Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine
Abstract
:1. Introduction
2. Structural and Synthetic Biology of Protein C-Mannosylation and C-Man-Trp
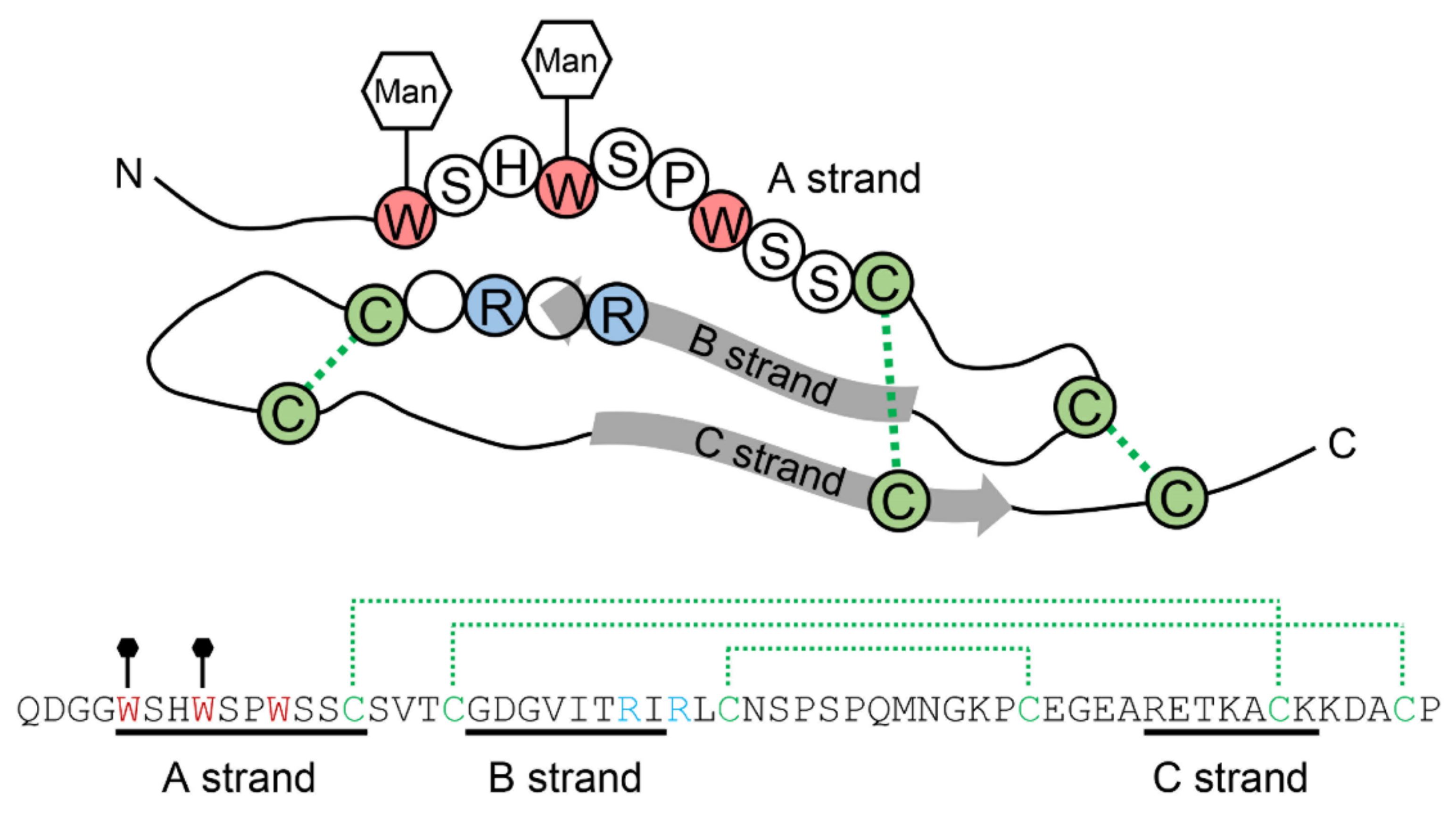
2.1. The Structure of C-Mannosylated Protein
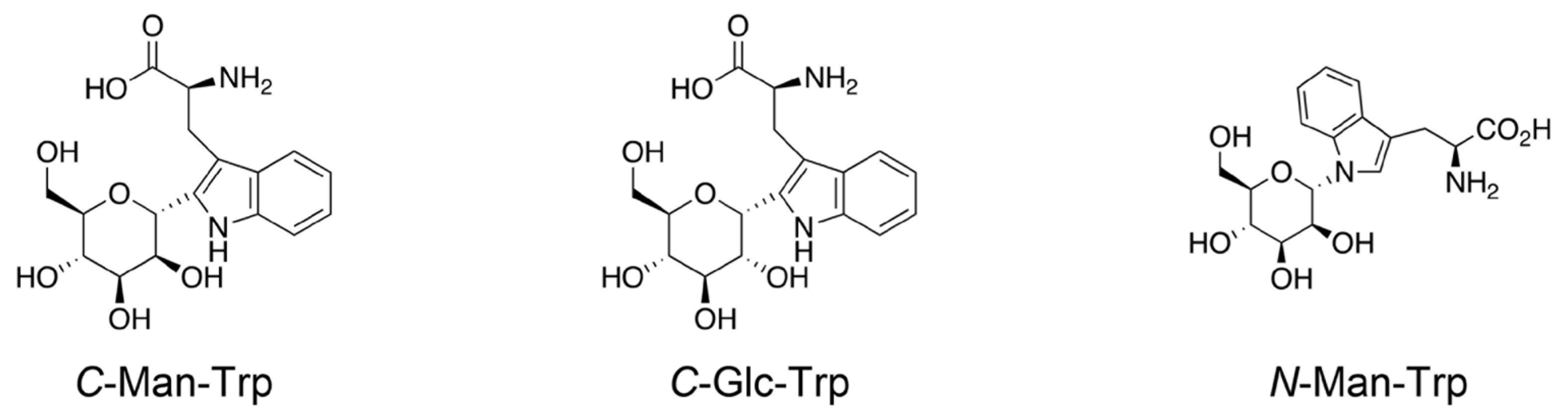
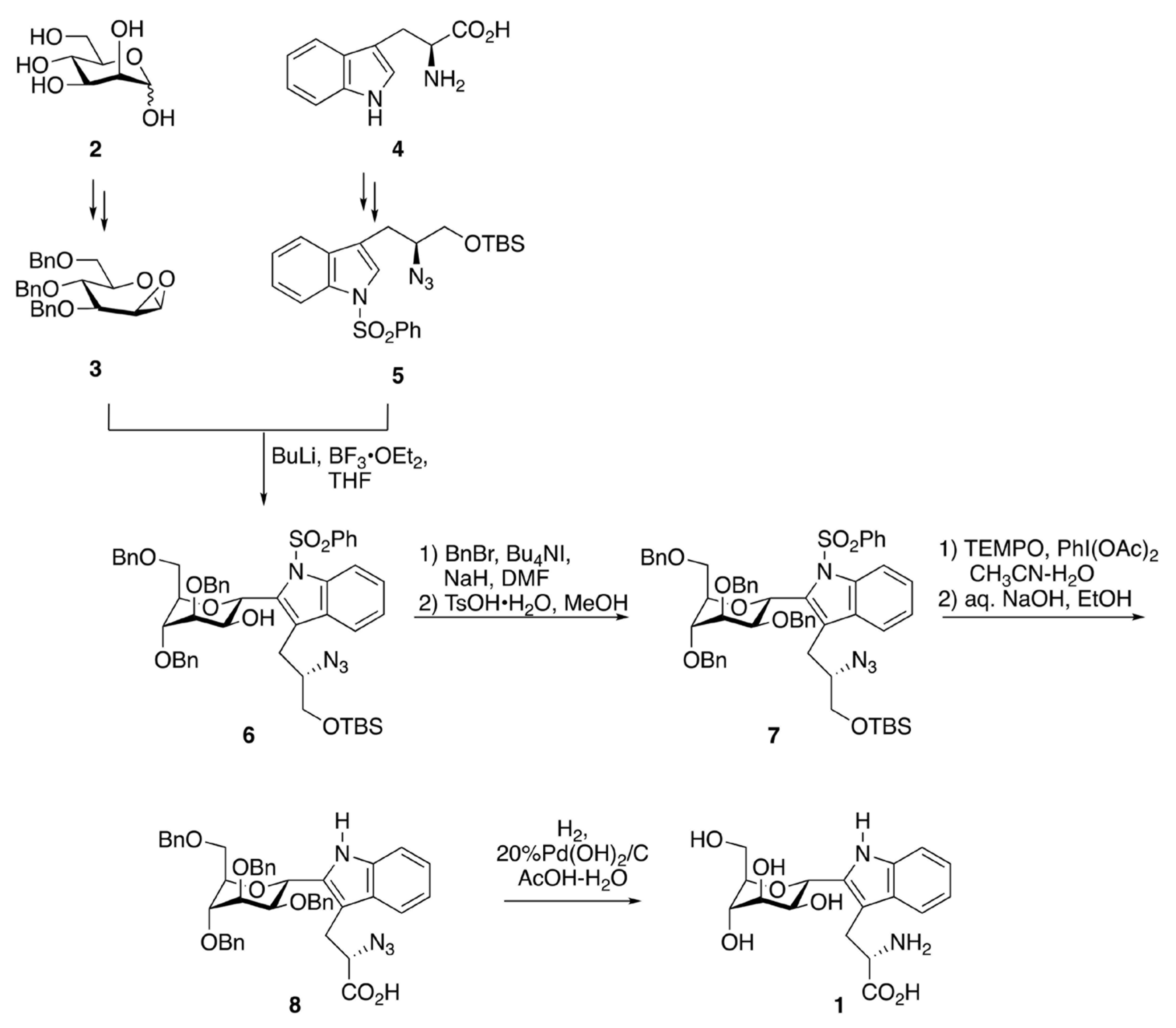
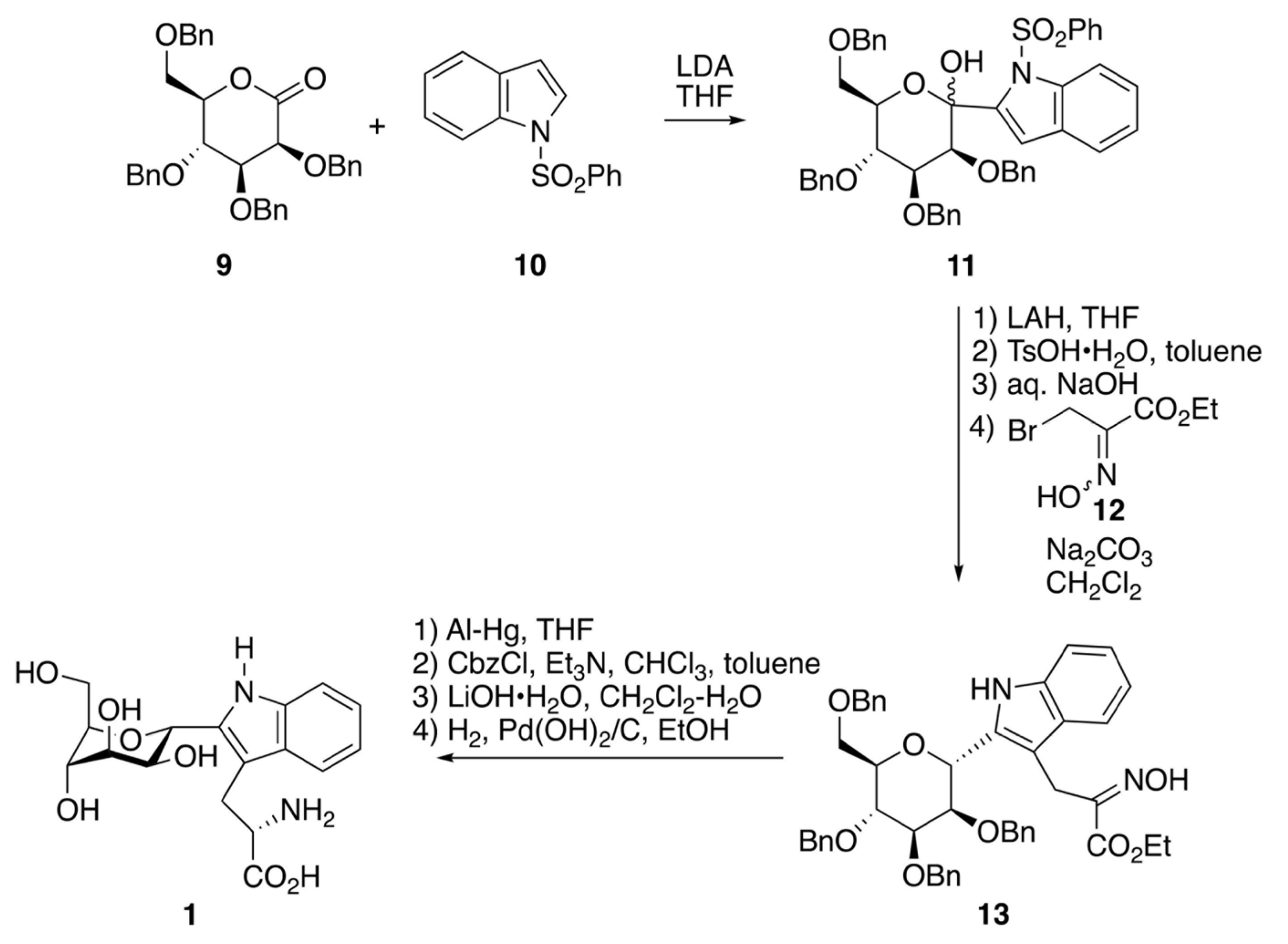
2.2. Synthetic Chemistry of C-Man Trp and the Related Molecules
3. Biochemical Aspects of Protein C-Mannosylation and C-Man-Trp
3.1. Detection and Identification of C-Mannosylation in Proteins
3.2. Analysis and Production of Monomeric C-Man-Trp
3.3. Protein C-Mannosylation in Mammals
3.3.1. The Substrate Proteins for C-Mannosylation
3.3.2. C-Mannosyltransferase
3.3.3. Mannose Donors for C-Mannosylation
3.3.4. Functions of Protein C-Mannosylation
3.4. Protein C-Mannosylation in Non-Mammals
4. Biomedical Topics of Protein C-Mannosylation and C-Man-Trp
4.1. Protein C-Mannosylation in Disease
4.1.1. Diseases by Defects in C-Mannosyltransferase
4.1.2. Diseases by Defects in Mannose Donors
4.1.3. Diseases Related to Protein C-Mannosylation
4.1.4. Research Use of Synthetic Peptides
4.2. C-Man-Trp in Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Furmanek, A.; Hofsteenge, J. Protein C-mannosylation: Facts and questions. Acta Biochim. Pol. 2000, 47, 781–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihara, Y.; Inai, Y.; Ikezaki, M.; Matsui, I.L.; Manabe, S.; Ito, Y. C-Mannosylation: Modification on tryptophan in cellular proteins. In Glycoscience: Biology and Medicine; Taniguchi, N., Endo, T., Hart, G.W., Seeberger, P.H., Wong, C.H., Eds.; Springer: Tokyo, Japan, 2015; Volume 2, pp. 1091–1099. [Google Scholar]
- Hofsteenge, J.; Müller, D.R.; de Beer, T.; Löffler, A.; Richter, W.J.; Vliegenthart, J.F. New type of linkage between a carbohydrate and a protein: C-glycosylation of a specific tryptophan residue in human RNase Us. Biochemistry 1994, 33, 13524–13530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gäde, G.; Kellner, R.; Rinehart, K.L.; Proefke, M.L. A tryptophan-substituted member of the AKH/RPCH family isolated from a stick insect corpus cardiacum. Biochem. Biophys. Res. Commun. 1992, 189, 1303–1309. [Google Scholar] [CrossRef]
- Horiuchi, K.; Yonekawa, O.; Iwahara, K.; Kanno, T.; Kurihara, T.; Fujise, Y. A hydrophilic tetrahydro-β-carboline in human urine. J. Biochem. 1994, 115, 362–366. [Google Scholar] [CrossRef]
- Gutsche, B.; Grun, C.; Scheutzow, D.; Herderich, M. Tryptophan glycoconjugates in food and human urine. Biochem. J. 1999, 343 Pt 1, 11–19. [Google Scholar] [CrossRef]
- Niwa, Y.; Simizu, S. C-mannnosylation: Previous Studies and Future Research Perspectives. Trends Glycosci. Glycotechnol. 2018, 30, E231–E238. [Google Scholar] [CrossRef]
- Löffler, A.; Doucey, M.A.; Jansson, A.M.; Müller, D.R.; de Beer, T.; Hess, D.; Meldal, M.; Richter, W.J.; Vliegenthart, J.F.; Hofsteenge, J. Spectroscopic and protein chemical analyses demonstrate the presence of C-mannosylated tryptophan in intact human RNase 2 and its isoforms. Biochemistry 1996, 35, 12005–12014. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.; Duquette, M.; Liu, J.H.; Dong, Y.; Zhang, R.; Joachimiak, A.; Lawler, J.; Wang, J.H. Crystal structure of the TSP-1 type 1 repeats: A novel layered fold and its biological implication. J. Cell Biol. 2002, 159, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Aleshin, A.E.; Schraufstatter, I.U.; Stec, B.; Bankston, L.A.; Liddington, R.C.; DiScipio, R.G. Structure of complement C6 suggests a mechanism for initiation and unidirectional, sequential assembly of membrane attack complex (MAC). J. Biol. Chem. 2012, 287, 10210–10222. [Google Scholar] [CrossRef] [Green Version]
- Hadders, M.A.; Bubeck, D.; Roversi, P.; Hakobyan, S.; Forneris, F.; Morgan, B.P.; Pangburn, M.K.; Llorca, O.; Lea, S.M.; Gros, P. Assembly and regulation of the membrane attack complex based on structures of C5b6 and sC5b9. Cell Rep. 2012, 1, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Aleshin, A.E.; DiScipio, R.G.; Stec, B.; Liddington, R.C. Crystal structure of C5b-6 suggests structural basis for priming assembly of the membrane attack complex. J. Biol. Chem. 2012, 287, 19642–19652. [Google Scholar] [CrossRef] [Green Version]
- Lovelace, L.L.; Cooper, C.L.; Sodetz, J.M.; Lebioda, L. Structure of human C8 protein provides mechanistic insight into membrane pore formation by complement. J. Biol. Chem. 2011, 286, 17585–17592. [Google Scholar] [CrossRef] [Green Version]
- Spicer, B.A.; Law, R.H.P.; CaradoC-Davies, T.T.; Ekkel, S.M.; Bayly-Jones, C.; Pang, S.S.; Conroy, P.J.; Ramm, G.; Radjainia, M.; Venugopal, H.; et al. The first transmembrane region of complement component-9 acts as a brake on its self-assembly. Nat. Commun. 2018, 9, 3266. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, M.; Nakayama, D.; Takeda, S.; Kokame, K.; Takagi, J.; Miyata, T. Crystal structure and enzymatic activity of an ADAMTS-13 mutant with the East Asian-specific P475S polymorphism. J. Thromb. Haemost. 2013, 11, 1399–1406. [Google Scholar] [CrossRef]
- Song, G.; Springer, T.A. Structures of the Toxoplasma gliding motility adhesin. Proc. Natl. Acad. Sci. USA 2014, 111, 4862–4867. [Google Scholar] [CrossRef] [Green Version]
- Van den Bos, R.M.; Pearce, N.M.; Granneman, J.; Brondijk, T.H.C.; Gros, P. Insights into enhanced complement activation by structures of properdin and its complex with the C-terminal domain of C3b. Front. Immunol. 2019, 10, 2097. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, D.V.; Gadeberg, T.A.F.; Thomas, C.; Wang, Y.; Joram, N.; Jensen, R.K.; Mazarakis, S.M.M.; Revel, M.; El Sissy, C.; Petersen, S.V.; et al. Structural basis for properdin oligomerization and convertase stimulation in the human complement system. Front. Immunol. 2019, 10, 2007. [Google Scholar] [CrossRef]
- Syed, R.S.; Reid, S.W.; Li, C.; Cheetham, J.C.; Aoki, K.H.; Liu, B.; Zhan, H.; Osslund, T.D.; Chirino, A.J.; Zhang, J.; et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature 1998, 395, 511–516. [Google Scholar] [CrossRef]
- Hamming, O.J.; Kang, L.; Svensson, A.; Karlsen, J.L.; Rahbek-Nielsen, H.; Paludan, S.R.; Hjorth, S.A.; Bondensgaard, K.; Hartmann, R. Crystal structure of interleukin-21 receptor (IL-21R) bound to IL-21 reveals that sugar chain interacting with WSXWS motif is integral part of IL-21R. J. Biol. Chem. 2012, 287, 9454–9460. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.; Waldhauer, I.; Nicolini, V.G.; Freimoser-Grundschober, A.; Nayak, T.; Vugts, D.J.; Dunn, C.; Bolijn, M.; Benz, J.; Stihle, M.; et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 2017, 6, e1277306. [Google Scholar] [CrossRef]
- Tamada, T.; Honjo, E.; Maeda, Y.; Okamoto, T.; Ishibashi, M.; Tokunaga, M.; Kuroki, R. Homodimeric cross-over structure of the human granulocyte colony-stimulating factor (GCSF) receptor signaling complex. Proc. Natl. Acad. Sci. USA 2006, 103, 3135–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, G.J.; Holloway, D.E.; Veluraja, K.; Acharya, K.R. Atomic resolution (0.98 Å) structure of eosinophil-derived neurotoxin. Biochemistry 2002, 41, 3341–3352. [Google Scholar] [CrossRef] [PubMed]
- Pronker, M.F.; Lemstra, S.; Snijder, J.; Heck, A.J.R.; Thies-Weesie, D.M.E.; Pasterkamp, R.J.; Janssen, B.J.C. Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat. Commun. 2016, 7, 13584. [Google Scholar] [CrossRef] [PubMed]
- Gallivan, J.P.; Dougherty, D.A. Cation-π interactions in structural biology. Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464. [Google Scholar] [CrossRef] [Green Version]
- Pääkkönen, K.; Tossavainen, H.; Permi, P.; Rakkolainen, H.; Rauvala, H.; Raulo, E.; Kilpeläinen, I.; Güntert, P. Solution structures of the first and fourth TSR domains of F-spondin. Proteins 2006, 64, 665–672. [Google Scholar] [CrossRef]
- Hofsteenge, J.; Huwiler, K.G.; Macek, B.; Hess, D.; Lawler, J.; Mosher, D.F.; Peter-Katalinic, J. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J. Biol. Chem. 2001, 276, 6485–6498. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.; Beccati, D.; Leeflang, B.R.; Vliegenthart, J.F.G. C-Mannosylation enhances the structural stability of human RNase 2. iScience 2020, 23, 101371. [Google Scholar] [CrossRef]
- Shcherbakova, A.; Preller, M.; Taft, M.H.; Pujols, J.; Ventura, S.; Tiemann, B.; Buettner, F.F.R.; Bakker, H. C-mannosylation supports folding and enhances stability of thrombospondin repeats. eLife 2019, 8, e52978. [Google Scholar] [CrossRef]
- John, A.; Järvå, M.A.; Shah, S.; Mao, R.; Chappaz, S.; Birkinshaw, R.W.; Czabotar, P.E.; Lo, A.W.; Scott, N.E.; Goddard-Borger, E.D. Yeast- and antibody-based tools for studying tryptophan C-mannosylation. Nat. Chem. Biol. 2021, 17, 428–437. [Google Scholar] [CrossRef]
- Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Total synthesis of aryl C-glycoside natural products: Strategies and tactics. Chem. Rev. 2018, 118, 1495–1598. [Google Scholar] [CrossRef]
- Zhu, F.; Walczak, M.A. Stereochemistry of transition metal complexes controlled by the metallo-anomeric effect. J. Am. Chem. Soc. 2020, 142, 15127–15136. [Google Scholar] [CrossRef]
- Manabe, S.; Ito, Y. Total synthesis of novel subclass of glyco-amino acid structure motif: C2-α-L-C-mannosylpyranosyl-L-tryptophan. J. Am. Chem. Soc. 1999, 121, 9754–9755. [Google Scholar] [CrossRef]
- Fujise, H.; Horiuchi, K.; Adachi, K.; Sano, H.; Suzuki, K. Novel Compound 2-amino-3-[2-(α-mannopyranosyl)indol-3-yl]propionic Acid, Process for Preparing the Same, and Method for Inspecting Function of Living Body with the Novel Compound. Patent WO99/09411, 25 February 1999. [Google Scholar]
- Isobe, M.; Nishizawa, R.; Hosokawa, S.; Nishikawa, T. Stereocontrolled synthesis and reactivity of sugar acetylenes. Chem. Commun. 1998, 2665–2676. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Nishikawa, T.; Koide, Y.; Kanakubo, A.; Yoshimura, H.; Isobe, M. Synthesis of β-analogues of C-mannosyltryptophan, a novel C-glycosylamino acid found in proteins. Org. Biomol. Chem. 2006, 4, 1268–1277. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, Y.; Zhu, W.; An, S.; Zhou, Q.; Zhu, S.-F.; He, G.; Liu, P.; Chen, G. Total synthesis of C-α-mannosyl tryptophan via palladium-catalyzed C–H glycosylation. CCS Chem. 2021, 3, 1729–1736. [Google Scholar] [CrossRef]
- Mao, R.; Xi, S.; Shah, S.; Roy, M.J.; John, A.; Lingford, J.P.; Gäde, G.; Scott, N.E.; Goddard-Borger, E.D. Synthesis of C-mannosylated glycopeptides enabled by Ni-catalyzed photoreductive cross-coupling reactions. J. Am. Chem. Soc. 2021, 143, 12699–12707. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Zanetta, J.P.; Pons, A.; Richet, C.; Huet, G.; Timmerman, P.; Leroy, Y.; Bohin, A.; Bohin, J.P.; Trinel, P.A.; Poulain, D.; et al. Quantitative gas chromatography/mass spectrometry determination of C-mannosylation of tryptophan residues in glycoproteins. Anal. Biochem. 2004, 329, 199–206. [Google Scholar] [CrossRef]
- Manabe, S.; Marui, Y.; Ito, Y. Total synthesis of mannosyl tryptophan and its derivatives. Chem. Eur. J. 2003, 9, 1435–1447. [Google Scholar] [CrossRef]
- Hinou, H.; Abe, Y.; Hayakawa, S.; Naruchi, K.; Fujitani, N.; Nishimura, S. Solid-phase synthesis of C-mannosylated glycopeptide on WSXWS motif of human erythropoietin receptor. Tetrahedron Lett. 2016, 57, 791–795. [Google Scholar] [CrossRef]
- Jonker, H.R.A.; Saxena, K.; Shcherbakova, A.; Tiemann, B.; Bakker, H.; Schwalbe, H. NMR spectroscopic characterization of the C-mannose conformation in a thrombospondin repeat using a selective labeling approach. Angew. Chem. Int. Ed. Engl. 2020, 59, 20659–20665. [Google Scholar] [CrossRef]
- De Beer, T.; Vliegenthart, J.F.; Löffler, A.; Hofsteenge, J. The hexopyranosyl residue that is C-glycosidically linked to the side chain of tryptophan-7 in human RNase Us is α-mannopyranose. Biochemistry 1995, 34, 11785–11789. [Google Scholar] [CrossRef]
- Hofsteenge, J.; Blommers, M.; Hess, D.; Furmanek, A.; Miroshnichenko, O. The four terminal components of the complement system are C-mannosylated on multiple tryptophan residues. J. Biol. Chem. 1999, 274, 32786–32794. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Hofsteenge, J. Properdin, the positive regulator of complement, is highly C-mannosylated. J. Biol. Chem. 2000, 275, 28569–28574. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, F.; Franc, V.; Halim, L.A.; Schellekens, H.; Heck, A.J. Hybrid mass spectrometry approaches in glycoprotein analysis and their usage in scoring biosimilarity. Nat. Commun. 2016, 7, 13397. [Google Scholar] [CrossRef]
- Gonzalez de Peredo, A.; Klein, D.; Macek, B.; Hess, D.; Peter-Katalinic, J.; Hofsteenge, J. C-mannosylation and O-fucosylation of thrombospondin type 1 repeats. Mol. Cell. Proteom. 2002, 1, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Cirksena, K.; Hütte, H.J.; Shcherbakova, A.; Thumberger, T.; Sakson, R.; Weiss, S.; Jensen, L.R.; Friedrich, A.; Todt, D.; Kuss, A.W.; et al. The C-mannosylome of human induced pluripotent stem cells implies a role for ADAMTS16 C-mannosylation in eye development. Mol. Cell. Proteom. 2021, 20, 100092. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, C.; Jia, W.; Yu, L.; Mo, M.; Wang, Q.; Huang, Y.; Lim, J.M.; Ishihara, M.; Wells, L.; et al. Structure of the F-spondin domain of mindin, an integrin ligand and pattern recognition molecule. EMBO J. 2009, 28, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Inai, Y.; Ueda, K.; Matsui, I.L.; Tajiri, M.; Minakata, S.; Wada, Y.; Ihara, Y. Role of C-mannosylation in the secretion of mindin. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129632. [Google Scholar] [CrossRef]
- Wang, L.W.; Leonhard-Melief, C.; Haltiwanger, R.S.; Apte, S.S. Post-translational modification of thrombospondin type-1 repeats in ADAMTS-like 1/punctin-1 by C-mannosylation of tryptophan. J. Biol. Chem. 2009, 284, 30004–30015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buettner, F.F.; Ashikov, A.; Tiemann, B.; Lehle, L.; Bakker, H.C. elegans DPY-19 is a C-mannosyltransferase glycosylating thrombospondin repeats. Mol. Cell 2013, 50, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shcherbakova, A.; Tiemann, B.; Buettner, F.F.; Bakker, H. Distinct C-mannosylation of netrin receptor thrombospondin type 1 repeats by mammalian DPY19L1 and DPY19L3. Proc. Natl. Acad. Sci. USA 2017, 114, 2574–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, J.; Su, J.; Ma, Z.; Ruan, C. The WXXW motif in the TSR1 of ADAMTS13 is important for its secretion and proteolytic activity. Thromb. Res. 2013, 131, 529–534. [Google Scholar] [CrossRef]
- Sorvillo, N.; Kaijen, P.H.; Matsumoto, M.; Fujimura, Y.; van der Zwaan, C.; Verbij, F.C.; Pos, W.; Fijnheer, R.; Voorberg, J.; Meijer, A.B. Identification of N-linked glycosylation and putative O-fucosylation, C-mannosylation sites in plasma derived ADAMTS13. J. Thromb. Haemost. 2014, 12, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Verbij, F.C.; Stokhuijzen, E.; Kaijen, P.H.P.; van Alphen, F.; Meijer, A.B.; Voorberg, J. Identification of glycans on plasma-derived ADAMTS13. Blood 2016, 128, e51–e58. [Google Scholar] [CrossRef]
- Hoppe, C.M.; Albuquerque-Wendt, A.; Bandini, G.; Leon, D.R.; Shcherbakova, A.; Buettner, F.F.R.; Izquierdo, L.; Costello, C.E.; Bakker, H.; Routier, F.H. Apicomplexan C-mannosyltransferases modify thrombospondin type I-containing adhesins of the TRAP family. Glycobiology 2018, 28, 333–343. [Google Scholar] [CrossRef]
- Albuquerque-Wendt, A.; Jacot, D.; Dos Santos Pacheco, N.; Seegers, C.; Zarnovican, P.; Buettner, F.F.R.; Bakker, H.; Soldati-Favre, D.; Routier, F.H. C-Mannosylation of Toxoplasma gondii proteins promotes attachment to host cells and parasite virulence. J. Biol. Chem. 2020, 295, 1066–1076. [Google Scholar] [CrossRef]
- Swearingen, K.E.; Lindner, S.E.; Shi, L.; Shears, M.J.; Harupa, A.; Hopp, C.S.; Vaughan, A.M.; Springer, T.A.; Moritz, R.L.; Kappe, S.H.; et al. Interrogating the Plasmodium sporozoite surface: Identification of surface-exposed proteins and demonstration of glycosylation on CSP and TRAP by mass spectrometry-based proteomics. PLoS Pathog. 2016, 12, e1005606. [Google Scholar] [CrossRef]
- Niwa, Y.; Suzuki, T.; Dohmae, N.; Simizu, S. Identification of DPY19L3 as the C-mannosyltransferase of R-spondin1 in human cells. Mol. Biol. Cell 2016, 27, 744–756. [Google Scholar] [CrossRef]
- Fujiwara, M.; Kato, S.; Niwa, Y.; Suzuki, T.; Tsuchiya, M.; Sasazawa, Y.; Dohmae, N.; Simizu, S. C-mannosylation of R-spondin3 regulates its secretion and activity of Wnt/β-catenin signaling in cells. FEBS Lett. 2016, 590, 2639–2649. [Google Scholar] [CrossRef] [Green Version]
- Morishita, S.; Suzuki, T.; Niwa, Y.; Dohmae, N.; Simizu, S. Dpy-19 like 3-mediated C-mannosylation and expression levels of RPE-spondin in human tumor cell lines. Oncol. Lett. 2017, 14, 2537–2544. [Google Scholar] [CrossRef]
- Mizuta, H.; Kuga, K.; Suzuki, T.; Niwa, Y.; Dohmae, N.; Simizu, S. C-mannosylation of R-spondin2 activates Wnt/β-catenin signaling and migration activity in human tumor cells. Int. J. Oncol. 2019, 54, 2127–2138. [Google Scholar] [CrossRef]
- Zhang, A.; Berardinelli, S.J.; Leonhard-Melief, C.; Vasudevan, D.; Liu, T.W.; Taibi, A.; Giannone, S.; Apte, S.S.; Holdener, B.C.; Haltiwanger, R.S. O-Fucosylation of ADAMTSL2 is required for secretion and is impacted by geleophysic dysplasia-causing mutations. J. Biol. Chem. 2020, 295, 15742–15753. [Google Scholar] [CrossRef]
- Miura, K.; Suzuki, T.; Sun, H.; Takada, H.; Ishizawa, Y.; Mizuta, H.; Dohmae, N.; Simizu, S. Requirement for C-mannosylation to be secreted and activated a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS4). Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129833. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Katayama, K.; Suzuki, T.; Dohmae, N.; Simizu, S. Regulation of N-glycosylation and secretion of Isthmin-1 by its C-mannosylation. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129840. [Google Scholar] [CrossRef]
- Neupane, S.; Goto, J.; Berardinelli, S.J.; Ito, A.; Haltiwanger, R.S.; Holdener, B.C. Hydrocephalus in mouse B3glct mutants is likely caused by defects in multiple B3GLCT substrates in ependymal cells and subcommissural organ. Glycobiology 2021. [Google Scholar] [CrossRef]
- Furmanek, A.; Hess, D.; Rogniaux, H.; Hofsteenge, J. The WSAWS motif is C-hexosylated in a soluble form of the erythropoietin receptor. Biochemistry 2003, 42, 8452–8458. [Google Scholar] [CrossRef]
- Siupka, P.; Hamming, O.T.; Kang, L.; Gad, H.H.; Hartmann, R. A conserved sugar bridge connected to the WSXWS motif has an important role for transport of IL-21R to the plasma membrane. Genes Immun. 2015, 16, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Sasazawa, Y.; Sato, N.; Suzuki, T.; Dohmae, N.; Simizu, S. C-Mannosylation of thrombopoietin receptor (C-Mpl) regulates thrombopoietin-dependent JAK-STAT signaling. Biochem. Biophys. Res. Commun. 2015, 468, 262–268. [Google Scholar] [CrossRef]
- Otani, K.; Niwa, Y.; Suzuki, T.; Sato, N.; Sasazawa, Y.; Dohmae, N.; Simizu, S. Regulation of granulocyte colony-stimulating factor receptor-mediated granulocytic differentiation by C-mannosylation. Biochem. Biophys. Res. Commun. 2018, 498, 466–472. [Google Scholar] [CrossRef]
- Krieg, J.; Gläsner, W.; Vicentini, A.; Doucey, M.A.; Löffler, A.; Hess, D.; Hofsteenge, J. C-Mannosylation of human RNase 2 is an intracellular process performed by a variety of cultured cells. J. Biol. Chem. 1997, 272, 26687–26692. [Google Scholar] [CrossRef] [Green Version]
- Doucey, M.A.; Hess, D.; Cacan, R.; Hofsteenge, J. Protein C-mannosylation is enzyme-catalysed and uses dolichyl-phosphate-mannose as a precursor. Mol. Biol. Cell 1998, 9, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Krieg, J.; Hartmann, S.; Vicentini, A.; Gläsner, W.; Hess, D.; Hofsteenge, J. Recognition signal for C-mannosylation of Trp-7 in RNase 2 consists of sequence Trp-x-x-Trp. Mol. Biol. Cell 1998, 9, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Doucey, M.A.; Hess, D.; Blommers, M.J.; Hofsteenge, J. Recombinant human interleukin-12 is the second example of a C-mannosylated protein. Glycobiology 1999, 9, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Perez-Vilar, J.; Randell, S.H.; Boucher, R.C. C-Mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology 2004, 14, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Gouyer, V.; Demouveaux, B.; Lacroix, G.; Valque, H.; Gottrand, F.; Desseyn, J.L. Non-C-mannosylable mucin CYS domains hindered proper folding and secretion of mucin. Biochem. Biophys. Res. Commun. 2018, 506, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Ervin, L.A.; Ball, L.E.; Crouch, R.K.; Schey, K.L. Phosphorylation and glycosylation of bovine lens MP20. Investig. Ophthalmol. Vis. Sci. 2005, 46, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Patwardhan, A.J.; Strittmatter, E.F.; Camp, D.G.; Smith, R.D.; Pallavicini, M.G. Comparison of normal and breast cancer cell lines using proteome, genome, and interactome data. J. Proteome Res. 2005, 4, 1952–1960. [Google Scholar] [CrossRef]
- Falzarano, D.; Krokhin, O.; Van Domselaar, G.; Wolf, K.; Seebach, J.; Schnittler, H.J.; Feldmann, H. Ebola sGP-the first viral glycoprotein shown to be C-mannosylated. Virology 2007, 368, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Munte, C.E.; Gäde, G.; Domogalla, B.; Kremer, W.; Kellner, R.; Kalbitzer, H.R. C-mannosylation in the hypertrehalosaemic hormone from the stick insect Carausius morosus. FEBS J. 2008, 275, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sagert, J.; Hwang, D.S.; Waite, J.H. Glycosylated hydroxytryptophan in a mussel adhesive protein from Perna viridis. J. Biol. Chem. 2009, 284, 23344–23352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, Y.; Niwa, Y.; Suzuki, T.; Dohmae, N.; Umezawa, K.; Simizu, S. C-mannosylation of human hyaluronidase 1: Possible roles for secretion and enzymatic activity. Int. J. Oncol. 2014, 45, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, S.; Murano, T.; Suzuki, T.; Uematsu, S.; Niwa, Y.; Sasazawa, Y.; Dohmae, N.; Bujo, H.; Simizu, S. Regulation of secretion and enzymatic activity of lipoprotein lipase by C-mannosylation. Biochem. Biophys. Res. Commun. 2017, 486, 558–563. [Google Scholar] [CrossRef]
- Osada, Y.; Suzuki, T.; Mizuta, H.; Mori, K.; Miura, K.; Dohmae, N.; Simizu, S. The fibrinogen C-terminal domain is seldom C-mannosylated but its C-mannosylation is important for the secretion of microfibril-associated glycoprotein 4. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129637. [Google Scholar] [CrossRef]
- Diem, S.; Bergmann, J.; Herderich, M. Tryptophan-N-glucoside in Fruits and Fruit Juices. J. Agric. Food Chem. 2000, 48, 4913–4917. [Google Scholar] [CrossRef]
- Li, J.S.; Cui, L.; Rock, D.L.; Li, J. Novel glycosidic linkage in Aedes aegypti chorion peroxidase: N-mannosyl tryptophan. J. Biol. Chem. 2005, 280, 38513–38521. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, S.; Inai, Y.; Minakata, S.; Manabe, S.; Ito, Y.; Ihara, Y. A novel assay for detection and quantification of C-mannosyl tryptophan in normal or diabetic mice. Sci. Rep. 2019, 9, 4675. [Google Scholar] [CrossRef]
- Minakata, S.; Inai, Y.; Manabe, S.; Nishitsuji, K.; Ito, Y.; Ihara, Y. Monomeric C-mannosyl tryptophan is a degradation product of autophagy in cultured cells. Glycoconj. J. 2020, 37, 635–645. [Google Scholar] [CrossRef]
- Garcia, A.; Lenis, L.A.; Jiménez, C.; Debitus, C.; Quiñoá, E.; Riguera, R. The occurrence of the human glycoconjugate C2-α-D-mannosylpyranosyl-L-tryptophan in marine ascidians. Org. Lett. 2000, 2, 2765–2767. [Google Scholar] [CrossRef]
- Gabant, M.; Martin, M.T.; Moriou, C.; Ermolenko, L.; Guerineau, V.; Retailleau, P.; Thoison, O.; Boury-Esnault, N.; Pérez, T.; Al-Mourabit, A. Axiphenylalaninium and axityrosinium, modified amino acids from the Mediterranean marine sponge Axinella polypoides. J. Nat. Prod. 2009, 72, 1875–1878. [Google Scholar] [CrossRef]
- Hossain, T.J.; Manabe, S.; Ito, Y.; Iida, T.; Kosono, S.; Ueda, K.; Hosomi, A.; Inoue, D.; Suzuki, T. Enrichment and characterization of a bacterial mixture capable of utilizing C-mannosyl tryptophan as a carbon source. Glycoconj. J. 2018, 35, 165–176. [Google Scholar] [CrossRef]
- Julenius, K. NetCGlyc 1.0: Prediction of mammalian C-mannosylation sites. Glycobiology 2007, 17, 868–876. [Google Scholar] [CrossRef]
- Niwa, Y.; Nakano, Y.; Suzuki, T.; Yamagishi, M.; Otani, K.; Dohmae, N.; Simizu, S. Topological analysis of DPY19L3, a human C-mannosyltransferase. FEBS J. 2018, 285, 1162–1174. [Google Scholar] [CrossRef]
- Albuquerque-Wendt, A.; Hütte, H.J.; Buettner, F.F.R.; Routier, F.H.; Bakker, H. Membrane topological model of glycosyltransferases of the GT-C superfamily. Int. J. Mol. Sci. 2019, 20, 4842. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.B.; Evans, P.J.; Hemming, F.W. Dolichol phosphates as acceptors of mannose from guanosine diphosphate mannose in liver systems. Biochem. J. 1971, 124, 957–959. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.B.; Hemming, F.W. The transfer of mannose from guanosine diphosphate mannose to dolichol phosphate and protein by pig liver endoplasmic reticulum. Biochem. J. 1972, 130, 77–93. [Google Scholar] [CrossRef] [Green Version]
- Waechter, C.J.; Lucas, J.J.; Lennarz, W.J. Membrane glycoproteins. I. Enzymatic synthesis of mannosyl phosphoryl polyisoprenol and its role as a mannosyl donor in glycoprotein synthesis. J. Biol. Chem. 1973, 248, 7570–7579. [Google Scholar] [CrossRef]
- Nakajima, K.; Kizuka, Y.; Yamaguchi, Y.; Hirabayashi, Y.; Takahashi, K.; Yuzawa, Y.; Taniguchi, N. Identification and characterization of UDP-mannose in human cell lines and mouse organs: Differential distribution across brain regions and organs. Biochem. Biophys. Res. Commun. 2018, 495, 401–407. [Google Scholar] [CrossRef]
- Guo, N.H.; Krutzsch, H.C.; Vogel, T.; Roberts, D.D. Interactions of a laminin-binding peptide from a 33-kDa protein related to the 67-kDa laminin receptor with laminin and melanoma cells are heparin-dependent. J. Biol. Chem. 1992, 267, 17743–17747. [Google Scholar] [CrossRef]
- Guo, N.H.; Krutzsch, H.C.; Nègre, E.; Vogel, T.; Blake, D.A.; Roberts, D.D. Heparin- and sulfatide-binding peptides from the type I repeats of human thrombospondin promote melanoma cell adhesion. Proc. Natl. Acad. Sci. USA 1992, 89, 3040–3044. [Google Scholar] [CrossRef] [Green Version]
- Schultz-Cherry, S.; Chen, H.; Mosher, D.F.; Misenheimer, T.M.; Krutzsch, H.C.; Roberts, D.D.; Murphy-Ullrich, J.E. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 1995, 270, 7304–7310. [Google Scholar] [CrossRef] [Green Version]
- Young, G.D.; Murphy-Ullrich, J.E. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-β complex. J. Biol. Chem. 2004, 279, 47633–47642. [Google Scholar] [CrossRef] [Green Version]
- Iruela-Arispe, M.L.; Lombardo, M.; Krutzsch, H.C.; Lawler, J.; Roberts, D.D. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation 1999, 100, 1423–1431. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.E.; Li, Z.; Kara, M.; Gardner, K.L.; Roberts, D.D. β1 integrin- and proteoglycan-mediated stimulation of T lymphoma cell adhesion and mitogen-activated protein kinase signaling by thrombospondin-1 and thrombospondin-1 peptides. J. Immunol. 1999, 163, 3621–3628. [Google Scholar]
- Bamdad, M.; Volle, D.; Dastugue, B.; Meiniel, A. α1β1-integrin is an essential signal for neurite outgrowth induced by thrombospondin type 1 repeats of SCO-spondin. Cell Tissue Res. 2004, 315, 15–25. [Google Scholar] [CrossRef]
- Bruel, A.; Touhami-Carrier, M.; Thomaidis, A.; Legrand, C. Thrombospondin-1 (TSP-1) and TSP-1-derived heparin-binding peptides induce promyelocytic leukemia cell differentiation and apoptosis. Anticancer Res. 2005, 25, 757–764. [Google Scholar]
- Yoshimura, A.; Zimmers, T.; Neumann, D.; Longmore, G.; Yoshimura, Y.; Lodish, H.F. Mutations in the Trp-Ser-X-Trp-Ser motif of the erythropoietin receptor abolish processing, ligand binding, and activation of the receptor. J. Biol. Chem. 1992, 267, 11619–11625. [Google Scholar] [CrossRef]
- Hilton, D.J.; Watowich, S.S.; Katz, L.; Lodish, H.F. Saturation mutagenesis of the WSXWS motif of the erythropoietin receptor. J. Biol. Chem. 1996, 271, 4699–4708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandini, G.; Albuquerque-Wendt, A.; Hegermann, J.; Samuelson, J.; Routier, F.H. Protein O- and C-Glycosylation pathways in Toxoplasma gondii and Plasmodium falciparum. Parasitology 2019, 146, 1755–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gas-Pascual, E.; Ichikawa, H.T.; Sheikh, M.O.; Serji, M.I.; Deng, B.; Mandalasi, M.; Bandini, G.; Samuelson, J.; Wells, L.; West, C.M. CRISPR/Cas9 and glycomics tools for Toxoplasma glycobiology. J. Biol. Chem. 2019, 294, 1104–1125. [Google Scholar] [CrossRef] [Green Version]
- López-Gutiérrez, B.; Cova, M.; Izquierdo, L. A Plasmodium falciparum C-mannosyltransferase is dispensable for parasite asexual blood stage development. Parasitology 2019, 146, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Honigberg, L.; Kenyon, C. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development 2000, 127, 4655–4668. [Google Scholar] [CrossRef]
- International Mouse Phenotyping Consortium. Available online: http://mousephenotype.org (accessed on 30 August 2021).
- Watanabe, K.; Takebayashi, H.; Bepari, A.K.; Esumi, S.; Yanagawa, Y.; Tamamaki, N. Dpy19l1, a multi-transmembrane protein, regulates the radial migration of glutamatergic neurons in the developing cerebral cortex. Development 2011, 138, 4979–4990. [Google Scholar] [CrossRef]
- Koscinski, I.; Elinati, E.; Fossard, C.; Redin, C.; Muller, J.; Velez de la Calle, J.; Schmitt, F.; Ben Khelifa, M.; Ray, P.F.; Kilani, Z.; et al. DPY19L2 deletion as a major cause of globozoospermia. Am. J. Hum. Genet. 2011, 88, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Haeuptle, M.A.; Hennet, T. Congenital disorders of glycosylation: An update on defects affecting the biosynthesis of dolichol-linked oligosaccharides. Hum. Mutat. 2009, 30, 1628–1641. [Google Scholar] [CrossRef] [Green Version]
- Buczkowska, A.; Swiezewska, E.; Lefeber, D.J. Genetic defects in dolichol metabolism. J. Inherit. Metab. Dis. 2015, 38, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Lefeber, D.J.; Schönberger, J.; Morava, E.; Guillard, M.; Huyben, K.M.; Verrijp, K.; Grafakou, O.; Evangeliou, A.; Preijers, F.W.; Manta, P.; et al. Deficiency of Dol-P-Man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am. J. Hum. Genet. 2009, 85, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Hendee, K.; Wang, L.W.; Reis, L.M.; Rice, G.M.; Apte, S.S.; Semina, E.V. Identification and functional analysis of an ADAMTSL1 variant associated with a complex phenotype including congenital glaucoma, craniofacial, and other systemic features in a three-generation human pedigree. Hum. Mutat. 2017, 38, 1485–1490. [Google Scholar] [CrossRef]
- Fernandez, I.Z.; Baxter, R.M.; Garcia-Perez, J.E.; Vendrame, E.; Ranganath, T.; Kong, D.S.; Lundquist, K.; Nguyen, T.; Ogolla, S.; Black, J.; et al. A novel human IL2RB mutation results in T and NK cell-driven immune dysregulation. J. Exp. Med. 2019, 216, 1255–1267. [Google Scholar] [CrossRef] [Green Version]
- Ihara, Y.; Manabe, S.; Kanda, M.; Kawano, H.; Nakayama, T.; Sekine, I.; Kondo, T.; Ito, Y. Increased expression of protein C-mannosylation in the aortic vessels of diabetic Zucker rats. Glycobiology 2005, 15, 383–392. [Google Scholar] [CrossRef]
- Stenina, O.I.; Krukovets, I.; Wang, K.; Zhou, Z.; Forudi, F.; Penn, M.S.; Topol, E.J.; Plow, E.F. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation 2003, 107, 3209–3215. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Ishikawa, M.; Isobe, M. Synthesis of a α-C-mannosyltryptophan derivative, naturally occurring C-glycosyl amino acid found in human ribonuclease. Synlett 1999, 1999, 123–125. [Google Scholar] [CrossRef]
- Muroi, E.; Manabe, S.; Ikezaki, M.; Urata, Y.; Sato, S.; Kondo, T.; Ito, Y.; Ihara, Y. C-Mannosylated peptides derived from the thrombospondin type 1 repeat enhance lipopolysaccharide-induced signaling in macrophage-like RAW264.7 cells. Glycobiology 2007, 17, 1015–1028. [Google Scholar] [CrossRef] [Green Version]
- Ihara, Y.; Manabe, S.; Ikezaki, M.; Inai, Y.; Matsui, I.S.; Ohta, Y.; Muroi, E.; Ito, Y. C-Mannosylated peptides derived from the thrombospondin type 1 repeat interact with Hsc70 to modulate its signaling in RAW264.7 cells. Glycobiology 2010, 20, 1298–1310. [Google Scholar] [CrossRef] [Green Version]
- Takahira, R.; Yonemura, K.; Yonekawa, O.; Iwahara, K.; Kanno, T.; Fujise, Y.; Hishida, A. Tryptophan glycoconjugate as a novel marker of renal function. Am. J. Med. 2001, 110, 192–197. [Google Scholar] [CrossRef]
- Yonemura, K.; Takahira, R.; Yonekawa, O.; Wada, N.; Hishida, A. The diagnostic value of serum concentrations of 2-(α-mannopyranosyl)-L-tryptophan for normal renal function. Kidney Int. 2004, 65, 1395–1399. [Google Scholar] [CrossRef] [Green Version]
- Niewczas, M.A.; Sirich, T.L.; Mathew, A.V.; Skupien, J.; Mohney, R.P.; Warram, J.H.; Smiles, A.; Huang, X.; Walker, W.; Byun, J.; et al. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: Metabolomic study. Kidney Int. 2014, 85, 1214–1224. [Google Scholar] [CrossRef] [Green Version]
- Sekula, P.; Goek, O.N.; Quaye, L.; Barrios, C.; Levey, A.S.; Romisch-Margl, W.; Menni, C.; Yet, I.; Gieger, C.; Inker, L.A.; et al. A metabolome-wide association study of kidney function and disease in the general population. J. Am. Soc. Nephrol. 2016, 27, 1175–1188. [Google Scholar] [CrossRef]
- Solini, A.; Manca, M.L.; Penno, G.; Pugliese, G.; Cobb, J.E.; Ferrannini, E. Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. J. Clin. Endocrinol. Metab. 2016, 101, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Sekula, P.; Dettmer, K.; Vogl, F.C.; Gronwald, W.; Ellmann, L.; Mohney, R.P.; Eckardt, K.U.; Suhre, K.; Kastenmuller, G.; Oefner, P.J.; et al. From discovery to translation: Characterization of C-mannosyltryptophan and pseudouridine as markers of kidney function. Sci. Rep. 2017, 7, 17400. [Google Scholar] [CrossRef] [PubMed]
- Niewczas, M.A.; Mathew, A.V.; Croall, S.; Byun, J.; Major, M.; Sabisetti, V.S.; Smiles, A.; Bonventre, J.V.; Pennathur, S.; Krolewski, A.S. Circulating modified metabolites and a risk of ESRD in patients with type 1 diabetes and chronic kidney disease. Diabetes Care 2017, 40, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbrenner, I.; Schultheiss, U.T.; Kotsis, F.; Schlosser, P.; Stockmann, H.; Mohney, R.P.; Schmid, M.; Oefner, P.J.; Eckardt, K.U.; Köttgen, A.; et al. Urine metabolite levels, adverse kidney outcomes, and mortality in CKD patients: A metabolome-wide association study. Am. J. Kidney Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Inai, Y.; Minakata, S.; Kishimoto, S.; Manabe, S.; Iwahashi, N.; Ino, K.; Ito, Y.; Akamizu, T.; Ihara, Y. Quantification of serum C-mannosyl tryptophan by novel assay to evaluate renal function and vascular complications in patients with type 2 diabetes. Sci. Rep. 2021, 11, 1946. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Moore, S.C.; Derkach, A.; Hua, X.; Liao, L.M.; Gu, F.; Mondul, A.M.; Sampson, J.N.; Albanes, D. Serum metabolomic profiling of all-cause mortality: A prospective analysis in the alpha-tocopherol, beta-carotene cancer prevention (ATBC) study cohort. Am. J. Epidemiol. 2018, 187, 1721–1732. [Google Scholar] [CrossRef] [Green Version]
- Iwahashi, N.; Inai, Y.; Minakata, S.; Sakurai, S.; Manabe, S.; Ito, Y.; Ino, K.; Ihara, Y. C-Mannosyl tryptophan increases in the plasma of patients with ovarian cancer. Oncol. Lett. 2020, 19, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and ovarian cancer: A comprehensive review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Menni, C.; Kastenmüller, G.; Petersen, A.K.; Bell, J.T.; Psatha, M.; Tsai, P.C.; Gieger, C.; Schulz, H.; Erte, I.; John, S.; et al. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int. J. Epidemiol. 2013, 42, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Livshits, G.; Malkin, I.; Bowyer, R.C.E.; Verdi, S.; Bell, J.T.; Menni, C.; Williams, F.M.K.; Steves, C.J. Multi-OMICS analyses of frailty and chronic widespread musculoskeletal pain suggest involvement of shared neurological pathways. Pain 2018, 159, 2565–2572. [Google Scholar] [CrossRef] [Green Version]
- Lustgarten, M.S.; Fielding, R.A. Metabolites associated with circulating interleukin-6 in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Pinto-Sanchez, M.I.; Bercik, P.; Britz-McKibbin, P. Metabolomics reveals elevated urinary excretion of collagen degradation and epithelial cell turnover products in irritable bowel syndrome patients. Metabolomics 2019, 15, 82. [Google Scholar] [CrossRef]
- Sovio, U.; McBride, N.; Wood, A.M.; Masconi, K.L.; Cook, E.; Gaccioli, F.; Charnock-Jones, D.S.; Lawlor, D.A.; Smith, G.C.S. 4-Hydroxyglutamate is a novel predictor of pre-eclampsia. Int. J. Epidemiol. 2020, 49, 301–311. [Google Scholar] [CrossRef]

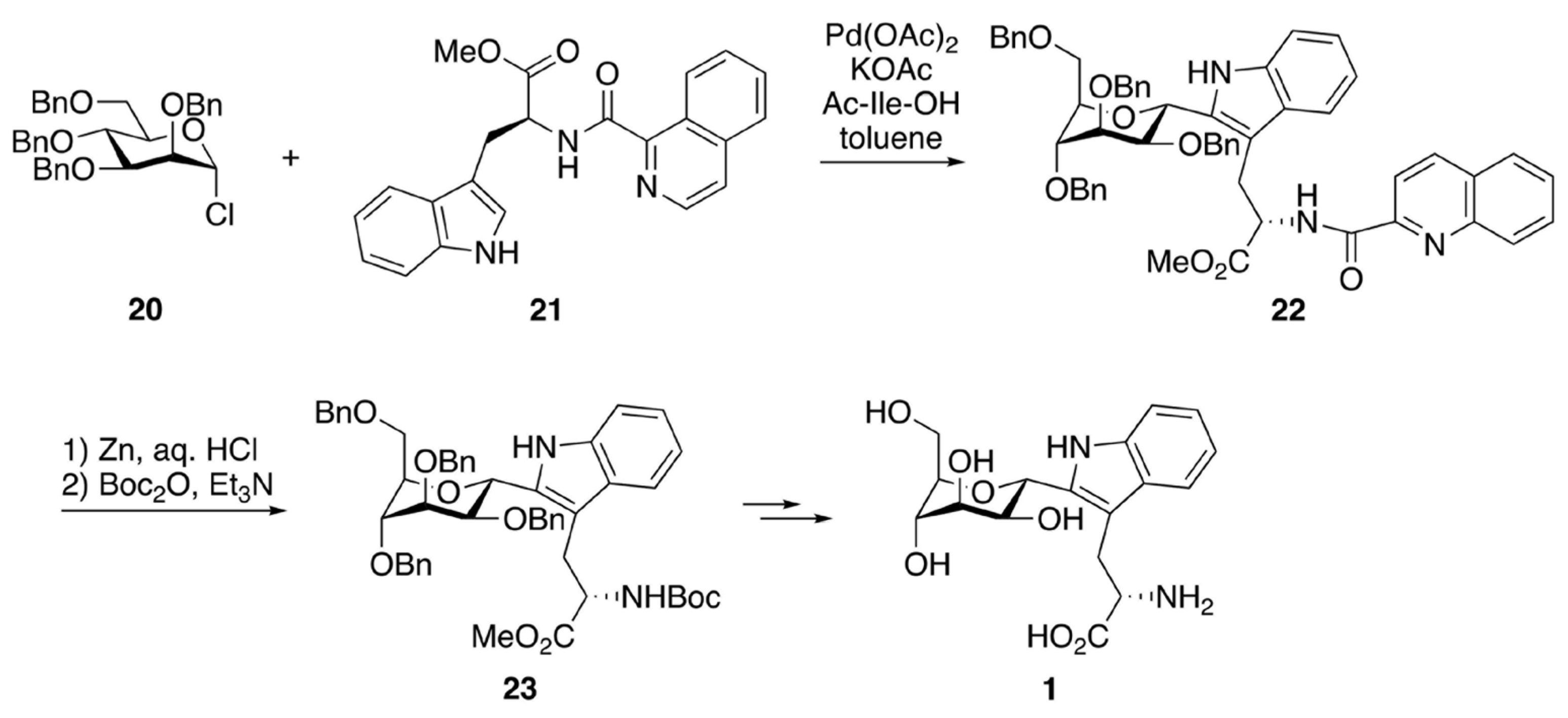
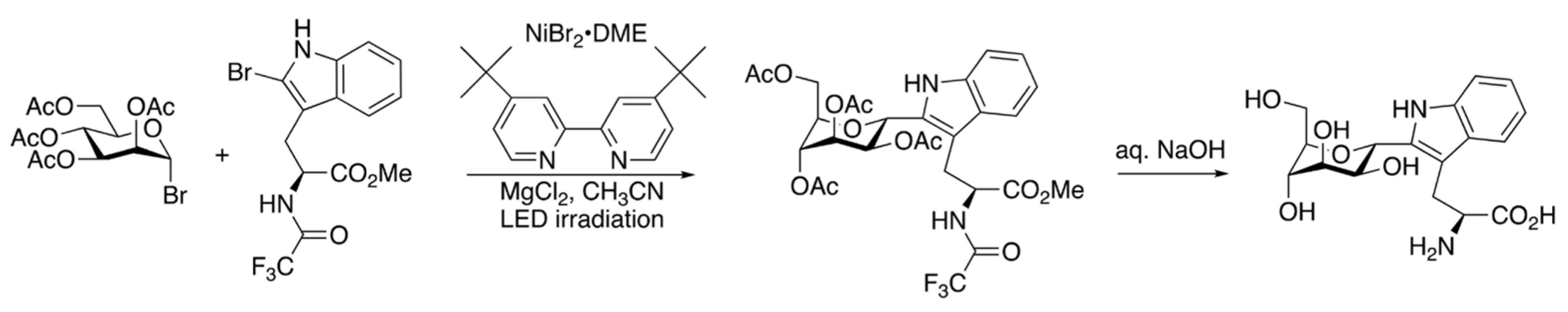
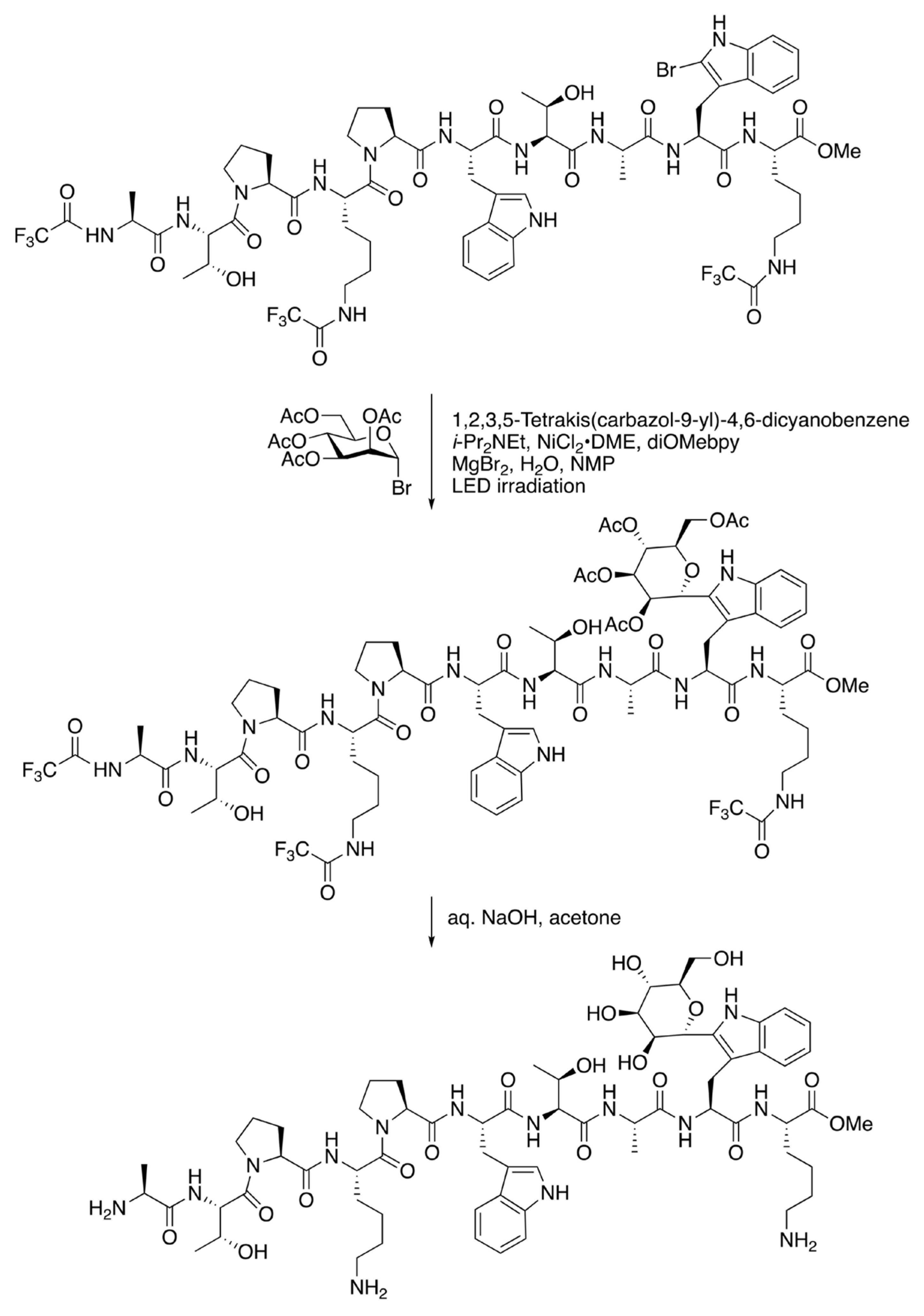
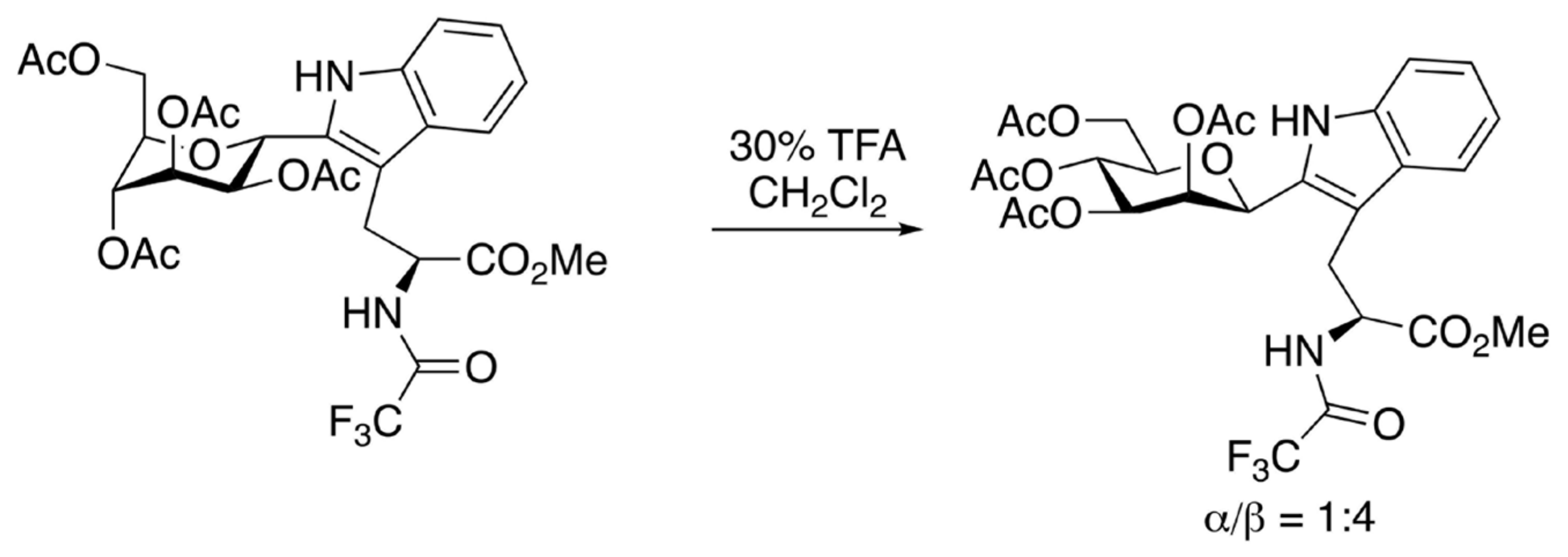
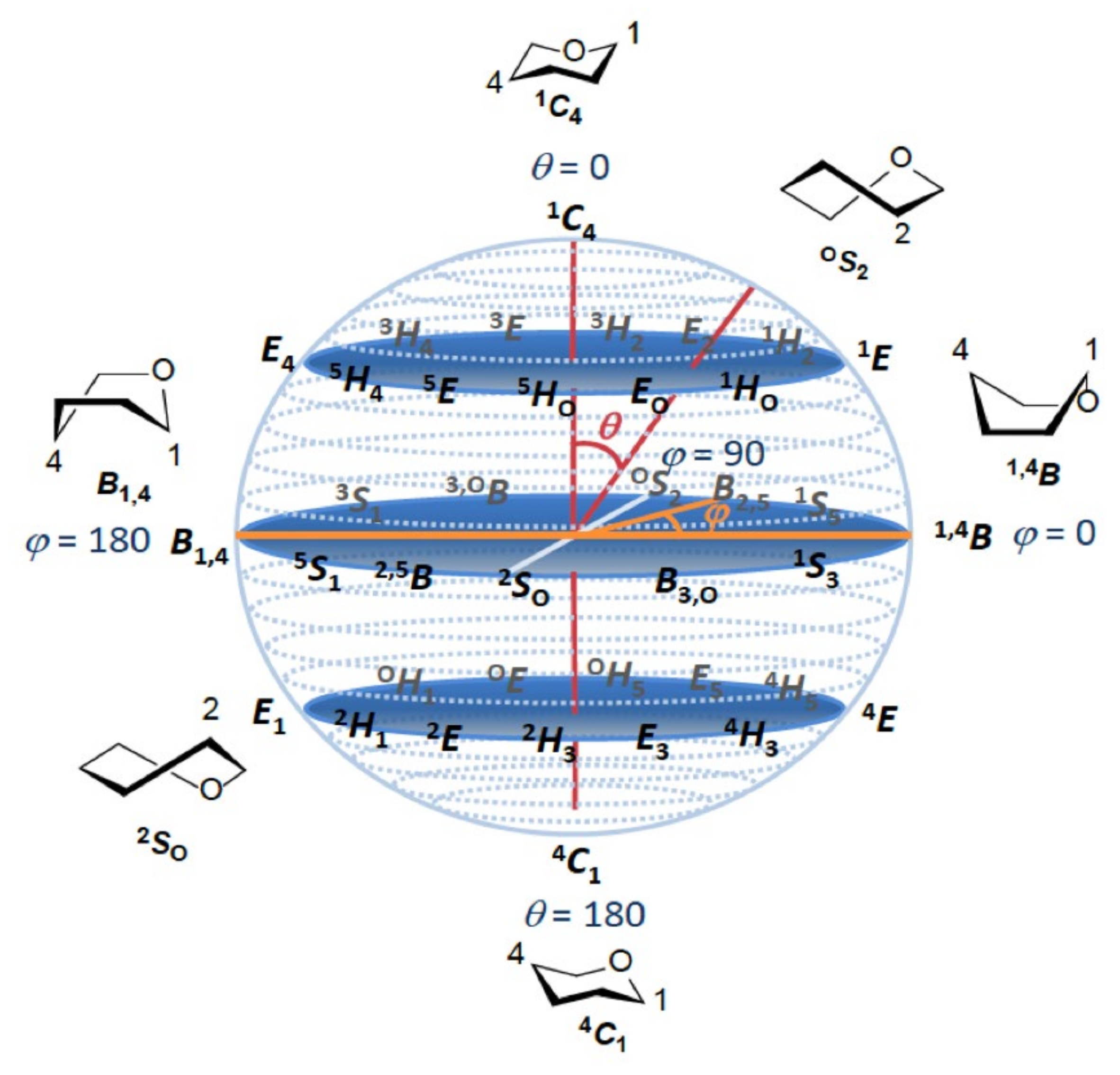
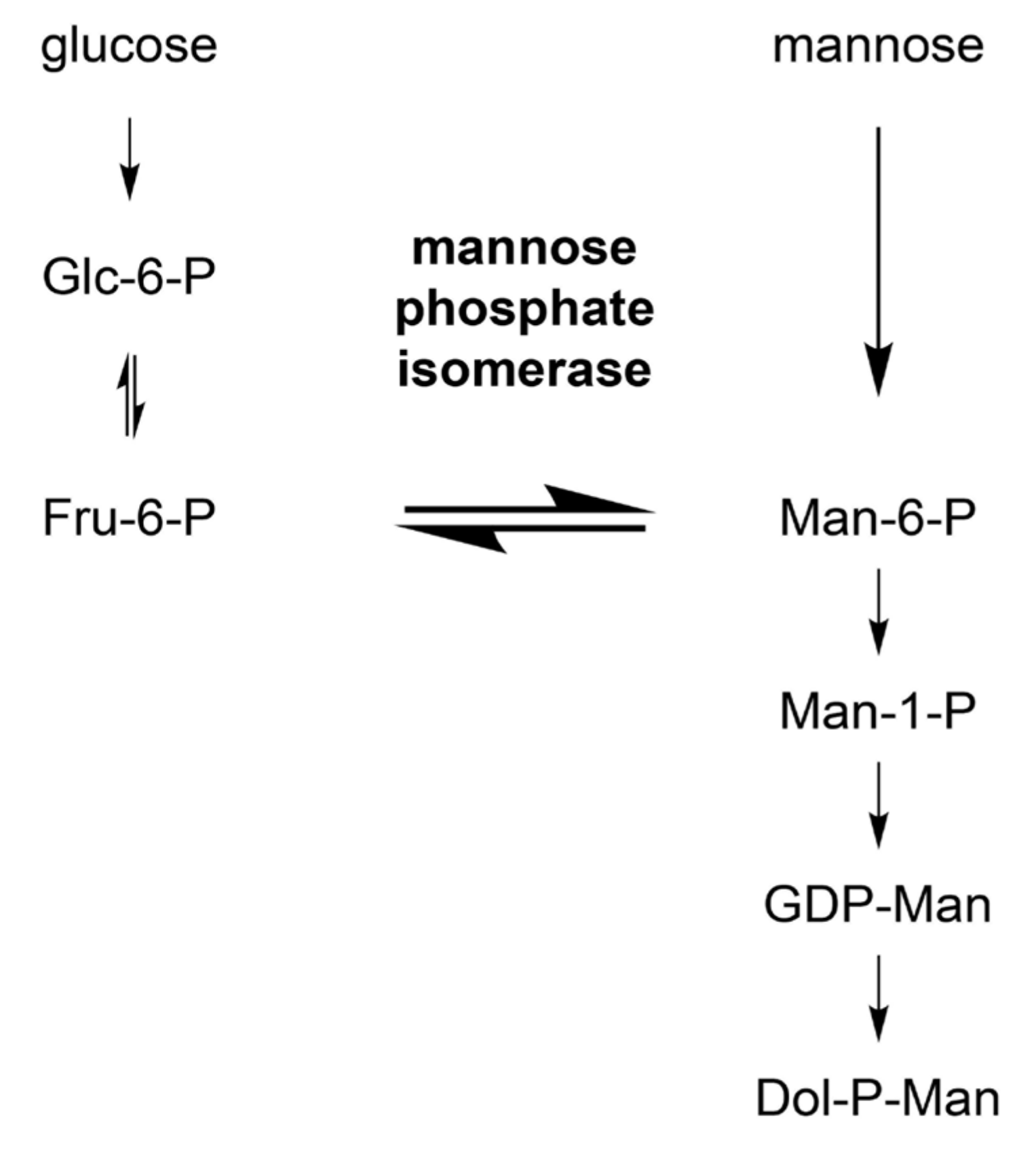
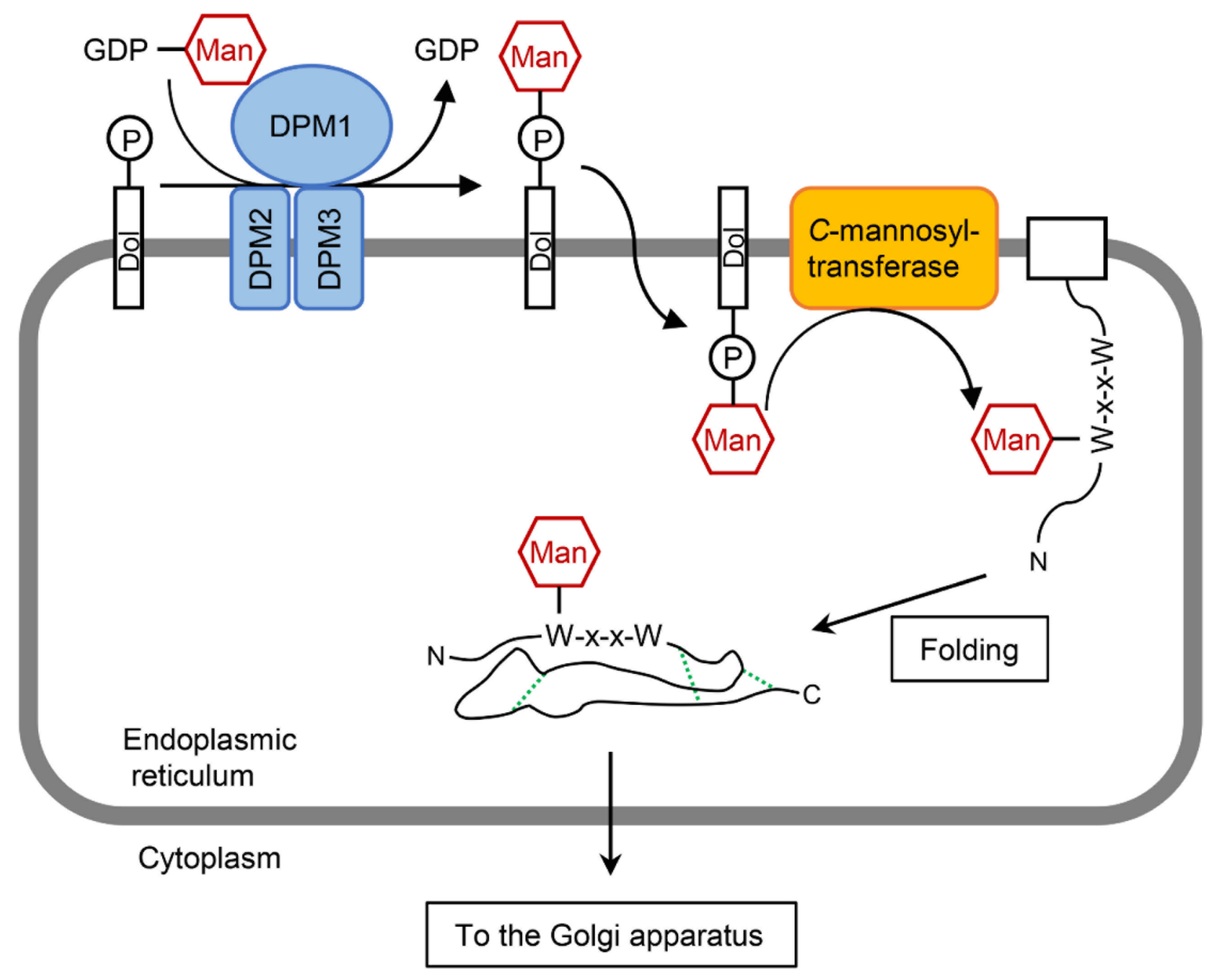
| Family | Proteins | Species | Primary Source/Recombinant Protein | Functions Affected by C-Mannosylation | References | UniProt Accession |
|---|---|---|---|---|---|---|
| TSR superfamily | Complements, C6 | Human | Plasma, serum | N/A | [10,11,12,13,14,46] | P13671 |
| C7 | Human | N/A | P10643 | |||
| C8 | Human | Plasma | P07357, P07358 | |||
| C9 | Human | Homologous overexpression | P02748 | |||
| Mouse | Heterologous overexpression | P06683 | ||||
| Properdin | Human | Plasma/homologous overexpression | N/A | [17,18,30,47,48] | P27918 | |
| Mouse | Brain | P11680 | ||||
| F-spondin | Rat | Heterologous overexpression | N/A | [1,30,49] | P35446 | |
| Mouse | Brain | Q8VCC9 | ||||
| Thrombospondin-1 (TSP-1) | Human | Platelet/homologous overexpression | Secretion | [27,50] | P07996 | |
| Mindin | Human | Heterologous overexpression | Molecular interaction, molecular interaction (-), redox-dependent folding, secretion | [30,51,52] | Q9BUD6 | |
| Mouse | Heterologous overexpression | Q8BMS2 | ||||
| ADAMTSL1 | Human | Heterologous overexpression | Secretion | [53] | Q8N6G6 | |
| ADAMTS5 | Human | Heterologous overexpression | N/A | [53] | Q9UNA0 | |
| UNC-5 | C.elegans | Heterologous overexpression | Secretion, folding, thermal stability | [29,30,54,55] | Q26261 | |
| Mouse | Heterologous overexpression | Q8K1S4 | ||||
| MIG-21 | C. elegans | Heterologous overexpression | Secretion | [54] | G1FC92 | |
| ADAMTS13 | Human | Plasma/homologous overexpression | Secretion | [56,57,58] | Q76LX8 | |
| MIC2 | T. gondii | RHΔku80Δhxgprt/heterologous overexpression | Secretion | [16,59,60] | S8F9G8 | |
| TRAP | P. falciparum | Sporozoites/heterologous overexpression | N/A | [59,61] | P16893 | |
| R-spondin1 | Human | Homologous or heterologous overexpression | Secretion, signal transduction | [30,62] | Q2MKA7 | |
| R-spondin3 | Human | Homologous overexpression | Secretion, signal transduction | [63] | Q9BXY4 | |
| RPE-spondin | Human | Homologous or heterologous overexpression | N/A | [30,64] | Q8IVN8 | |
| Mouse | Brain | Q3UPR9 | ||||
| R-spondin2 | Human | Homologous overexpression | Secretion (dependent on the type of cell lines), cell migration | [65] | Q6UXX9 | |
| ADAMTSL2 | Mouse | Heterologous overexpression | N/A | [66] | Q7TSK7 | |
| ADAMTS4 | Human | Homologous overexpression | Secretion, enzyme activity | [67] | O75173 | |
| Isthmin-1 | Human | Homologous overexpression | Secretion, N-glycosylation | [68] | B1AKI9 | |
| ADAMTS20 | Mouse | Brain | N/A | [30] | P59511 | |
| Thrombospondin type-1 domain- containing protein 7A (Thsd7a) | Mouse | Brain | N/A | [30] | Q69ZU6 | |
| Thrombospondin type-1 domain- containing protein 7B (Thsd7b) | Mouse | Brain | N/A | [30] | Q6P4U0 | |
| Adhesion G protein-coupled receptor B1 (Adgrb1) | Mouse | Brain | N/A | [30] | Q3UHD1 | |
| Hemicentin-1 (Hmcn1) | Mouse | Brain | N/A | [30] | D3YXG0 | |
| Semaphorin-5A (Sema5a) | Mouse | Brain | N/A | [30] | Q62217 | |
| Subcommissural organ-spondin (SCO-spondin) | Mouse | Heterologous overexpression | N/A | [69] | Q8CG65 | |
| ADAMTS16 | Human | Heterologous overexpression | Secretion | [50] | Q8TE57 | |
| Cytokine receptor type I family | EPO receptor | Human | Heterologous overexpression | Thermal stability | [30,70] | P19235 |
| Mouse | Heterologous overexpression | P14753 | ||||
| IL-21 receptor | Human | Homologous or heterologous overexpression | Cell surface expression | [20,30,71] | Q9HBE5 | |
| TPO receptor | Human | Homologous overexpression | Cell surface expression, signal transduction | [72] | P40238 | |
| IL-2 receptor | Human | N/A | N/A | [21] | P14784 | |
| G-CSF receptor | Human | Homologous or heterologous overexpression | Signal transduction | [73] | Q99062 | |
| Others | RNase 2 | Human | Urine/homologous or heterologous overexpression | Structural stability, thermal stability | [3,8,28,30,74,75,76] | P10153 |
| IL-12B | Human | Heterologous overexpression | Thermal stability | [30,77] | P29460 | |
| Mucins (MUC5AC, MUC5B) | Human | Heterologous overexpression | Secretion | [78,79] | P98088, Q9HC84 | |
| Membrane protein 20 | Bovine | Lens | N/A | [80] | P20274 | |
| EEF1A1 | Human | Breast carcinoma cell lines | N/A | [81] | P68104 | |
| sGP | Zaire ebolavirus | Heterologous overexpression | Secretion (not affected) | [82] | P60170 | |
| Hypertrehalosaemic hormone | C. morosus | Corpora cardiaca | Inhibition of aggregation | [83] | P62542 | |
| Pvfp-1 | Perna viridis | Mussel feet | N/A | [84] | A1X158 | |
| Hyaluronidase 1 (HYAL1) | Human | Homologous overexpression | Secretion (-), enzyme activity (-) | [85] | Q12794 | |
| Myelin-associated glycoprotein (MAG) | Mouse | Heterologous overexpression | Molecular interaction (-) | [24] | P20917 | |
| Lipoprotein lipase | Human | Homologous overexpression | Secretion, enzyme activity | [86] | P06858 | |
| Ribitol-5-phosphate xylosyltransferase 1 (Rxylt1) | Mouse | Brain | N/A | [30] | Q8VDX6 | |
| Protein Aster-B (Gramd1b) | Mouse | Brain | N/A | [30] | Q80TI0 | |
| Microfibril-associated glycoprotein 4 (MFAP4) | Human | Homologous overexpression | Secretion | [87] | P55083 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minakata, S.; Manabe, S.; Inai, Y.; Ikezaki, M.; Nishitsuji, K.; Ito, Y.; Ihara, Y. Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine. Molecules 2021, 26, 5258. https://doi.org/10.3390/molecules26175258
Minakata S, Manabe S, Inai Y, Ikezaki M, Nishitsuji K, Ito Y, Ihara Y. Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine. Molecules. 2021; 26(17):5258. https://doi.org/10.3390/molecules26175258
Chicago/Turabian StyleMinakata, Shiho, Shino Manabe, Yoko Inai, Midori Ikezaki, Kazuchika Nishitsuji, Yukishige Ito, and Yoshito Ihara. 2021. "Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine" Molecules 26, no. 17: 5258. https://doi.org/10.3390/molecules26175258
APA StyleMinakata, S., Manabe, S., Inai, Y., Ikezaki, M., Nishitsuji, K., Ito, Y., & Ihara, Y. (2021). Protein C-Mannosylation and C-Mannosyl Tryptophan in Chemical Biology and Medicine. Molecules, 26(17), 5258. https://doi.org/10.3390/molecules26175258






