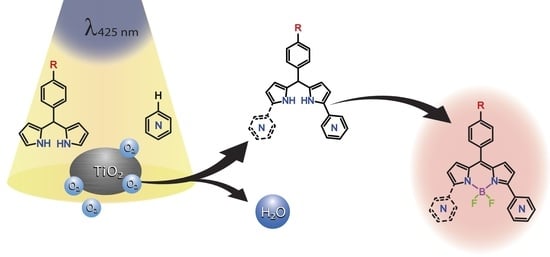
Oxidative C-H/C-H Coupling of Dipyrromethanes with Azines by TiO2-Based Photocatalytic System. Synthesis of New BODIPY Dyes and Their Photophysical and Electrochemical Properties
Abstract
:1. Introduction
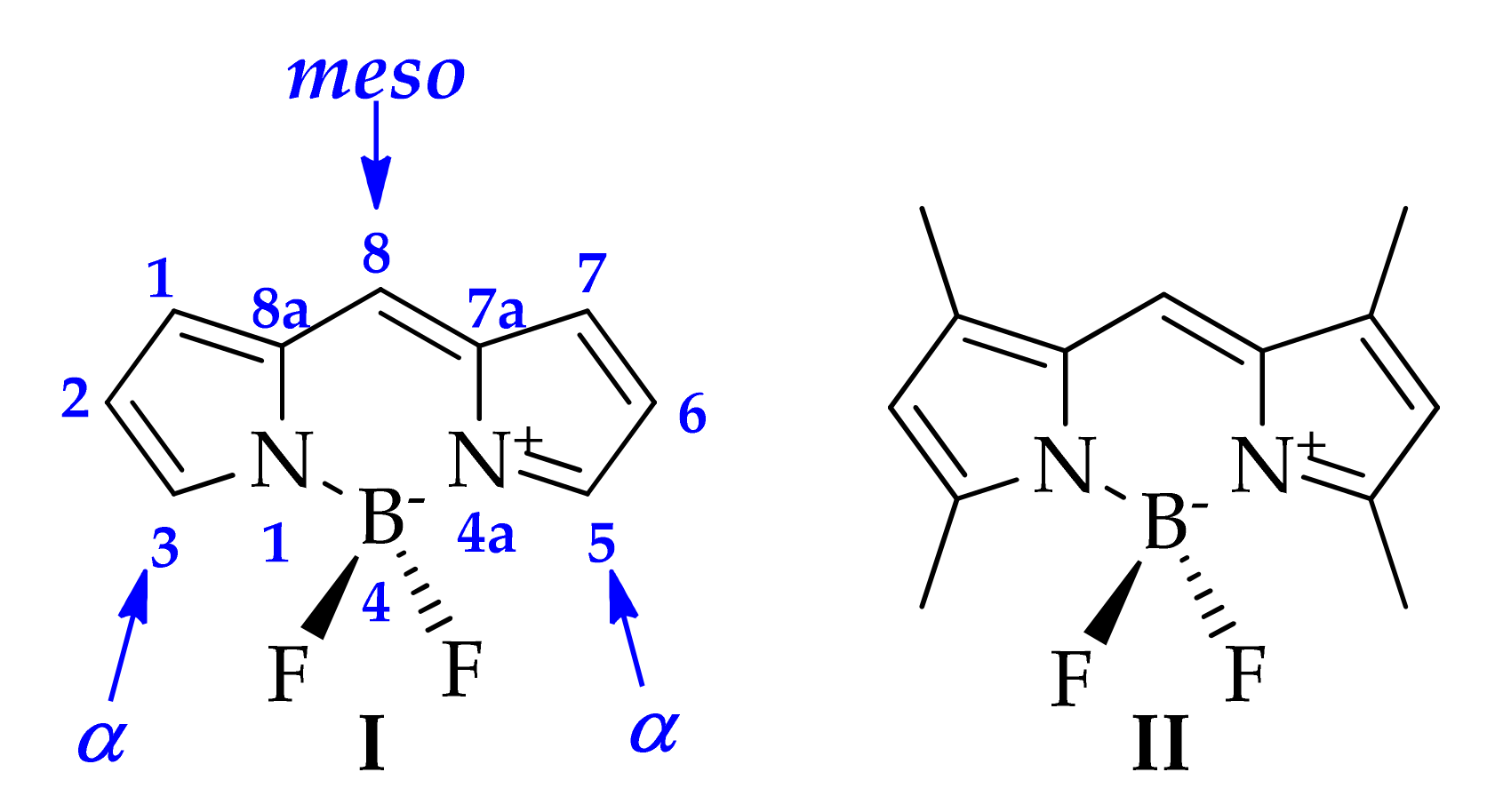
2. Results
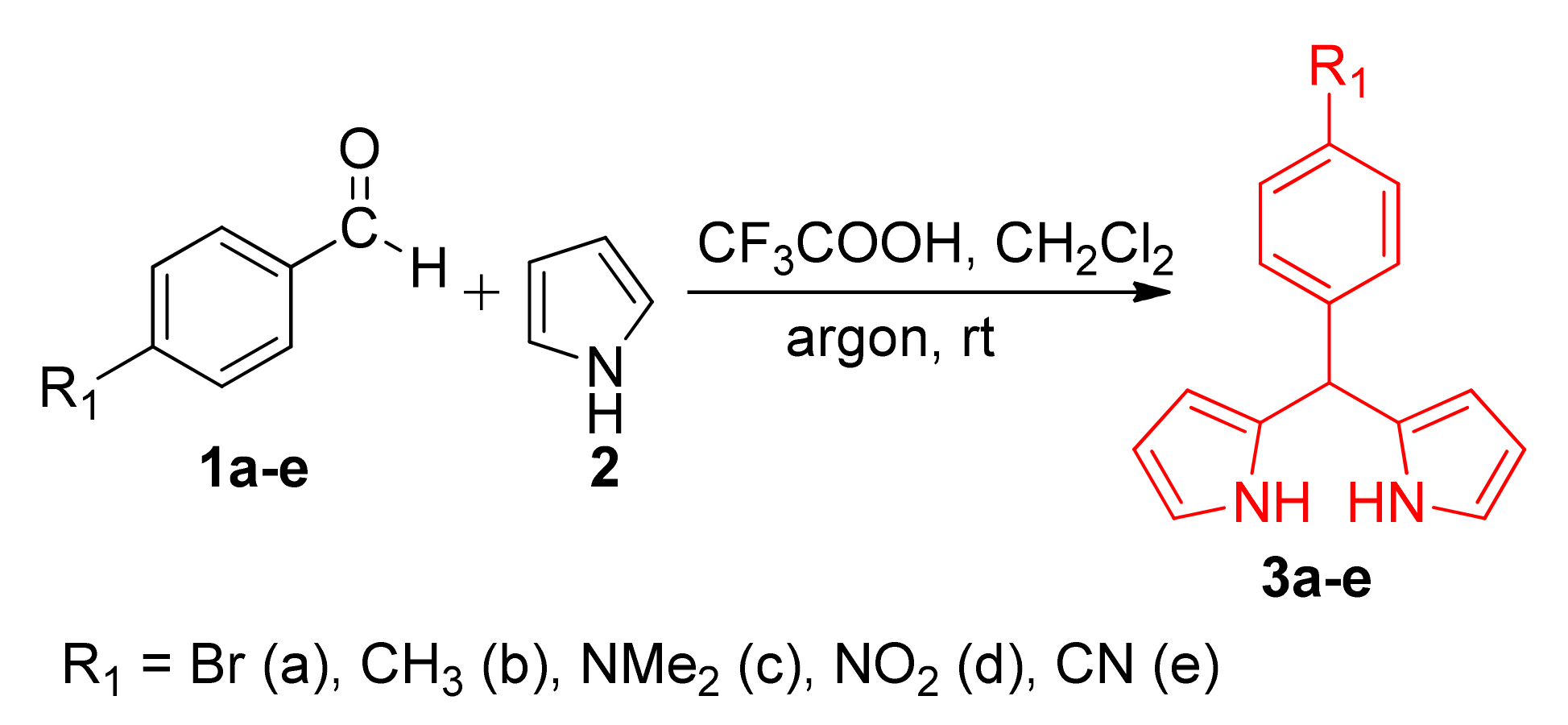
2.1. Synthesis
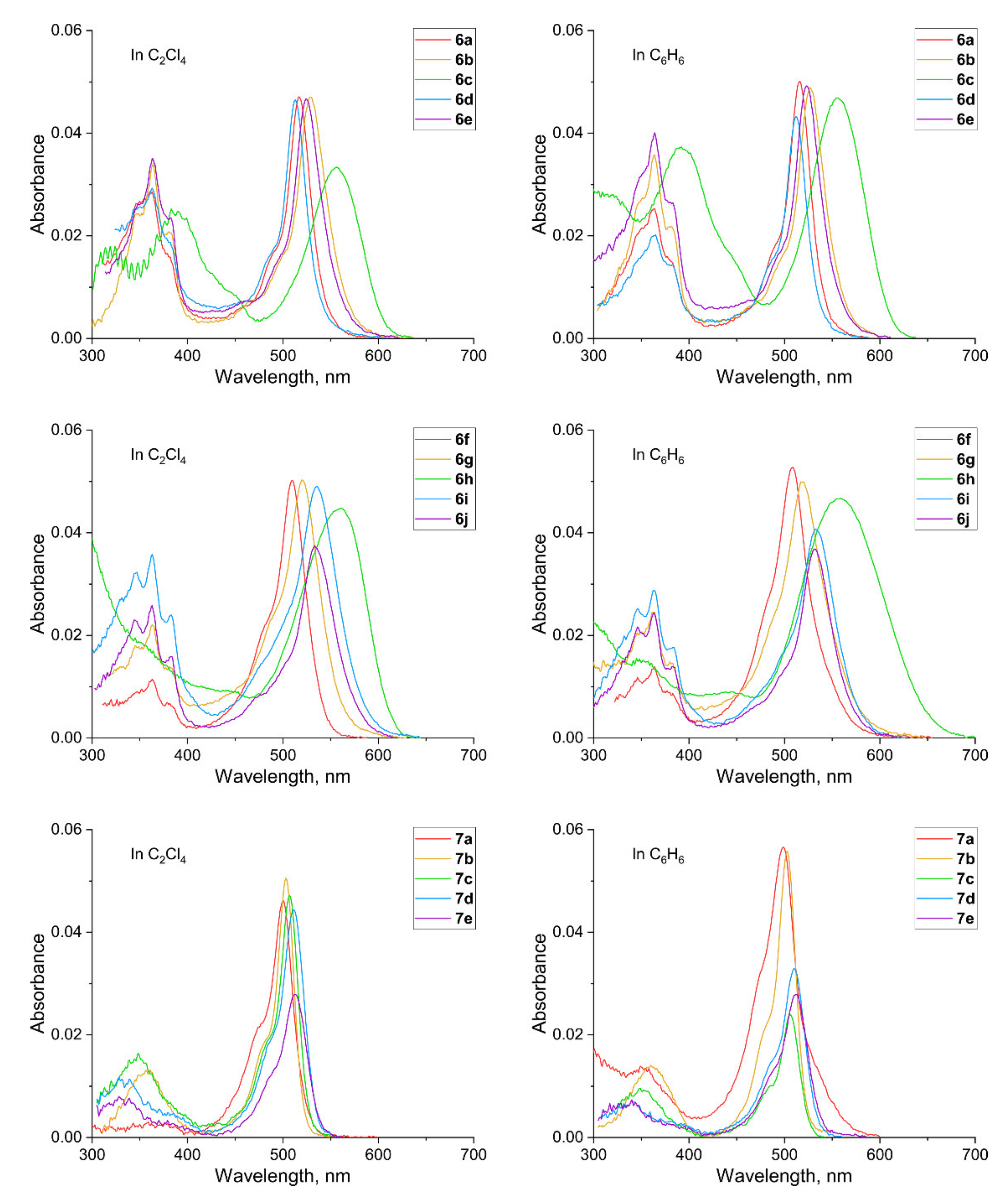
2.2. Photophysical Properties
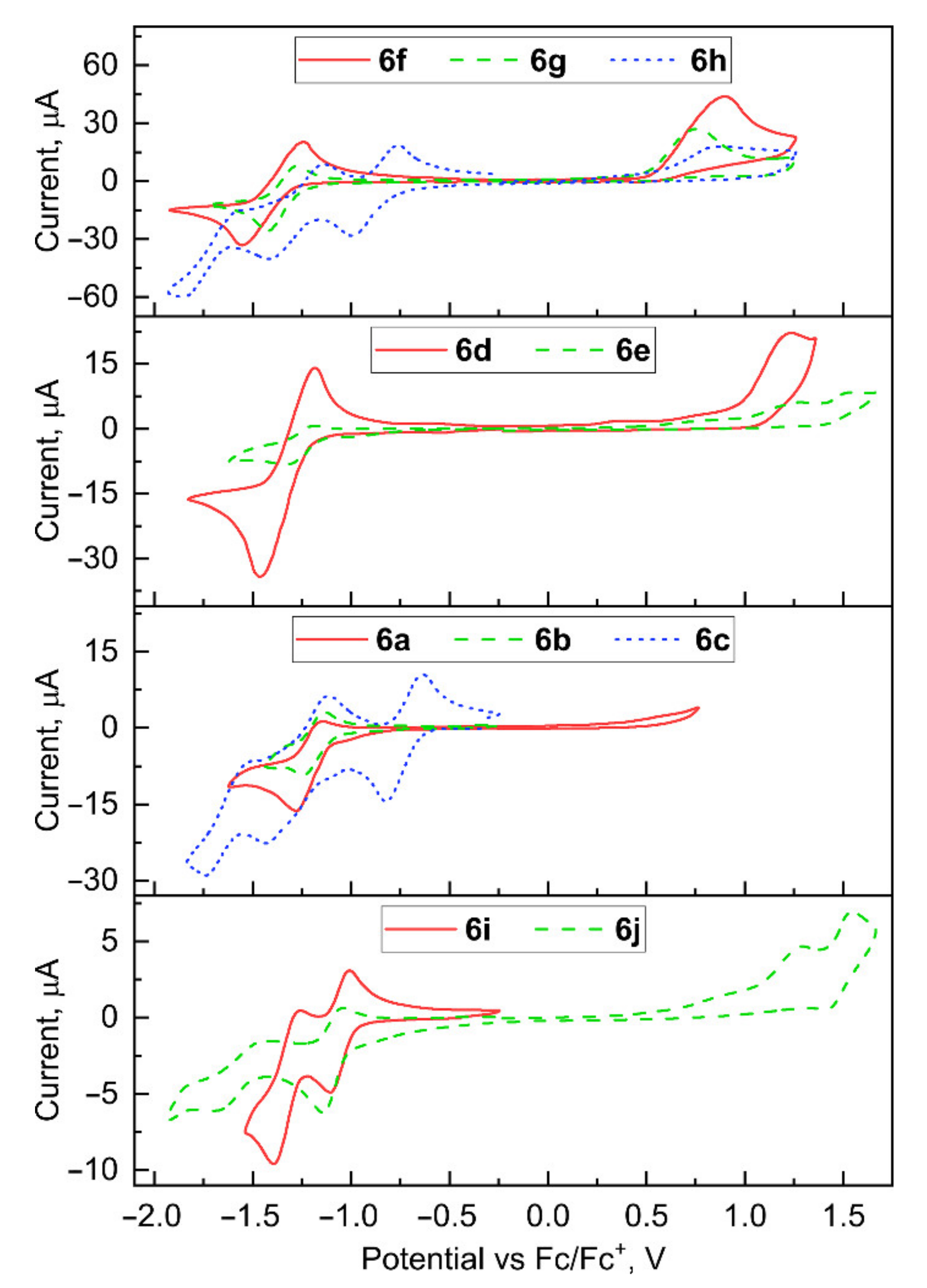
2.3. Electrochemical Properties
3. Materials and Methods
3.1. General
3.2. Optical Measurements
3.3. Electrochemical Measurements
3.4. General Synthesis of Dipyrromethanes 5a–g
3.5. General Procedure for BODIPYs 6a–g
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Treibs, A.; Kreuzer, F.-H. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- De Wael, E.V.; Pardoen, J.A.; van Koeveringe, J.A.; Lugtenburg, J. Pyrromethene-BF2 Complexes (4,4′-Difluoro-4-Bora-3a,4a-Diaza-s-Indacenes). Synthesis and Luminescence Properties. Recl. Trav. Chim. Pays-Bas 1977, 96, 306–309. [Google Scholar] [CrossRef]
- Wories, H.J.; Koek, J.H.; Lodder, G.; Lugtenburg, J.; Fokkens, R.; Driessen, O.; Mohn, G.R. A Novel Water-Soluble Fluorescent Probe: Synthesis, Luminescence and Biological Properties of the Sodium Salt of the 4-Sulfonato-3,3′,5,5′-Tetramethyl-2,2′-Pyrromethen-1,1′-BF2 Complex. Recl. Trav. Chim. Pays-Bas 1985, 104, 288–291. [Google Scholar] [CrossRef]
- Pavlopoulos, T.G.; Shah, M.; Boyer, J.H. Laser Action from a Tetramethylpyrromethene-BF2 Complex. Appl. Opt. 1988, 27, 4998–4999. [Google Scholar] [CrossRef]
- Haugland, R.P.; Kang, H.C. Chemically Reactive Dipyrrometheneboron Difluoride Dyes. U.S. Patent 4,774,339, 27 September 1988. [Google Scholar]
- Kang, H.C.; Haugland, R.P.; Fisher, P.J.; Prendergast, F.G. Spectral Properties Of 4-Sulfonato-3,3′,5,5′-Tetramethy1-2,Z-Pyrronlethen-1,1′-Borondifluoride Complex (Bodipy), Its Sodium Salt, and Protein Derivatives. In New Technologies in Cytometry; SPIE: Bellingham, WA, USA, 1989; Volume 1063, pp. 68–73. [Google Scholar]
- Johnson, I.D.; Kang, H.C.; Haugland, R.P. Fluorescent Membrane Probes Incorporating Dipyrrometheneboron Difluoride Fluorophores. Anal. Biochem. 1991, 198, 228–237. [Google Scholar] [CrossRef]
- Tram, K.; Yan, H.; Jenkins, H.A.; Vassiliev, S.; Bruce, D. The Synthesis and Crystal Structure of Unsubstituted 4,4-Difluoro-4-Bora-3a,4a-Diaza-s-Indacene (BODIPY). Dyes Pigment. 2009, 82, 392–395. [Google Scholar] [CrossRef]
- Arroyo, I.J.; Hu, R.; Merino, G.; Tang, B.Z.; Peña-Cabrera, E. The Smallest and One of the Brightest. Efficient Preparation and Optical Description of the Parent Borondipyrromethene System. J. Org. Chem. 2009, 74, 5719–5722. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Wang, L.; Verbelen, B.; Tonnelé, C.; Beljonne, D.; Lazzaroni, R.; Leen, V.; Dehaen, W.; Boens, N. UV–Vis Spectroscopy of the Coupling Products of the Palladium-Catalyzed C–H Arylation of the BODIPY Core. Photochem. Photobiol. Sci. 2013, 12, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Prlj, A.; Vannay, L.; Corminboeuf, C. Fluorescence Quenching in BODIPY Dyes: The Role of Intramolecular Interactions and Charge Transfer. Helv. Chim. Acta 2017, 100, e1700093. [Google Scholar] [CrossRef]
- Li, F.; Yang, S.I.; Ciringh, Y.; Seth, J.; Martin, C.H.; Singh, D.L.; Kim, D.; Birge, R.R.; Bocian, D.F.; Holten, D.; et al. Design, Synthesis, and Photodynamics of Light-Harvesting Arrays Comprised of a Porphyrin and One, Two, or Eight Boron-Dipyrrin Accessory Pigments. J. Am. Chem. Soc. 1998, 120, 10001–10017. [Google Scholar] [CrossRef]
- Lai, R.Y.; Bard, A.J. Electrogenerated Chemiluminescence 71. Photophysical, Electrochemical, and Electrogenerated Chemiluminescent Properties of Selected Dipyrromethene−BF2 Dyes. J. Phys. Chem. B 2003, 107, 5036–5042. [Google Scholar] [CrossRef]
- Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T. Design and Synthesis of a Library of BODIPY-Based Environmental Polarity Sensors Utilizing Photoinduced Electron-Transfer-Controlled Fluorescence ON/OFF Switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Hinkeldey, B.; Wild, M.; Jung, G. Synthesis of the Core Compound of the BODIPY Dye Class: 4,4′-Difluoro-4-Bora-(3a,4a)-Diaza-s-Indacene. J. Fluoresc. 2009, 19, 755–758. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, Y.; Xu, B. Enhance the Fluorescence and Singlet Oxygen Generation Ability of BODIPY: Modification on the Meso-Phenyl Unit with Electron Withdrawing Groups. J. Photochem. Photobiol. A Chem. 2017, 349, 197–206. [Google Scholar] [CrossRef]
- Umezawa, K.; Nakamura, Y.; Makino, H.; Citterio, D.; Suzuki, K. Bright, Color-Tunable Fluorescent Dyes in the Visible–Near-Infrared Region. J. Am. Chem. Soc. 2008, 130, 1550–1551. [Google Scholar] [CrossRef]
- Gabe, Y.; Urano, Y.; Kikuchi, K.; Kojima, H.; Nagano, T. Highly Sensitive Fluorescence Probes for Nitric Oxide Based on Boron Dipyrromethene Chromophore Rational Design of Potentially Useful Bioimaging Fluorescence Probe. J. Am. Chem. Soc. 2004, 126, 3357–3367. [Google Scholar] [CrossRef] [PubMed]
- Baruah, M.; Qin, W.; Vallée, R.A.L.; Beljonne, D.; Rohand, T.; Dehaen, W.; Boens, N. A Highly Potassium-Selective Ratiometric Fluorescent Indicator Based on BODIPY Azacrown Ether Excitable with Visible Light. Org. Lett. 2005, 7, 4377–4380. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, P.; Li, P.; Xie, T.; Yu, F.; Tang, B. The Synthesis of Polarity-Sensitive Fluorescent Dyes Based on the BODIPY Chromophore. Dyes Pigment. 2011, 89, 217–222. [Google Scholar] [CrossRef]
- Zhao, W.; Xiao, F.; Jin, G.; Li, B. Design, Synthesis and Photophysical Studies of BODIPY-o, m, p-Phenylenediamine-Based Probes: Insights into Their Responsiveness under Different PH Conditions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120118. [Google Scholar] [CrossRef]
- Mula, S.; Ray, A.K.; Banerjee, M.; Chaudhuri, T.; Dasgupta, K.; Chattopadhyay, S. Design and Development of a New Pyrromethene Dye with Improved Photostability and Lasing Efficiency: Theoretical Rationalization of Photophysical and Photochemical Properties. J. Org. Chem. 2008, 73, 2146–2154. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, N.; Li, Y.; Yang, Z.; Chen, L.; Sun, T.; Xie, Z. Comparative Study of Two Near-Infrared Coumarin–BODIPY Dyes for Bioimaging and Photothermal Therapy of Cancer. J. Mater. Chem. B 2019, 7, 4717–4724. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.; Killoran, J.; O’Shea, C.; Kenna, T.; Gallagher, W.M.; O’Shea, D.F. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, X.; Zong, S.; Tang, C.; Cui, Y.; Zheng, Q. BODIPY-Doped Silica Nanoparticles with Reduced Dye Leakage and Enhanced Singlet Oxygen Generation. Sci. Rep. 2015, 5, 12602. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Cheng, Q.; Dang, H.; Qian, H.; Teng, C.; Xie, K.; Yan, L. Amino Modified Iodinated BODIPY Photosensitizer for Highly Efficient NIR Imaging-Guided Photodynamic Therapy with Ultralow Dose. Dyes Pigment. 2021, 194, 109611. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-Red to near Infrared Analyte-Responsive Fluorescent Probes Based on Organic Fluorophore Platforms for Fluorescence Imaging. Chem. Soc. Rev. 2012, 42, 622–661. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W.; Wang, L.; Robeyns, K.; Qin, W.; Tang, X.; Beljonne, D.; Tonnelé, C.; Paredes, J.M.; et al. Visible Absorption and Fluorescence Spectroscopy of Conformationally Constrained, Annulated BODIPY Dyes. J. Phys. Chem. A 2012, 116, 9621–9631. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural Modification Strategies for the Rational Design of Red/NIR Region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [Green Version]
- Poronik, Y.M.; Yakubovskyi, V.P.; Shandura, M.P.; Vlasenko, Y.G.; Chernega, A.N.; Kovtun, Y.P. 3,5-Bis(Benzothiazolyl)-Substituted BODIPY Dyes. Eur. J. Org. Chem. 2010, 2010, 2746–2752. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Trofimov, B.A.; Mikhaleva, A.I.; Zorina, N.V.; Protzuk, N.I.; Petrushenko, K.B.; Ushakov, I.A.; Dvorko, M.Y.; Méallet-Renault, R.; Clavier, G.; et al. Synthesis and Optical Properties of 2-(Benzo[b]Thiophene-3-Yl)Pyrroles and a New BODIPY Fluorophore (BODIPY = 4,4-Difluoro-4-Bora-3a,4a-Diaza-s-Indacene). Chem. Eur. J. 2009, 15, 5823–5830. [Google Scholar] [CrossRef]
- Lakhe, D.; Jairaj, K.K.; Pradhan, M.; Ladiwala, U.; Agarwal, N. Synthesis and Photophysical Studies of Heteroaryl Substituted-BODIPy Derivatives for Biological Applications. Tetrahedron Lett. 2014, 55, 7124–7129. [Google Scholar] [CrossRef]
- Dixit, S.; Mahaddalkar, T.; Lopus, M.; Agarwal, N. Synthesis, Photophysical Studies of Positional Isomers of Heteroaryl BODIPYs, and Biological Evaluation of Di-Pyrrolyl BODIPY on Human Pancreatic Cancer Cells. J. Photochem. Photobiol. A Chem. 2018, 353, 368–375. [Google Scholar] [CrossRef]
- Peña-Cabrera, E.; Aguilar-Aguilar, A.; González-Domínguez, M.; Lager, E.; Zamudio-Vázquez, R.; Godoy-Vargas, J.; Villanueva-García, F. Simple, General, and Efficient Synthesis of Meso-Substituted Borondipyrromethenes from a Single Platform. Org. Lett. 2007, 9, 3985–3988. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Jiao, L.; Feng, Y.; Yu, C.; Chen, N.; Wei, Y.; Mu, X.; Hao, E. Regioselective and Stepwise Syntheses of Functionalized BODIPY Dyes through Palladium-Catalyzed Cross-Coupling Reactions and Direct C–H Arylations. J. Org. Chem. 2016, 81, 6281–6291. [Google Scholar] [CrossRef]
- Zhao, N.; Xuan, S.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Stepwise Polychlorination of 8-Chloro-BODIPY and Regioselective Functionalization of 2,3,5,6,8-Pentachloro-BODIPY. J. Org. Chem. 2015, 80, 8377–8383. [Google Scholar] [CrossRef] [Green Version]
- Leen, V.; Gonzalvo, V.Z.; Deborggraeve, W.M.; Boens, N.; Dehaen, W. Direct Functionalization of BODIPY Dyes by Oxidative Nucleophilic Hydrogen Substitution at the 3- or 3,5-Positions. Chem. Commun. 2010, 46, 4908–4910. [Google Scholar] [CrossRef]
- Verbelen, B.; Boodts, S.; Hofkens, J.; Boens, N.; Dehaen, W. Radical C–H Arylation of the BODIPY Core with Aryldiazonium Salts: Synthesis of Highly Fluorescent Red-Shifted Dyes. Angew. Chem. Int. Ed. 2015, 54, 4612–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, S.; Patil, M.; Agarwal, N. Ferrocene Catalysed Heteroarylation of BODIPy and Reaction Mechanism Studies by EPR and DFT Methods. RSC Adv. 2016, 6, 47491–47497. [Google Scholar] [CrossRef]
- Utepova, I.A.; Trestsova, M.A.; Chupakhin, O.N.; Charushin, V.N.; Rempel, A.A. Aerobic Oxidative C–H/C–H Coupling of Azaaromatics with Indoles and Pyrroles in the Presence of TiO2 as a Photocatalyst. Green Chem. 2015, 17, 4401–4410. [Google Scholar] [CrossRef]
- Utepova, I.A.; Chupakhin, O.N.; Trestsova, M.A.; Musikhina, A.A.; Kucheryavaya, D.A.; Charushin, V.N.; Rempel, A.A.; Kozhevnikova, N.S.; Valeeva, A.A.; Mikhaleva, A.I.; et al. Direct Functionalization of the C–H Bond in (Hetero)Arenes: Aerobic Photoinduced Oxidative Coupling of Azines with AromatiC-Nucleophiles (SNH-Reactions) in the Presence of a CdS/TiO2 Photocatalyst. Russ. Chem. Bull. 2016, 65, 445–450. [Google Scholar] [CrossRef]
- Utepova, I.A.; Trestsova, M.A.; Kucheryavaya, D.A.; Tsmokalyuk, A.N.; Chupakhin, O.N.; Charushin, V.N.; Rempel, A.A. Mechanistic Study of the Direct Oxidative Photocatalytic Aerobic CH/CH Coupling of Azines with Heteroarenes. J. Photochem. Photobiol. A Chem. 2019, 368, 85–89. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lindsey, J.S. One-Flask Synthesis of Meso-Substituted Dipyrromethanes and Their Application in the Synthesis of Trans-Substituted Porphyrin Building Blocks. Tetrahedron 1994, 50, 11427–11440. [Google Scholar] [CrossRef]
- Littler, B.J.; Miller, M.A.; Hung, C.-H.; Wagner, R.W.; O’Shea, D.F.; Boyle, P.D.; Lindsey, J.S. Refined Synthesis of 5-Substituted Dipyrromethanes. J. Org. Chem. 1999, 64, 1391–1396. [Google Scholar] [CrossRef]
- Malatesti, N.; Smith, K.; Savoie, H.; Greenman, J.; Boyle, R.W. Synthesis and in Vitro Investigation of Cationic 5,15-Diphenyl Porphyrin-Monoclonal Antibody Conjugates as Targeted Photodynamic Sensitisers. Int. J. Oncol. 2006, 28, 1561–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargrove, A.E.; Reyes, R.N.; Riddington, I.; Anslyn, E.V.; Sessler, J.L. Boronic Acid Porphyrin Receptor for Ginsenoside Sensing. Org. Lett. 2010, 12, 4804–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorosheva, I.B.; Rempel, A.A.; Trestsova, M.A.; Utepova, I.A.; Chupakhin, O.N. Synthesis of a TiO2 Photocatalyst for Dehydrogenative Cross-Coupling of (Hetero)Arenes. Inorg. Mater. 2019, 55, 155–161. [Google Scholar] [CrossRef]
- Basumatary, B.; Raja Sekhar, A.; Ramana Reddy, R.V.; Sankar, J. Corrole-BODIPY Dyads: Synthesis, Structure, and Electrochemical and Photophysical Properties. Inorg. Chem. 2015, 54, 4257–4267. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, D.; Raghav, D.; Sujatha, S.; Kumar, H.H.; Rathinasamy, K.; Arunkumar, C. Synthesis, Structure, Photophysical, Electrochemical Properties and Antibacterial Activity of Brominated BODIPYs. RSC Adv. 2016, 6, 80808–80824. [Google Scholar] [CrossRef]
- Benniston, A.C.; Clift, S.; Harriman, A. Intramolecular Charge-Transfer Interactions in a Julolidine–Bodipy Molecular Assembly as Revealed via 13C-NMR Chemical Shifts. J. Mol. Struct. 2011, 985, 346–354. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Guo, P.; Qian, X. A Dual Channel Chemodosimeter for Hg2+ and Ag+ Using a 1,3-Dithiane Modified BODIPY. New J. Chem. 2012, 36, 1621–1625. [Google Scholar] [CrossRef]
 | |||||
|---|---|---|---|---|---|
| Entry | Acid | Solution | Temperature, °C | 5a [%] 2 | 5b [%] 2 |
| 1 | CH3COOH | - | rt | 23 | 0 |
| 2 | CH3COOH | - | 120 | 0 | 0 |
| 3 | CF3COOH | CH2Cl2 | rt | 0 | 0 |
| 4 | BF3OEt2 | CH2Cl2 | rt | 0 | 0 |
| 5 | CH3COOH | CH2Cl2 | rt | 48 | 0 |
| 6 | CH3COOH | CH2Cl2 | 30 | 40 | 10 |
| 7 | CH3COOH | CH2Cl2 | 45 | 10 | 40 |
| 8 | CH3COOH | CH2Cl2 | 50 | 0 | 63 |
| 9 | CH3COOH | CH2Cl2 | >50 | 0 | 0 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | Additive | Solution | Temperature, °C | Yield, % |
| 5a | Br | acridine | H | CH3COOH | CH2Cl2 | rt | 48 1 |
| 5b | Br | acridine | acridine | CH3COOH | CH2Cl2 | 50 | 63 1 |
| 5c | Br | 6-phenyl-[1,2,5]oxadiazolo[3,4-b]pyrazine | 6-phenyl-[1,2,5]oxadiazolo[3,4-b]pyrazine | CH3COOH | CH2Cl2 | rt | 57 1 |
| 5d | CH3 | acridine | H | CH3COOH | CH2Cl2 | rt | 60 1 |
| 5e | CH3 | acridine | acridine | CH3COOH | CH2Cl2 | 50 | 76 1 |
| 5f | NMe2 | acridine | H | CH3COOH | CH2Cl2 | rt | 59 1 |
| 5g | NMe2 | acridine | acridine | CH3COOH | CH2Cl2 | 50 | 38 1 |
| 5h | NMe2 | 6-phenyl-[1,2,5]oxadiazolo[3,4-b]pyrazine | 6-phenyl-[1,2,5]oxadiazolo[3,4-b]pyrazine | CH3COOH | CH2Cl2 | rt | 36 1 |
| 5i | NO2 | acridine | acridine | CH3COOH | CH2Cl2 | rt | 49 1 |
| 5j | CN | acridine | acridine | CH3COOH | CH2Cl2 | rt | 79 1 |
| 5k | Br | acridine hydrochloride | acridine hydrochloride | - | n-BuOH | rt | 85 2 |
| 5l | CH3 | acridine hydrochloride | acridine hydrochloride | - | n-BuOH | rt | 80 2 |
| 5m | NMe2 | acridine hydrochloride | acridine hydrochloride | - | n-BuOH | rt | 82 2 |
| 5n | NO2 | acridine hydrochloride | acridine hydrochloride | - | n-BuOH | rt | 85 2 |
| 5o | CN | acridine hydrochloride | acridine hydrochloride | - | n-BuOH | rt | 85 2 |
 | |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | Base | Yield, % 1 |
| 6a | Br | acridine | H | NEt3 | 32 |
| 6b | Br | acridine | acridine | DIPEA | 38 |
| 6c | Br | 6-phenyl-[1,2,5]oxadiazolo[3,4-b]pyrazine | 6-phenyl-[1,2,5]oxadiazolo[3,4-b]pyrazine | DIPEA | 42 |
| 6d | CH3 | acridine | H | NEt3 | 46 |
| 6e | CH3 | acridine | acridine | NEt3 | 70 |
| 6f | NMe2 | acridine | NEt3 | 59 | |
| 6g | NMe2 | acridine | acridine | NEt3 | 56 |
| 6h | NMe2 | 6-phenyl-[1,2,5]oxadiazolo[3,4-b]pyrazine | 6-phenyl-[1,2,5]oxadiazolo[3,4-b]pyrazine | NEt3 | 52 |
| 6i | NO2 | acridine | acridine | NEt3 | 48 |
| 6j | CN | acridine | acridine | DIPEA | 46 |
| Compound | Solvent | λabs/nm | λem/nm | φ |
|---|---|---|---|---|
| 7a | C2Cl4 | 500 | 536 | 0.284 |
| 7a | benzene | 499 | 635 | 0.049 |
| 6f | C2Cl4 | 510 | 570 | 0.319 |
| 6f | benzene | 508 | 637 | 0.054 |
| 6g | C2Cl4 | 520 | 600 | 0.289 |
| 6g | benzene | 519 | 634 | 0.068 |
| 6h | benzene | 557 | 782 | 0.0025 |
| 7b | C2Cl4 | 503 | 519 | 0.063 |
| 7b | benzene | 503 | 519 | 0.063 |
| 6d | C2Cl4 | 513 | 576 | 0.155 |
| 6d | benzene | 512 | 581 | 0.140 |
| 6e | C2Cl4 | 524 | 611 | 0.249 |
| 6e | benzene | 523 | 614 | 0.228 |
| 7c | C2Cl4 | 507 | 525 | 0.027 |
| 7c | benzene | 506 | 525 | 0.036 |
| 6a | C2Cl4 | 517 | 589 | 0.077 |
| 6a | benzene | 516 | 594 | 0.082 |
| 6b | C2Cl4 | 529 | 626 | 0.160 |
| 6b | benzene | 527 | 628 | 0.160 |
| 6c | C2Cl4 | 556 | 613 | 0.70 |
| 6c | benzene | 555 | 619 | 0.81 |
| 7d | C2Cl4 | 511 | 535 | 0.0072 |
| 7d | benzene | 510 | 537 | 0.0078 |
| 6j | C2Cl4 | 533 | 640 | 0.055 |
| 6j | benzene | 512 | 544 | 0.061 |
| 7e | C2Cl4 | 513 | 541 | 0.0058 |
| 7e | benzene | 512 | 544 | 0.0064 |
| 6i | C2Cl4 | 535 | 646 | 0.036 |
| 6i | benzene | 533 | 649 | 0.041 |
| Compound | E[onset, red vs. Fc/Fc+], V | ELUMO 1, eV | E[onset, ox vs. Fc/Fc+], V | EHOMO 2, eV | Egap, eV |
|---|---|---|---|---|---|
| 6a | −1.09 | −4.01 | - | ||
| 6b | −1.08 | −4.02 | - | ||
| 6c | −0.61 | −4.49 | - | ||
| 6d | −1.16 | −3.94 | 0.99 | −6.09 | 2.15 |
| 6e | −1.13 | −3.97 | 1.02 | 6.12 | 2.15 |
| 6f | −1.24 | −3.86 | 0.50 | −5.60 | 1.74 |
| 6g | −1.14 | −3.96 | 0.42 | −5.52 | 1.56 |
| 6h | −0.72 | −4.38 | 0.48 | −5.58 | 1.2 |
| 6i | −0.97 | −4.13 | - | ||
| 6j | −0.96 | −4.14 | 1.07 | 6.17 | 2.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trestsova, M.A.; Utepova, I.A.; Chupakhin, O.N.; Semenov, M.V.; Pevtsov, D.N.; Nikolenko, L.M.; Tovstun, S.A.; Gadomska, A.V.; Shchepochkin, A.V.; Kim, G.A.; et al. Oxidative C-H/C-H Coupling of Dipyrromethanes with Azines by TiO2-Based Photocatalytic System. Synthesis of New BODIPY Dyes and Their Photophysical and Electrochemical Properties. Molecules 2021, 26, 5549. https://doi.org/10.3390/molecules26185549
Trestsova MA, Utepova IA, Chupakhin ON, Semenov MV, Pevtsov DN, Nikolenko LM, Tovstun SA, Gadomska AV, Shchepochkin AV, Kim GA, et al. Oxidative C-H/C-H Coupling of Dipyrromethanes with Azines by TiO2-Based Photocatalytic System. Synthesis of New BODIPY Dyes and Their Photophysical and Electrochemical Properties. Molecules. 2021; 26(18):5549. https://doi.org/10.3390/molecules26185549
Chicago/Turabian StyleTrestsova, Maria A., Irina A. Utepova, Oleg N. Chupakhin, Maksim V. Semenov, Dmitry N. Pevtsov, Lyubov M. Nikolenko, Sergey A. Tovstun, Anna V. Gadomska, Alexander V. Shchepochkin, Gregory A. Kim, and et al. 2021. "Oxidative C-H/C-H Coupling of Dipyrromethanes with Azines by TiO2-Based Photocatalytic System. Synthesis of New BODIPY Dyes and Their Photophysical and Electrochemical Properties" Molecules 26, no. 18: 5549. https://doi.org/10.3390/molecules26185549
APA StyleTrestsova, M. A., Utepova, I. A., Chupakhin, O. N., Semenov, M. V., Pevtsov, D. N., Nikolenko, L. M., Tovstun, S. A., Gadomska, A. V., Shchepochkin, A. V., Kim, G. A., Razumov, V. F., Dorosheva, I. B., & Rempel, A. A. (2021). Oxidative C-H/C-H Coupling of Dipyrromethanes with Azines by TiO2-Based Photocatalytic System. Synthesis of New BODIPY Dyes and Their Photophysical and Electrochemical Properties. Molecules, 26(18), 5549. https://doi.org/10.3390/molecules26185549