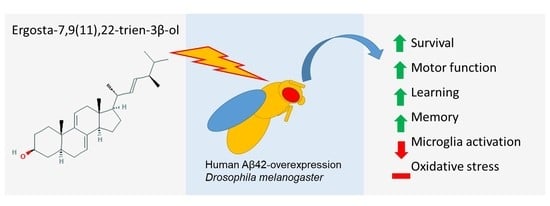
Ergosta-7,9(11),22-trien-3β-ol Rescues AD Deficits by Modulating Microglia Activation but Not Oxidative Stress
Abstract
:1. Introduction
2. Results
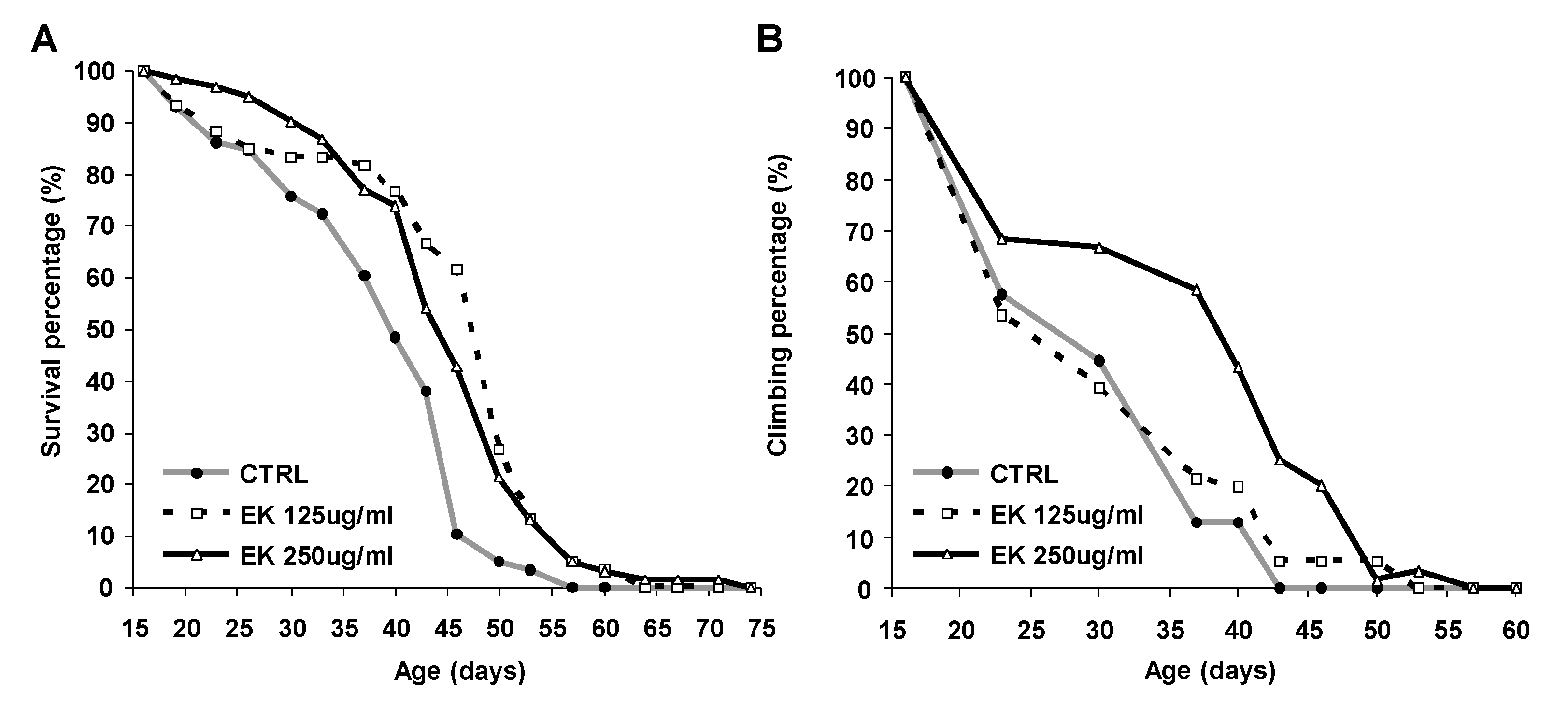
2.1. Ergosta-7,9(11),22-trien-3β-ol Rescued Survival and Climbing of AD Drosophila
2.2. Ergosta-7,9(11),22-trien-3β-ol Rescued Learning and One-Hour Memory of AD Drosophila
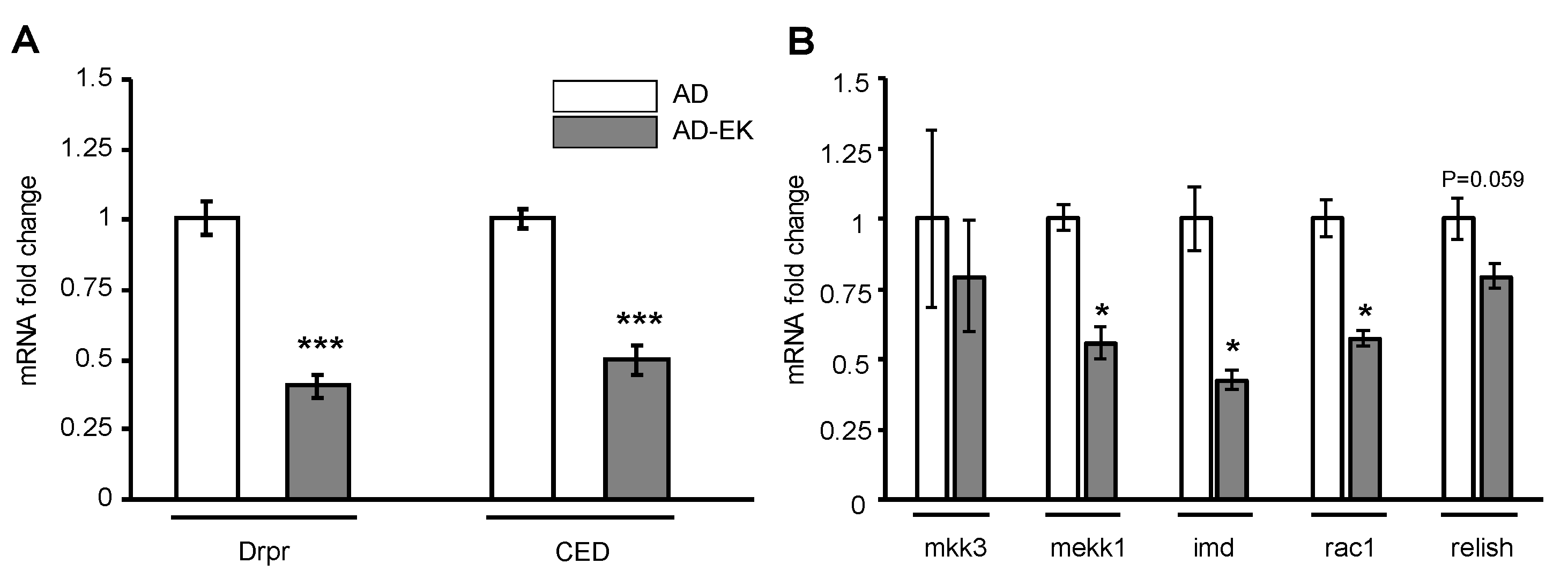
2.3. Ergosta-7,9(11),22-trien-3β-ol Modulated Microglial Activation in AD Drosophila
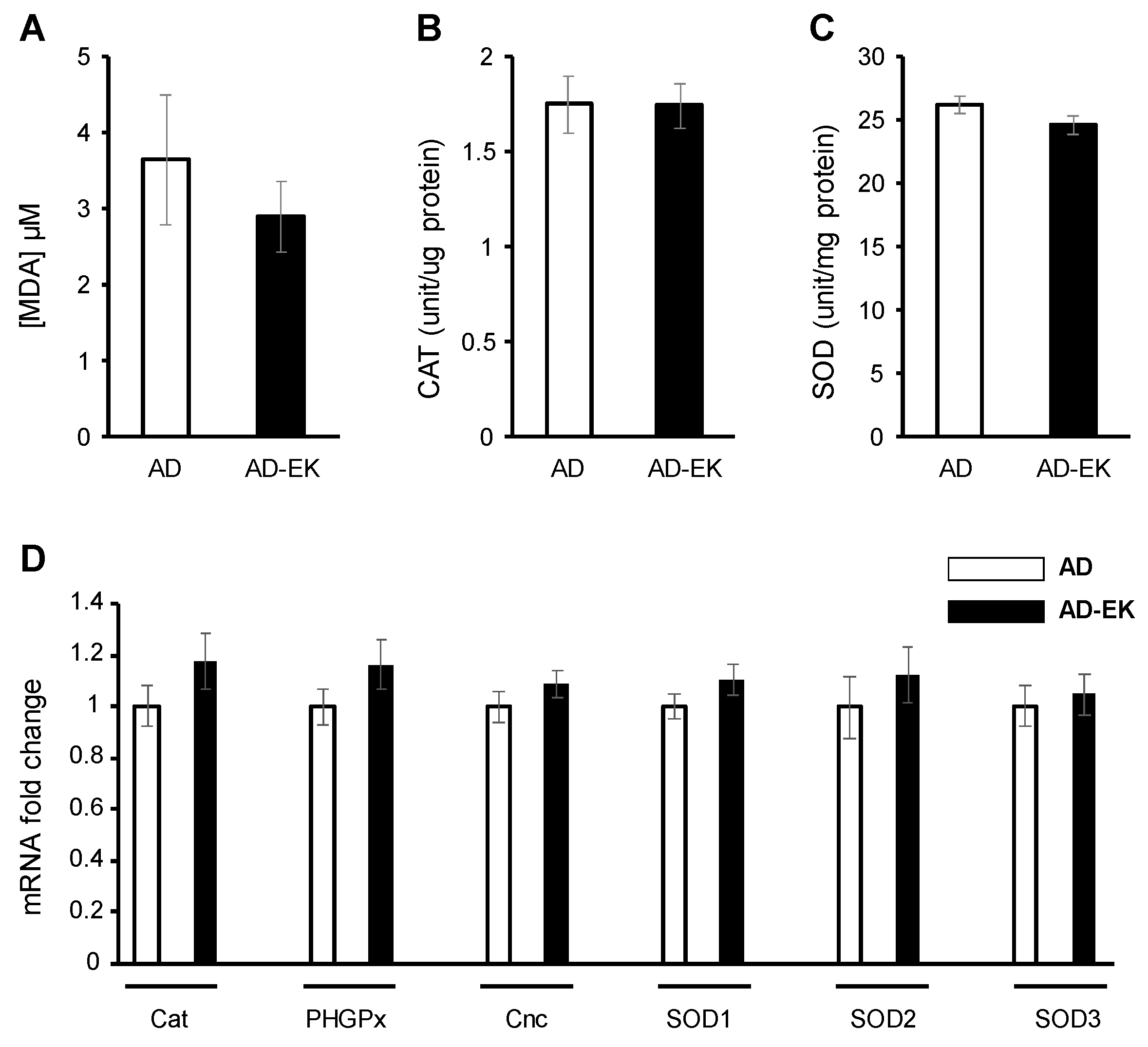
2.4. Ergosta-7,9(11),22-trien-3β-ol Did Not Modulate Ubiquitous Oxidative Stress in AD Drosophila
3. Discussion
4. Materials and Methods
4.1. Fly Stock Maintenance and Lifespan Measurement
4.2. Ergosta-7,9(11),22-trien-3β-ol (Ek100)
4.3. Antigeotaxis Assays
4.4. Drosophila Learning and Memory Platform (T-Maze)
4.5. Quantitative PCR
4.6. Oxidative Stress Assays
4.7. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Davidson, J.W. The Island of Formosa, Past and Present: History, People, Resources, and Commercial Prospects. Tea, Camphor, Sugar, Gold, Coal, Sulphur, Economical Plants, and Other Productions; Macmillan & Company: New York, NY, USA, 1903. [Google Scholar]
- Tsai, Z.; Liaw, S. The Use and the Effect of Ganoderma; Sheng-Yun Publishers, Inc.: Taichung, Taiwan, 1985; pp. 116–117. [Google Scholar]
- Chen, C.-J. Study on solid cultivation and bioactivity of Antrodia camphorata. Fung. Sci. 2001, 16, 65–72. [Google Scholar]
- Geethangili, M.; Tzeng, Y.-M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid.-Based Complement. Altern. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogura, S.; Jakovljevic, M.M. Global population aging-health care, social and economic consequences. Front. Public Health 2018, 6, 335. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—A critical review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Agostinho, P.; A Cunha, R.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Galasko, D.; Montine, T.J. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark. Med. 2010, 4, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.-H.; Lin, T.-Y.; You, Y.-J.; Wen, K.-C.; Sung, P.-J.; Chiang, H.-M. Antiinflammatory and antiphotodamaging effects of ergostatrien-3β-ol, isolated from Antrodia camphorata, on hairless mouse skin. Molecules 2016, 21, 1213. [Google Scholar] [CrossRef] [Green Version]
- Chao, T.-Y.; Hsieh, C.-C.; Hsu, S.-M.; Wan, C.-H.; Lian, G.-T.; Tseng, Y.-H.; Kuo, Y.-H.; Hsieh, S.-C. Ergostatrien-3β-ol (EK100) from Antrodia camphorata Attenuates Oxidative Stress, Inflammation, and Liver Injury In Vitro and In Vivo. Prev. Nutr. Food Sci. 2021, 26, 58. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Tung, Y.-T.; Kuo, Y.-H.; Liao, J.-W.; Tsai, H.-C.; Chong, K.-Y.; Chen, H.-L.; Chen, C.-M. Anti-Inflammatory Effects of Antrodia camphorata and its Active Compound, Ergostatrien-3β-ol, in a Mouse Skin Ischemia Model. FASEB J. 2015, 29, LB444. [Google Scholar]
- Chang, W.-H.; Chen, M.C.; Cheng, I.H. Antroquinonol lowers brain amyloid-β levels and improves spatial learning and memory in a transgenic mouse model of Alzheimer’s disease. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-M.; Sung, H.-C.; Kuo, Y.-H.; Hsu, Y.-J.; Huang, C.-C.; Liang, H.-L. The Effects of Ergosta-7, 9 (11), 22-trien-3β-ol from Antrodia camphorata on the Biochemical Profile and Exercise Performance of Mice. Molecules 2019, 24, 1225. [Google Scholar] [CrossRef] [Green Version]
- Finelli, A.; Kelkar, A.; Song, H.-J.; Yang, H.; Konsolaki, M. A model for studying Alzheimer’s Aβ42-induced toxicity in Drosophila melanogaster. Mol. Cell. Neurosci. 2004, 26, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Hahm, E.-T.; Waro, G.; Song, Q.; Vo-Ba, D.-A.; Licursi, A.; Bao, H.; Ganoe, L.; Finch, K.; Tsunoda, S. Linking aβ42-induced hyperexcitability to neurodegeneration, learning and motor deficits, and a shorter lifespan in an Alzheimer’s model. PLoS Genet. 2015, 11, e1005025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rival, T.; Page, R.M.; Chandraratna, D.S.; Sendall, T.J.; Ryder, E.; Liu, B.; Lewis, H.; Rosahl, T.; Hider, R.; Camargo, L. Fenton chemistry and oxidative stress mediate the toxicity of the β-amyloid peptide in a Drosophila model of Alzheimer’s disease. Eur. J. Neurosci. 2009, 29, 1335–1347. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Speese, S.D.; Logan, M.A. Glial draper rescues Aβ toxicity in a Drosophila model of Alzheimer’s disease. J. Neurosci. 2017, 37, 11881–11893. [Google Scholar] [CrossRef] [Green Version]
- Jeibmann, A.; Paulus, W. Drosophila melanogaster as a model organism of brain diseases. Int. J. Mol. Sci. 2009, 10, 407–440. [Google Scholar] [CrossRef] [PubMed]
- Brookmeyer, R.; Corrada, M.M.; Curriero, F.C.; Kawas, C. Survival following a diagnosis of Alzheimer disease. Arch. Neurol. 2002, 59, 1764–1767. [Google Scholar] [CrossRef]
- Lam, L.C.; Tang, W.; Leung, V.; Chiu, H.F. Behavioral profile of Alzheimer’s disease in Chinese elderly–a validation study of the Chinese version of the Alzheimer’s disease behavioral pathology rating scale. Int. J. Geriatr. Psych. 2001, 16, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; McGeer, P.L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimer’s Dis. 2008, 13, 359–369. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.M.; Beach, M.G.; Porpiglia, E.; Sheehan, A.E.; Watts, R.J.; Freeman, M.R. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 2006, 50, 869–881. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Stankovic, N.D.; Teodorczyk, M.; Ploen, R.; Zipp, F.; Schmidt, M.H. Microglia–blood vessel interactions: A double-edged sword in brain pathologies. Acta Neuropathol. 2016, 131, 347–363. [Google Scholar] [CrossRef]
- Konishi, H.; Kiyama, H. Microglial TREM2/DAP12 signaling: A double-edged sword in neural diseases. Front. Cell. Neurosci. 2018, 12, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Lalli, G.; Schott, J.M.; Hardy, J.; De Strooper, B. Aducanumab: A new phase in therapeutic development for Alzheimer’s disease? EMBO Mol. Med. 2021, 13, e14781. [Google Scholar]
- Takata, K.; Kitamura, Y.; Saeki, M.; Terada, M.; Kagitani, S.; Kitamura, R.; Fujikawa, Y.; Maelicke, A.; Tomimoto, H.; Taniguchi, T. Galantamine-induced amyloid-β clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J. Biol. Chem. 2010, 285, 40180–40191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrekar-Colucci, S.; Karlo, J.C.; Landreth, G.E. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J. Neurosci. 2012, 32, 10117–10128. [Google Scholar] [CrossRef] [Green Version]
- McGeer, P.L.; McGeer, E.G. Targeting microglia for the treatment of Alzheimer’s disease. Expert Opin. Ther. Targets 2015, 19, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Song, W.M.; Colonna, M. The identity and function of microglia in neurodegeneration. Nat. Immunol. 2018, 19, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Lin, C.-H.; Shih, C.-C. Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 2015, 63, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, E.; Lints, F.A. Hypergravity and aging in Drosophila melanogaster. 4. Climbing activity. Gerontology 1992, 38, 59–64. [Google Scholar] [CrossRef]
- Waddell, S.; Quinn, W.G. Flies, genes, and learning. Ann. Rev. Neurosci. 2001, 24, 1283–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-P.; Kuo, Y.-H.; Cheng, J.; Chang, L.-Z.; Chang, M.-S.; Su, L.-W.; Chuang, T.-N.; Lin, W.-Y. Ergosta-7,9(11),22-trien-3β-ol Rescues AD Deficits by Modulating Microglia Activation but Not Oxidative Stress. Molecules 2021, 26, 5338. https://doi.org/10.3390/molecules26175338
Liu H-P, Kuo Y-H, Cheng J, Chang L-Z, Chang M-S, Su L-W, Chuang T-N, Lin W-Y. Ergosta-7,9(11),22-trien-3β-ol Rescues AD Deficits by Modulating Microglia Activation but Not Oxidative Stress. Molecules. 2021; 26(17):5338. https://doi.org/10.3390/molecules26175338
Chicago/Turabian StyleLiu, Hsin-Ping, Yueh-Hsiung Kuo, Jack Cheng, Li-Zhong Chang, Meng-Shiun Chang, Li-Wen Su, Tsai-Ni Chuang, and Wei-Yong Lin. 2021. "Ergosta-7,9(11),22-trien-3β-ol Rescues AD Deficits by Modulating Microglia Activation but Not Oxidative Stress" Molecules 26, no. 17: 5338. https://doi.org/10.3390/molecules26175338
APA StyleLiu, H.-P., Kuo, Y.-H., Cheng, J., Chang, L.-Z., Chang, M.-S., Su, L.-W., Chuang, T.-N., & Lin, W.-Y. (2021). Ergosta-7,9(11),22-trien-3β-ol Rescues AD Deficits by Modulating Microglia Activation but Not Oxidative Stress. Molecules, 26(17), 5338. https://doi.org/10.3390/molecules26175338