An All-Solid-State Coaxial Structural Battery Using Sodium-Based Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Cells
2.2. Electrochemical Analysis of the Cells
2.3. Mechanical Test and Cell Post-Mortem Electrochemical Analysis
3. Results and Discussion
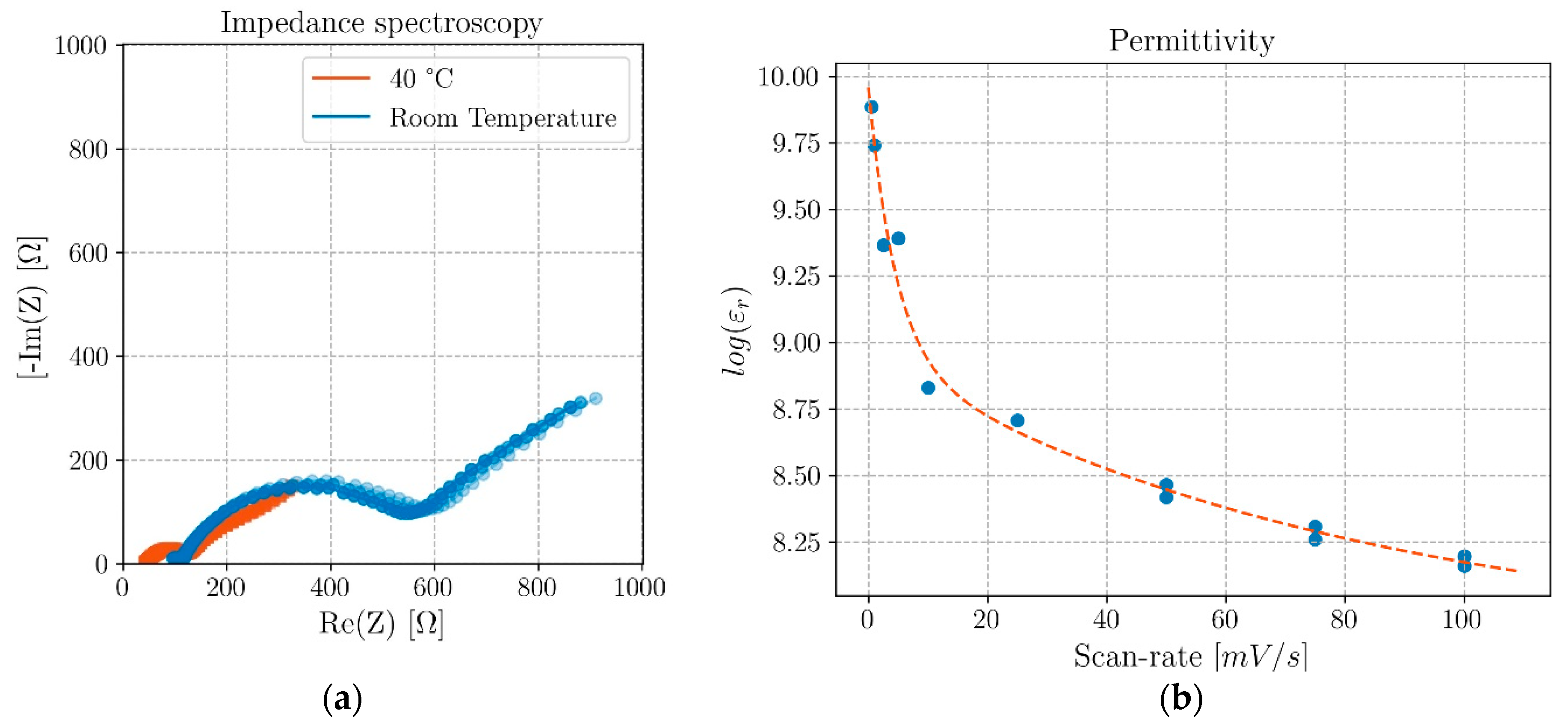
3.1. Electrochemical Test Results
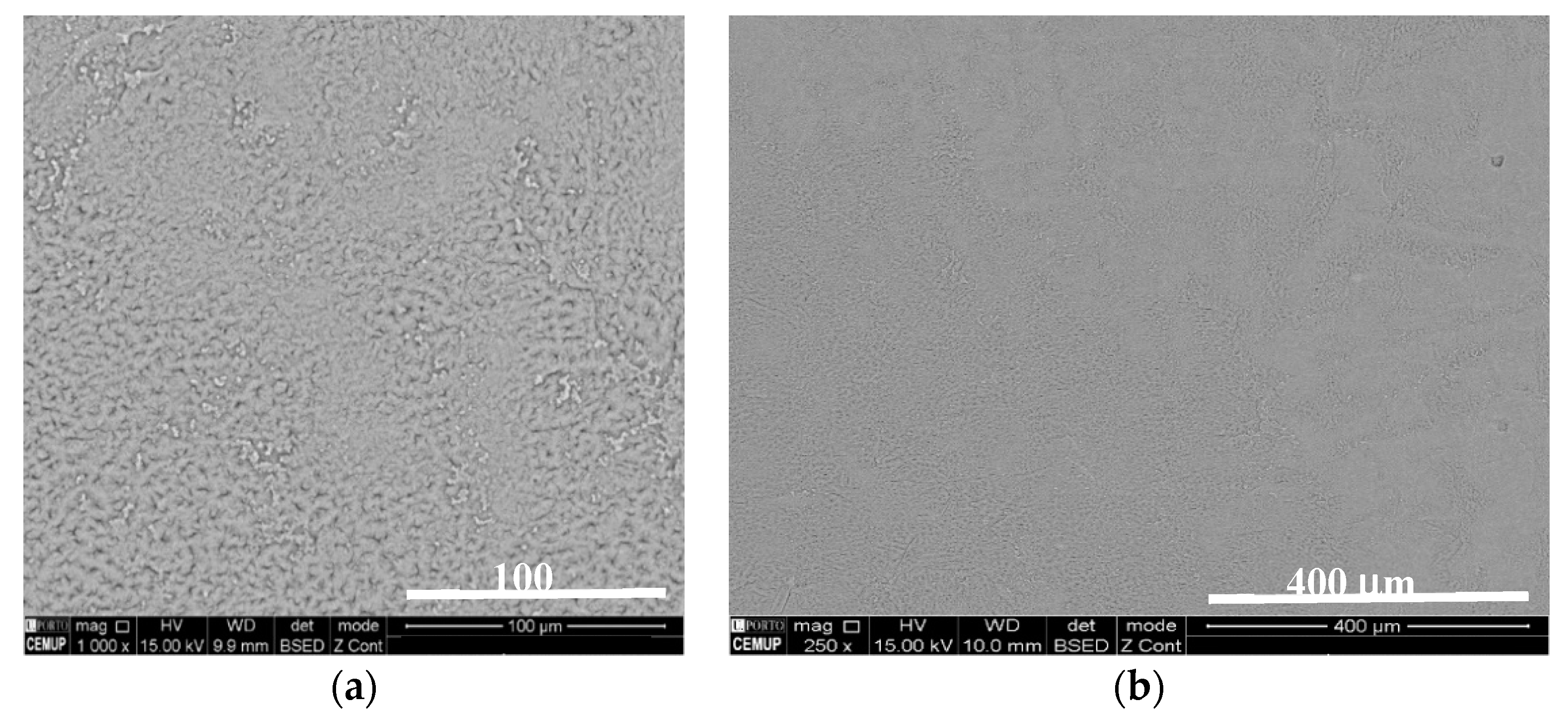
3.2. Mechanical Test Results and Post-Mortem Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef]
- Ferreira, A.D.B.; Nóvoa, P.R.; Marques, A.T. Multifunctional Material Systems: A state-of-the-art review. Compos. Struct. 2016, 151, 3–35. [Google Scholar] [CrossRef]
- Thomas, J.P.; Qidwai, M.A. Mechanical design and performance of composite multifunctional materials. Acta Mater. 2004, 52, 2155–2164. [Google Scholar] [CrossRef]
- Ekstedt, S.; Wysocki, M.; Asp, L.E. Structural batteries made from fibre reinforced composites. Plast. Rubber Compos. 2010, 39, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.C.; Aglietti, G.S. Structural performance of a multifunctional spacecraft structure based on plastic lithiumion batteries. Acta Astronaut. 2010, 67, 424–439. [Google Scholar] [CrossRef] [Green Version]
- Pereira, T.; Guo, Z.; Nieh, S.; Arias, J.; Hahn, H.T. Energy Storage Structural Composites: A Review. J. Compos. Mater. 2009, 43, 549–560. [Google Scholar] [CrossRef]
- Asp, L.E.; Greenhalgh, E.S. Structural power composites. Compos. Sci. Technol. 2014, 101, 41–61. [Google Scholar] [CrossRef]
- Asp, L.E.; Johansson, M.; Lindbergh, G.; Xu, J.; Zenkert, D. Structural battery composites: A review. Funct. Compos. Struct. 2019, 1, 042001. [Google Scholar] [CrossRef]
- Danzi, F.; Salgado, R.M.; Oliveira, J.E.; Arteiro, A.; Camanho, P.P.; Braga, M.H. Structural Batteries: A Review. Molecules 2021, 26, 2203. [Google Scholar] [CrossRef]
- Thomas, J.P.; Qidwai, M.A. The design and application of multifunctional structure-battery materials systems. J. Miner. Met. Mater. Soc. 2005, 57, 18–24. [Google Scholar] [CrossRef]
- Thomas, J.; Qidwai, S.; WR Pogue, I.; Pham, G. Multifunctional structure-battery composites for marine systems. J. Compos. Mater. 2013, 47, 5–26. [Google Scholar] [CrossRef]
- Galos, J.; Khatibi, A.A.; Mouritz, A.P. Vibration and acoustic properties of composites with embedded lithium-ion polymer batteries. Compos. Struct. 2019, 220, 677–686. [Google Scholar] [CrossRef]
- Galos, J.; Best, A.; Mouritz, A. Multifunctional sandwich composites containing embedded lithium-ion polymer batteries under bending loads. Mater. Des. 2020, 185, 108228. [Google Scholar] [CrossRef]
- Galos, J.; Fredriksson, C.; Das, R. Multifunctional sandwich panel design with lithium-ion polymer batteries. J. Sandw. Struct. Mater. 2020. [Google Scholar] [CrossRef]
- Ladpli, P.; Nardari, R.; Kopsaftopoulos, F.; Chang, F.K. Multifunctional energy storage composite structures with embedded lithium-ion batteries. J. Power Sources 2019, 414, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Kjell, M.H.; Jacques, E.; Zenkert, D.; Behm, M.; Lindbergh, G. PAN-Based Carbon Fiber Negative Electrodes for Structural Lithiumion Batteries. J. Electrochem. Soc. 2011, 158, A1455. [Google Scholar] [CrossRef] [Green Version]
- Jacques, E.; Kjell, M.H.; Zenkert, D.; Lindbergh, G.; Behm, M.; Willgert, M. Impact of electrochemical cycling on the tensile properties of carbon fibres for structural lithium-ion composite batteries. Compos. Sci. Technol. 2012, 72, 792–798. [Google Scholar] [CrossRef] [Green Version]
- Jacques, E.; Kjell, M.H.; Zenkert, D.; Lindbergh, G. The effect of lithium-intercalation on the mechanical properties of carbon fibres. Carbon 2014, 68, 725–733. [Google Scholar] [CrossRef]
- Hagberg, J.; Leijonmarck, S.; Lindbergh, G. High Precision Coulometry of Commercial PAN-Based Carbon Fibers as Electrodes in Structural Batteries. J. Electrochem. Soc. 2016, 163, A1790–A1797. [Google Scholar] [CrossRef]
- Ihrner, N.; Johannisson, W.; Sieland, F.; Zenkert, D.; Johansson, M. Structural lithium ion battery electrolytes via reaction induced phase-separation. J. Mater. Chem. A 2017, 5, 25652–25659. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.M.; Ihrner, N.; Zenkert, D.; Johansson, M. Bicontinuous Electrolytes via Thermally Initiated Polymerization for Structural Lithium Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 4362–4369. [Google Scholar] [CrossRef] [Green Version]
- Asp, L.E.; Bouton, K.; Carlstedt, D.; Duan, S.; Harnden, R.; Johannisson, W.; Johansen, M.; Johansson, M.K.G.; Lindbergh, G.; Liu, F.; et al. A structural battery and its multifunctional performance. Adv. Energy Sustain. Res. 2021. [Google Scholar] [CrossRef]
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon fiber reinforced structural lithium-ion battery composite: Multifunctional power integration for CubeSats. Energy Storage Mater. 2020, 24, 676–681. [Google Scholar] [CrossRef]
- Moyer, K.; Boucherbil, N.A.; Zohair, M.; Eaves-Rathert, J.; Pint, C.L. Polymer reinforced carbon fiber interfaces for high energy density structural lithium-ion batteries. Sustain. Energy Fuels 2020, 4, 2661–2668. [Google Scholar] [CrossRef]
- Pyo, J.; Park, H.W.; Jang, M.S.; Choi, J.S.; Sathish Kumar, S.K.; Kim, C.G. Tubular laminated composite structural battery. Compos. Sci. Technol. 2021, 208, 108646. [Google Scholar] [CrossRef]
- Braga, M.H.; Ferreira, J.A.; Stockhausen, V.; Oliveira, J.E.; El-Azab, A. Novel Li3ClO based glasses with superionic properties for lithium batteries. J. Mater. Chem. A 2014, 2, 5470–5480. [Google Scholar] [CrossRef] [Green Version]
- Braga, M.H.; Oliveira, J.E.; Kai, T.; Murchison, A.J.; Bard, A.J.; Goodenough, J.B. Extraordinary dielectric properties at heterojunctions of amorphous ferroelectrics. J. Am. Chem. Soc. 2018, 140, 17968–17976. [Google Scholar] [CrossRef]
- North Thin Ply Technology: Product Datasheet-ThinPreg 736LT/CF. NTPT Data Sheet 2021. Available online: https://www.thinplytechnology.com/pl/datasheets (accessed on 27 August 2021).
- Braga, M.H.; Murchison, A.J.; Ferreira, J.A.; Singh, P.; Goodenough, J.B. Glass-amorphous alkali-ion solid electrolytes and their performance in symmetrical cells. Energy Environ. Sci. 2016, 9, 948–954. [Google Scholar] [CrossRef]
- Braga, M.H.; Oliveira, J.E.; Murchison, A.J.; Goodenough, J.B. Performance of a ferroelectric glass electrolyte in a self-charging electrochemical cell with negative capacitance and resistance. Appl. Phys. Rev. 2020, 7, 011406. [Google Scholar] [CrossRef]
- Braga, M.H. Coherence in the Ferroelectric A3ClO (A = Li, Na) Family of Electrolytes. Materials 2021, 14, 2398. [Google Scholar] [CrossRef] [PubMed]
- Rivello, R. Theory and Analysis of Flight Structures, 1st ed.; McGraw-Hill: London, UK, 1969. [Google Scholar]
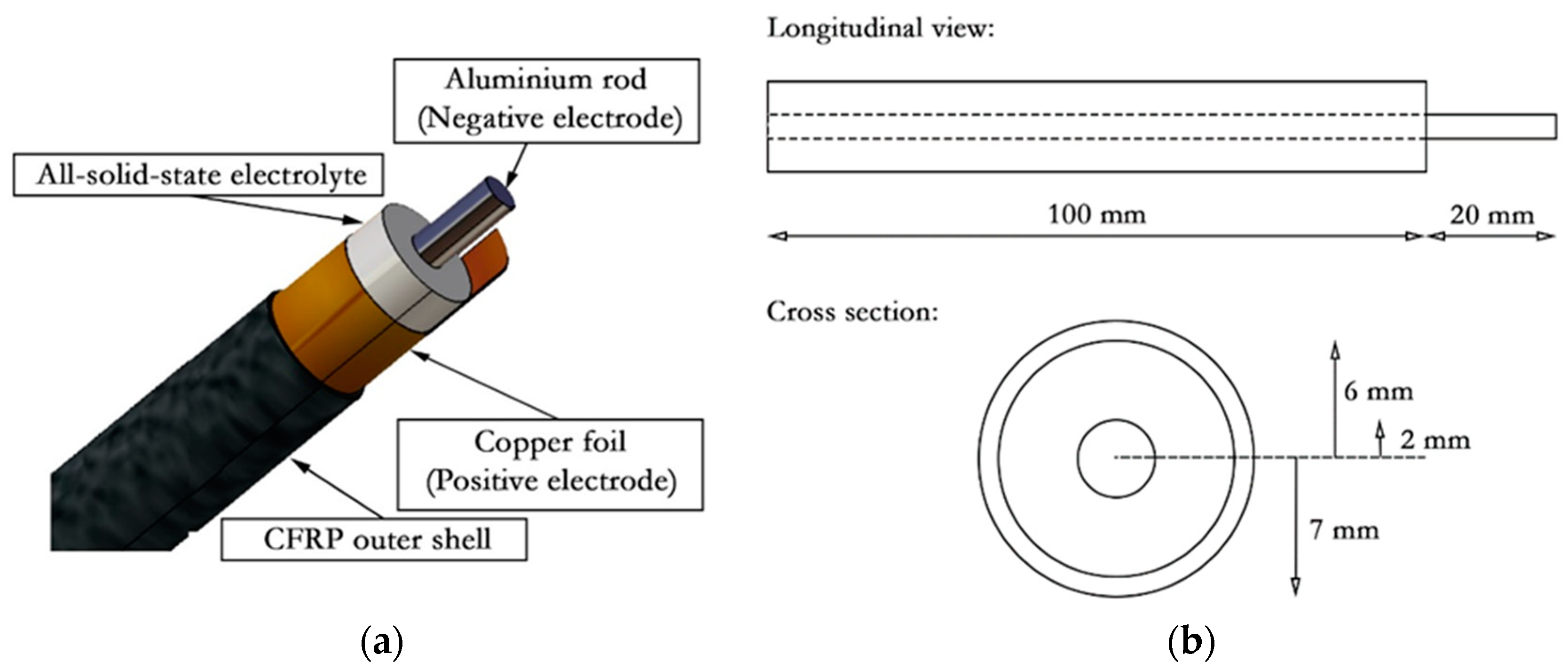

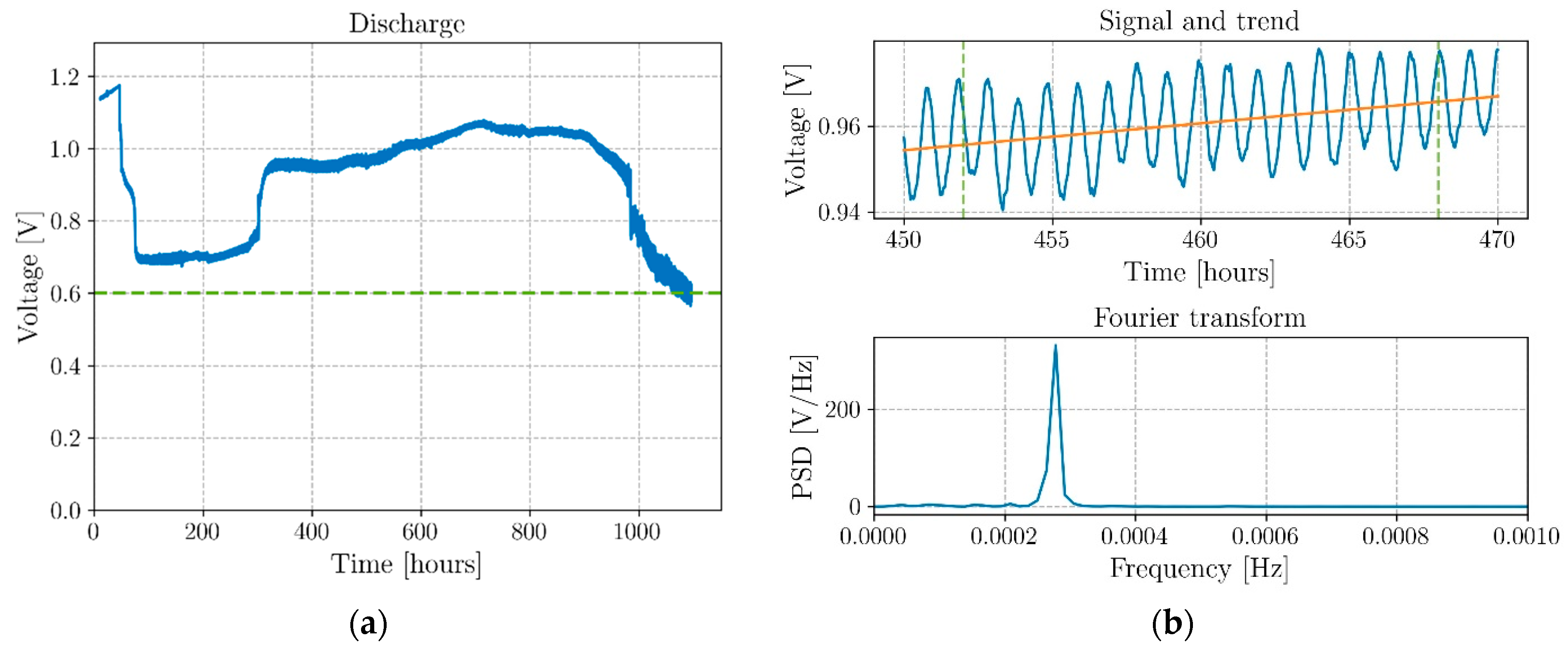
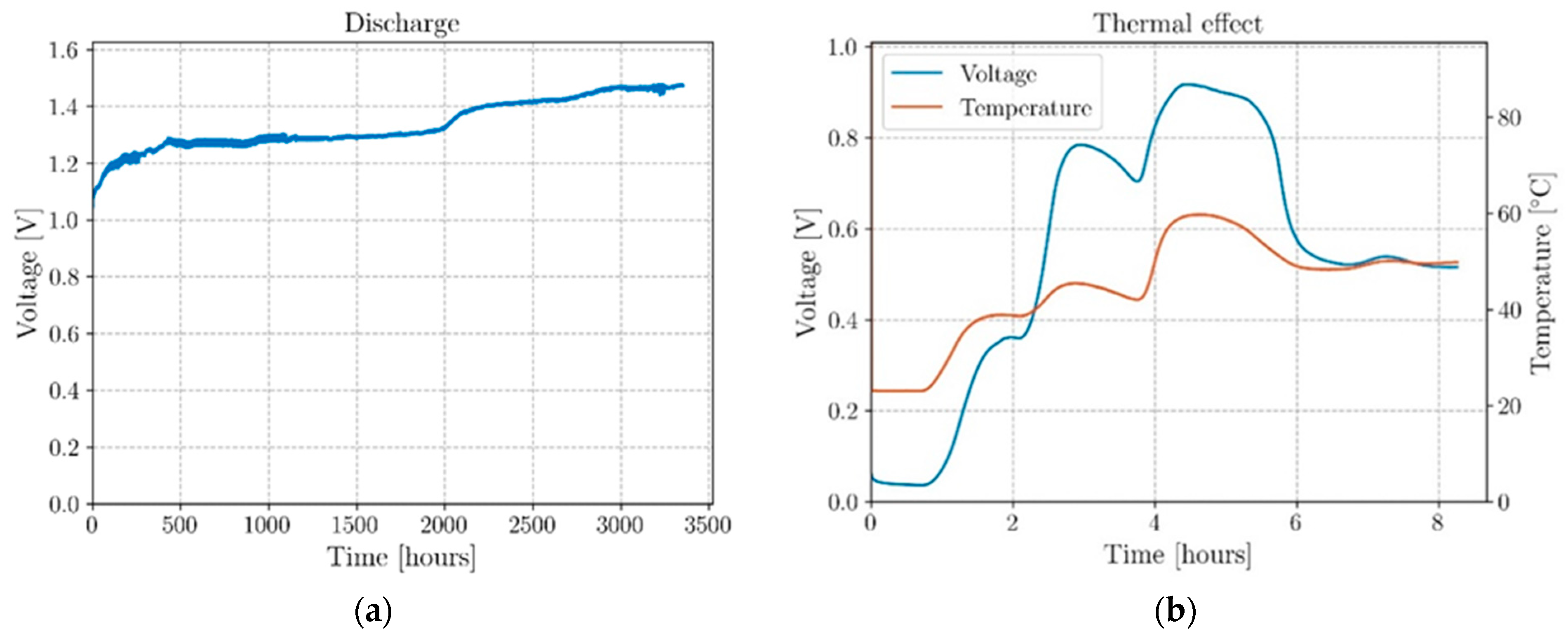


| Authors | Electrochemical Cell Components | Manufacturing | Specific Energy(Wh/kg) | Discharge Voltage (V) | |
|---|---|---|---|---|---|
| Ladpli et al. | [16] | NMC on Al foil (60/10 μm) Graphite on Cu foil (96/10 μm) LiPF6 in EC/DMC/DEC on trilayer polyolefin separator (PP/PE) (25 μm) Liquid electrolyte Not safe | CFRP shell with interlocking rivets with embedded customized cell | 123–139 (No rivets) | 3.7 |
| Asp et al. | [23] | Carbon fibers (65 μm) LiFePO4 on Al foil (50/30 μm) 0.4 M LiBoB/0.6 M LiTf on Whatman GF/A separator (185 μm) ionic liquid (Safe, low ionic conductivity) | Cell components stacking in a pouch bag Structural electrolyte addition Sealing and curing | 11.6 | 2.8 |
| Asp et al. | [23] | Carbon fibers (125 μm) LiFePO4 on Al foil (50/30 μm) 0.4 M LiBoB/0.6 M LiTf on glass fiber separator (70 μm) ionic liquid Safe, low ionic conductivity | Cell components stacking in a pouch bag Structural electrolyte addition Sealing and curing | 23.6 | 2.8 |
| Moyer et al. | [24] | LiFePO4 on carbon fiber Graphite on carbon fiber LiTFSI in EMIMBF4 on Whatman glass fiber separator ionic liquid Safe, low ionic conductivity | Battery layup in carbon fiber shells Epoxy resin impregnation and curing | 35 | N.A. |
| Moyer et al. | [25] | LiFePO4 on carbon fiber Graphite on carbon fiber LiPF6 in EC/DEC Celgard 2525 separator Liquid electrolyte Not safe | Battery layup in carbon fiber shells with PAN-coated interfaces Epoxy resin impregnation and curing | 52 (max. 58) | 2.5 |
| Pyo et al. | [26] | LiFePO4 on Al foil, Li4Ti5O12 on Al foil, LiPF6 in EC/DMC on Double-layer glass fabric 2116 separator Liquid electrolyte with LTO anode but still not fully safe | Tubular structure and battery components stacking and curing PDMS application and curing Electrolyte filling Final sealing | 3 (One tubular laminated composite battery) | 1.8 |
| Full Cell | Full Cell No CFRP | CFRP Shell | Positive Electrode (Cu) | CFRP + Cu | Na+-Electrolyte + PVac (80%/20%) | ||
|---|---|---|---|---|---|---|---|
| Mass | (g) | 21.6 | 15.2 | 6.4 | 0.83 | 7.22 | 10.5 |
| Specific capacity | (mAh/g) | 41.0 | 58.1 | - | 1067 | 123 | 82.2 |
| Energy density | (Wh/kg) | 38.0 | 75.8 | - | 989 | 114 | 78.1 |
| Specific energy | (Wh/L) | 56.2 | 76.3 | - | - | - | 83.9 |
| Cost | (€/kWh) | <1200 a | <10 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danzi, F.; Camanho, P.P.; Braga, M.H. An All-Solid-State Coaxial Structural Battery Using Sodium-Based Electrolyte. Molecules 2021, 26, 5226. https://doi.org/10.3390/molecules26175226
Danzi F, Camanho PP, Braga MH. An All-Solid-State Coaxial Structural Battery Using Sodium-Based Electrolyte. Molecules. 2021; 26(17):5226. https://doi.org/10.3390/molecules26175226
Chicago/Turabian StyleDanzi, Federico, Pedro Ponces Camanho, and Maria Helena Braga. 2021. "An All-Solid-State Coaxial Structural Battery Using Sodium-Based Electrolyte" Molecules 26, no. 17: 5226. https://doi.org/10.3390/molecules26175226
APA StyleDanzi, F., Camanho, P. P., & Braga, M. H. (2021). An All-Solid-State Coaxial Structural Battery Using Sodium-Based Electrolyte. Molecules, 26(17), 5226. https://doi.org/10.3390/molecules26175226







