Caffeic Acid on Metabolic Syndrome: A Review
Abstract
1. Introduction
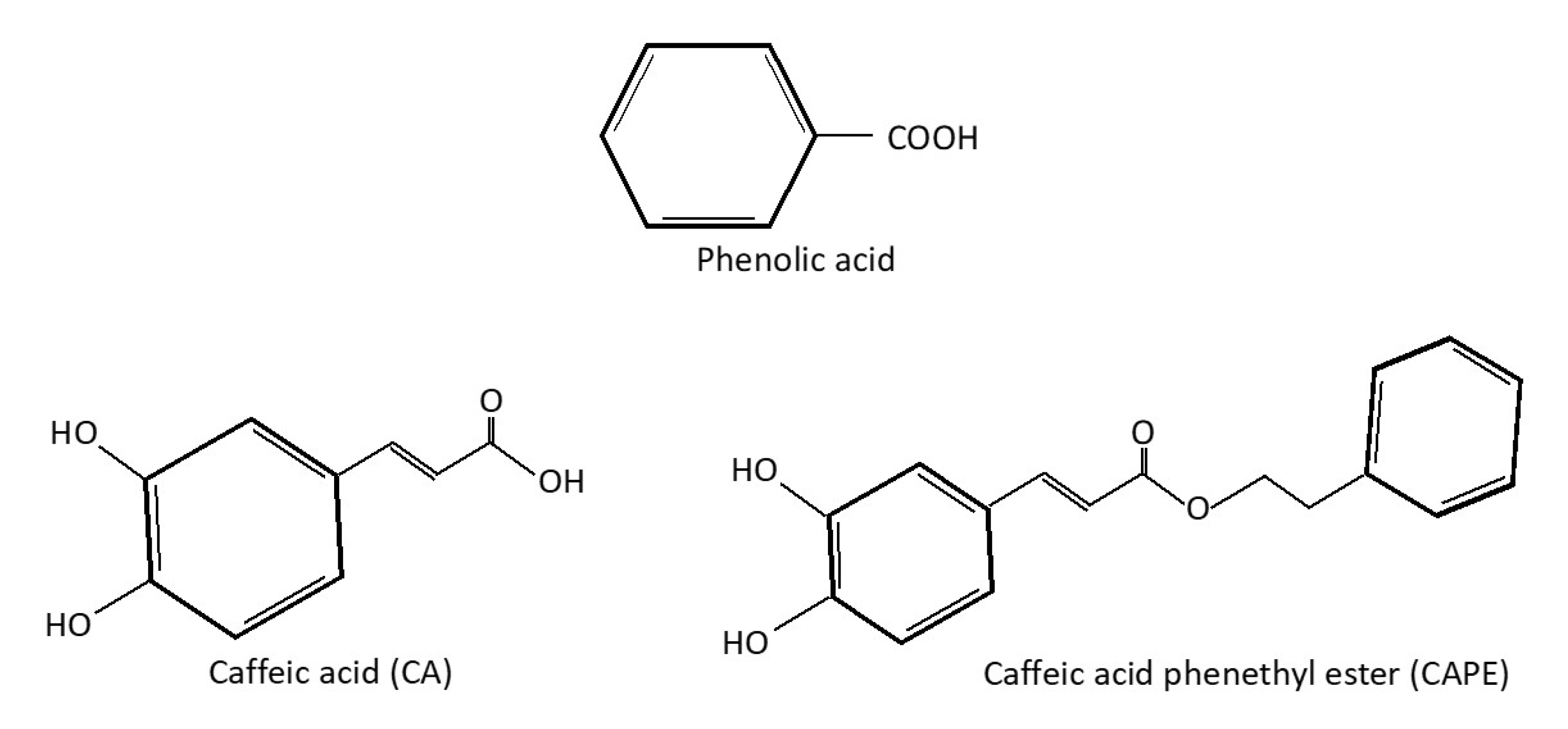
2. Caffeic Acid as a Phenolic Compound
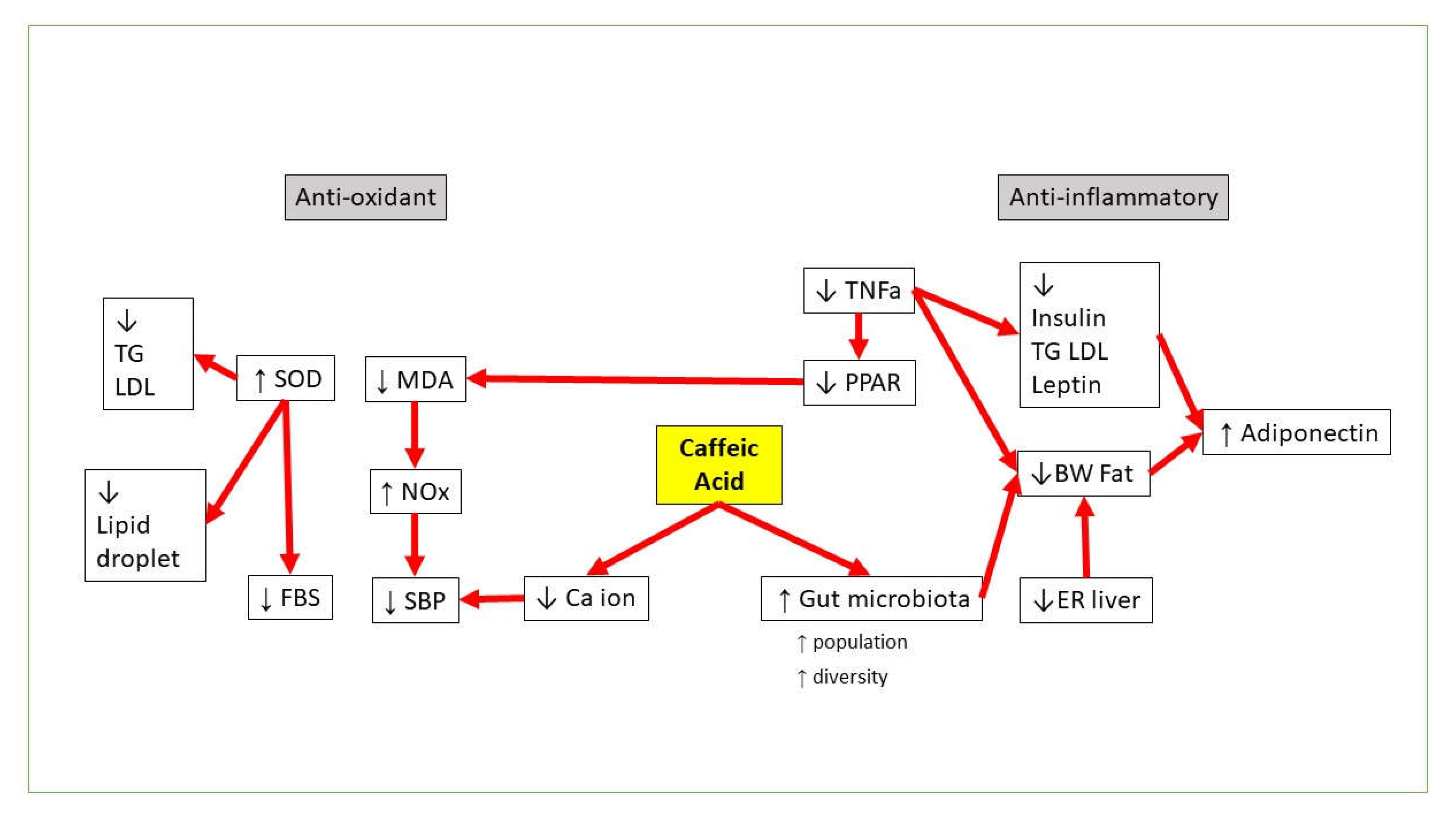
3. CA vs. Obesity
4. CA vs. Hyperglycemia and Insulin Resistance
5. CA vs. Dyslipidemia
6. CA vs. Hypertension
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 107. [Google Scholar] [CrossRef]
- Manaf, M.R.A.; Nawi, A.M.; Tauhid, N.M.; Othman, H.; Rahman, M.R.A.; Yusoff, H.M.; Safian, N.; Ng, P.Y.; Manaf, Z.A.; Kadir, N.B.A.; et al. Prevalence of metabolic syndrome and its associated risk factors among staffs in a Malaysian public university. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Ando, K.; Fujita, T. Metabolic syndrome and oxidative stress. Free Radic. Biol. Med. 2009, 47, 213–218. [Google Scholar] [CrossRef]
- Martinez-Ferran, M.; De La Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic Impacts of Confinement during the COVID-19 Pandemic due to Modified Diet and Physical Activity Habits. Nutrients 2020, 12, 1549. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- EEckel, R.H.; Alberti, K.G.M.M.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Li, A.; Zheng, N.; Ding, X. Mitochondrial abnormalities: A hub in metabolic syndrome-related cardiac dysfunction caused by oxidative stress. Heart Fail. Rev. 2021, 1–8. [Google Scholar] [CrossRef]
- Etchegoyen, M.; Nobile, M.H.; Baez, F.; Posesorski, B.; González, J.; Lago, N.; Milei, J.; Otero-Losada, M. Metabolic Syndrome and Neuroprotection. Front. Neurosci. 2018, 12, 196. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.L.; Imperatore, G.; Bennett, P.H.; Knowler, W.C. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 2002, 51, 3120–3127. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A.; Jankovic, A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021, 42, 101887. [Google Scholar] [CrossRef]
- Quinn, K.J.; Shah, N.H. A dataset quantifying polypharmacy in the United States. Sci. Data 2017, 4, 170167. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, e162750. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Kumar, S.R.; Ramli, E.S.M.; Nasir, N.A.A.; Ismail, N.H.M.; Fahami, N.A.M. Preventive Effect of Naringin on Metabolic Syndrome and Its Mechanism of Action: A Systematic Review. Altern. Med. 2019, 2019, e9752826. [Google Scholar] [CrossRef]
- Azuma, K.; Ippoushi, K.; Nakayama, M.; Ito, H.; Higashio, H.; Terao, J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000, 48, 5496–5500. [Google Scholar] [CrossRef] [PubMed]
- Manish, P.; Wei Ling, L.; Seong Lin, T.; Mohamad Fairuz, Y. Flavonoids and its Neuroprotective Effects on Brain Ischemia and Neurodegenerative Diseases. Curr. Drug Targets 2018, 19, 1710–1720. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef] [PubMed]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Adv. Pharmacol. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hydroxycinnamic acids on gut microbiota and health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 710–737. [Google Scholar] [CrossRef]
- Göçer, H.; Gülçin, I. Caffeic acid phenethyl ester (CAPE): Correlation of structure and antioxidant properties. Int. J. Food Sci. Nutr. 2011, 62, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Filipe, H.; Sousa, C.; Marquês, J.T.; Vila-Viçosa, D.; de Granada-Flor, A.; Viana, A.; Santos, M.; Machuqueiro, M.; de Almeida, R.F. Differential targeting of membrane lipid domains by caffeic acid and its ester derivatives. Free Radic. Biol. Med. 2018, 115, 232–245. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Armutcu, F.; Akyol, S.; Ustunsoy, S.; Turan, F.F. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Exp. Ther. Med. 2015, 9, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- KKępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. BioMed Res. Int. 2018, 2018, e7413504. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sung, Y.-H.; Lee, H.-H.; Ko, I.-G.; Kim, S.-E.; Shin, M.-S.; Kim, B.-K. Postnatal treadmill exercise alleviates short-term memory impairment by enhancing cell proliferation and suppressing apoptosis in the hippocampus of rat pups born to diabetic rats. J. Exerc. Rehabil. 2014, 10, 209–217. [Google Scholar] [CrossRef]
- Koga, M.; Nakagawa, S.; Kato, A.; Kusumi, I. Caffeic acid reduces oxidative stress and microglial activation in the mouse hippocampus. Tissue Cell 2019, 60, 14–20. [Google Scholar] [CrossRef]
- Pereira, P.; De Oliveira, P.A.; Ardenghi, P.; Rotta, L.; Henriques, J.A.P.; Picada, J.N. Neuropharmacological analysis of caffeic acid in rats. Basic Clin. Pharmacol. Toxicol. 2006, 99, 374–378. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Chin, K.-Y.; Zarkasi, K.A.; Ahmad, F. The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet. Environ. Res. Public Health 2019, 16, 4987. [Google Scholar] [CrossRef] [PubMed]
- Kolgazi, M.; Cilingir, S.; Yilmaz, O.; Gemici, M.; Yazar, H.; Ozer, S.; Acikel-Elmas, M.; Arbak, S.; Suyen, G.G. Caffeic acid attenuates gastric mucosal damage induced by ethanol in rats via nitric oxide modulation. Chem.-Biol. Interact. 2021, 334, 109351. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: Synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-C.; Chuang, S.-T.; Lin, Y.-C.; Chuang, C.-F.; Chi, T.-C.; Chiu, H.-L.; Kuo, Y.-H.; Su, M.-J. Caffeic Acid Phenylethyl Amide Protects against the Metabolic Consequences in Diabetes Mellitus Induced by Diet and Streptozocin. Evid.-Based Complement. Altern. Med. 2012, 2012, e984780. [Google Scholar] [CrossRef]
- Shin, E.J.; Jo, S.; Choi, H.-K.; Choi, S.; Byun, S.; Lim, T.-G. Caffeic Acid Phenethyl Ester Inhibits UV-Induced MMP-1 Expression by Targeting Histone Acetyltransferases in Human Skin. Int. J. Mol. Sci. 2019, 20, 3055. [Google Scholar] [CrossRef]
- Honecker, J.; Weidlich, D.; Heisz, S.; Lindgren, C.M.; Karampinos, D.C.; Claussnitzer, M.; Hauner, H. A distribution-centered approach for analyzing human adipocyte size estimates and their association with obesity-related traits and mitochondrial function. Int. J. Obes. 2021, 45, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Jayarathne, S.; Koboziev, I.; Park, O.-H.; Oldewage-Theron, W.; Shen, C.-L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 327–343. [Google Scholar] [CrossRef]
- Jensen, M.D. Visceral Fat: Culprit or Canary? Endocrinol. Metab. Clin. N. Am. 2020, 49, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.J.; Arusi, E.; Vinik, A.I.; Johnson, D.A. The Role and Influence of Gut Microbiota in Pathogenesis and Management of Obesity and Metabolic Syndrome. Front. Endocrinol. 2014, 5, 47. [Google Scholar] [CrossRef]
- Nie, J.; Chang, Y.; Li, Y.; Zhou, Y.; Qin, J.; Sun, Z.; Li, H. Caffeic Acid Phenethyl Ester (Propolis Extract) Ameliorates Insulin Resistance by Inhibiting JNK and NF-κB Inflammatory Pathways in Diabetic Mice and HepG2 Cell Models. J. Agric. Food Chem. 2017, 65, 9041–9053. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Cao, S.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.; Wang, S.; Shoulin, W. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget 2016, 7, 31790–31799. [Google Scholar] [CrossRef]
- Vanella, L.; Tibullo, D.; Godos, J.; Pluchinotta, F.R.; Di Giacomo, C.; Sorrenti, V.; Acquaviva, R.; Russo, A.; Volti, G.L.; Barbagallo, I. Caffeic Acid Phenethyl Ester Regulates PPAR’s Levels in Stem Cells-Derived Adipocytes. PPAR Res. 2016, 2016, e7359521. [Google Scholar] [CrossRef] [PubMed]
- Mariana, B.D.; Tiago, L.S.; Ramon, R.P.P.B.D.M.; Jamile, M.F.; Tiago, S.M.; Richard, R.C.M.; Hector, G.R.; Dânya, B.L.; Alice, M.C.M.; Maria, G.R.D.Q. Caffeic acid reduces lipid accumulation and reactive oxygen species production in adipocytes. Afr. J. Pharm. Pharmacol. 2018, 12, 263–268. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Dueñas, M.; Muñoz-Gonzalez, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.; Bartolomé, B. A Survey of Modulation of Gut Microbiota by Dietary Polyphenols. BioMed Res. Int. 2015, 2015, e850902. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ge, J.; He, X.; Sheng, Y.; Zheng, S.; Zhang, C.; Xu, W.; Huang, K. Caffeic acid reduces body weight by regulating gut microbiota in diet-induced-obese mice. J. Funct. Foods 2020, 74, 104061. [Google Scholar] [CrossRef]
- Mu, H.-N.; Zhou, Q.; Yang, R.-Y.; Tang, W.-Q.; Li, H.-X.; Wang, S.-M.; Li, J.; Chen, W.-X.; Dong, J. Caffeic acid prevents non-alcoholic fatty liver disease induced by a high-fat diet through gut microbiota modulation in mice. Food Res. Int. 2021, 143, 110240. [Google Scholar] [CrossRef]
- Freeman, A.M.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK507839/ (accessed on 12 August 2021).
- Nolan, C.J.; Ruderman, N.B.; Kahn, S.E.; Pedersen, O.; Prentki, M. Insulin Resistance as a Physiological Defense Against Metabolic Stress: Implications for the Management of Subsets of Type 2 Diabetes. Diabetes 2015, 64, 673–686. [Google Scholar] [CrossRef]
- Hashim, K.-N.; Chin, K.-Y.; Ahmad, F. The Mechanism of Honey in Reversing Metabolic Syndrome. Molecules 2021, 26, 808. [Google Scholar] [CrossRef]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2018, 124, 410–417. [Google Scholar] [CrossRef]
- Nasry, M.R.; Abo-Youssef, A.M.; Zaki, H.F.; El-Denshary, E.-E.-D.S. Effect of caffeic acid and pioglitazone in an experimental model of metabolic syndrome. Int. J.Sci. Res. Publ. 2015, 5, 1–9. [Google Scholar]
- JJung, U.J.; Lee, M.-K.; Park, Y.B.; Jeon, S.-M.; Choi, M.-S. Antihyperglycemic and Antioxidant Properties of Caffeic Acid in db/db Mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef]
- Eid, H.M.; Thong, F.; Nachar, A.; Haddad, P.S. Caffeic acid methyl and ethyl esters exert potential antidiabetic effects on glucose and lipid metabolism in cultured murine insulin-sensitive cells through mechanisms implicating activation of AMPK. Pharm. Biol. 2017, 55, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, Q.; Qu, H.; Song, X.; Hu, J.; Xu, X.; Zhang, L.; Yin, S. Stress adaptation is associated with insulin resistance in women with gestational diabetes mellitus. Nutr. Diabetes 2020, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, V.; Sanchez, N.; Clark, E.L.M.; Miller, R.L.; Casamassima, M.; Verros, M.; Conte, I.; Ruiz-Jaquez, M.; Gulley, L.D.; Johnson, S.A.; et al. Associations of adverse childhood experiences with stress physiology and insulin resistance in adolescents at risk for adult obesity. Dev. Psychobiol. 2021, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, J.; Radmilovic, A.; Montina, T.D.; Metz, G.A.S. Cardiorenal metabolic biomarkers link early life stress to risk of non-communicable diseases and adverse mental health outcomes. Sci. Rep. 2020, 10, 13295. [Google Scholar] [CrossRef]
- Choudhary, S.; Mourya, A.; Ahuja, S.; Sah, S.P.; Kumar, A. Plausible anti-inflammatory mechanism of resveratrol and caffeic acid against chronic stress-induced insulin resistance in mice. Inflammopharmacology 2016, 24, 347–361. [Google Scholar] [CrossRef]
- Gonna, H.; Ray, K.K. The importance of dyslipidaemia in the pathogenesis of cardiovascular disease in people with diabetes. Diabetes Obes. Metab. 2019, 21, 6–16. [Google Scholar] [CrossRef]
- Pappan, N.; Rehman, A. Dyslipidemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK560891/ (accessed on 12 August 2021).
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Lee, E.S.; Huh, J.H.; Chung, C.H. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet–induced obese mice by regulating autophagy. Nutrition 2018, 55–56, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gun, A.; Ozer, M.K.; Bilgiç, S.; Kocaman, N.; Ozan, G. Effect of Caffeic Acid Phenethyl Ester on Vascular Damage Caused by Consumption of High Fructose Corn Syrup in Rats. Oxidative Med. Cell. Longev. 2016, 2016, e3419479. [Google Scholar] [CrossRef] [PubMed]
- Bocco, B.M.L.D.C.; Fernandes, G.W.; Lorena, F.; Cysneiros, R.; Christoffolete, M.; Grecco, S.; Lancellotti, C.; Romoff, P.; Lago, J.H.G.; Bianco, A.; et al. Combined treatment with caffeic and ferulic acid from Baccharis uncinella C. DC. (Asteraceae) protects against metabolic syndrome in mice. Braz. J. Med Biol. Res. 2016, 49, e5003. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jembrek, M.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef]
- Chao, C.-Y.; Mong, M.-C.; Chan, K.-C.; Yin, M.-C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G. Hypercholesterolemia, angiotensin converting enzyme and ecto-enzymes of purinergic system: Ameliorative properties of caffeic and chlorogenic acid in hypercholesterolemic rats. J. Food Biochem. 2018, 42, e12604. [Google Scholar] [CrossRef]
- Hassan, N.A.; El-Bassossy, H.M.; Mahmoud, M.; Fahmy, A. Caffeic acid phenethyl ester, a 5-lipoxygenase enzyme inhibitor, alleviates diabetic atherosclerotic manifestations: Effect on vascular reactivity and stiffness. Chem.-Biol. Interact. 2014, 213, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kim, H.-H.; Cho, H.-J.; Bae, J.-S.; Yu, Y.-B.; Park, H.-J. Antiplatelet effects of caffeic acid due to Ca2+ mobilizationinhibition via cAMP-dependent inositol-1, 4, 5-trisphosphate receptor phosphorylation. J. Atheroscler. Thromb. 2014, 21, 23–37. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Q.; Liu, Y.-Y.; Sun, K.; Fan, J.-Y.; Wang, C.-S.; Han, J.-Y. Inhibitory effect of caffeic acid on ADP-induced thrombus formation and platelet activation involves mitogen-activated protein kinases. Sci. Rep. 2015, 5, 13824. [Google Scholar] [CrossRef]
- Nam, G.S.; Nam, K.-S.; Park, H.-J. Caffeic Acid Diminishes the Production and Release of Thrombogenic Molecules in Human Platelets. Biotechnol. Bioprocess Eng. 2018, 23, 641–648. [Google Scholar] [CrossRef]
- Mendoza, M.F.; Kachur, S.M.; Lavie, C.J. Hypertension in obesity. Curr. Opin. Cardiol. 2020, 35, 389–396. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.D.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef]
- Surikow, S.; Nguyen, T.; Stafford, I.; Horowitz, J. Inhibition of Nitric Oxide Synthase: Impact on Cardiovascular Injury and Mortality in a Model of Takotsubo Syndrome. Heart Lung Circ. 2019, 28, S132. [Google Scholar] [CrossRef][Green Version]
- Oboh, G.; Ojueromi, O.O.; Ademosun, A.O.; Omayone, T.P.; Oyagbemi, A.A.; Ajibade, T.O.; Adedapo, A.A. Effects of caffeine and caffeic acid on selected biochemical parameters in L-NAME-induced hypertensive rats. J. Food Biochem. 2021, 45, 13384. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, L.; Gao, P.; Liu, D.; Zhu, Z. Caffeic Acid Ameliorates Angiotensin II-induced Increase In Blood Pressure by Activating Vascular Sarco-/Endoplasmic Reticulum CA-ATPASE2A. J. Hypertens. 2021, 39, e248. [Google Scholar] [CrossRef]
- Li, P.-G.; Xu, J.-W.; Ikeda, K.; Kobayakawa, A.; Kayano, Y.; Mitani, T.; Ikami, T.; Yamori, Y. Caffeic Acid Inhibits Vascular Smooth Muscle Cell Proliferation Induced by Angiotensin II in Stroke-Prone Spontaneously Hypertensive Rats. Hypertens. Res. 2005, 28, 369–377. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lassalle-Claux, G.; Touaibia, M.; Rupasinghe, H.V. Antihypertensive effect of caffeic acid and its analogs through dual renin–angiotensin–aldosterone system inhibition. Eur. J. Pharmacol. 2014, 730, 125–132. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G. Caffeic acid and chlorogenic acid: Evaluation of antioxidant effect and inhibition of key enzymes linked with hypertension. J. Food Biochem. 2018, 42, e12541. [Google Scholar] [CrossRef]
- Castro, M.F.V.; Stefanello, N.; Assmann, C.E.; Baldissarelli, J.; Bagatini, M.D.; da Silva, A.D.; da Costa, P.; Borba, L.; da Cruz, I.B.M.; Morsch, V.M.; et al. Modulatory effects of caffeic acid on purinergic and cholinergic systems and oxi-inflammatory parameters of streptozotocin-induced diabetic rats. Life Sci. 2021, 277, 119421. [Google Scholar] [CrossRef]
- Xu, W.; Luo, Q.; Wen, X.; Xiao, M.; Mei, Q. Antioxidant and anti-diabetic effects of caffeic acid in a rat model of diabetes. Trop. J. Pharm. Res. 2020, 19, 1227–1232. [Google Scholar] [CrossRef]
- Matboli, M.; Eissa, S.; Ibrahim, D.; Hegazy, M.; Imam, S.S.; Habib, E.K. Caffeic Acid Attenuates Diabetic Kidney Disease via Modulation of Autophagy in a High-Fat Diet/Streptozotocin- Induced Diabetic Rat. Sci. Rep. 2017, 7, 2263. [Google Scholar] [CrossRef]
- Salmas, R.E.; Gulhan, M.F.; Durdagi, S.; Sahna, E.; Abdullah, H.I.; Selamoglu, Z. Effects of propolis, caffeic acid phenethyl ester, and pollen on renal injury in hypertensive rat: An experimental and theoretical approach. Cell Biochem. Funct. 2017, 35, 304–314. [Google Scholar] [CrossRef]
- Taher, M.A.; Hussain, D.A.A.; Hasan, H.F.; Fahmi, Z.M.; Luaibi, O.K.; Ali, M.G. Hypolipidemic Effect of Caffeic Acid Isolated From Arctium Lappa Cultivated In Iraq, in Hyperlipidemic Rat Model. Iraqi J. Pharm. Sci. 2015, 24, 18–24. [Google Scholar]
| Pathological Induction/State | Dose of CA or Its Derivates and Administration Route | Duration of Treatment | Observations | Reference |
|---|---|---|---|---|
| Diet-induced MetS with HFD in male Wistar rats | 40 mg/kg via oral gavage | 6 weeks | Reduced: —Insulin —HOMA-IR —Leptin —TNFα —IL-6 —IL-8 —Total cholesterol, TG,VLDLc, LDLc,HDLc Increased: —Adiponectin | [61] |
| Diet-induced hypercholesterolemic rats | 10 and 15 mg/kg | 21 days | Reduced: —Total cholesterol —TG —LDL —HDL (With dose 15 mg/kg showing better results) Increased: —Plasma and heart SOD activity | [77] |
| Nω-Nitro-L-argininge-methylester (L-NAME)-induced hypertensive in male Wistar rats | 5 mg/kg and 25 mg/kg via oral gavage | 20 days | Reduced: —SBP —MDA Increased: —ACE activity —NOx level | [85] |
| Surgically implanted mini osmotic pumps filled with Ang II solution in wild type mice and SERCA2a knockout mice | 0.05% CA in drinking water | 8 weeks | CA was able to: —Relax mesenteric artery —Smooth norepinephrine-induced vasoconstriction —Reduced intracellular Ca2+ ions —Bind to SERCA forming strong hydrogen bonds —Significantly attenuated AngII-induced hypertension. However, CA failed to do so in SERCA2a knockout mice | [86] |
| HFD obesity-induced C57BL/6J mice | 50 mg/kg via oral gavage | 12 weeks | Reduced serum insulin | [56] |
| Alloxan-induced type-1 diabetic in Swiss albino mice | 50 mg/kg intraperitoneal injection | 7 days | Protective effects on liver and kidneys Hypoglycemic and hypolipidemic properties. | [75] |
| STZ-induced diabetic male Wistar rats | 10 and 50 mg/kg via oral gavage (diluted in canola oil) | 30 days | Reduced —FBS —oxidative stress parameters (lipid peroxidation, reactive species production, protein oxidation, and MPO activity). | [90] |
| STZ-induced diabetic rats | orally | 5 weeks | Increased: —serum insulin level —GSH, CAT, and SOD levels Reduced: —Blood glucose level Histologically seen normal islet morphology in CA administered diabetic rats. | [91] |
| STZ and high-fat high-fructose-diet-induced CD1 (ICR) mice | 10 mg/kg/day of CAPA orally | 2 weeks | Reduced —Body weight increase —Plasma retinol binding protein 4 (RBP4) —Adiponectin level —TNFα in liver Preserved glucose tolerance Prevented glucose intolerance Preserved basal coronary flow | [40] |
| Insulin-resistant adipocytes ASCs exposed to high glucose levels | Decreased lipid droplets and radical oxygen species formation. Increased insulin sensitivity (showed reduction in pro-inflammatory cytokines level and increased adiponectins). | [51] | ||
| HFD inducing NAFLD in C57BL/6J mice | 0.08% or 0.16% CA added to pellet diet | 8 weeks | Reduced body weight in both concentrations. Positively altered the community compositional structure of gut microbiota. | [57] |
| Non-insulin-dependent DM (NIDDM) and insulin-resistant (IR) mice models | 15 and 30 mg/kg CAPE dissolved in PEG-400 given via oral gavage. | 5 weeks | Improved: —Insulin sensitivity —Hyperlipidemia —Peroxisome-proliferator-activated receptor-α (PPAR-α) —TNFα —Glucose consumption —Glucose uptake —Glycogen content —Oxidative stress level —Decreased level of glucose-6-phosphotase expression (G6Pase). | [49] |
| HFD-induced obesity in mice | 50 mg/kg/day orally | 10 weeks | Reduced: —Body weight —Liver weight —Liver lipid accumulation —Levels of ER stress markers in the liver Improved glucose intolerance and insulin sensitivity. | [72] |
| High fructose corn syrup- induced vascular dysfunction in Sprague Dawley rats | 50 mmol/kg intraperitoneal injection | 2 weeks | Reduced SBP Increased NO synthase production. Significant reduction in TC and LDL. No significant change to HDL nor TG. | [73] |
| Chronic restraint stress-induced insulin resistance in LACA mice | 5 and 10 mg/kg intraperitoneal injections | 30 days | Reduces: —Fasting blood sugar —Systemic inflammation —Oxidative stress —Improved insulin sensitivity | [68] |
| HFD-induced MetS in C57 mice | A combination of ferulic acid (50 mg/kg/day) with CA 0.9 mg/kg/day via subcutaneous injection | 40 days | Prevents obesity. Reverts hyperglycemia. Reverts dyslipidemia. Reverts hepatic steatosis. | [74] |
| High-fat-diet and STZ-induced diabetic male Wistar rats | 40 mg/kg via oral gavage | 8 weeks | Improved albumin excretion by kidneys. Improved blood glucose Reduced renal mesangial matrix extension. CA results were seen better in reversing the diabetic nephropathy in comparison to prevention. | [92] |
| L-NAME-induced Sprague Dawley rats | 50 µmol/kg/day intraperitoneally | 14 days | Kidney tissue analysis shows that CA was: —Unable to preserve PON1 activity —Unable to reduce NF-κB significantly | [93] |
| Hyperlipidemic Wistar Albino | 20 mg/kg/day | 30 days | Significantly reduced: —Total cholesterol —TG —HDL-c | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. https://doi.org/10.3390/molecules26185490
Muhammad Abdul Kadar NN, Ahmad F, Teoh SL, Yahaya MF. Caffeic Acid on Metabolic Syndrome: A Review. Molecules. 2021; 26(18):5490. https://doi.org/10.3390/molecules26185490
Chicago/Turabian StyleMuhammad Abdul Kadar, Nellysha Namela, Fairus Ahmad, Seong Lin Teoh, and Mohamad Fairuz Yahaya. 2021. "Caffeic Acid on Metabolic Syndrome: A Review" Molecules 26, no. 18: 5490. https://doi.org/10.3390/molecules26185490
APA StyleMuhammad Abdul Kadar, N. N., Ahmad, F., Teoh, S. L., & Yahaya, M. F. (2021). Caffeic Acid on Metabolic Syndrome: A Review. Molecules, 26(18), 5490. https://doi.org/10.3390/molecules26185490






