Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer
Abstract
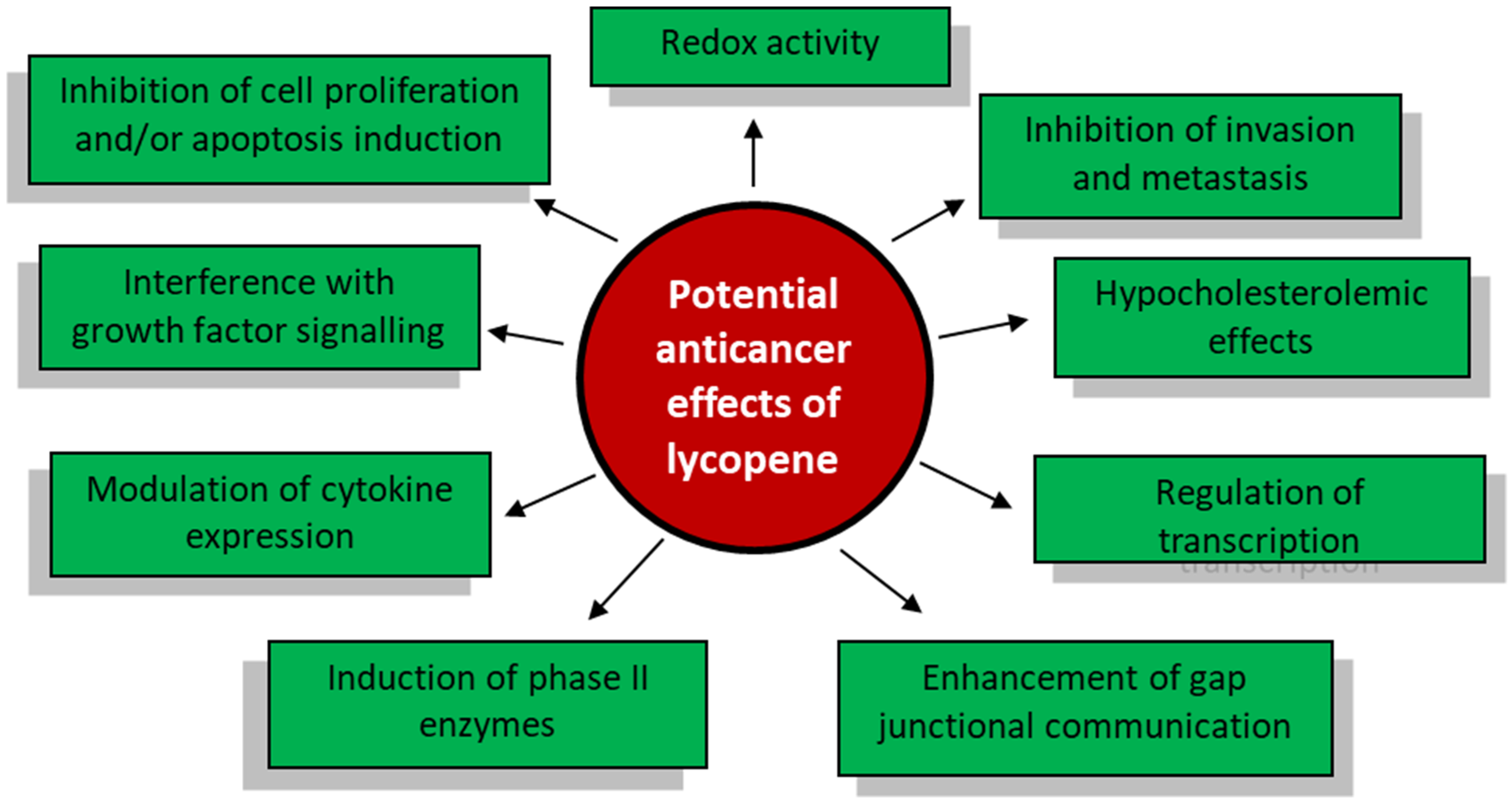
:1. Introduction
2. The Antioxidant Properties of Lycopene
2.1. Molecular Mechanisms
2.1.1. Modulatory Effect on Lipid Peroxidation and DNA Damage
2.1.2. Modulation of Antioxidant Responsive Elements (ARE) and Nrf2
2.1.3. Expression Modulation of P450
2.1.4. Inhibition on iNOS and COX-2 Expression
2.1.5. Downregulation of NF-kB Modulation
3. The Antineoplastic Properties of Lycopene
3.1. Effect on Cancer Cell Proliferation and Growth
3.2. Molecular Mechanism of Lycopene on Prostate Cancer Cells
3.3. Effects of Lycopene on the Cell Cycle
3.4. Effects of Lycopene on Cell Viability
3.5. Effects of Lycopene on Apoptosis
4. Clinical Case Studies
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Beynon, R.A.; Richmond, R.C.; Santos Ferreira, D.L.; Ness, A.R.; May, M.; Smith, G.D.; Vincent, E.E.; Adams, C.; Ala-Korpela, M.; Wurtz, P.; et al. Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: The ProDiet randomised controlled trial. Int. J. Cancer 2019, 144, 1918–1928. [Google Scholar] [CrossRef] [Green Version]
- Lane, J.A.; Er, V.; Avery, K.N.L.; Horwood, J.; Cantwell, M.; Caro, G.P.; Crozier, A.; Smith, G.D.; Donovan, J.L.; Down, L.; et al. ProDiet: A Phase II Randomized Placebo-controlled Trial of Green Tea Catechins and Lycopene in Men at Increased Risk of Prostate Cancer. Cancer Prev. Res. 2018, 11, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef] [Green Version]
- Hausladen, A.; Stamler, J.S. Nitrosative stress. Methods Enzym. 1999, 300, 389–395. [Google Scholar] [CrossRef]
- Halliwell, B.; Aruoma, O.I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991, 281, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Krinsky, N.I. The antioxidant and biological properties of the carotenoids. Ann. N. Y. Acad. Sci. 1998, 854, 443–447. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Ma, Y.; Qu, Y.; Lu, X.; Luo, H. Effects of Dietary Lycopene Supplementation on Plasma Lipid Profile, Lipid Peroxidation and Antioxidant Defense System in Feedlot Bamei Lamb. Asian-Australas J. Anim. Sci. 2015, 28, 958–965. [Google Scholar] [CrossRef]
- Matos, H.R.; Di Mascio, P.; Medeiros, M.H. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. Arch. Biochem. Biophys. 2000, 383, 56–59. [Google Scholar] [CrossRef]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W., Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E.; Rhodes, L.E. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: A randomized controlled trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef]
- Bhuvaneswari, V.; Velmurugan, B.; Balasenthil, S.; Ramachandran, C.R.; Nagini, S. Chemopreventive efficacy of lycopene on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Fitoterapia 2001, 72, 865–874. [Google Scholar] [CrossRef]
- Trejo-Solis, C.; Pedraza-Chaverri, J.; Torres-Ramos, M.; Jimenez-Farfan, D.; Cruz Salgado, A.; Serrano-Garcia, N.; Osorio-Rico, L.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Based Complement. Altern. Med. 2013, 2013, 705121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar]
- Lian, F.; Wang, X.D. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int. J. Cancer 2008, 123, 1262–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic. Biol. Med. 2009, 47, 659–667. [Google Scholar] [CrossRef]
- Yang, P.M.; Chen, H.Z.; Huang, Y.T.; Hsieh, C.W.; Wung, B.S. Lycopene inhibits NF-kappaB activation and adhesion molecule expression through Nrf2-mediated heme oxygenase-1 in endothelial cells. Int. J. Mol. Med. 2017, 39, 1533–1540. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharm. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Ma, Q. NRF2 cysteine residues are critical for oxidant/electrophile-sensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol. Pharmacol. 2009, 76, 1265–1278. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Zangar, R.C.; Davydov, D.R.; Verma, S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharm. 2004, 199, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Danielson, P.B. The cytochrome P450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr. Drug Metab. 2002, 3, 561–597. [Google Scholar] [CrossRef] [PubMed]
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and Roles in Cancer, Diabetes and Atherosclerosis. Asian Pac. J. Cancer Prev. 2018, 19, 2057–2070. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. Carotenoids in Aquatic Ecosystems and Aquaculture: A Colorful Business with Implications for Human Health. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.F.; Bae, S.H.; Kwon, M.J.; Park, J.B.; Choi, H.D.; Shin, W.G.; Bae, S.K. Inhibitory effects of astaxanthin, beta-cryptoxanthin, canthaxanthin, lutein, and zeaxanthin on cytochrome P450 enzyme activities. Food Chem. Toxicol. 2013, 59, 78–85. [Google Scholar] [CrossRef]
- Astorg, P.; Gradelet, S.; Berges, R.; Suschetet, M. Dietary lycopene decreases the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutr. Cancer 1997, 29, 60–68. [Google Scholar] [CrossRef]
- Breinholt, V.; Lauridsen, S.T.; Daneshvar, B.; Jakobsen, J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000, 154, 201–210. [Google Scholar] [CrossRef]
- De Stefano, D.; Maiuri, M.C.; Simeon, V.; Grassia, G.; Soscia, A.; Cinelli, M.P.; Carnuccio, R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur. J. Pharmacol. 2007, 566, 192–199. [Google Scholar] [CrossRef]
- Rafi, M.M.; Yadav, P.N.; Reyes, M. Lycopene inhibits LPS-induced proinflammatory mediator inducible nitric oxide synthase in mouse macrophage cells. J. Food Sci. 2007, 72, S069-074. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Maccarrone, M. Chronic Inflammatory Disorders and Their Redox Control: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 2605–2641. [Google Scholar] [CrossRef]
- Kretz-Remy, C.; Bates, E.E.; Arrigo, A.P. Amino acid analogs activate NF-kappaB through redox-dependent IkappaB-alpha degradation by the proteasome without apparent IkappaB-alpha phosphorylation. Consequence on HIV-1 long terminal repeat activation. J. Biol. Chem. 1998, 273, 3180–3191. [Google Scholar] [CrossRef] [Green Version]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Huang, T.F.; Chen, B.H.; Shieh, J.M.; Wu, P.H.; Wu, W.B. Lycopene inhibits TNF-alpha-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur. J. Pharm. 2008, 586, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.E.; Karrasch, T.; Muhlbauer, M.; Allard, B.; Narula, A.; Herfarth, H.H.; Jobin, C. Tomato lycopene extract prevents lipopolysaccharide-induced NF-kappaB signaling but worsens dextran sulfate sodium-induced colitis in NF-kappaBEGFP mice. PLoS ONE 2009, 4, e4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palozza, P.; Colangelo, M.; Simone, R.; Catalano, A.; Boninsegna, A.; Lanza, P.; Monego, G.; Ranelletti, F.O. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.M.; Huang, S.M.; Liu, C.L.; Hu, M.L. Apo-8’-lycopenal induces expression of HO-1 and NQO-1 via the ERK/p38-Nrf2-ARE pathway in human HepG2 cells. J. Agric. Food Chem. 2012, 60, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharm. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Pirayesh Islamian, J.; Mehrali, H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: An overview. Cell J. 2015, 16, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, A.J.; Oliveira, F.L.; Martins, N.B.; Maia Gde, A.; Martucci, R.B.; Borojevic, R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012, 12, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palozza, P.; Simone, R.E.; Catalano, A.; Mele, M.C. Tomato lycopene and lung cancer prevention: From experimental to human studies. Cancers 2011, 3, 2333–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharoni, Y.; Danilenko, M.; Dubi, N.; Ben-Dor, A.; Levy, J. Carotenoids and transcription. Arch. Biochem. Biophys. 2004, 430, 89–96. [Google Scholar] [CrossRef]
- Ribas, V.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palozza, P.; Parrone, N.; Catalano, A.; Simone, R. Tomato Lycopene and Inflammatory Cascade: Basic Interactions and Clinical Implications. Curr. Med. Chem. 2010, 17, 2547–2563. [Google Scholar] [CrossRef]
- Chen, M.; Huang, J. The expanded role of fatty acid metabolism in cancer: New aspects and targets. Precis. Clin. Med. 2019, 2, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharm. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [Green Version]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Stahl, W.; von Laar, J.; Martin, H.D.; Emmerich, T.; Sies, H. Stimulation of gap junctional communication: Comparison of acyclo-retinoic acid and lycopene. Arch. Biochem. Biophys. 2000, 373, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Kapral, M.; Wawszczyk, J.; Jurzak, M.; Hollek, A.; Weglarz, L. The effect of inositol hexaphosphate on the expression of selected metalloproteinases and their tissue inhibitors in IL-1beta-stimulated colon cancer cells. Int. J. Colorectal. Dis. 2012, 27, 1419–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.S.; Lee, H.J. Inhibitory effects of lycopene on the adhesion, invasion, and migration of SK-Hep1 human hepatoma cells. Exp. Biol. Med. (Maywood) 2006, 231, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Shih, M.K.; Chuang, C.H.; Hu, M.L. Lycopene inhibits cell migration and invasion and upregulates Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 cells. J. Nutr. 2005, 135, 2119–2123. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Bowen, P.E. Effects of tomato paste extracts on cell proliferation, cell-cycle arrest and apoptosis in LNCaP human prostate cancer cells. Biofactors 2005, 23, 75–84. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Butler, W.; Huang, J. Neuroendocrine cells of the prostate: Histology, biological functions, and molecular mechanisms. Precis. Clin. Med. 2021. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Wang, X.; Wang, S.; Meng, S.; Zhu, Y.; Liang, Z.; Zheng, X.; Xie, L. Tomato consumption and prostate cancer risk: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 37091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Jin, T.; Zeng, X.; Wang, J.S. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. J. Nutr. 2005, 135, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.T. Prevalent mutations in prostate cancer. J. Cell. Biochem. 2006, 97, 433–447. [Google Scholar] [CrossRef]
- Wertz, K. Lycopene effects contributing to prostate health. Nutr. Cancer 2009, 61, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Goo, Y.A.; Li, Z.; Pajkovic, N.; Shaffer, S.; Taylor, G.; Chen, J.; Campbell, D.; Arnstein, L.; Goodlett, D.R.; van Breemen, R.B. Systematic investigation of lycopene effects in LNCaP cells by use of novel large-scale proteomic analysis software. Proteomics Clin. Appl. 2007, 1, 513–523. [Google Scholar] [CrossRef]
- Pathak, S.K.; Sharma, R.A.; Steward, W.P.; Mellon, J.K.; Griffiths, T.R.; Gescher, A.J. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: Targets for chemopreventive strategies. Eur. J. Cancer 2005, 41, 61–70. [Google Scholar] [CrossRef]
- Qiu, X.; Yuan, Y.; Vaishnav, A.; Tessel, M.A.; Nonn, L.; van Breemen, R.B. Effects of lycopene on protein expression in human primary prostatic epithelial cells. Cancer Prev. Res. (Phila) 2013, 6, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Barber, N.J.; Zhang, X.; Zhu, G.; Pramanik, R.; Barber, J.A.; Martin, F.L.; Morris, J.D.; Muir, G.H. Lycopene inhibits DNA synthesis in primary prostate epithelial cells in vitro and its administration is associated with a reduced prostate-specific antigen velocity in a phase II clinical study. Prostate Cancer Prostatic Dis. 2006, 9, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharm. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Ivanov, N.I.; Cowell, S.P.; Brown, P.; Rennie, P.S.; Guns, E.S.; Cox, M.E. Lycopene differentially induces quiescence and apoptosis in androgen-responsive and -independent prostate cancer cell lines. Clin. Nutr. 2007, 26, 252–263. [Google Scholar] [CrossRef]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.; Prall, O.W.; Levy, J.; Sharoni, Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.Y.; Cho, H.J.; Pai, M.H.; Chen, Y.H. Concomitant supplementation of lycopene and eicosapentaenoic acid inhibits the proliferation of human colon cancer cells. J. Nutr. Biochem. 2009, 20, 426–434. [Google Scholar] [CrossRef]
- Velmurugan, B.; Mani, A.; Nagini, S. Combination of S-allylcysteine and lycopene induces apoptosis by modulating Bcl-2, Bax, Bim and caspases during experimental gastric carcinogenesis. Eur. J. Cancer Prev. 2005, 14, 387–393. [Google Scholar] [CrossRef]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp. Biol. Med. (Maywood) 2005, 230, 171–179. [Google Scholar] [CrossRef]
- Kumar, N.B.; Besterman-Dahan, K.; Kang, L.; Pow-Sang, J.; Xu, P.; Allen, K.; Riccardi, D.; Krischer, J.P. Results of a Randomized Clinical Trial of the Action of Several Doses of Lycopene in Localized Prostate Cancer: Administration Prior to Radical Prostatectomy. Clin. Med. Urol. 2008, 1, 1–14. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Sharifi, R.; Viana, M.; Pajkovic, N.; Zhu, D.; Yuan, L.; Yang, Y.; Bowen, P.E.; Stacewicz-Sapuntzakis, M. Antioxidant effects of lycopene in African American men with prostate cancer or benign prostate hyperplasia: A randomized, controlled trial. Cancer Prev. Res. (Phila) 2011, 4, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Magbanua, M.J.; Roy, R.; Sosa, E.V.; Weinberg, V.; Federman, S.; Mattie, M.D.; Hughes-Fulford, M.; Simko, J.; Shinohara, K.; Haqq, C.M.; et al. Gene expression and biological pathways in tissue of men with prostate cancer in a randomized clinical trial of lycopene and fish oil supplementation. PLoS ONE 2011, 6, e24004. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Forbes, K.M.; Hassed, C. Lycopene for the prevention of prostate cancer. Cochrane Database Syst. Rev. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Takkouche, B.; Caamano-Isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 340–345. [Google Scholar]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzocco, S.; Singla, R.K.; Capasso, A. Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer. Molecules 2021, 26, 5333. https://doi.org/10.3390/molecules26175333
Marzocco S, Singla RK, Capasso A. Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer. Molecules. 2021; 26(17):5333. https://doi.org/10.3390/molecules26175333
Chicago/Turabian StyleMarzocco, Stefania, Rajeev K. Singla, and Anna Capasso. 2021. "Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer" Molecules 26, no. 17: 5333. https://doi.org/10.3390/molecules26175333
APA StyleMarzocco, S., Singla, R. K., & Capasso, A. (2021). Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer. Molecules, 26(17), 5333. https://doi.org/10.3390/molecules26175333







