3,4-thiadiazolen. Chem. Ber. 1994, 127, 2527–2539. [Google Scholar] [CrossRef]



| μ | η | ω | N | |
|---|---|---|---|---|
| 1a | −5.45 | 3.81 | 3.91 | 1.76 |
| 1b | −5.33 | 3.46 | 4.11 | 2.06 |
| 1c | −3.00 | 5.60 | 0.81 | 3.32 |
| 1d | −2.90 | 5.59 | 0.75 | 3.42 |
| 2a | −4.24 | 3.49 | 2.58 | 3.14 |
| 2b | −4.17 | 3.71 | 2.35 | 3.09 |
| 2c | −3.82 | 3.90 | 1.87 | 3.35 |
| 2d | −3.80 | 3.91 | 1.84 | 3.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlostoń, G.; Kula, K.; Jasiński, R. A DFT Study on the Molecular Mechanism of Additions of Electrophilic and Nucleophilic Carbenes to Non-Enolizable Cycloaliphatic Thioketones. Molecules 2021, 26, 5562. https://doi.org/10.3390/molecules26185562
Mlostoń G, Kula K, Jasiński R. A DFT Study on the Molecular Mechanism of Additions of Electrophilic and Nucleophilic Carbenes to Non-Enolizable Cycloaliphatic Thioketones. Molecules. 2021; 26(18):5562. https://doi.org/10.3390/molecules26185562
Chicago/Turabian StyleMlostoń, Grzegorz, Karolina Kula, and Radomir Jasiński. 2021. "A DFT Study on the Molecular Mechanism of Additions of Electrophilic and Nucleophilic Carbenes to Non-Enolizable Cycloaliphatic Thioketones" Molecules 26, no. 18: 5562. https://doi.org/10.3390/molecules26185562
APA StyleMlostoń, G., Kula, K., & Jasiński, R. (2021). A DFT Study on the Molecular Mechanism of Additions of Electrophilic and Nucleophilic Carbenes to Non-Enolizable Cycloaliphatic Thioketones. Molecules, 26(18), 5562. https://doi.org/10.3390/molecules26185562








