Chemical Composition and Antioxidant, Anti-Inflammatory, and Enzyme Inhibitory Activities of an Endemic Species from Southern Algeria: Warionia saharae
Abstract
1. Introduction
2. Results and Discussion
2.1. UHPLC-DAD-ESI-MS/MSn Characterization of W. saharae Extract
2.2. GC–MS Characterization of W. saharae Extract
2.3. Total Bioactive Content
2.4. Antioxidant Activities
2.5. Anti-Inflammatory Activity
2.6. Enzyme Inhibitory Activities
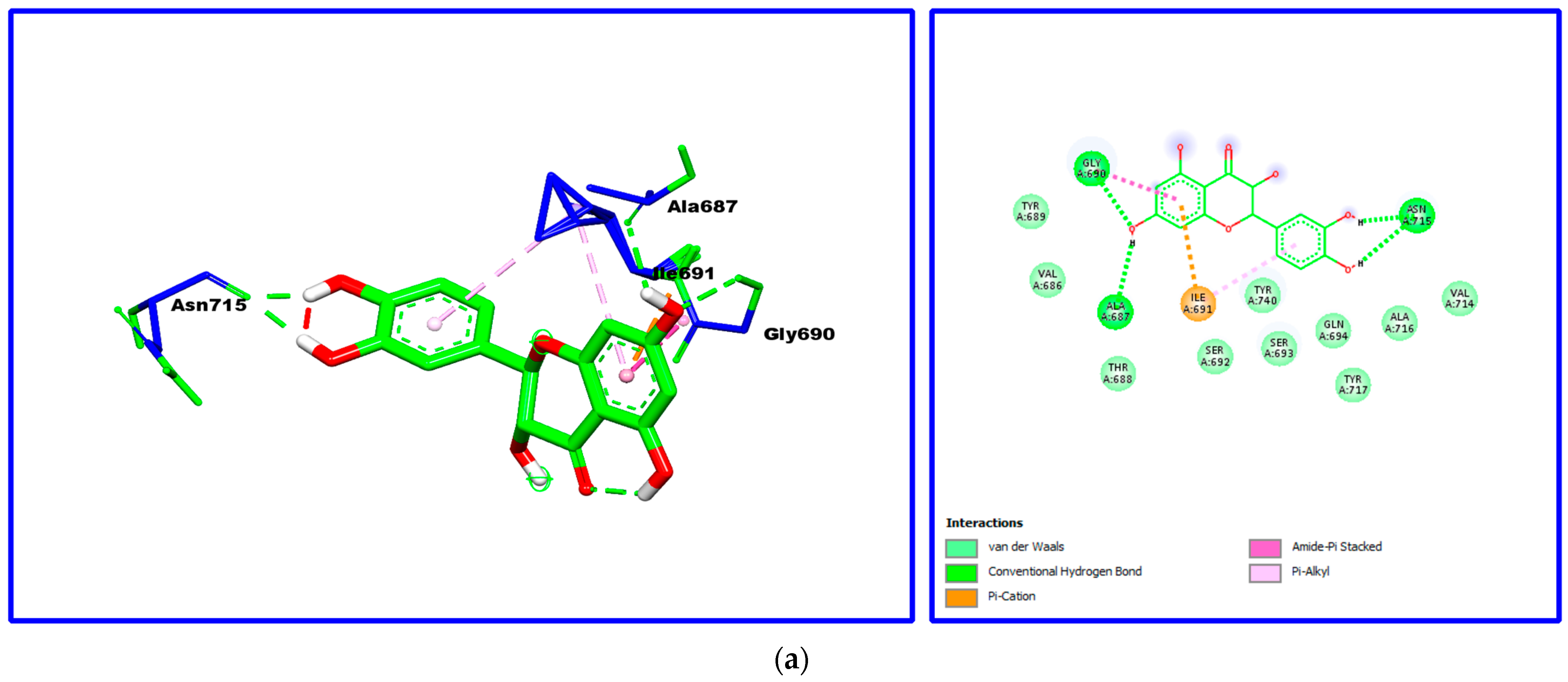
2.7. Molecular Docking
3. Materials and Methods
3.1. Chemicals
3.2. Extract Preparation
3.3. Total Bioactive Content
3.3.1. Total Phenolic Content
3.3.2. Total Flavonoid Content
3.4. UHPLC-DAD-ESI-MS/MSn Characterization of W. saharae Extract
3.5. GC–MS Characterization of W. saharae Extract
3.6. Antioxidant Activities
3.6.1. Determination of 1,1-Diphenyl-2-Picrylhydrazyl Radical Scavenging Activity
3.6.2. Determination of 2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid) Scavenging Activity
3.6.3. Galvinoxyl Radical Scavenging Activity
3.6.4. Ferric Reducing Power Assay
3.6.5. Cupric Ion Reducing Antioxidant Capacity Assay
3.7. Anti-Inflammatory Activity
3.8. Inhibition of Enzymatic Activities
3.8.1. Inhibition of α-Glucosidase Activity
3.8.2. Inhibition of α-Amylase Activity
3.8.3. Inhibition of Acetylcholinesterase Activity
3.9. Molecular Docking Study
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Das, A.; Chaudhuri, D.; Sarkar, R.; Ghate, N.B.; Panja, S.; Mandal, N. Plants of Indian traditional medicine with antioxidant activity. In Nutritional Antioxidant Therapies: Treatments and Perspectives; Al-Gubory, K.H., Laher, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 27–64. [Google Scholar]
- Joshee, N.; Dhekney, S.A.; Parajuli, P. Medicinal Plants: From Farm to Pharmacy; Springer International Publishing: New York, NY, USA, 2019; p. 439. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, biological role, and therapeutic applications. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin, Germany, 2013; pp. 2665–2691. [Google Scholar] [CrossRef]
- Liu, C.M.; Kao, C.L.; Wu, H.M.; Li, W.J.; Huang, C.T.; Li, H.T.; Chen, C.Y. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Oliveira, M.E.B.S.; Cardoso, F.C.I. Advances and challenges on the in vitro production of secondary metabolites from medicinal plants. Hortic. Bras. 2019, 37, 124–132. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Kabtni, S.; Sdouga, D.; Bettaib Rebey, I.; Mattew Save, N.T.F.; Fauconnier, M.L.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 8293. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Ozenda, P. Flore du Sahara Septentrional et Central; CNRS: Paris, France, 1958; p. 444. [Google Scholar]
- Katinas, L.; Pruski, J.; Gisela Sancho, G.; Tellería, M.C. The subfamily Mutisioideae (Asteraceae). Bot. Rev. 2008, 74, 469–716. [Google Scholar] [CrossRef]
- Bellakhdar, J.; Baayaoui, A.; Kazdari, A.; Marechal, J. Herboristes et medecine traditionnelle à Tissint, oasis presaharien du sud Marocain (province de Tata). Al Biruniya 1986, 3, 7–50. [Google Scholar]
- Ajebli, M.; Eddouks, M. Flavonoid-enriched extract from desert plant Warionia saharae improves glucose and cholesterol levels in diabetic rats. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 28–39. [Google Scholar] [CrossRef]
- Beghalia, M.; Ghalem, S.; Allali, H. Comparison of the inhibitory capacity of two groups of pure natural extract on the crystallization of two types of material compound urinary stones in vitro study. IOP Conf. Ser. Mater. Sci. Eng. 2015, 92, 012025. [Google Scholar] [CrossRef]
- Amezouar, F.; Badri, W.; Hsaine, M.; Bourhim, N.; Fougrach, H. Chemical composition, antioxidant and antibacterial activities of leaves essential oil and ethanolic extract of Moroccan Warionia saharae Benth. & Coss. J. Appl. Pharm. Sci. 2012, 2, 212–217. [Google Scholar] [CrossRef]
- Sellam, K.; Ramchoun, M.; Alem, C.; Khallouki, F.; El Moualij, B.; El Rhaffari, L. Chemical composition, antioxidant and antimicrobial activities of essential oil of Warionia saharae from Oases of Marocco. In Gas Chromatography–Biochemicals, Narcotics and Essential Oils; Salih, B., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 10; pp. 213–220. [Google Scholar] [CrossRef]
- Znini, M.; Cristofari, G.; Majidi, L.; Paolini, J.; Desjobert, J.M.; Costa, J. Essential oil composition and antifungal activity of Pulicaria mauritanica Coss., against postharvest phytopathogenic fungi in apples. LWT-Food Sci. Technol. 2013, 54, 564–569. [Google Scholar] [CrossRef]
- Mezhoud, S.; Derbré, S.; Ameddah, S.; Mekkiou, R.; Boumaza, O.; Seghiri, R.; Benayache, S.; Richomme, P.; Benayache, F. Antioxidant activity and chemical constituents of Warionia saharae Benth. & Coss. (Compositae) from Algeria. Int. J. Med. Arom. Plants. 2012, 2, 509–513. [Google Scholar]
- El-Ouady, F.; Eddouks, M. Warionia saharae induces antihypertensive and vasorelaxant activities through nitric oxide and KATP channels pathways in rats. J. Complement. Integr. Med. 2019, 26, 17. [Google Scholar] [CrossRef]
- Boittier, E.D.; Tang, Y.Y.; Buckley, M.E.; Schuurs, Z.P.; Richard, D.J.; Gandhi, N.S. Assessing molecular docking tools to guide targeted drug discovery of CD38 inhibitors. Int. J. Mol. Sci. 2020, 21, 5183. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Luo, C.; Wang, X.; Gao, G.; Wang, L.; Li, Y.; Sun, C. Identification and quantification of free, conjugate and total phenolic compounds in leaves of 20 sweet potato cultivars by HPLC–DAD and HPLC–ESI–MS/MS. Food Chem. 2013, 141, 2697–2706. [Google Scholar] [CrossRef]
- Rahmouni, N.; Pinto, D.C.G.A.; Beghidja, N.; Benayache, S.; Silva, A.M.S. Scabiosa stellata L. phenolic content clarifies its antioxidant activity. Molecules 2018, 23, 1285. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Arraez-Roman, D.; Segura-Carretero, A.; Menendez, J.A.; Menendez-Gutierrez, M.P.; Micol, V.; Fernandez-Gutierrez, A. Qualitative screening of phenolic compounds in olive leaf extracts by hyphenated liquid chromatography and preliminary evaluation of cytotoxic activity against human breast cancer cells. Anal. Bioanal. Chem. 2010, 397, 643–654. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of Sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Quirantes-Pin, R.; Amessis-Ouchemoukh, N.; Khodir, M.; Segura-Carretero, A.; Fernandez-Gutierrez, A. A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. J. Pharm. Biomed. Anal. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Boukhalkhal, S.; Gourine, N.; Pinto, D.C.G.A.; Silva, A.M.S.; Yousfi, M. UHPLC-DAD-ESI-MSn profiling variability of the phenolic constituents of Artemisia campestris L. populations growing in Algeria. Biocatal. Agric. Biotechnol. 2020, 23, 101483. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of Chrysanthemum flower (Chrysanthemum morifolium ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.A.; Domingues, M.R.M.; Silvestre, A.J.D.; Pascoal Neto, C. Characterization of phenolic components in polar extracts of Eucalyptus globules Labill. Bark by high-performance liquid chromatography mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef]
- Vuković, N.L.; Vukić, M.D.; Đelić, G.T.; Kacaniova, M.M.; Cvijović, M. The investigation of bioactive secondary metabolites of the methanol extract of Eryngium amethystinum. Kragujevac J. Sci. 2018, 40, 113–129. [Google Scholar] [CrossRef]
- Guedes, J.A.C.; Alves Filho, E.G.; Silva, M.F.S.; Rodrigues, T.H.S.; Ramires, C.M.C.; Lima, M.A.C.; Silva, G.S.; Pessoa, C.Ó.; Canuto, K.M.; Brito, E.S.; et al. GC-MS-Based metabolomic profiles combined with chemometric tools and cytotoxic activities of non-polar leaf extracts of Spondias mombin L. and Spondias tuberosa Arr. Cam. J. Braz. Chem. Soc. 2020, 31, 331–340. [Google Scholar] [CrossRef]
- Jahan, I.; Tona, M.R.; Sharmin, S.; Sayeed, M.A.; Tania, F.Z.; Paul, A.; Chy, M.N.U.; Rakib, A.; Bin Emran, T.; Simal-Gandara, J. GC-MS Phytochemical profiling, pharmacological properties, and in silico studies of Chukrasia velutina leaves: A novel source for bioactive agents. Molecules 2020, 25, 3536. [Google Scholar] [CrossRef]
- Tankiewicz, M.; Olkowska, E.; Berg, A.; Wolska, L. Advancement in determination of phthalate metabolites by gas chromatography eliminating derivatization step. Front. Chem. 2020, 7, 928. [Google Scholar] [CrossRef]
- Engel, B.; Suralik, P.; Marchetti-Deschmann, M. Critical considerations for trimethylsilyl derivatives of 24 primary metabolites measured by gas chromatography–tandem mass spectrometry. Sep. Sci. Plus. 2020, 3, 407–418. [Google Scholar] [CrossRef]
- Pautova, A.; Khesina, Z.; Getsina, M.; Sobolev, P.; Revelsky, A.; Beloborodova, N. Determination of tryptophan metabolites in serum and cerebrospinal fluid samples using microextraction by packed sorbent, silylation and GC-MS detection. Molecules 2020, 25, 3258. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Lei, X.; Cheng, S.; Peng, H.; He, Q.; Zhu, H.; Xu, M.; Wang, Q.; Liu, L.; Zhang, C.; Zhou, Q.; et al. Anti-inflammatory effect of Zanthoxylum bungeanum-cake-separated moxibustion on rheumatoid arthritis rats. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 45–52. [Google Scholar] [CrossRef]
- Wang, X.; Ding, G.; Liu, B.; Wang, Q. Flavonoids and antioxidant activity of rare and endangered fern: Isoetes sinensis. PLoS ONE 2020, 15, e0232185. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear Factor-Kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Fardoun, M.M.; Maaliki, D.; Halabi, N.; Iratni, R.; Bitto, A.; Baydoun, E.; Eid, A.H. Flavonoids in adipose tissue inflammation and atherosclerosis: One arrow, two targets. Clin. Sci. 2020, 134, 1403–1432. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in Kandelia obovata under cadmium and zinc stress. Chemosphere 2020, 249, 126341. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety-chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Zeghad, N.; Ahmed, E.; Belkhiri, A.; Heyden, Y.V.; Demeyer, K. Antioxidant activity of Vitis vinifera, Punica granatum, Citrus aurantium and Opuntia ficus indica fruits cultivated in Algeria. Heliyon 2019, 5, e01575. [Google Scholar] [CrossRef] [PubMed]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2016, 50, 687–695. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szczepaniak, O.; Szymanowska-Powałowska, D.; Piechocka, J.; Szulc, P.; Dziedziński, M. Antioxidant potential of various solvent extract from Morus alba fruits and its major polyphenols composition. Ciência Rural. 2020, 50, 20190371. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef]
- Salehi, B.; Azzini, E.; Zucca, P.; Varoni, E.M.; Kumar, N.V.A.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Peluso, I.; et al. Plant-derived bioactives and oxidative stress-related disorders: A key trend towards healthy aging and longevity promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Dorman, H.J.D.; Laakso, I.; Hiltunen, R. Chemical composition and antioxidative activity of Moldavian balm (Dracocephalum moldavica L.) extracts. LWT-Food Sci. Technol. 2007, 40, 1655–1663. [Google Scholar] [CrossRef]
- Khatri, D.; Chhetri, S.B.B. Reducing Sugar, Total Phenolic Content, and Antioxidant Potential of Nepalese Plants. BioMed Res. Int. 2020, 2020, 7296859. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006, 41, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Benslama, A.; Harrar, A. Free radicals scavenging activity and reducing power of two Algerian Sahara medicinal plants extracts. Int. J. Herb. Med. 2016, 4, 158–161. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Nagai, C.; Crozier, A. On-line HPLC analysis of the antioxidant activity of phenolic compounds in brewed, paper-filtered coffee. Braz. J. Plant. Physiol. 2006, 18, 253–262. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and cytoprotective effects of the di-O-caffeoylquinic acid family: The mechanism, structure-activity relationship, and conformational effect. Molecules 2018, 23, 222. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Jiang, Q.; Wei, G.; Lin, L.; Li, C.; Ou, X.; Yang, L.; Xie, Y.; Fu, Z.; et al. The mechanism of (+) taxifolin’s protective antioxidant effect for •OH-treated bone marrow-derived mesenchymal stem cells. Cell. Mol. Biol. Lett. 2017, 22, 31. [Google Scholar] [CrossRef]
- Zu, Y.; Wu, W.; Zhao, X.; Li, Y.; Wang, W.; Zhong, C.; Zhang, Y.; Zhao, X. Enhancement of solubility, antioxidant ability and bioavailability of taxifolin nanoparticles by liquid antisolvent precipitation technique. Int. J. Pharmaceut. 2014, 471, 366–376. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Li, G.; Zhou, J.; Sun, M.; Cen, J.; Xu, J. Role of luteolin extracted from Clerodendrum cyrtophyllum Turcz leaves in protecting HepG2 cells from TBHP-induced oxidative stress and its cytotoxicity, genotoxicity. J. Funct. Foods 2020, 74, 104196. [Google Scholar] [CrossRef]
- Orhan, I.E.; Senol, F.S.; Ozturk, N.; Celik, S.A.; Pulur, A.; Kan, Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. Food Chem. Toxicol. 2013, 59, 96–103. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, J.; Yang, F.; Wu, J.; Cai, R.; Wang, T.; Zhang, J. Effect of luteolin on the methylation status of the OPCML gene and cell growth in breast cancer cells. Exp. Ther. Med. 2018, 16, 3186–3194. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Muramatsu, D.; Uchiyama, H.; Kida, H.; Iwai, A. In vitro anti-inflammatory and anti-lipid accumulation properties of taxifolin-rich extract from the Japanese larch, Larix kaempferi. Heliyon 2020, 6, e05505. [Google Scholar] [CrossRef]
- Lankatillake, C.; Huynh, T.; Dias, D.A. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant. Methods 2019, 15, 105. [Google Scholar] [CrossRef]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-derived bioactive peptides: A treatment to cure diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2014, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Proenca, C.; Freitasa, M.; Ribeiroa, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tome, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ruan, Y.T.; Li, Y.; Chen, J.G.; Yin, Z.P.; Zhang, Q.F. In vitro and in vivo inhibitory activity of taxifolin on three digestive enzymes. Int. J. Biol. Macromol. 2020, 150, 31–37. [Google Scholar] [CrossRef]
- Nyambe-Silavwe, H.; Williamson, G. Chlorogenic and phenolic acids are only very weak inhibitors of human salivary α-amylase and rat intestinal maltase activities. Food Res. Int. 2018, 113, 452–455. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Tomé, S.M.; Oliveir, E.F.T.; Viegas, M.F.; Araújo, A.N.; Ramo, M.J.; Silva, A.M.S.; Fernandes, P.A.; et al. Evaluation of a flavonoids library for inhibition of pancreatic α-amylase towards a structure–activity relationship. J. Enzym. Inhib. Med. Chem. 2019, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac. J. Clin. Nutr. 2006, 15, 433–441. [Google Scholar]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Ding, X.; Ouyang, M.A.; Liu, X.; Wang, R.Z. Acetylcholinesterase inhibitory activities of flavonoids from the leaves of Ginkgo biloba against brown plant hopper. J. Chem. 2013, 2013, 645086. [Google Scholar] [CrossRef]
- Gocer, H.; Topal, F.; Topal, M.; Küçük, M.; Teke, D.; Gülçin, İ.; Alwasel, S.; Supuran, C.T. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J. Enzym. Inhib. Med. Chem. 2016, 31, 441–447. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G. Analysis of the activity of hydroxycinnamic acids from green and roasted coffee extracts as acetylcholinesterase inhibitors using an isothermal method of titration calorimetry. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2019, 50, 15–24. [Google Scholar] [CrossRef]
- Anwar, J.; Spanevello, M.; Thomé, G.; Stefanello, N.; Schmatz, R.; Gutierres, J.; Vieira, J.; Baldissarelli, J.; Carvalho, F.B.; da Rosa, M.M.; et al. Effects of caffeic acid on behavioral parameters and on the activity of acetylcholinesterase in different tissues from adult rats. Pharmacol. Biochem. Behav. 2012, 103, 386–394. [Google Scholar] [CrossRef]
- Rezg, R.; Mornagui, B.; El-Fazaa, S.; Gharbi, N. Caffeic acid attenuates malathion induced metabolic disruption in rat liver, involvement of acetylcholinesterase activity. Toxicology 2008, 250, 27–31. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu reagent. Met. Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Türkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007, 101, 267–273. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Cavaleiro, J.A.S. Lipophilic extractives of the inner and outer barks of Eucalyptus globulus. Holzforschung 2002, 56, 372–379. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Bensouici, C.; Kabouche, A.; Karioti, A.; Ozturk, M.; Duru, M.E.; Bilia, A.R.; Kabouche, Z. Compounds from Sedum caeruleum with antioxidant, anticholinesterase and antibacterial activities. Pharm. Biol. 2016, 54, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity, methods in enzymology. Methods Enzymol. 2001, 335, 157–166. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, Using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Válega, M.; Silva, A.M.S.; Cardoso, S.M. Metabolites and biological activities of Thymus zygis, Thymus pulegioides, and Thymus fragrantissimus grown under organic cultivation. Molecules 2018, 23, 1514. [Google Scholar] [CrossRef]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as functional ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.C.; Wickramaratne, D.B.M. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherston, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide protein data bank. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera? A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S. Discovery Studio Modeling Environment; Release 4.5; Dassault Systèmes: San Diego, CA, USA, 2017. [Google Scholar]

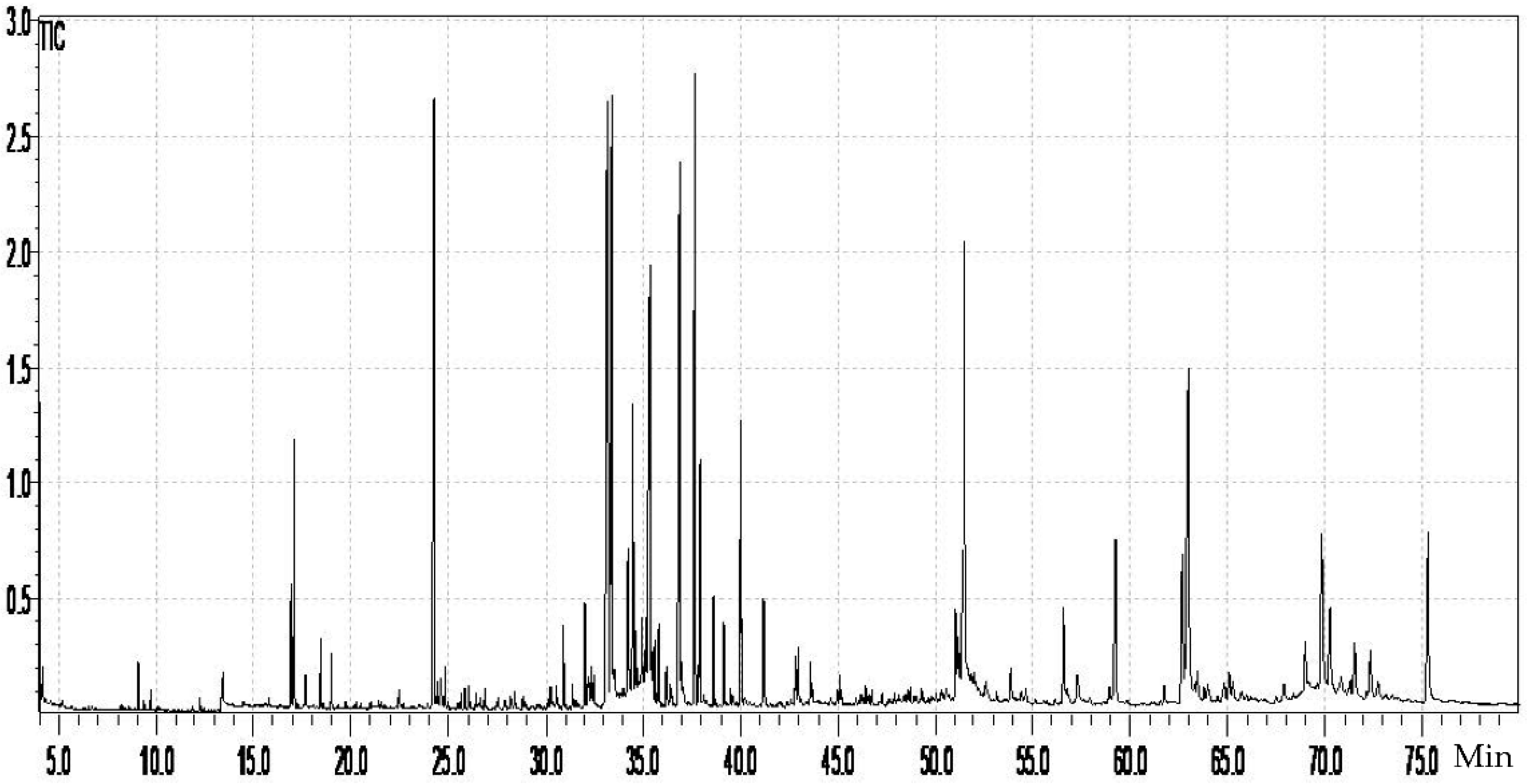

| N° | RT | Λmax | [M − H]− | MS2 (m/z) | Identified Compound | Quantification (µg/mg Extract) |
|---|---|---|---|---|---|---|
| 1 | 1.53 | 256 | 215 | 179,160 | Caffeic acid adduct with chloride | NQ |
| 2 | 1.75 | 194 | 137 | 110,82 | Hydroxybenzoic acid | 0.855 ± 0.037 |
| 3 | 6.58 | 204,224,250,289,340 | 339 | 177 | Esculetin-6-O-glucoside | NQ |
| 4 | 8.05 | 325,238,218 | 353 | 191,179 | 5-O-CQA | 10.333 ± 2.360 |
| 5 | 8.29 | 203,225,337 | 353 | 191,179 | 3-O-CQA | 1.537 ± 0.239 |
| 6 | 8.46 | 202,225,298,342 | 353 | 191,179,173,135 | 4-O-CQA | 3.039 ± 0.420 |
| 7 | 8.84 | 299,235,322 | 179 | 135 | Caffeic acid | 1.380 ± 0.790 |
| 8 | 9.06 | 260 | 536 | - | Unknown | NQ |
| 9 | 10.54 | 198,229,288 | 515 | 353 | O-diCQA isomer | 1.631 ± 0.430 |
| 10 | 10.96 | 234,288,330 | 477 | 301 | Quercetin glucuronide | 0.560 ± 0.110 |
| 11 | 11.23 | 232,290 | 515 | 353,335,179 | 3,5-O-diCQA | 1.280 ± 0.190 |
| 12 | 11.76 | 261 | 609 | 459,315,299 | Unknown | NQ |
| 13 | 12.03 | 204,256,353 | 463 | 301,300 | Quercetin-O-hexoside | 2.460 ± 0.790 |
| 14 | 12.15 | 227,289 | 303 | 285,175,125 | Taxifolin | 62.800 ± 16.690 |
| 15 | 12.47 | 231,290 | 303 | 285,177 | Taxifolin isomer | 1.540 ± 0.590 |
| 16 | 12.60 | 236,256,268,341 | 477 | 315,300 | Isorhamnetin-O-hexoside | 0.640 ± 0.130 |
| 17 | 12.78 | 220,241,324 | 515 | 353,335,172,191 | 3,4-O-diCQA | 4.185 ± 1.340 |
| 18 | 13.03 | 220,241,324 | 515 | 353,335,191 | 1,3-O-diCQA | 43.120 ± 17.390 |
| 19 | 13.67 | 219,242,326 | 515 | 353,299,255,203 | 4,5-O-diCQA | 9.660 ± 2.970 |
| 20 | 15.97 | 207,254,351 | 285 | 241,217,199,175 | Luteolin | 7.410 ± 0.530 |
| 21 | 17.91 | 245,274,332 | 299 | 284 | 3′-O-Methylluteolin (Chrysoeriol) | 0.740 ± 0.520 |
| 22 | 18.07 | 246,342 | 299 | 284 | Luteolin 4′-methyl ether (Diosmetin) | Tr |
| 23 | 18.43 | 296,234 | 315 | 300 | Isorhamnetin | 3.435 ± 1.390 |
| 24 | 18.76 | 200,230,290 | 299 | 271,255,243 | Naringenin derivative | NQ |
| Families | Peak | Rt (min) | Identified Compound * | Quantification |
|---|---|---|---|---|
| Carboxylic acids and esters Total=124.6 µg/mg | 4 | 24.259 | Malic acid | 50.79 ± 5.67 |
| 11 | 33.568 | Citric acid | 4.01 ± 1.98 | |
| 13 | 34.517 | Quinic acid | 18.53 ± 2.19 | |
| 14 | 34.960 | 2-n-Propyl valerate | 4.63 ± 0.47 | |
| 23 | 37.945 | D-Gluconic acid | 17.11 ± 1.37 | |
| 28 | 41.191 | Caffeic acid | 6.02 ± 0.72 | |
| 37 | 62.690 | Chlorogenic acid 1 | 19.89 ± 2.89 | |
| 39 | 65.073 | Chlorogenic acid 2 | 3.62 ± 0.77 | |
| Fatty acids Total=11.364 µg/mg | 3 | 18.452 | Butanedioic acid | 3.58 ± 0.56 |
| 25 | 39.147 | Palmitic acid | 5.73 ± 0.12 | |
| 29 | 42.814 | Linoelaidic acid | 1.056 ± 0.80 | |
| 30 | 42.961 | Oleic acid | 0.98 ± 0.11 | |
| Alcohols Total=128.520 µg/mg | 2 | 17.099 | Glycerol | 12.03 ± 2.07 |
| 5 | 30.892 | D-arabitol | 2.17 ± 0.66 | |
| 16 | 35.335 | Dulcitol | 31.99 ± 1.93 | |
| 21 | 36.916 | Myo-inositol | 60.91 ± 6.91 | |
| 24 | 38.630 | Scyllo-Inositol | 4.24 ± 0.90 | |
| 26 | 40.015 | Myo-Inositol | 17.18 ± 3.16 | |
| Sugars Total=214.735 µg/mg | 6 | 32.041 | Ribonic acid | Tr |
| 7 | 32.222 | D-Talose | Tr | |
| 8 | 32.379 | D-Tagatose | Tr | |
| 9 | 33.230 | D-Fructose (isomer 1) a | 51.21 ± 14.36 | |
| 10 | 33.442 | D-Fructose (isomer 2) a | 32.19 ± 19.31 | |
| 12 | 34.249 | D-Galactose (isomer 1) b | 2.18 ± 0.14 | |
| 17 | 35.393 | D-Galactose (isomer 2) b | 34.57 ± 0.46 | |
| 18 | 35.615 | D-Galactose (isomer 3) b | Tr | |
| 22 | 37.677 | D-Galactose (isomer 4) b | 19.81 ± 4.66 | |
| 31 | 51.024 | D-Fructose (isomer 3) a | Tr | |
| 32 | 51.153 | D-Turanose | Tr | |
| 33 | 51.515 | Sucrose derivative | 36.27 ± 12.46 | |
| 34 | 53.916 | D-Trehalose | Tr | |
| 35 | 56.602 | β-Lyxose | 0.075 ± 0.01 | |
| 38 | 62.999 | D-Glucose (isomer 1) c | 26.06 ± 4.58 | |
| 40 | 68.978 | D-Gulose | Tr | |
| 41 | 69.824 | Sucrose | 12.37 ± 0.03 | |
| 43 | 71.578 | D-Glucose (isomer 2) c | Tr | |
| 44 | 72.381 | D-Glucose (isómer 3) c | Tr | |
| Other compounds | 1 | 16.930 | Phosphoric acid | NQ |
| 15 | 35.172 | Gluconolactone | NQ | |
| 36 | 59.258 | Catechin | NQ | |
| 27 | 40.082 | Esculetin | NQ | |
| 19 | 35.829 | Acrylsaeure | NQ | |
| 42 | 70.226 | 5-Methyluridine | NQ |
| Sample | Radical Scavenging Activity IC50 (μg/mL) | Radical Scavenging Activity A0.5 (μg/mL) | |||
|---|---|---|---|---|---|
| DPPH● | ABTS●+ | Galvinoxyl | FRP | CUPRAC | |
| W. saharae | 7.12 ± 0.09 | 4.19 ± 0.35 | 3.55 ± 0.08 | 31.21 ± 0.14 | 3.84 ± 0.16 |
| Trolox | 5.12 ± 0.21 | 3.21 ± 0.06 | 4.31 ± 0.05 | 5.25 ± 0.20 | 8.69 ± 0.14 |
| BHA | 6.14 ± 0.41 | 1.81 ± 0.10 | 5.38 ± 0.06 | 7.99 ± 0.87 | 6.62 ± 0.05 |
| BHT | 12.99 ± 0.41 | 1.29 ± 0.30 | 3.32 ± 0.18 | >200 | 8.97 ± 3.94 |
| Ascorbic acid | 4.39 ± 0.01 | 3.04 ± 0.05 | 5.02 ± 0.01 | 3.62 ± 0.29 | 8.31 ± 0.15 |
| Sample | Enzyme Inhibitory Activity | ||
|---|---|---|---|
| α-Glucosidase IC50 (μg/mL) | α-Amylase (% inhibition) | Acetylcholinesterase (% inhibition) | |
| W. saharae extract | 23.52 ± 6.33 A | 38.41 ± 5.51 a | 28.578 ± 2.979 b |
| Acarbose (μg/mL) | 405.77 ± 34.83 B | ND | ND |
| Compound | BE | Amino Acid Involved in Interaction |
|---|---|---|
| Taxifolin | −5.89 | Asn715, Ile691, Ala687, Gly690. |
| 4,5-O-Dicaffeoylquinic acid | −4.02 | His206, Ile691, Asn415, Tyr717, Ser693, Ser692, Gly690, Gln219, Val686, Gln208. |
| 1,3-O-Dicaffeoylquinic acid | −4.17 | Met801, Gln533, Tyr561, Arg536, Asn668, Asp802. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rechek, H.; Haouat, A.; Hamaidia, K.; Allal, H.; Boudiar, T.; Pinto, D.C.G.A.; Cardoso, S.M.; Bensouici, C.; Soltani, N.; Silva, A.M.S. Chemical Composition and Antioxidant, Anti-Inflammatory, and Enzyme Inhibitory Activities of an Endemic Species from Southern Algeria: Warionia saharae. Molecules 2021, 26, 5257. https://doi.org/10.3390/molecules26175257
Rechek H, Haouat A, Hamaidia K, Allal H, Boudiar T, Pinto DCGA, Cardoso SM, Bensouici C, Soltani N, Silva AMS. Chemical Composition and Antioxidant, Anti-Inflammatory, and Enzyme Inhibitory Activities of an Endemic Species from Southern Algeria: Warionia saharae. Molecules. 2021; 26(17):5257. https://doi.org/10.3390/molecules26175257
Chicago/Turabian StyleRechek, Habiba, Ammar Haouat, Kaouther Hamaidia, Hamza Allal, Tarek Boudiar, Diana C. G. A. Pinto, Susana M. Cardoso, Chawki Bensouici, Noureddine Soltani, and Artur M. S. Silva. 2021. "Chemical Composition and Antioxidant, Anti-Inflammatory, and Enzyme Inhibitory Activities of an Endemic Species from Southern Algeria: Warionia saharae" Molecules 26, no. 17: 5257. https://doi.org/10.3390/molecules26175257
APA StyleRechek, H., Haouat, A., Hamaidia, K., Allal, H., Boudiar, T., Pinto, D. C. G. A., Cardoso, S. M., Bensouici, C., Soltani, N., & Silva, A. M. S. (2021). Chemical Composition and Antioxidant, Anti-Inflammatory, and Enzyme Inhibitory Activities of an Endemic Species from Southern Algeria: Warionia saharae. Molecules, 26(17), 5257. https://doi.org/10.3390/molecules26175257









