Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions
Abstract
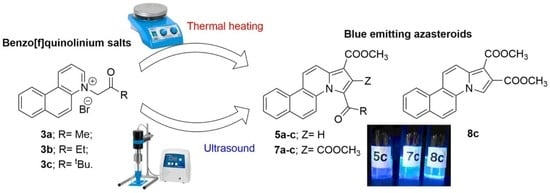
1. Introduction
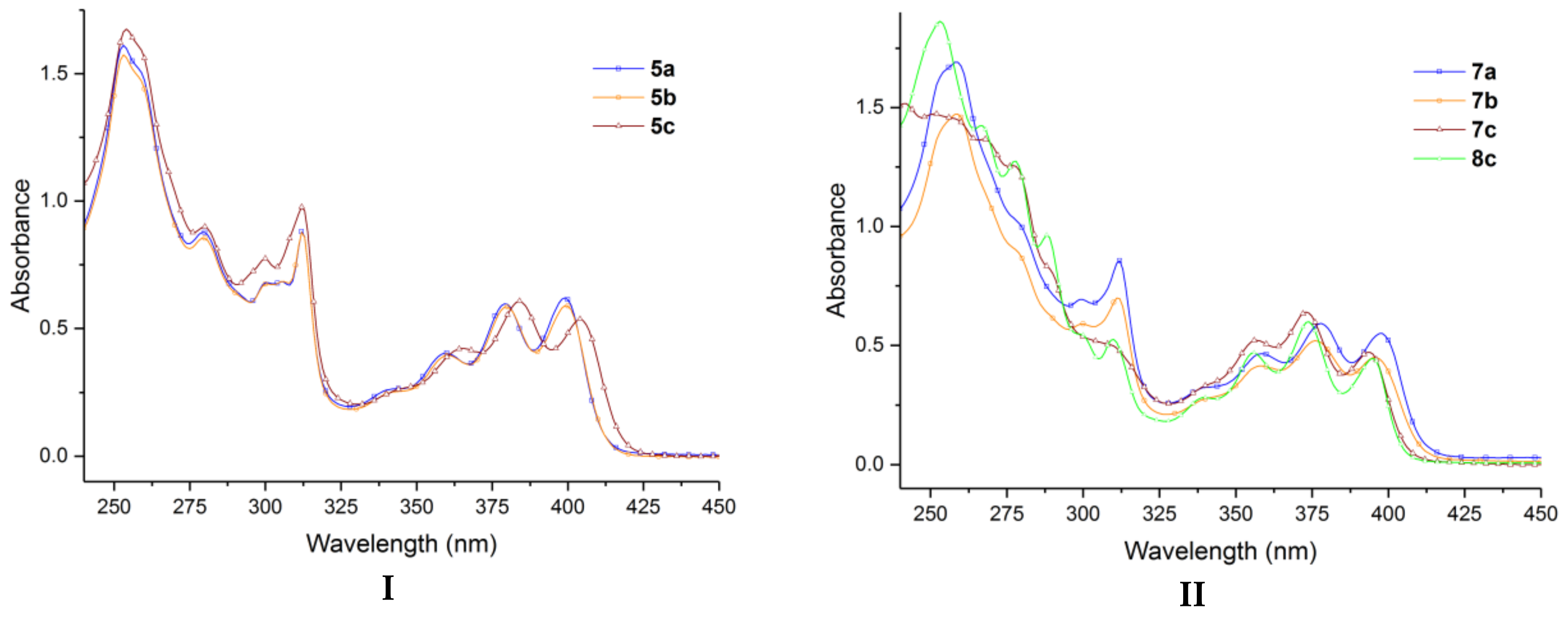
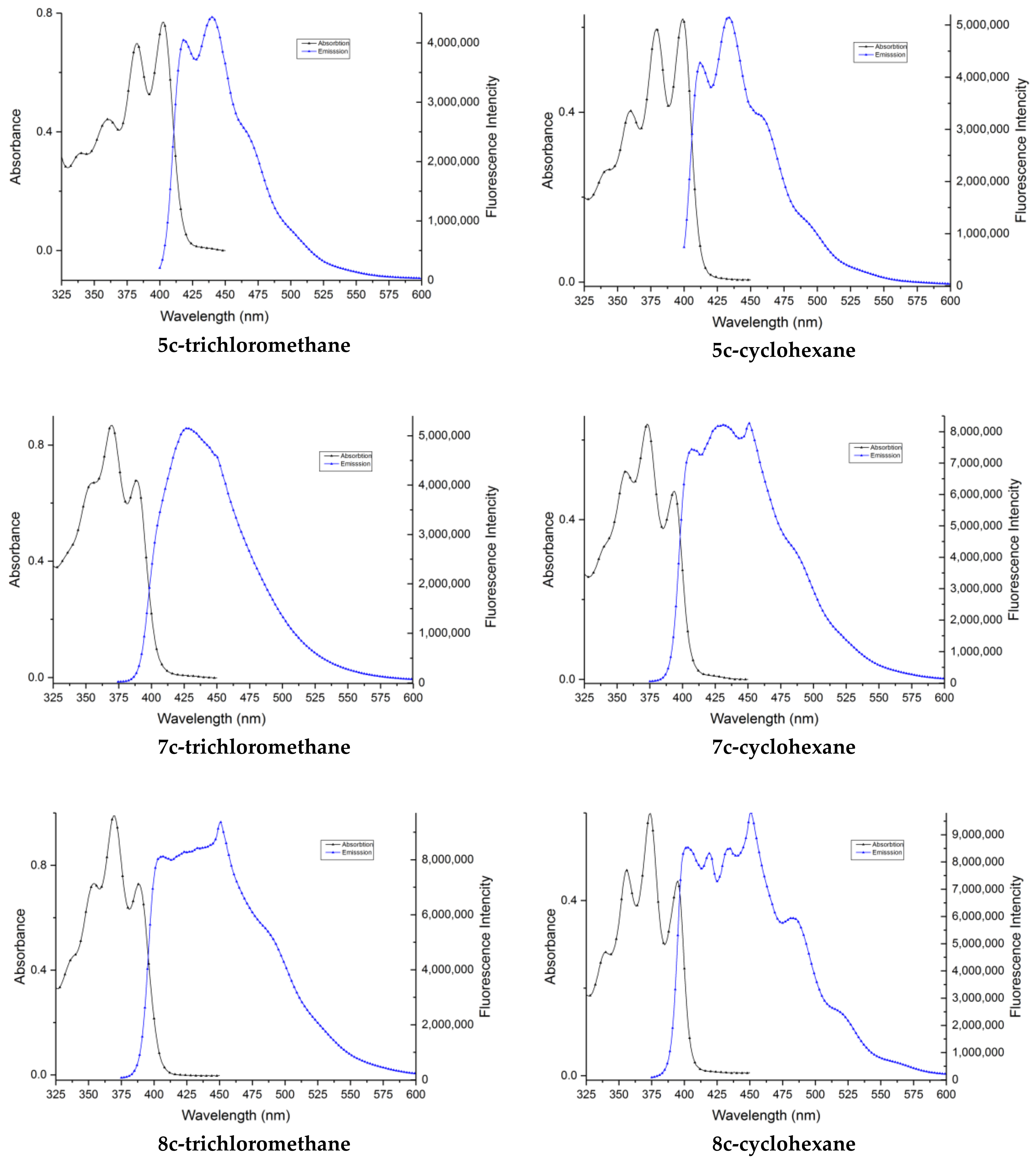
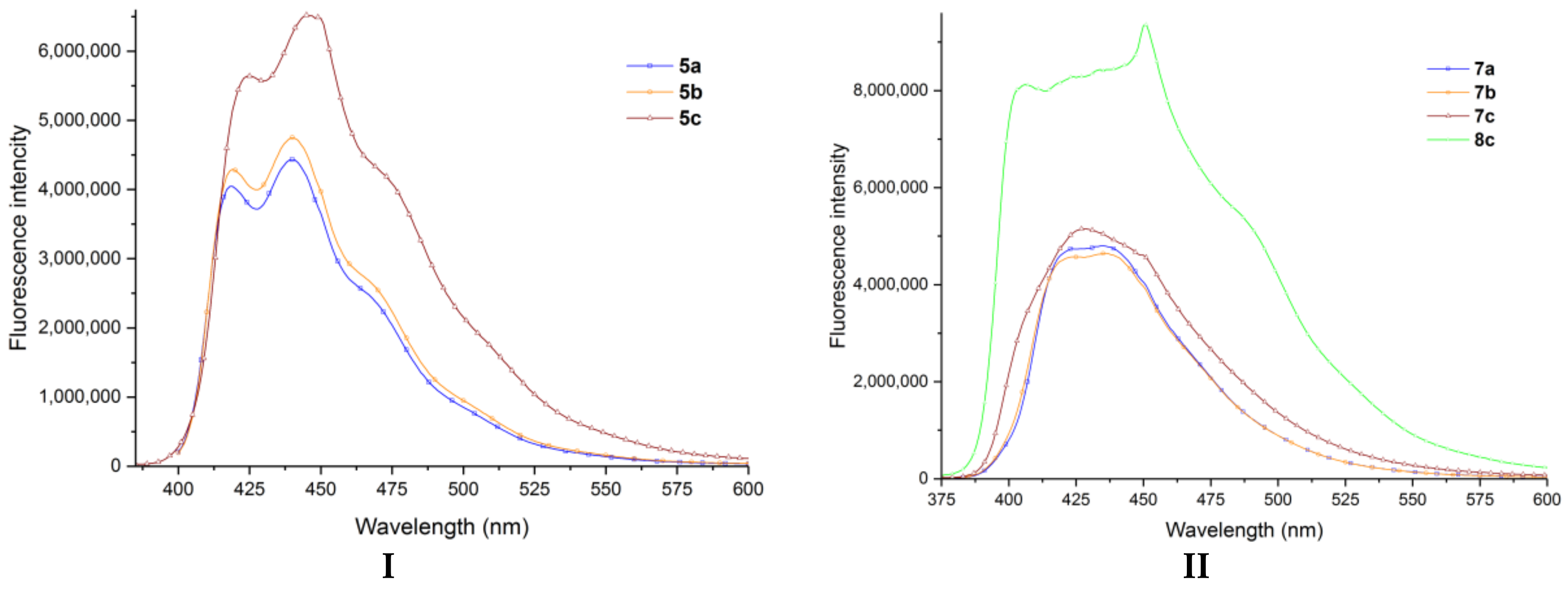
2. Results and Discussion
3. Experimental Section
3.1. General Procedure
3.1.1. General Procedure for Synthesis of Azasteroids Derivatives, 5a–b, 7a–b and 8c under Conventional TH Conditions
3.1.2. General Procedure for Synthesis of Azasteroids Derivatives, 5a–b, 7a–b and 8c under US Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, R.; Panda, G. An overview of synthetic approaches for heterocyclic steroids. Tetrahedron 2013, 69, 2853–2884. [Google Scholar] [CrossRef]
- Karnik, A.V.; Malviya, N.J.; Kulkarni, A.M.; Jadhav, B.L. Synthesis and in vitro antibacterial activity of novel heterocyclic derivatives of 18-nor-equilenin. Eur. J. Med. Chem. 2006, 41, 891–895. [Google Scholar] [CrossRef]
- Burbiel, J.; Bracher, F. Azasteroids as antifungals. Steroids 2003, 68, 587–594. [Google Scholar] [CrossRef]
- Robinson, A.J.; DeLucca, I.; Drummond, S.; Boswell, G.A. Steroidal nitrone inhibitors of 5α-reductase. Tetrahedron Lett. 2003, 44, 4801–4804. [Google Scholar] [CrossRef]
- Singh, J.; Singh, R.; Gupta, P.; Rai, S.; Ganesher, A.; Badrinarayan, P.; Sastry, G.N.; Konwar, R.; Panda, G. Targeting progesterone metabolism in breast cancer with L-proline derived new 14-azasteroids. Bioorg. Med. Chem. 2017, 25, 4452–4463. [Google Scholar] [CrossRef]
- Thomas, S.T.; Yang, X.; Sampson, N.S. Inhibition of the M. tuberculosis 3β-hydroxysteroid dehydrogenase by azasteroids. Bioorganic Med. Chem. Lett. 2011, 21, 2216–2219. [Google Scholar] [CrossRef]
- Bagnich, S.A.; Gulyakevich, O.V.; Mikhalchuk, A.L. Spectral-Luminescent properties of 12-oximino derivatives of 8-AZA-D-homogona-12,17a-diones and their concentration dependence. J. Fluoresc. 2008, 18, 277–283. [Google Scholar] [CrossRef]
- Borisevich, N.A.; Raichyonok, T.F.; Sukhodola, A.A.; Tolstorozhev, G.B. Delayed fluorescence and phosphorescence of 8-aza-d-homogonane in the gas and condensed phases. J. Fluoresc. 2006, 16, 649–653. [Google Scholar] [CrossRef]
- Akhrem, A.A.; Borisevich, N.A.; Gulyakevich, O.V.; Mikhalchuk, A.L.; Raichyonok, T.F.; Tikhomirov, S.A.; Tolstorozhev, G.B. Specific fluorescence properties and picosecond transient absorption of 8-azasteroids. J. Fluoresc. 1999, 9, 357–361. [Google Scholar]
- Li, Z.; Askim, J.R.; Suslick, K.S. The optoelectronic nose: Colorimetric and fluorometric sensor arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Queralto, N.; Berliner, A.N.; Goldsmith, B.; Martino, R.; Rhodes, P.; Lim, S.H. Detecting cancer by breath volatile organic compound analysis: A review of array-based sensors. J. Breath Res. 2014, 8, 027112. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Amariucai-Mantu, D.; Mangalagiu, V.; Antoci, V.; Maftei, D.; Mangalagiu, I.I.; Zbancioc, G. Microwave assisted reactions of fluorescent pyrrolodiazine building blocks. Molecules 2019, 24, 3760. [Google Scholar] [CrossRef]
- Tisler, M. Structure and reactivity correlation of bicyclic 10-π electron systems with bridgehead nitrogen. Pure Appl. Chem. 1980, 52, 1611–1621. [Google Scholar] [CrossRef][Green Version]
- Maftei, D.; Zbancioc, G.; Humelnicu, I.; Mangalagiu, I. Conformational effects on the lowest excited states of benzoyl-pyrrolopyridazine: Insights from PCM time-dependent DFT. J. Phys. Chem. A 2013, 117, 3165–3175. [Google Scholar] [CrossRef]
- Zbancioc, G.; Mangalagiu, I.I. Pyrrolopyridazine derivatives as blue organic luminophores: Synthesis and properties. Part 2. Tetrahedron 2010, 66, 278–282. [Google Scholar] [CrossRef]
- Zbancioc, G.; Huhn, T.; Groth, U.; Deleanu, C.; Mangalagiu, I.I. Pyrrolodiazine derivatives as blue organic luminophores: Synthesis and properties. Part 3. Tetrahedron 2010, 66, 4298–4306. [Google Scholar] [CrossRef][Green Version]
- Shingare, M.S.; Shingate, B.B. Ultrasound in synthetic applications and organic chemistry. In Handbook on Applications of Ultrasound: Sonochemistry for Sustainability, 1st ed.; Chen, D., Sharma, S.K., Mudhoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439842065. [Google Scholar]
- Mason, T.J.; Peters, D. Practical Sonochemistry. Power Ultrasound Uses and Applications, 2nd ed.; Ellis Horwood: Chichester, UK, 2002; ISBN 9781898563839. [Google Scholar]
- Bejan, V.; Moldoveanu, C.; Mangalagiu, I.I. Ultrasounds assisted reactions of steroid analogous of anticipated biological activities. Ultrason. Sonochem. 2009, 16, 312–315. [Google Scholar] [CrossRef]
- Zbancioc, G.; Zbancioc, A.M.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of dihydroxyacetophenone derivatives with or without 1,2-diazine skeleton. Ultrason. Sonochem. 2014, 21, 802–811. [Google Scholar] [CrossRef]
- Zbancioc, G.; Moldoveanu, C.; Mangalagiu, I.I. Ultrasound assisted synthesis of imidazolium salts: An efficient way to ionic liquids. Ultrason. Sonochem. 2015, 23, 376–384. [Google Scholar] [CrossRef]
- Antoci, V.; Moldoveanu, C.; Danac, R.; Mangalagiu, V.; Zbancioc, G. Huisgen 3+2 dipolar cycloadditions of phthalazinium ylides to activated symmetric and non-symmetric alkynes. Molecules 2020, 25, 4416. [Google Scholar] [CrossRef]
- Mantu, D.; Moldoveanu, C.; Nicolescu, A.; Deleanu, C.; Mangalagiu, I.I. A facile synthesis of pyridazinone derivatives under ultrasonic irradiation. Ultrason. Sonochem. 2009, 16, 312–315. [Google Scholar] [CrossRef]
- Borsoi, A.F.; Paz, J.D.; Pissinate, K.; Rambo, R.S.; Pestana, V.Z.; Bizarro, C.V.; Basso, L.A.; Machado, P. Ultrasound-Assisted synthesis of 4-alkoxy-2-methylquinolines: An efficient method toward antitubercular drug candidates. Molecules 2021, 26, 1215. [Google Scholar] [CrossRef]
- Banik, B.K.; Sahoo, B.M.; Kumar, B.V.V.R.; Panda, K.C.; Jena, J.; Mahapatra, M.K.; Borah, P. Green synthetic approach: An efficient eco-friendly tool for synthesis of biologically active oxadiazole derivatives. Molecules 2021, 26, 1163. [Google Scholar] [CrossRef]
- Rodríguez-Villar, K.; Yépez-Mulia, L.; Cortés-Gines, M.; Aguilera-Perdomo, J.D.; Quintana-Salazar, E.A.; Olascoaga Del Angel, K.S.; Cortés-Benítez, F.; Palacios-Espinosa, J.F.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis, antiprotozoal activity and cheminformatic analysis of 2-phenyl-2H-indazole derivatives. Molecules 2021, 26, 2145. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK; New York, NY, USA, 1988. [Google Scholar]
- Moldoveanu, C.; Zbancioc, G.; Mantu, D.; Maftei, D.; Mangalagiu, I.I. The cycloaddition of the benzimidazolium ylides with alkynes: New mechanistic insights. PLoS ONE 2016, 11, e0156129. [Google Scholar] [CrossRef][Green Version]
- Zbancioc, A.M.; Miron, A.; Tuchilus, C.; Rotinberg, P.; Mihai, C.T.; Mangalagiu, I.I.; Zbancioc, G. Synthesis and in vitro analysis of novel dihydroxyacetophenone derivatives with antimicrobial and antitumor activities. Med. Chem. 2014, 10, 476–483. [Google Scholar] [CrossRef]
- Antoci, V.; Mantu, D.; Cozma, D.G.; Usru, C.; Mangalagiu, I.I. Hybrid anticancer 1,2-diazine derivatives with multiple mechanism of action. Part 3. Med. Hypotheses 2014, 82, 11–15. [Google Scholar] [CrossRef]
- Lungu, C.N.; Bratanovici, B.I.; Grigore, M.M.; Antoci, V.; Mangalagiu, I.I. Hybrid Imidazole-Pyridne Derivatives: An Approach to Nevel Anticancer DNA Intercalators. Curr. Med. Chem. 2020, 27, 154–169. [Google Scholar] [CrossRef]
- Antoci, V.; Cucu, D.; Zbancioc, G.; Moldoveanu, C.; Mangalagiu, V.; Amariucai-Mantu, D.; Aricu, A.; Mangalagiu, I.I. Bis-(imidazole/benzimidazole)-pyridine derivatives: Synthesis, structure and antimycobacterial activity. Future Med. Chem. 2020, 12, 207–222. [Google Scholar] [CrossRef]
- Antoci, V.; Oniciuc, L.; Amariucai-Mantu, D.; Moldoveanu, C.; Mangalagiu, V.; Amarandei, A.M.; Lungu, C.N.; Dunca, S.; Mangalagiu, I.I.; Zbancioc, G. Benzoquinoline derivatives: A straightforward and efficient route to antibacterial and antifungal agents. Pharmaceuticals 2021, 14, 335. [Google Scholar] [CrossRef]




| Compound Data Availability Statement | Ultrasounds | Conventional TH | |||
|---|---|---|---|---|---|
| Reaction Time, (h) | Yield, % | Reaction Time, (h) | Yield, % | ||
| 5a | 2 | 80 | 48 | 65 | |
| 5b | 81 | 69 | |||
| 5c | 78 | 64 | |||
| 7a | 71 | 54 | |||
| 7b | 69 | 54 | |||
| 7c | 65 | 38 | |||
| 8c | 5 | 13 | |||
| Compound | Fluorescence (λmax, nm) | Absorption (λmax, nm) | ||
|---|---|---|---|---|
| Cyclohexane | Trichloromethane | Cyclohexane | Trichloromethane | |
| 5a | 434 | 439 | 399 | 403 |
| 5b | 434 | 440 | 399 | 402 |
| 5c | 440 | 445 | 384 | 386 |
| 7a | 432 | 432 | 378 | 381 |
| 7b | 432 | 433 | 376 | 375 |
| 7c | 451 | 430 | 373 | 370 |
| 8c | 450 | 449 | 374 | 369 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldoveanu, C.; Mangalagiu, I.; Zbancioc, G. Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions. Molecules 2021, 26, 5098. https://doi.org/10.3390/molecules26165098
Moldoveanu C, Mangalagiu I, Zbancioc G. Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions. Molecules. 2021; 26(16):5098. https://doi.org/10.3390/molecules26165098
Chicago/Turabian StyleMoldoveanu, Costel, Ionel Mangalagiu, and Gheorghita Zbancioc. 2021. "Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions" Molecules 26, no. 16: 5098. https://doi.org/10.3390/molecules26165098
APA StyleMoldoveanu, C., Mangalagiu, I., & Zbancioc, G. (2021). Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions. Molecules, 26(16), 5098. https://doi.org/10.3390/molecules26165098