Tissue-Specific Regulation of HNK-1 Biosynthesis by Bisecting GlcNAc
Abstract
1. Introduction
2. Results
2.1. HNK-1 Expression Is Inhibited by Bisecting GlcNAc in Mouse Brain but Not in Kidney
2.2. GlcAT-S Is Fully Active toward N-glycans with Bisecting GlcNAc
2.3. Different Effects of Bisecting GlcNAc on Intracellular Activity of GlcAT-P and GlcAT-S
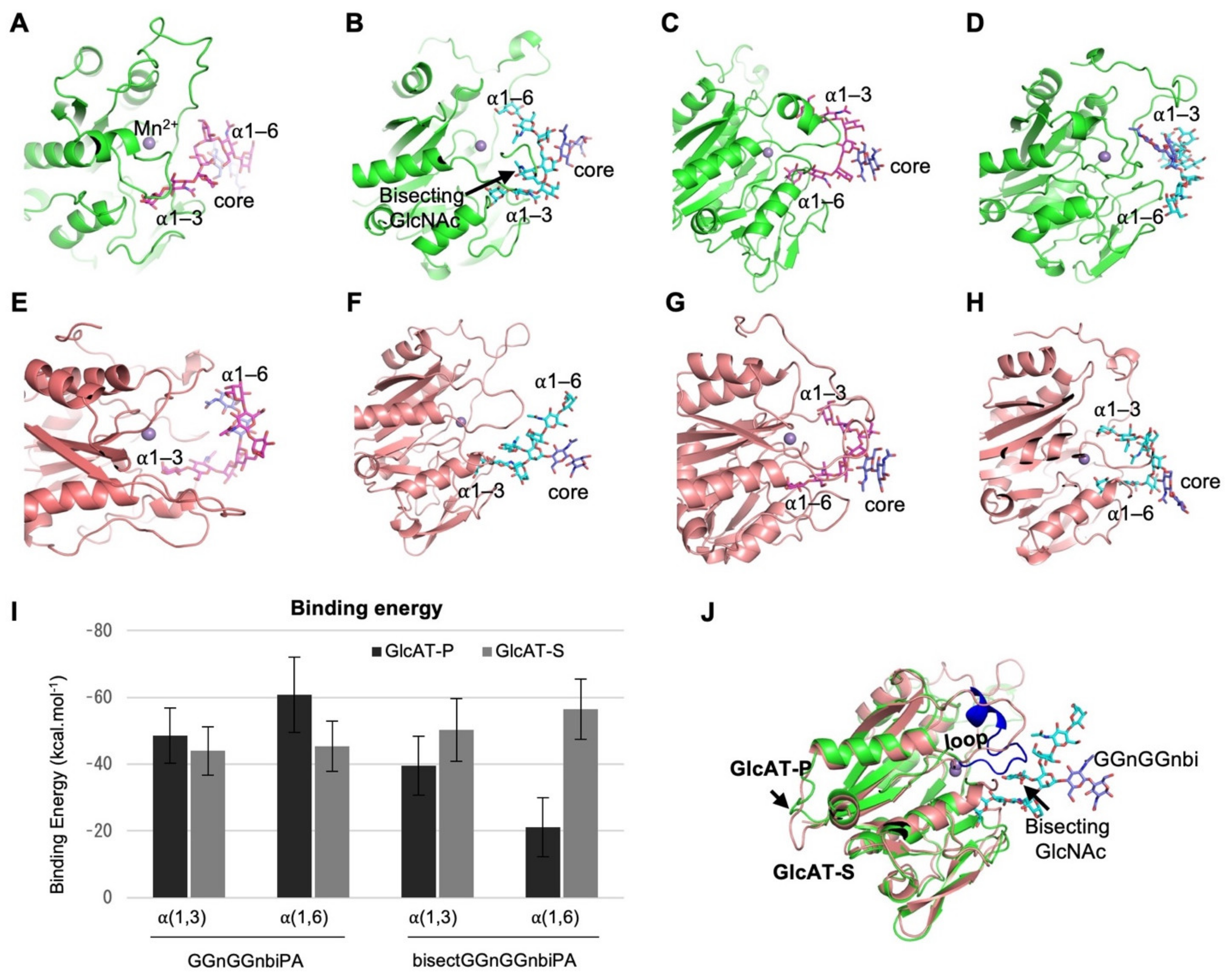
2.4. Molecular Dynamics (MD) Simulations of the Binding between GlcATs and N-glycans with or without Bisecting GlcNAc
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal Experiments
4.3. Preparation of Membrane and Soluble Fractions
4.4. Western and Lectin Blotting, and CBB Staining
4.5. Immunofluorescence Staining
4.6. Cell Culture and Transfection
4.7. Plasmid Construction
4.8. Purification of Recombinant Enzyme
4.9. Glycosyltransferase Activity Assay
4.10. Generation of GnT-III KO Neuro2A Cells
4.11. Building GlcAT/N-Glycan Complex Models
4.12. Molecular Dynamics and Binding Energy Calculation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kizuka, Y. Glycans and cancer: Role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 2015, 126, 11–51. [Google Scholar]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Kizuka, Y.; Kitazume, S.; Taniguchi, N. N-glycan and Alzheimer’s disease. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2015; pp. 99–111. [Google Scholar]
- Aebi, M. N-linked protein glycosylation in the ER. Biochim. Biophys. Acta 2013, 1833, 2430–2437. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Nishikawa, A.; Ihara, Y.; Hatakeyama, M.; Kangawa, K.; Taniguchi, N. Purification, cDNA cloning, and expression of UDP-N-acetylglucosamine: Beta-D-mannoside beta-1,4N-acetylglucosaminyltransferase III from rat kidney. J. Biol. Chem. 1992, 267, 18199–18204. [Google Scholar] [CrossRef]
- Gu, J.; Nishikawa, A.; Tsuruoka, N.; Ohno, M.; Yamaguchi, N.; Kangawa, K.; Taniguchi, N. Purification and characterization of UDP-N-acetylglucosamine: Alpha-6-D-mannoside beta 1-6N-acetylglucosaminyltransferase (N-acetylglucosaminyltransferase V) from a human lung cancer cell line. J. Biochem. 1993, 113, 614–619. [Google Scholar] [CrossRef]
- Oguri, S.; Minowa, M.T.; Ihara, Y.; Taniguchi, N.; Ikenaga, H.; Takeuchi, M. Purification and characterization of UDP-N-acetylglucosamine: Alpha1,3-D-mannoside beta1,4-N-acetylglucosaminyltransferase (N-acetylglucosaminyltransferase-IV) from bovine small intestine. J. Biol. Chem. 1997, 272, 22721–22727. [Google Scholar] [CrossRef]
- Koyota, S.; Ikeda, Y.; Miyagawa, S.; Ihara, H.; Koma, M.; Honke, K.; Shirakura, R.; Taniguchi, N. Down-regulation of the alpha-Gal epitope expression in N-glycans of swine endothelial cells by transfection with the N-acetylglucosaminyltransferase III gene. Modulation of the biosynthesis of terminal structures by a bisecting GlcNAc. J. Biol. Chem. 2001, 276, 32867–32874. [Google Scholar] [CrossRef]
- Miyoshi, E.; Uozumi, N.; Noda, K.; Hayashi, N.; Hori, M.; Taniguchi, N. Expression of alpha1-6 fucosyltransferase in rat tissues and human cancer cell lines. Int. J. Cancer 1997, 72, 1117–1121. [Google Scholar] [CrossRef]
- Kizuka, Y.; Nakano, M.; Miura, Y.; Taniguchi, N. Epigenetic regulation of neural N-glycomics. Proteomics 2016, 16, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Taniguchi, N. Neural functions of bisecting GlcNAc. Glycoconj. J. 2018, 35, 345–351. [Google Scholar] [CrossRef]
- Kizuka, Y.; Kitazume, S.; Fujinawa, R.; Saito, T.; Iwata, N.; Saido, T.C.; Nakano, M.; Yamaguchi, Y.; Hashimoto, Y.; Staufenbiel, M.; et al. An aberrant sugar modification of BACE1 blocks its lysosomal targeting in Alzheimer’s disease. EMBO Mol. Med. 2015, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Nakano, M.; Kitazume, S.; Saito, T.; Saido, T.C.; Taniguchi, N. Bisecting GlcNAc modification stabilizes BACE1 protein under oxidative stress conditions. Biochem. J. 2016, 473, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Nishikawa, A.; Ihara, Y.; Taniguchi, S.; Taniguchi, N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc. Natl. Acad Sci. USA 1995, 92, 8754–8758. [Google Scholar] [CrossRef]
- Song, Y.; Aglipay, J.A.; Bernstein, J.D.; Goswami, S.; Stanley, P. The bisecting GlcNAc on N-glycans inhibits growth factor signaling and retards mammary tumor progression. Cancer Res. 2010, 70, 3361–3371. [Google Scholar] [CrossRef]
- Nakano, M.; Mishra, S.K.; Tokoro, Y.; Sato, K.; Nakajima, K.; Yamaguchi, Y.; Taniguchi, N.; Kizuka, Y. Bisecting GlcNAc Is a General Suppressor of Terminal Modification of N-glycan. Mol. Cell Proteom. 2019, 18, 2044–2057. [Google Scholar] [CrossRef]
- Kizuka, Y.; Oka, S. Regulated expression and neural functions of human natural killer-1 (HNK-1) carbohydrate. Cell Mol. Life Sci. 2012, 69, 4135–4147. [Google Scholar] [CrossRef]
- Morise, J.; Takematsu, H.; Oka, S. The role of human natural killer-1 (HNK-1) carbohydrate in neuronal plasticity and disease. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Terayama, K.; Oka, S.; Seiki, T.; Miki, Y.; Nakamura, A.; Kozutsumi, Y.; Takio, K.; Kawasaki, T. Cloning and functional expression of a novel glucuronyltransferase involved in the biosynthesis of the carbohydrate epitope HNK-1. Proc. Natl. Acad Sci. USA 1997, 94, 6093–6098. [Google Scholar] [CrossRef] [PubMed]
- Seiki, T.; Oka, S.; Terayama, K.; Imiya, K.; Kawasaki, T. Molecular cloning and expression of a second glucuronyltransferase involved in the biosynthesis of the HNK-1 carbohydrate epitope. Biochem. Biophys. Res. Commun. 1999, 255, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Bakker, H.; Friedmann, I.; Oka, S.; Kawasaki, T.; Nifant’ev, N.; Schachner, M.; Mantei, N. Expression cloning of a cDNA encoding a sulfotransferase involved in the biosynthesis of the HNK-1 carbohydrate epitope. J. Biol. Chem. 1997, 272, 29942–29946. [Google Scholar] [CrossRef]
- Yamamoto, S.; Oka, S.; Inoue, M.; Shimuta, M.; Manabe, T.; Takahashi, H.; Miyamoto, M.; Asano, M.; Sakagami, J.; Sudo, K.; et al. Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J. Biol. Chem. 2002, 277, 27227–27231. [Google Scholar] [CrossRef]
- Tagawa, H.; Kizuka, Y.; Ikeda, T.; Itoh, S.; Kawasaki, N.; Kurihara, H.; Onozato, M.L.; Tojo, A.; Sakai, T.; Kawasaki, T.; et al. A non-sulfated form of the HNK-1 carbohydrate is expressed in mouse kidney. J. Biol. Chem. 2005, 280, 23876–23883. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Kobayashi, K.; Kakuda, S.; Nakajima, Y.; Itoh, S.; Kawasaki, N.; Oka, S. Laminin-1 is a novel carrier glycoprotein for the nonsulfated HNK-1 epitope in mouse kidney. Glycobiology 2008, 18, 331–338. [Google Scholar] [CrossRef][Green Version]
- Nagae, M.; Kanagawa, M.; Morita-Matsumoto, K.; Hanashima, S.; Kizuka, Y.; Taniguchi, N.; Yamaguchi, Y. Atomic visualization of a flipped-back conformation of bisected glycans bound to specific lectins. Sci. Rep. 2016, 6, 22973. [Google Scholar] [CrossRef]
- Yabuno, K.; Morise, J.; Kizuka, Y.; Hashii, N.; Kawasaki, N.; Takahashi, S.; Miyata, S.; Izumikawa, T.; Kitagawa, H.; Takematsu, H.; et al. A Sulfated Glycosaminoglycan Linkage Region is a Novel Type of Human Natural Killer-1 (HNK-1) Epitope Expressed on Aggrecan in Perineuronal Nets. PLoS ONE 2015, 10, e0144560. [Google Scholar]
- Kakuda, S.; Sato, Y.; Tonoyama, Y.; Oka, S.; Kawasaki, T. Different acceptor specificities of two glucuronyltransferases involved in the biosynthesis of HNK-1 carbohydrate. Glycobiology 2005, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, S.; Oka, S.; Kawasaki, T. Purification and characterization of two recombinant human glucuronyltransferases involved in the biosynthesis of HNK-1 carbohydrate in Escherichia coli. Protein Expr. Purif. 2004, 35, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, S.; Shiba, T.; Ishiguro, M.; Tagawa, H.; Oka, S.; Kajihara, Y.; Kawasaki, T.; Wakatsuki, S.; Kato, R. Structural basis for acceptor substrate recognition of a human glucuronyltransferase, GlcAT-P, an enzyme critical in the biosynthesis of the carbohydrate epitope HNK-1. J. Biol. Chem. 2004, 279, 22693–22703. [Google Scholar] [CrossRef] [PubMed]
- Shiba, T.; Kakuda, S.; Ishiguro, M.; Morita, I.; Oka, S.; Kawasaki, T.; Wakatsuki, S.; Kato, R. Crystal structure of GlcAT-S, a human glucuronyltransferase, involved in the biosynthesis of the HNK-1 carbohydrate epitope. Proteins 2006, 65, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Isaji, T.; Lu, Y.; Gu, W.; Kondo, M.; Fukuda, T.; Du, Y.; Gu, J. Roles of N-acetylglucosaminyltransferase III in epithelial-to-mesenchymal transition induced by transforming growth factor beta1 (TGF-beta1) in epithelial cell lines. J. Biol. Chem. 2012, 287, 16563–16574. [Google Scholar] [CrossRef]
- Taniguchi, N.; Kizuka, Y.; Takamatsu, S.; Miyoshi, E.; Gao, C.; Suzuki, K.; Kitazume, S.; Ohtsubo, K. Glyco-redox, a link between oxidative stress and changes of glycans: Lessons from research on glutathione, reactive oxygen and nitrogen species to glycobiology. Arch. Biochem. Biophys. 2016, 595, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Morita, I.; Kakuda, S.; Takeuchi, Y.; Kawasaki, T.; Oka, S. HNK-1 (human natural killer-1) glyco-epitope is essential for normal spine morphogenesis in developing hippocampal neurons. Neuroscience 2009, 164, 1685–1694. [Google Scholar] [CrossRef]
- Morita, I.; Kakuda, S.; Takeuchi, Y.; Itoh, S.; Kawasaki, N.; Kizuka, Y.; Kawasaki, T.; Oka, S. HNK-1 glyco-epitope regulates the stability of the glutamate receptor subunit GluR2 on the neuronal cell surface. J. Biol. Chem. 2009, 284, 30209–30217. [Google Scholar] [CrossRef]
- Garcia-Ayllon, M.S.; Botella-Lopez, A.; Cuchillo-Ibanez, I.; Rabano, A.; Andreasen, N.; Blennow, K.; Avila, J.; Saez-Valero, J. HNK-1 Carrier Glycoproteins Are Decreased in the Alzheimer’s Disease Brain. Mol. Neurobiol. 2017, 54, 188–199. [Google Scholar] [CrossRef]
- Thomas, S.N.; Soreghan, B.A.; Nistor, M.; Sarsoza, F.; Head, E.; Yang, A.J. Reduced neuronal expression of synaptic transmission modulator HNK-1/neural cell adhesion molecule as a potential consequence of amyloid beta-mediated oxidative stress: A proteomic approach. J. Neurochem. 2005, 92, 705–717. [Google Scholar] [CrossRef]
- Huang, Y.F.; Aoki, K.; Akase, S.; Ishihara, M.; Liu, Y.S.; Yang, G.; Kizuka, Y.; Mizumoto, S.; Tiemeyer, M.; Gao, X.D.; et al. Global mapping of glycosylation pathways in human-derived cells. Dev. Cell 2021, 56, 1195–1209.e7. [Google Scholar] [CrossRef]
- Mkhikian, H.; Mortales, C.L.; Zhou, R.W.; Khachikyan, K.; Wu, G.; Haslam, S.M.; Kavarian, P.; Dell, A.; Demetriou, M. Golgi self-correction generates bioequivalent glycans to preserve cellular homeostasis. eLife 2016, 5, e14814. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Tanaka, H. Molecular differentiation of the otic vesicle and neural tube in the chick embryo demonstrated by monoclonal antibodies. Neurosci. Res. 1988, 6, 131–142. [Google Scholar] [CrossRef]
- Priatel, J.J.; Sarkar, M.; Schachter, H.; Marth, J.D. Isolation, characterization and inactivation of the mouse Mgat3 gene: The bisecting N-acetylglucosamine in asparagine-linked oligosaccharides appears dispensable for viability and reproduction. Glycobiology 1997, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Matsui, T.; Takematsu, H.; Kozutsumi, Y.; Kawasaki, T.; Oka, S. Physical and functional association of glucuronyltransferases and sulfotransferase involved in HNK-1 biosynthesis. J. Biol. Chem. 2006, 281, 13644–13651. [Google Scholar] [CrossRef]
- Takamatsu, S.; Korekane, H.; Ohtsubo, K.; Oguri, S.; Park, J.Y.; Matsumoto, A.; Taniguchi, N. N-acetylglucosaminyltransferase (GnT) assays using fluorescent oligosaccharide acceptor substrates: GnT-III, IV, V, and IX (GnT-Vb). Methods Mol. Biol. 2013, 1022, 283–298. [Google Scholar]
- Kuhn, B.; Benz, J.; Greif, M.; Engel, A.M.; Sobek, H.; Rudolph, M.G. The structure of human alpha-2,6-sialyltransferase reveals the binding mode of complex glycans. Acta Crystallogr. D Biol. Crystallogr. 2013, 69 Pt 9, 1826–1838. [Google Scholar] [CrossRef]
- Li, P.; Song, L.F.; Merz, K.M., Jr. Parameterization of highly charged metal ions using the 12-6-4 LJ-type nonbonded model in explicit water. J. Phys. Chem. B 2015, 119, 883–895. [Google Scholar] [CrossRef]
- Nagae, M.; Mishra, S.K.; Neyazaki, M.; Oi, R.; Ikeda, A.; Matsugaki, N.; Akashi, S.; Manya, H.; Mizuno, M.; Yagi, H.; et al. 3D structural analysis of protein O-mannosyl kinase, POMK, a causative gene product of dystroglycanopathy. Genes Cells 2017, 22, 348–359. [Google Scholar] [CrossRef]
- Nagae, M.; Mishra, S.K.; Hanashima, S.; Tateno, H.; Yamaguchi, Y. Distinct roles for each N-glycan branch interacting with mannose-binding type Jacalin-related lectins Orysata and Calsepa. Glycobiology 2017, 27, 1120–1133. [Google Scholar] [CrossRef]
- Mishra, S.K.; Koca, J. Assessing the Performance of MM/PBSA, MM/GBSA, and QM-MM/GBSA Approaches on Protein/Carbohydrate Complexes: Effect of Implicit Solvent Models, QM Methods, and Entropic Contributions. J. Phys. Chem. B 2018, 122, 8113–8121. [Google Scholar] [CrossRef]
- Murugan, N.A.; Nordberg, A.; Agren, H. Different Positron Emission Tomography Tau Tracers Bind to Multiple Binding Sites on the Tau Fibril: Insight from Computational Modeling. ACS Chem. Neurosci. 2018, 9, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawade, H.; Morise, J.; Mishra, S.K.; Tsujioka, S.; Oka, S.; Kizuka, Y. Tissue-Specific Regulation of HNK-1 Biosynthesis by Bisecting GlcNAc. Molecules 2021, 26, 5176. https://doi.org/10.3390/molecules26175176
Kawade H, Morise J, Mishra SK, Tsujioka S, Oka S, Kizuka Y. Tissue-Specific Regulation of HNK-1 Biosynthesis by Bisecting GlcNAc. Molecules. 2021; 26(17):5176. https://doi.org/10.3390/molecules26175176
Chicago/Turabian StyleKawade, Haruka, Jyoji Morise, Sushil K. Mishra, Shuta Tsujioka, Shogo Oka, and Yasuhiko Kizuka. 2021. "Tissue-Specific Regulation of HNK-1 Biosynthesis by Bisecting GlcNAc" Molecules 26, no. 17: 5176. https://doi.org/10.3390/molecules26175176
APA StyleKawade, H., Morise, J., Mishra, S. K., Tsujioka, S., Oka, S., & Kizuka, Y. (2021). Tissue-Specific Regulation of HNK-1 Biosynthesis by Bisecting GlcNAc. Molecules, 26(17), 5176. https://doi.org/10.3390/molecules26175176





